Abstract
Serotype 5 and 8 capsular polysaccharides predominate among clinical isolates of Staphylococcus aureus. The results of experiments in animal models of infection have revealed that staphylococcal capsules are important in the pathogenesis of S. aureus infections. The capsule enhances staphylococcal virulence by impeding phagocytosis, resulting in bacterial persistence in the bloodstream of infected hosts. S. aureus capsules also promote abscess formation in rats. Although the capsule has been shown to modulate S. aureus adherence to endothelial surfaces in vitro, animal studies suggest that it also promotes bacterial colonization and persistence on mucosal surfaces. S. aureus capsular antigens are surface associated, limited in antigenic specificity, and highly conserved among clinical isolates. With the emergence of vancomycin-resistant S. aureus in the United States in 2002, new strategies are needed to combat staphylococcal infections. Purified serotype 5 and 8 capsular polysaccharides offer promise as target antigens for a vaccine to prevent staphylococcal infections, although the inclusion of other antigens is likely to be essential in the development of an effective S. aureus vaccine. The genetics and mechanisms of capsule biosynthesis are complex, and much work remains to enhance our understanding of capsule biosynthesis and its regulation.
INTRODUCTION
Staphylococcus aureus is an opportunistic bacterial pathogen responsible for a diverse spectrum of human and animal diseases. S. aureus is associated with asymptomatic colonization of the skin and mucosal surfaces of normal humans. However, Staphylococcus is also a major cause of wound infections and has the invasive potential to induce osteomyelitis, endocarditis, and bacteremia, leading to secondary infections in any of the major organ systems. Staphylococcal infections occur most frequently when the skin or mucosal barriers are breached, following insertion of a foreign body, and in hosts with compromised immune systems.
Because of the prevalence of antibiotic-resistant strains and the recent emergence of clinical isolates resistant to vancomycin (77), control of S. aureus has become increasingly difficult. Staphylococcus plays a major role in nosocomial infections and recently has been acknowledged as an important cause of community-acquired infections (11, 35, 40, 50). Communityacquired S. aureus infections often occur in otherwise healthy individuals who lack the expected risk factors for S. aureus infections, e.g., recent hospitalization or surgery, residence in a long-term-care facility, or use of injected drugs. It is postulated that strains causing these community-acquired infections have a high virulence potential because of their ability to cause disease in immunocompetent hosts (22, 39).
The bacterial components and secreted products that affect the pathogenesis of S. aureus infections are numerous (93) and include surface-associated adhesins, a capsular polysaccharide, exoenzymes, and exotoxins. This constellation of bacterial products allows staphylococci to adhere to eukaryotic membranes, resist opsonophagocytosis, lyse eukaryotic cells, and trigger the production of a cascade of host immunomodulating molecules. Because of the multifactorial nature of staphylococcal infections and the functional redundancy of S. aureus adhesins and exoproteins, it has been difficult to sort out the role that individual virulence determinants play in the pathogenic process.
Definition of S. aureus Encapsulation
Microorganisms that cause invasive disease commonly produce extracellular capsular polysaccharides (95). Capsules enhance microbial virulence by rendering the bacterium resistant to phagocytosis. Capsule production by S. aureus was first described in 1931 by Gilbert (38). However, since capsule detection methods were crude (India ink negative staining, colony morphology on agar plates and in serum-soft agar, and lack of cell-associated clumping factor), very few strains of S. aureus were recognized as capsule positive. These highly encapsulated strains (typified by strains M and Smith diffuse) produced mucoid colonies, resisted phagocytosis, and were virulent for mice (13, 56, 76, 121, 122).
In 1982 Karakawa and Vann proposed a new capsular polysaccharide typing scheme for S. aureus based upon the preparation of absorbed rabbit antiserum to prototype S. aureus strains (52). These investigators were the first to report that most S. aureus strains were encapsulated, and they described eight capsular serotypes. The heavily encapsulated strains M and Smith diffuse were assigned to serotypes 1 and 2, respectively. Strains of these two serotypes produce mucoid colonies on solid medium, and they are rarely encountered among clinical isolates (4, 49, 52, 64, 103). Isolates belonging to the remaning serotypes produce nonmucoid colonies on solid medium, and their colony morphology is indistinguishable from that of strains lacking a capsule. Some investigators have referred to nonmucoid, encapsulated S. aureus isolates as microencapsulated to distinguish them from the atypical mucoid strains.
