Abstract
Amodiaquine (AQ) is currently being used as a partner drug in combination with artesunate for treatment of uncomplicated malaria in most endemic countries of Africa. In the absence of molecular markers of artemisinin resistance, molecular markers of resistance to AQ may be useful for monitoring the development and spread of parasites resistance to Artesunate-Amodiaquine combination. This study was designed to assess the potential role of polymorphisms on pfcrt and pfmdr1 genes and parasite in vitro susceptibility for epidemiological surveillance of amodiaquine resistance in Plasmodium falciparum. The modified schizont inhibition assay was used to determine in vitro susceptibility profiles of 98 patients' isolates of Plasmodium falciparum to amodiaquine. Polymorphisms on parasites pfcrt and pfmdr1 genes were determined with nested PCR followed by sequencing.
The geometric mean (GM) of AQ 50% inhibitory concentration (IC-50) in the 97 P. falciparum isolates was 20.48nM (95% CI 16.53–25.36nM). Based on the cut-off value for AQ in vitro susceptibility, 87% (84) of the P. falciparum isolates were sensitive to AQ (GM IC-50= 16.32nM; 95%CI 13.3–20.04nM) while 13% were resistant to AQ in vitro (GM IC-50= 88.73nM; 95%CI 69.67–113.0nM). Molecular analysis showed presence of mutant CVIET pfcrt haplotype, mutant pfmdr1Tyr86 allele and the double mutant CVIET pfcrt haplotype+pfmdr1Tyr86 in 72%, 49% and 35% respectively. The GM IC-50 of isolates harboring the wild-type pfcrt CVMNK haplotype+ pfmdr1Asn86 allele (3.93nM; 95%CI 1.82–8.46) was significantly lower (p=0.001) than those isolates harboring the double mutant pfcrtCVIET haplotype+pfmdr1Tyr86 allele (50.40nM; 95%CI 40.17–63.24).
Results from this study suggest that polymorphisms in pfcrt and pfmdr1 genes are important for AQ resistance and therefore may be useful for epidemiological surveillance of P. falciparum resistance to AQ.
Keywords: Plasmodium falciparum, Amodiaquine, Resistance, mutation, pfcrt, pfmdr1
Introduction
The widespread of parasite resistance to chloroquine (CQ) and sulphadoxine-pyrimethamine (SP) has been associated with increasing childhood morbidity and mortality in sub Saharan Africa (Trape, 2001). Artemisinin based combination therapies (ACTs) are currently recommended for the treatment of uncomplicated falciparum malaria (WHO, 2001). Nearly all malaria endemic countries of the world have already changed their first line treatment to ACTs (WHO, 2010a). In Nigeria, one of the ACTs in use as first line treatment of uncomplicated falciparum is Artesunate-Amodiaquine (AS-AQ). The rationale behind the use of AS-AQ is the rapid reduction of parasite biomass and fever in patients by AS and as well reduces chances of development of amodiaquine (AQ) resistance in the parasites (WHO, 2001). However there are concerns that parasites from this area might have quickly develop resistance to AQ due to the high level of CQ resistance (Happi et al., 2006a).
Very little is known about the mechanism or epidemiology of AQ resistance since its use as a therapeutic antimalarial drug (Meshnick and Alker, 2005). In vitro studies have found correlation between resistance to CQ and AQ, since the two drugs are chemically related (Ochong et al., 2003). Several in vitro (Childs et al., 1989; Basco and Le Bras, 1993) and clinical (Bloland and Ruebush, 1996; White, 1996; Sowunmi et al., 2001; Schellenberg et al., 2002) reports have shown cross-resistance between CQ and AQ or its active metabolite, desethylamodiaquine. A tool for monitoring emergence and spread of AQ resistance is required, as its increasing use will likely increase reduced susceptibility to this drug. This tool would be useful for monitoring the development and spread of parasites resistance to AS-AQ since molecular markers of artemisinin resistance are yet to be discovered.
The molecular mechanisms of cross-resistance between CQ and AQ are yet to be addressed. However, it has been argued that because CQ and AQ are structural analogs with likely common mode of action, they are also likely to have a common mechanism of resistance (Bray et al., 1998; Ginsburg et al., 1998). Some apparent cross-resistance suggest that molecular markers linked to CQ resistance might be useful for monitoring AQ resistance (Ochong et al., 2003). Two P. falciparum genes namely Plasmodium falciparum multidrug resistance 1 (pfmdr1) gene which encodes the Pgh1 protein and Plasmodium falciparum chloroquine resistance transporter (pfcrt) gene which encodes parasite trans-membrane protein Pfcrt, have been reported to play important roles in CQ resistance. Point mutations in these two genes have been associated with in vitro CQ resistance in laboratory parasite clones or field isolates (Duraisingh et al., 1997; Fidock et al., 2000; Reed et al., 2000; Durand et al., 2001; Thomas et al., 2002; Lim et al., 2003; Folarin et al., 2008) and in vivo in most malaria endemic countries (Adagu et al., 1996; Djimde et al., 2001; Dorsey et al., 2001; Maguire et al., 2001; Tinto et al., 2005; Happi et al., 2006b).
In vitro tests of parasite drug sensitivity can serve as an early warning system for the emergence of drug resistance because of its relative simplicity and low cost when compared with in vivo assessment (WHO 2005). This study investigates the association between in vitro AQ resistance in P. falciparum and polymorphisms on CQ resistance markers (pfcrt and pfmdr1 genes) in isolates obtained from children with falciparum malaria in Nigeria.
1. Material and methods
2.1. Study site
The study was carried out at the Malaria Research Laboratory clinic, College of Medicine, University of Ibadan, Nigeria. Malaria in Ibadan is hyperendemic, where transmission occurs all year round but is more intense during the rainy season from April to October (Happi et al., 2006b).