Serotyping studies of staphylococcal isolates from diverse strain collections representing several geographic regions have revealed that serotype 5 and 8 isolates account for ≈25% and 50%, respectively, of isolates recovered from humans (4, 49, 64, 103). Moreover, these two serotypes are prevalent among isolates from clinical infections as well as from commensal sources. Serotype 5 and 8 capsules are also made by S. aureus strains isolated from cows, rabbits, poultry, pigs, and horses (20, 43, 92, 104, 110). The type 5 and 8 capsules were localized to the cell surface of clinical isolates by immunoelectron microscopy with type-specific monoclonal antibodies to stabilize and visualize the polysaccharides (49, 103). In 1985, Sompolinsky et al. (103) serotyped a large collection of S. aureus isolates from different sources and included three new serotypes in their collection, bringing the number of putative capsular serotypes to 11.
Strains that do not react with antibodies to capsule types 1, 2, 5, or 8 are currently referred to as nontypeable (2, 4, 49, 103), since neither the prototype strains nor antiserum to the other putative serotypes is available.
Biochemistry
Capsules or microcapsules from at least 18 S. aureus strains have been described and at least partially characterized (122). Each contains hexosaminuronic acids. Biochemical characterizations have been performed on polysaccharides purified from only four of the 11 putative capsule types. Strain M expresses a type 1 capsule with the following structure: (→4)-α-d-GalNAcA-(1→4)-α-d-GalNAcA-(1→3)-α-d-FucNAc-(1→)n; a taurine residue is amide linked to every fourth d-GalNAcA residue (70, 80). Two other strains, D (52) and SA1 mucoid (65), produce capsules that are serologically and biochemically similar to that produced by strain M. The Smith diffuse capsule of serotype 2 has the structure: (→4)-β-d-GlcNAcA-(1→4)-β-dGlcNAcA-(l-alanyl)-(1→)n (44).
Type 5 and 8 capsular polysaccharides (CP5 and CP8, respectively) purified from the prototype strains Reynolds and Becker, respectively, are structurally very similar to each other and to the capsule made by strain T, described previously by Wu and Park (123). Type 5 has the structure (→4)-3-O-Ac-β-d-ManNAcA-(1→4)-α-l-FucNAc-(1→3)-β-d-FucNAc-(1→)n (32, 78), and type 8 has the structure (→3)-4-O-Ac-β-d-ManNAcA-(1→3)-α-l-FucNAc-(1→3)-β-d-FucNAc-(1→)n (33). Type 5 and 8 polysaccharides differ only in the linkages between the sugars and in the sites of O-acetylation of the mannosaminuronic acid residues, yet they are serologically distinct. The structure of CP4 was never elucidated, although the prototype serotype 4 strain 7007 has been shown to react with antibodies to CP5. CP4 is most likely a polysaccharide with a trisaccharide structure identical to that of CP5 but differing in the presence, absence, or location of the O-acetyl moieties on the N-acetylmannosaminuronic acid residues (113). The linkage(s) that anchors any of the S. aureus capsular polysaccharides to the staphylococcal cell wall remains undefined.
HIGHLY ENCAPSULATED SEROTYPE 1 AND 2 STRAINS
Serotype 1 and 2 Capsules Impede Phagocytic Killing
Highly encapsulated S. aureus isolates have all the attributes of classic encapsulated bacterial pathogens. Serotype 1 and 2 strains resist in vitro opsonophagocytic killing by human polymorphonuclear leukocytes (6, 62, 87, 116, 122, 124). In nonimmune serum, complement (C3b fragments) and antibodies directed to S. aureus cell wall components are deposited on the bacterial cell wall beneath the capsular layer (116, 122). The capsule impedes the interaction between cell wall-bound C3b or immunoglobulin and receptors for these molecules on the phagocytic cell. As a result, the bacterium evades phagocytic uptake. In the presence of antibodies specific for the capsule, C3b and antibody are deposited throughout the capsular matrix and on the bacterial surface, making them available for recognition by receptors on the phagocyte (6, 116). In the presence of specific capsular antibodies, mucoid strains are efficiently taken up by phagocytes and killed in vitro (62, 87, 116, 122, 124).