2.2. Patients Selection, and Sample Collection
Children aged 6 months to 12 years with microscopic confirmation of P. falciparum malaria infections were enrolled after clinical examination in a large clinical efficacy study. Informed consent for participation in the study was obtained from parents/guardians of children under the age of 10 years, while assent was obtained from each patient between the ages of 10–12 years. The Joint UI/UCH Institutional Review Committee (IRC) approved the study protocol.
Five milliliter (5ml) of venous blood was obtained from each child enrolled into the study for in vitro sensitivity to antimalarial drugs and cryopreservation. Finger pricked blood sample was obtained for thin and thick blood film to determine the parasite density. In addition, finger pricked blood sample was also spotted on 3MM Whatman filter paper for the molecular analysis. Thick and thin blood films prepared from finger prick were Giemsa-stained and examined by light microscopy under an oil immersion objective at X1000 magnification. Parasitemia in thick film was estimated by counting asexual parasites relative to 200 leukocytes. The parasite density was calculated assuming leukocyte count of 6000/μl of blood (Sowunmi et al, 1995; WHO, 2010b).
2.3. Determination of in-vitro Sensitivity of Patient Isolates to Amodiaquine
In vitro sensitivity of each patient isolate of P. falciparum to amodiaquine (AQ) was determined using a modification of the schizont inhibition assay (Folarin et al., 2008). Briefly, a template containing three-fold serial dilutions of the working solution of AQ (10750nM) was prepared in a 96-well microtiter plate. Wells in row H served as controls without drug. Test plates were derived from each template by transferring 25μl of the drug dilutions to each plate. Two hundred microliters (200μl) of 1ml-parasitized blood diluted in 19ml of culture medium (RPMI 1640+ HEPES and sodium bicarbonate) was transferred into each well of the plate. Plates containing parasites suspension with different concentrations of AQ were incubated at 37°C for 24 to 36 hours in a plexiglass chambers containing a gas mixture (5% O2, 5% CO2, 90% N2). The final concentration of AQ in wells A – G ranged from 1075nM to 1.5nM.
The assay was terminated when at least 60% of parasites in the control wells (Row H) were schizonts. Each well in a column of 96-well microtiter plate was harvested onto glass slides as thick smears, air dried and stained with Giemsa. Parasites development to schizont was determined by counting the number of schizonts against 200 white blood cells in each smear using X100 oil immersion objective of a light microscope. Concentration-response data were analyzed by a non-linear regression analysis. The 50% inhibitory concentrations (IC-50) of AQ on P. falciparum isolates were calculated and scatter plots were performed using GrahpPad® Prism version 4.0 for windows software (GraphPad® software, San Diego, LA, USA, www.graphpad.com).
P. falciparum isolates with AQ IC-50 ≤ 60nM was considered sensitive to AQ while IC-50 > 60nM was considered AQ resistant isolates (WHO 1997; Basco et al., 2002; Ferreira et al., 2007).
2.4. DNA extraction
Parasite genomic DNA was extracted from filter paper blood samples using saponin lysis with phenol-chloroform extraction method (Snounou et al., 1993). Briefly, 1X PBS with 0.05% saponin was added to small pieces of blood impregnated filter paper and incubated at 37°C for two hours. The Supernatant was discarded and lysis buffer (40mM of Tris-HCl pH 8.0, 80mM of EDTA, pH 8.0, 0.2% SDS) with 150μL Proteinase K was added to the pellet. The pellet in the lysis buffer was incubated at 37°C for two hours. After digestion, phenol-chloroform v/v was added to the sample and centrifuged for DNA purification. The supernatant was discarded and the pellet re-suspended in 50μL of milliQ-distilled water.
2.5. PCR detection of mutations on codons 72–76 and 86 of pfcrt and pfmdr1 genes in Plasmodium falciparum
Polymorphisms around codons 72–76 pfcrt haplotype and 86 of pfmdr1 genes were analyzed. Other loci on pfmdr1 gene were not analyzed because previous reports (Happi et al., 2006a; Happi et al., 2009) in the same study site have shown the rarity/absence of other pfmdr1 polymorphisms in isolates from Ibadan, Nigeria. Thus, region of the parasite genomic DNA spanning codons 76 and 86 of pfcrt and pfmdr1 genes respectively were amplified by nested PCR according to the method of Djimde and others (Djimde et al., 2001) in SN5333 Master cycler (Eppendorf, Germany). The PCR reaction consisting of 1X PCR buffer (10mM Tris-HCl PH 9.0, 50mM KCl), 250uM dNTPs, 1.5mM MgCl2, 50pM each of forward and reverse primers and 2U of Taq polymerase was performed in final volume of 25μl. Two microliters (2μl) of the genomic DNA was used as template for the primary amplification while 1μl of the primary PCR product was used for the nest PCR. DNA from two laboratory adapted P. falciparum clones, 3D7 (Wild-type) and Dd2 (mutant) were used as negative and positive controls respectively for K76T polymorphism on pfcrt gene. The K1 and 7G8 laboratory strains of P. falciparum were used for negative and positive controls respectively for N86Y polymorphism on pfmdr1 gene.
2.6. Direct DNA sequence analysis
The nested PCR amplification products for each gene were purified using Wizard SV Gel and PCR Clean-Up System kit (Promega, UK) and sequenced directly on an ABI PRISM® 3100-Avant Genetic Analyzer with BigDye Terminator v3.1 Cycle Sequencing kit according to the manufacturer's instructions. The sequence products were analyzed for polymorphisms using 3D7 sequence obtained from PlasmoDB (www.Plasmodb.org) as control in BLAST analysis.
2.7. Data Analysis
For each isolate, the number of schizonts with three or more nuclei over a total of 200 WBC was counted. The IC-50 is defined as the drug concentration able to inhibit 50% of the parasite growth compared with drug free control wells. Data were expressed as the geometric mean IC-50 (GM IC-50) and the 95% confidence intervals were computed. Data were entered in SPSS 16.0. The proportions of isolates displaying AQ resistance were compared by the χ2 test for trend. The GM IC-50 for each of the genotype was compared for significant difference using the Epi Info 6.0. A value of p<0.05 was considered statistically significant.
The sequence analysis was performed using FinchTV (Geospiza) for sequence chromatogram visualization and the mutations were localized using Mutation Surveyor (Softgenetics). The sequence of 3D7 was used as reference and was obtained from PlasmoDB (http://plasmodb.org/plasmo/).
3. Results
3.1. In vitro susceptibility of P. falciparum isolates to Amodiaquine
In vitro susceptibility testing for AQ was successful in 97 of 107 (91%) of P falciparum isolates analyzed. The geometric mean of parasite density at presentation was 38650 parasites/ul blood (95%CI 32333–46204).
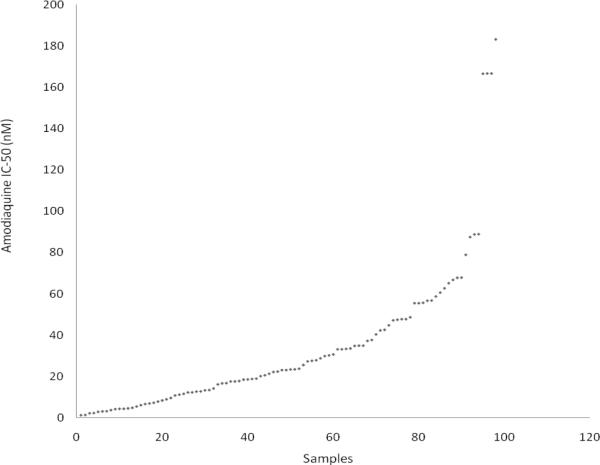
In vitro susceptibility testing of P. falciparum isolates showed a wide range of response to AQ with IC-50 ranging from 1.25nM to 183.20nM (Fig 1).
Fig 1.
Scatter plot of Amodiaquine IC-50 of Plasmodium falciparum isolates obtained children with uncomplicated malaria infection
Based on the IC-50 threshold for AQ in vitro resistance (WHO 1997; Basco et al., 2002; Ferreira et al., 2007), 87% (84 of 97) of the P. falciparum isolates had IC-50 < 60nM (GM IC-50=16.32nM; 95%CI 13.3–20.03nM) and were therefore considered AQ in vitro sensitive. Thirteen percent (13%) of the isolates analyzed showed IC-50 > 60nM (GM IC-50= 88.73nM; 95%CI 69.67–113nM) and were classified as AQ resistant.
3.2. Prevalence of pfcrt and pfmdr1 polymorphisms in P. falciparum isolates
Nested PCR followed by direct DNA sequencing was used to detect polymorphisms around codons 72–76 and 86 of pfcrt and pfmdr1 genes respectively. Of the 107 parasites genomic DNA sequenced for polymorphisms, 69 and 95 were successful for pfcrt and pfmdr1 genes respectively. Sequence analysis of pfcrt haplotype (codons 72–76) showed the presence of wild-type CVMNK pfcrt haplotype in 23% of the P. falciparum isolates analyzed. Mutant CVIET and CVMNT pfcrt haplotypes were present in 72% and 5% of the isolates respectively. Wild-type pfmdr1Asn86 allele was present in 51% (49/95) of the isolates, while the mutant pfmdr1Tyr86 allele was present in 49% of parasites analyzed.
The wild type CVMNK pfcrt haplotype+pfmdr1Asn86 allele was present in 12% (8/69) of the P. falciparum isolates while 35% harbored the mutant CVIET pfcrt haplotype+pfmdr1Tyr86. Four percent (4%) of the parasite isolates analyzed harbored the CVMNT pfcrt haplotype+pfmdr1Asn86 allele while only one (1) isolates harbored the CVMNT pfcrt haplotype+pfmdr1Tyr86
3.3. Association between the presence of pfcrt and pfmdr1 polymorphisms and reduced AQ in vitro susceptibility
The AQ GM IC-50 in 48 P. falciparum isolates harboring wild-type pfmdr1Asn86 allele was 11.97nM (95%CI 9.69–17.0nM). All of these isolates were sensitive to AQ in vitro. Mutant pfmdr1Tyr86 allele was present in 47 P. falciparum isolates and showed increased IC-50. Of these 47 isolates, 72% (34) were sensitive to AQ in vitro (GM IC=50 26.18nM; 95%CI 19.91–34.42nM), while 28% (13) were resistant to the drug (GM IC-50=88.73nM; 95%CI 69.67–113.0nM) [Fig 2a]. AQ resistant parasites harbouring the pfmdr1Tyr86 allele showed significantly higher IC-50 (p=0.0001) compared to sensitive ones (Table 1).
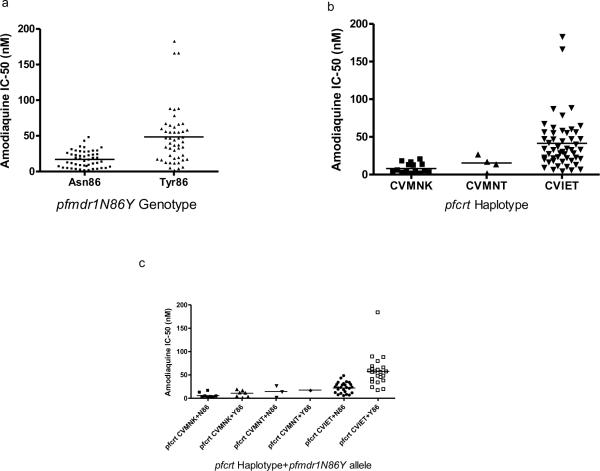
Fig 2.
Distribution of Amodiaquine IC-50 of P. falciparum isolates and the pfmdr1N86Y genotype (a), pfcrt haplotype (b) and pfcrt haplotype+pfmdr1N86Y genotype (c)
Table 1.
Geometric mean IC-50 of Amodiaquine sensitive and resistant P. falciparum isolates and pfcrt and pfmdr1 polymorphisms
| Gene/Allele/Haplotype | In vitro amodiaquine sensitive isolates (n) | In-vitro Amodiaquine resistant isolates (n) | p-value |
|---|---|---|---|
| Pfmdr1Asn86 | 11.97nM (48) | - | - |
| Pfmdr1Tyr86 | 26.18nM (34) | 88.73nM (13) | 0.0001 |
| C72-V73-M74-N75-K76 (CVMNK) | 5.65nM (15) | - | - |
| C72-V73-M74-N75-T76 (CVMNT) | 11.20nM (4) | - | - |
| C72-V73-I74-E75-T76 (CVIET) | 25.03nM (42) | 81.19nM (8) | 0.0001 |
| CVMNK+pfmdr1Asn86 | 3.93nM (8) | - | - |
| CVMNK+pfmdr1 Tyr86 | 8.56nM (7) | - | - |
| CVMNT+pfmdr1Asn86 | 9.63nM (3) | - | - |
| CVMNT+pfmdr1 Tyr86 | 17.6nM (1) | - | - |
| CVIET+pfmdr1Asn86 | 18.88nM (26) | - | - |
| CVIET+pfmdr1 Tyr86 | 39.55nM (16) | 81.19nM (8) | 0.0001 |
Lys–Lysine; Asn–Asparagine; 72–76–Aminoacid positions on pfcrt; CVMNK–Cysteine, Valine, Methionine, Asparagine, Lysine; CVIET – Cysteine, Valine, Isoleucine, Glutamate, Threonine.
Fifteen (15) isolates harbored the wild-type pfcrt CVMNK haplotype and were all sensitive to AQ in vitro (GM IC-50= 5.65nM; 95%CI 3.37–9.48nM). Mutant pfcrt CVMNT (GM IC-50=11.20nM; 95%CI 2.30–27.30nM) and CVIET (GM IC50=30.21nM; 95%CI 24.39–37.42nM) haplotypes were found in 4 and 50 isolates respectively. All the four isolates with the mutant pfcrt CVMNT were sensitive to AQ in vitro, while 16% (8/50) with CVIET haplotype were resistant to AQ (GM IC-50=81.19nM; 60.06–109.8nM) [Fig2b].
Correlation analysis carried out between the presence of double mutant CVIET pfcrt haplotype+pfmdr1Tyr86 allele and parasite susceptibility to AQ showed increased IC-50 (GM IC-50=50.40nM; 95%CI 40.17–63.24nM) in 24 isolates harboring these pfcrt and pfmdr1 mutations. Sixty six percent (16/24) of these double mutants (CVIET pfcrt + pfmdr1Tyr86) were sensitive to AQ in vitro (GM IC-50=39.55nM; 95% CI 31.98–48.92nM) while 34% (8/24) were resistant to AQ (GM IC-50=81.19nM; 95%CI 60.06–109.8nM) [Fig 2c]. Eight (8) P. falciparum isolates harbored the double wild-type CVMNK pfcrt haplotype+pfmdr1Asn86 allele and were highly sensitive to AQ in vitro (GM IC-50=3.93nM; 95%CI 1.82–8.46nM) [Fig 2c]. All isolates harboring either mutant CVIET pfcrt haplotype+wild-type pfmdr1Asn86 (26) or wild type CVMNK pfcrt haplotype+mutant pfmdr1Tyr86 (7) allele were sensitive to AQ (GM IC-50=18.88nM; 95%CI 14.68–24.30nM and GM IC-50= 8.56nM; 95%CI 4.00–18.31nM respectively) [Fig 2c]. However, there was a significant difference (p<0.005) between the GM IC-50 of isolates harboring the pfcrt wild-type CVMNK haplotype+pfmdr1Asn86 allele and those with mutant pfcrt CVIET haplotype+pfmdr1Tyr86 allele (Fig 2c). Three of the four isolates harboring the CVMNT pfcrt haplotype also harbored the wild-type pfmdr1Asn86 (GM IC-50=9.63nM), and one had the CVMNT pfcrt haplotype+mutant pfmdr1Tyr86 allele (IC-50= 17.60nM) [Fig2c].
4. Discussion
This study demonstrates increasing trend of in vitro resistance of Plasmodium falciparum to AQ and the association between parasites' in vitro reduced sensitivity to AQ and polymorphisms on pfcrt and pfmdr1 genes in a clinical setting of Southwest Nigeria.
As many countries have adopted the use of ACTs to increase treatment efficacy and to delay the emergence of drug resistant parasites, monitoring parasites' susceptibility to the individual components of the combinations is therefore necessary in order to provide evidence based signs of resistance to components of ACTs. This monitoring can be done either by in vitro susceptibility testing or molecular markers. In this study, we demonstrated that 13% of P. falciparum isolates collected from children were resistant to AQ with an IC-50 >60nM according to the threshold suggested by the WHO guideline (WHO, 1997). Although in vitro response cannot be directly extrapolated to predict in vivo response, a similar level of in vivo resistance (13%) to AQ was previously reported in an AQ clinical efficacy study from the same area (Happi et al., 2006a). Similar reports have been made in other malaria endemic areas of Africa (Holmgren et al., 2006). The high level of in vitro parasites resistance to AQ as shown in this study could be due to the extensive use of CQ and the selection of chloroquine resistant parasites in the study area over the past 50 years. Cross-resistance between CQ and AQ have been observed in other malaria endemic areas (Olliaro and Mussano, 2003) of Africa. Cross-resistance between chemically similar drugs may be a source of concern for the long-term viability of AQ as a component of ACTs, especially in areas where CQ has been used extensively and CQ resistance has been reported.
Assessing polymorphisms in potential candidate genes of drug resistance is of utmost relevance to establish their role in modulating drug responses and to predict epidemiological dynamics of resistance. A high prevalence of the mutant pfmdr1Tyr86 alleles and CVIET pfcrt haplotype were observed in the P. falciparum isolates analyzed. This is consistent with rates (60–100%) observed in previous study from the same area (Happi et al., 2006b) and other malaria endemic areas (Djimde et al., 2001; Johnson et al., 2004; Anderson et al., 2005; Holmgren et al., 2006; Nsobya et al., 2007). This high baseline prevalence of CQ resistance associated alleles are also in line with report from East and west Africa, where there has been long use of CQ (Sidhu et al., 2002) leading to selection of these alleles in the parasite population.
The GM IC-50 of P. falciparum isolates with mutant CVIET pfcrt haplotype was observed to be 5 fold higher than that observed in isolates with wild-type CVMNK pfcrt haplotype (Fig 2b). The significant difference (p<0.05) in the GM IC-50 suggests that mutations at condons 72–76 of pfcrt may be primarily important in reducing the susceptibility of Plasmodium falciparum to AQ. This is supported by the fact that all the 15 P. falciparum isolates with the CVMNK wild-type pfcrt haplotype are sensitive to AQ in vitro (Table 1). The presence of mutant CVIET pfcrt haplotype allele in P. falciparum isolates with significantly high IC-50 further confirmed its primary involvement parasites reduced susceptibility to AQ, while other SNPs on the same gene or other P. falciparum genes may be important in modulating the resistance of the parasite to AQ. The mutant CVIET haplotype has been reported to have emerged from Southeast Asia and spread to Africa (Anderson et al., 2005), and has also been suggested to confer some level of cross resistance to AQ/DEAQ in vitro (Warhurst, 2003). Molecular studies have shown that SVMNT haplotype is sufficient to confer resistance to AQ/DEAQ (Sidhu et al., 2002), however this allele is only detected in parasite population from South America, Papua New Giunea and only recently in Tanzania where AQ monotherapy has been widely used due to high sulphadoxine-pyrimethamine failure (Alifrangis et al., 2006). It can therefore be suggested that this haplotype might be selected by artesunate-amodiaquine combination in malaria endemic areas where this ACT is been used for treatment of acute uncomplicated malaria.
Our study showed the presence of the mutant CVMNT pfcrt haplotype in Nigerian isolates of Plasmodium falciparum. Although all the isolates harboring this haplotype are sensitive to AQ in vitro, their GM IC-50 is 2 fold higher than those with CVMNK haplotype. It is likely that reduced susceptibility to AQ is a stepwise process whereby polymorphisms accumulate first on codon 72–76 stretch of the pfcrt gene, and followed by additional mutations in the same gene or in other parasite genes including pfmdr1. A proposed AQ/DEAQ resistance pathway suggest a drive from the wild-type CVMNK pfcrt haplotype via CQ resistance associated pfcrt haplotype CVIET (Ariey et al., 2006) and then via further selection or import to the putatively DEAQ resistance associated pfcrt haplotype SVMNT (Alifrangis et al., 2006; Holmgren et al., 2007). It is also possible that the CVMNT haplotype is first selected from the CVMNK haplotype and followed by CVIET. It is however not clear if SVMNT will be selected in P. falciparum isolates from Africa as AQ pressure increases.
In addition to pfcrt polymorphisms, observation from this study showed that the N86Y polymorphism on pfmdr1 gene might play a modulating role in reducing the susceptibility of parasites to AQ. P. falciparum isolates harboring the mutant pfmdr1Tyr86 allele showed a 3-fold increase and a significantly high GM IC-50 compared to isolates with wild-type pfmdr1Asn86 allele. Previous studies from the same study site have shown not only the rarity of other pfmdr1 polymorphisms (at codons 184 and 1246) in Plasmodium falciparum isolates (Happi et al., 2009), but also the selection and modulatory role of the pfmdr1 N86Y mutation in AQ resistance (Happi et al., 2006b). Other studies in other part of Africa have reported the selection of Y86-Y184-Y1246 pfmdr1 haplotype in patients who failed treatment with AQ (Nsobya et al., 2007; Humphreys et al., 2007). Interestingly, a study from Madagascar (Rason et al., 2007) has reported highly CQ and AQ sensitive Plasmodium falciparum isolates with mutant pfmdr1Tyr86, without the mutant pfcrtThr76 allele. Overall, these reports from malaria endemic areas of Africa confirm the primary and modulatory roles of mutation(s) in pfcrt and pfmdr1 genes respectively in AQ resistance.
It has been suggested that since AQ and CQ have similar chemical structures and likely common mode of action (Bray et al., 1998; Ginsburg et al., 1998), these two compounds may have similar molecular mechanisms of resistance (Happi et al., 2006b). Our data show that the double mutant CVIET pfcrt haplotype+pfmdr1Tyr86 allele that has been severally reported to be good CQ resistance marker (Bray et al., 1998; Fidock et al., 2000; Djimde et al., 2001; Sidhu et al., 2002; Happi et al., 2006a) is also associated with reduced susceptibility/resistance to AQ in P. falciparum. These observations are also similar to results from Colombia (Echeverry et al., 2007). Although, it is also obvious from this study and other previous reports (Holmgren et al., 2007) that additional polymorphisms in these two genes or other genes may be involved in reduced parasite sensitivity to AQ. There is an urgent need for the identification and evaluation of these Plasmodium falciparum polymorphisms.
A regular monitoring of the in vitro drug response and/or molecular markers is warranted for surveillance of drug resistance especially in this current era of ACTs. In vitro drug susceptibility testing is a laboratory tool that can determine the parasite's response to individual drugs without interference of host factors. A major technical limitation of the in vitro testing is that the parasite phenotype is largely determined by the predominant parasite population as infections in this area is mostly polyclonal as reported in previous studies from the same area (Happi et al., 2003, 2004). If equal proportions of drug susceptible parasites and drug resistant parasites are tested, the resulting IC-50 may tend to reflect the response of the resistant parasites. Despite this limitation, in vitro drug susceptibility still remains an important component of research on surveillance of drug resistance.
Molecular markers have proven to be very useful and effective in predicting drug resistance in many settings of Africa and even in situation of epidemics (Djimde et al., 2004). Although, there are still variations in the predictive value of the molecular markers of resistance to antimalarial drugs in some peculiar areas of Africa like Madagascar (Rason et al., 2007).
In conclusion our study shows increase in parasites with reduced susceptibility/resistance to AQ in vitro in Southwestern Nigeria. The association observed between AQ in vitro resistance and double mutant CVIET pfcrt haplotype+pfmdr1Tyr86 allele may contribute to understanding the long known cross-resistance observed between AQ and CQ and could be useful for monitoring the spread of AQ resistance even as it is used as a partner drug with artemisinin derivatives.
Research Highlights
There is increasing trend in reduced susceptibility of P. falciparum to Amodiaquine.
Double mutant CVIET pfcrt haplotype+pfmdr1Tyr86 allele is associated with AQ resistance in vitro.
These may serve as epidemiological tool for monitoring the spread of AQ resistance as it is a partner drug in the ACTs.
There is a need to evaluate the role of other SNPs on these genes or other genes in AQ resistance
Acknowledgements
The authors thank all the patients, their parents or guardians for volunteering to participate in the study and our laboratory staff Mr M.O. Olatunde for assistance with the study.
This study was supported by the NIH/Fogarty International Centre, The European Union Developing Countries Clinical Trial Partnership (EDCTP), the UNICEF/UNDP/World Bank/WHO/TDR, and the Multilateral Initiative for Malaria in Africa (MIM)/TDR. Christian T. Happi is the 2011 recipient of the Exxon-Mobil Corporation Malaria Research Leadership Award and is supported by the EDCTP Grant Award no. TA2007/40200016 for Senior Research Fellowship; the Fogarty International Research Collaboration Award (FIRCA) no. NIH RO3TW007757-03 and the UNICEF/UNDP/World Bank/WHO/TDR Grant ID A50337. Grace O Gbotosho is supported by the MIM/TDR project ID A20239.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adagu IS, Dias F, Pinheiro L, Rombo L, do Rosario V, Warhurst DC. Guinea Bissau: association of chloroquine resistance of Plasmodium falciparum with the Tyr86 allele of the multiple drug-resistance gene Pfmdr1. Trans. R. Soc. Trop. Med. Hyg. 1996;90:90–91. doi: 10.1016/s0035-9203(96)90491-5. [DOI] [PubMed] [Google Scholar]
- Alifrangis M, Dalgaard MB, Lusingu JP, Vestergaard LS, Staalsoe T, Jensen AT, Enevold A, Ronn AM, Khalil IF, warhurst DC, Lemnge MM, Theander TG, Bygbjerg IC. Occurrence of the Southeast Asian/South American SVMNT haplotype of the chloroquine resistance transporter gene in Plasmodium falciparum in Tanzania. J. Infect. Dis. 2006;193:1738–1741. doi: 10.1086/504269. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Nair S, Qin H, Singlam S, Brockman A, Paiphun L, Nosten F. Are transporter genes other than the chloroquine resistance locus (pfcrt) and multidrug resistance gene (pfmdr) associated with antimalarial drug resistance? Antimicrob. Agents Chemother. 2005;49:2180–2188. doi: 10.1128/AAC.49.6.2180-2188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariey F, Fandeur T, Durand R, Randrianarivelojosi M, Jambou R, Legrand E, Ekala MT, Bouchier C, Cojean S, Duchemin JB, Robert V, Le Bras J, Mercereau-Puijalon O. Invasion pf Africa by a single pfcrt allele of South East Asian type. Malar. J. 2006;5:34. doi: 10.1186/1475-2875-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basco LK, Le Bras J. In vitro activity of monodesethylamodiaquine and amopyroquine against African isolates and clones of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 1993;48:120–125. doi: 10.4269/ajtmh.1993.48.120. [DOI] [PubMed] [Google Scholar]
- Basco LK, Ndouga M, Keundjian A, Ringwald P. Molecular epidemiology of malaria in Cameroon. IX. Characteristics of recrudescent and persistent Plasmodium falciparum infections after chloroquine and amodiaquine treatment in children. Am. J. Trop. Med. Hyg. 2002;66:117–123. doi: 10.4269/ajtmh.2002.66.117. [DOI] [PubMed] [Google Scholar]
- Bloland PB, Ruebush TK. Amodiaquine. Lancet. 1996;348:1659–1660. doi: 10.1016/S0140-6736(05)65723-6. [DOI] [PubMed] [Google Scholar]
- Bray PG, Mungthin M, Ridley RG, Ward SA. Access to hematin: the basis of chloroquine resistance. Mol. Pharmacol. 1998;54:170–179. doi: 10.1124/mol.54.1.170. [DOI] [PubMed] [Google Scholar]
- Childs GE, Boudreau EF, Milhous WK, Wimonwattratee T, Pooyindee N, Pang L, Davidson DE., Jr. A comparison of the in vitro activities of amodiaquine and desethylamodiaquine against isolates of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 1989;40:7–11. doi: 10.4269/ajtmh.1989.40.7. [DOI] [PubMed] [Google Scholar]
- Djimde A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourte Y, Dicko A, Su XZ, Nomura T, Fidock DA, Wellems TE, Plowe CV, Coulibaly D. A molecular marker for chloroquine-resistant falciparum malaria. N. Engl. J. Med. 2001;344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- Djimde AA, Dolo A, Ouattara A, Diakite S, Plowe CV, Doumbo OK. Molecular diagnosis of resistance to antimalarial drugs during epidemics and in war zones. J. Infect. Dis. 2004;190:853–855. doi: 10.1086/422758. [DOI] [PubMed] [Google Scholar]
- Dorsey G, Kamya MR, Singh A, Rosenthal PJ. Polymorphisms in the Plasmodium falciparum pfcrt and pfmdr-1 genes and clinical response to chloroquine in Kampala, Uganda. J. Infect. Dis. 2001;183:1417–1420. doi: 10.1086/319865. [DOI] [PubMed] [Google Scholar]
- Duraisingh MT, Drakeley CJ, Muller O, Bailey R, Snounou G, Targett GA, Greenwood BM, Warhurst DC. Evidence for selection for the tyrosine-86 allele of the pfmdr 1 gene of Plasmodium falciparum by chloroquine and amodiaquine. Parasitology. 1997;114:205–211. doi: 10.1017/s0031182096008487. [DOI] [PubMed] [Google Scholar]
- Durand R, Jafari S, Vauzelle J, Delabre JF, Jesic Z, Le Bras J. Analysis of pfcrt point mutations and chloroquine susceptibility in isolates of Plasmodium falciparum. Mol. Biochem. Parasitol. 2001;114:95–102. doi: 10.1016/s0166-6851(01)00247-x. [DOI] [PubMed] [Google Scholar]
- Echeverry DF, Holmgren G, Murillo C, Higuita JC, Bjorkman A, Gil JP, Osorio L. Short report: polymorphisms in the pfcrt and pfmdr1 genes of Plasmodium falciparum and in vitro susceptibility to amodiaquine and desethylamodiaquine. Am. J. Trop. Med. Hyg. 2007;77:1034–1038. [PubMed] [Google Scholar]
- Ferreira ID, Lopes D, Martinelli A, Ferreira C, do Rosário VE, Cravo P. In vitro assessment of artesunate, artemether and amodiaquine susceptibility and molecular analysis of putative resistance associated mutations of Plasmodium falciparum from Sao Tome and Prıncipe. Trop. Med. Int. Health. 2007;12:353–362. doi: 10.1111/j.1365-3156.2006.01789.x. [DOI] [PubMed] [Google Scholar]
- Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LM, Sidhu AB, Naude B, Deitsch KW, Su XZ, Wootton JC, Roepe PD, Wellems TE. Mutations in the P. falciparum digestive vacuole transmembrane protein Pfcrt and evidence for their role in chloroquine resistance. Mol. Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folarin OA, Gbotosho GO, Sowunmi A, Olorunsogo OO, Oduola AM, Happi TC. Chloroquine Resistant Plasmodium falciparum in Nigeria: Relationship between pfcrt and pfmdr1 Polymorphisms, In-Vitro Resistance and Treatment Outcome. Open Trop. Med. J. 2008;1:74–82. doi: 10.2174/1874315300801010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H, Famin O, Zhang J, Krugliak M. Inhibition of glutathione-dependent degradation of heme by chloroquine and amodiaquine as a possible basis for their antimalarial mode of action. Biochem. Pharmacol. 1998;56:1305–1313. doi: 10.1016/s0006-2952(98)00184-1. [DOI] [PubMed] [Google Scholar]
- Happi CT, Gbotosho GO, Folarin OA, Bolaji OM, Sowunmi A, Kyle DE, Milhous W, Wirth DF, Oduola AM. Association between mutations in Plasmodium falciparum chloroquine resistance transporter and P. falciparum multidrug resistance 1 genes and in vivo amodiaquine resistance in P. falciparum malaria-infected children in Nigeria. Am. J. Trop. Med. Hyg. 2006a;75:155–161. [PubMed] [Google Scholar]
- Happi CT, Gbotosho GO, Folarin OA, Sowunmi A, Bolaji OM, Fateye BA, Kyle DE, Milhous W, Wirth DF, Oduola AM. Linkage disequilibrium between two distinct loci in chromosomes 5 and 7 of Plasmodium falciparum and in vivo chloroquine resistance in Southwest Nigeria. Parasitol. Res. 2006b;100:141–148. doi: 10.1007/s00436-006-0246-4. [DOI] [PubMed] [Google Scholar]
- Happi CT, Gbotosho GO, Sowunmi A, Falade CO, Akinboye DO, Gerena L, Kyle DE, Milhous W, Wirth DF, Oduola AM. Molecular analysis of Plasmodium falciparum recrudescent malaria infections in children treated with chloroquine in Nigeria. Am. J. Trop. Med. Hyg. 2004;70:20–26. [PubMed] [Google Scholar]
- Happi TC, Thomas SM, Gbotosho GO, Falade CO, Akinboye DO, Gerena L, Hudson T, Sowunmi A, Kyle DE, Milhous W, Wirth DF, Oduola AM. Point mutations in the pfcrt and pfmdr-1 genes of Plasmodium falciparum and clinical response to chloroquine, among malaria patients from Nigeria. Ann. Trop. Med. Parasitol. 2003;97:439–451. doi: 10.1179/000349803235002489. [DOI] [PubMed] [Google Scholar]
- Happi CT, Gbotosho GO, Folarin OA, Sowunmi A, Hudson T, O'Neil M, Milhous W, Wirth DF, Oduola AM. Selection of Plasmodium falciparum multidrug resistance gene 1 alleles in asexual stages and gametocytes by artemether-lumefantrine in Nigerian children with uncomplicated falciparum malaria. Antimicrob. Agents Chemother. 2009;53:888–893. doi: 10.1128/AAC.00968-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren G, Gil JP, Ferreira PM, Veiga MI, Obonyo CO, Bjorkman A. Amodiaquine resistant Plasmodium falciparum malaria in vivo is associated with selection of pfcrt 76T and pfmdr1 86Y. Infect. Genet. Evol. 2006;6:309–314. doi: 10.1016/j.meegid.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Humphreys GS, Merinopoulos I, Ahmed J, Whitty CJM, Mutabingwa TK, Sutherland CJ, hallett RL. Amodiquine and Arthemether-Lumefantrine select distinct alleles of the pfmdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrob. Agents. Chemother. 2007;51:991–997. doi: 10.1128/AAC.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DJ, Fidock DA, Mungthin M, Lakshmanan V, Sidhu AB, Bray PG, Ward SA. Evidence for a central role for pfcrt in conferring Plasmodium falciparum resistance to diverse antimalarial agents. Mol. Cell. 2004;15:867–877. doi: 10.1016/j.molcel.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim P, Chy S, Ariey F, Incardona S, Chim P, Sem R, Denis MB, Hewitt S, Hoyer S, Socheat D, Merecreau-Puijalon O, Fandeur T. pfcrt polymorphism and chloroquine resistance in Plasmodium falciparum strains isolated in Cambodia. Antimicrob. Agents. Chemother. 2003;47:87–94. doi: 10.1128/AAC.47.1.87-94.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JD, Susanti AI, Krisin, Sismadi P, Fryauff DJ, Baird JK. The T76 mutation in the pfcrt gene of Plasmodium falciparum and clinical chloroquine resistance phenotypes in Papua, Indonesia. Ann. Trop. Med. Parasitol. 2001;95:559–572. doi: 10.1080/00034980120092516. [DOI] [PubMed] [Google Scholar]
- Meshnick SR, Alker AP. Amodiaquine and combination chemotherapy for malaria. Am. J. Trop. Med. Hyg. 2005;73:821–823. [PubMed] [Google Scholar]
- Nsobya SL, Dokomajilar C, Joloba M, Dorsey G, Rosenthal PJ. Resistance-mediating Plasmodium falciparum pfcrt and pfmdr1 alleles after treatment with artesunate-amodiaquine in Uganda. Antimicrob. Agents. Chemother. 2007;51:3023–3025. doi: 10.1128/AAC.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochong EO, van den Broek IV, Keus K, Nzila A. Short report: association between chloroquine and amodiaquine resistance and allelic variation in the Plasmodium falciparum multiple drug resistance 1 gene and the chloroquine resistance transporter gene in isolates from the upper Nile in southern Sudan. Am. J. Trop. Med. Hyg. 2003;69:184–187. [PubMed] [Google Scholar]
- Olliaro P, Mussano P. Amodiaquine for treating malaria. Cochrane Database Syst. Rev. 2003;(2):CD000016. doi: 10.1002/14651858.CD000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rason MA, Andrianantenaina HB, Ariey F, Raveloson A, Domarle O, Randrianarivelojosia M. Prevalent pfmdr1 n86y mutant Plasmodium falciparum in Madagascar despite absence of pfcrt mutant strains. Am. J. Trop. Med. Hyg. 2007;76:1079–1083. [PubMed] [Google Scholar]
- Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- Schellenberg D, Kahigwa E, Drakeley C, Malende A, Wigayi J, Msokame C, Aponte JJ, Tanner M, Mshinda H, Menendez C, Alonso PL. The safety and efficacy of sulfadoxinepyrimethamine, amodiaquine, and their combination in the treatment of uncomplicated Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 2002;67:17–23. doi: 10.4269/ajtmh.2002.67.17. [DOI] [PubMed] [Google Scholar]
- Sidhu AB, Verdier-Pinard D, Fidock DA. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science. 2002;298:210–213. doi: 10.1126/science.1074045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol. Biochem. Parasitol. 1993;58:283–292. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- Sowunmi A, Akindele JA, Balogun MA. Leukocytes counts in falciparum malaria in African children from an endemic area. Afr. J. Med. med. Sci. 1995;24:145–149. [PubMed] [Google Scholar]
- Sowunmi A, Ayede AI, Falade AG, Ndikum VN, Sowunmi CO, Adedeji AA, Falade CO, Happi TC, Oduola AM. Randomized comparison of chloroquine and amodiaquine in the treatment of acute, uncomplicated, Plasmodium falciparum malaria in children. Ann. Trop. Med. Parasitol. 2001;95:549–558. doi: 10.1080/00034980120092507. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Ndir O, Dieng T, Mboup S, Wypij D, Maguire JH, Wirth DF. In vitro chloroquine susceptibility and PCR analysis of pfcrt and pfmdr1 polymorphisms in Plasmodium falciparum isolates from Senegal. Am. J. Trop. Med. Hyg. 2002;66:474–480. doi: 10.4269/ajtmh.2002.66.474. [DOI] [PubMed] [Google Scholar]
- Tinto H, Sanou B, Dujardin JC, Ouedraogo JB, Overmier CV, Erhart A, Marck EV, Guiguemde TR, D'Alessandro U. Usefulness of the Plasmodium falciparum chloroquine resistance transporter T76 genotype failure index for the estimation of in vivo chloroquine resistance in Burkina Faso. Am. J. Trop. Med. Hyg. 2005;73:171–173. [PubMed] [Google Scholar]
- Trape JF. The public health impact of chloroquine resistance in Africa. Am. J. Trop. Med. Hyg. 2001;64(Suppl):12–17. doi: 10.4269/ajtmh.2001.64.12. [DOI] [PubMed] [Google Scholar]
- warhurst DC. Polymorphism in the Plasmodium falciparum chloroquine resistance transporter protein links verapamil enhancement of chloroquine sensitivity with the clinical efficacy of amodiaquine. Malar. J. 2003;2:31. doi: 10.1186/1475-2875-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ. Can amodiaquine be resurrected? Lancet. 1996;348:1184–1185. doi: 10.1016/S0140-6736(05)65475-X. [DOI] [PubMed] [Google Scholar]
- White NJ. Qinghaosu (artemisinin): the price of success. Science. 2001;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- World Health Organisation . Instructions for the use of the in vitro Micro test kit for the assessment of the response of Plasmodium falciparum to chloroquine, mefloquine, quinine, amodiaquine, sulfadoxine/pyrimethamine and artemisinin. WHO; Geneva: 1997. Document CTD/MAL/97.20. [Google Scholar]
- World Health Organization . Antimalarial drug combination therapy. Report of a WHO Technical consultation. World Health Organisation; Geneva, Switzerland: 2001. [Google Scholar]
- World Health Organization . Susceptibility of Plasmodium falciparum to antimalarial drugs. World Health Organization; Geneva: 2005. Report on Global monitoring 1996–2004. [Google Scholar]
- World Health Organization . New malaria guideline for treatment of malaria. Second Edition. 2010a. http://www.who.int/publications/news/news. [Google Scholar]
- World Health Organization Basic Malaria Microscopy. 2010b Learners Guide - 2010 http://www.searo.who.int/LinkFiles/Malaria_malaria_microscopy_Learners_guide2010.pdf.