Role of Serotype 1 and 2 Capsules in Virulence
When inoculated by the intraperitoneal route, highly encapsulated (serotypes 1 and 2) strains of S. aureus, like strains M and Smith diffuse, are more virulent for mice than are nonmucoid strains. This difference in virulence has been shown with spontaneous nonmucoid variant strains (13, 56, 76, 98, 101) as well as with isogenic strains that have been derived by genetic methods (62, 71). Mucoid isolates are not effectively phagocytosed within the peritoneal cavity, and thus these highly encapsulated S. aureus are free to replicate extracellularly. Multiplication of the staphylococci is accompanied by the production of alpha toxin, resulting in mortality within 24 to 48 h of bacterial challenge (13, 56).
To determine whether the size of the S. aureus capsule influenced virulence in vivo, our group prepared transposon-induced mutants from a highly encapsulated serotype 1 strain of S. aureus (62). Using mouse models of staphylococcal infection, we compared the virulence of the wild-type, highly encapsulated parent strain (SA1 mucoid) with that of two nonmucoid mutants, one microencapsulated (producing less capsule than the parental strain) and the other nonencapsulated. When challenged by the intraperitoneal route, strain SA1 mucoid had a 50% lethal dose (LD5o) for mice of 2.1 × 104 CFU. The microencapsulated and nonencapsulated mutant strains had comparable LD5o values (≈2 × 108 CFU) that were similar to those of serotype 5 and 8 isolates of S. aureus (1) and >3,000-fold higher than that of the highly encapsulated parental strain. In mice challenged intravenously with the bacterial strains, the nonmucoid mutants were cleared more readily from the bloodstream and kidneys than strain SA1 mucoid. In an in vitro assay, only the highly encapsulated strain demonstrated antibody-dependent, complement-mediated opsonophagocytosis by human leukocytes. The microencapsulated and nonencapsulated mutants were opsonized for phagocytosis by preimmune serum with complement activity that lacked antibodies to the capsule. These studies not only established the importance of the type 1 capsule in staphylococcal virulence but showed that the amount of surface-associated capsular polysaccharide was critical in the host-parasite interaction.
In contrast to the enhanced virulence of the mucoid serotype 1 strain of S. aureus in the murine lethality or bacteremia model, the same organism was poorly virulent in the rat model of catheter-induced endocarditis (82). The inoculum dose of S. aureus SA1 mucoid required to induce infection in 50% of catheterized rats (ID50) was ≈4 × 106 CFU, a dose 1,000-fold higher than that reported for serotype 5 and 8 isolates (7). Likewise, serotype 5 and 8 S. aureus strains were less virulent in the endocarditis infection model than were isogenic mutant strains that were devoid of capsule production. In dose-response experiments, the acapsular mutants showed ID50 values ≈10-fold lower than those of the parental serotype 5 and 8 strains (7). Staphylococcal adherence to the damaged heart valve is critical to initiate infection in the S. aureus endocarditis model. The inverse correlation between capsule production and infectivity in this model suggests that the capsule may be masking adhesins that have been shown to be important determinants of virulence in endocarditis (57, 79).
Protection Afforded by Crude Capsule Preparations
Capsular antigens were among the earliest target antigens in vaccine studies designed to protect against staphylococcal infections. Mice immunized intraperitoneally with a single dose of live or killed cells of strain Smith diffuse, a highly encapsulated serotype 2 isolate of S. aureus, were protected against a lethal challenge dose (108 CFU) of the homologous strain prepared in saline and injected intraperitoneally (23, 25). Immunization with a heterologous strain (Cowan I, serotype 8) was not protective against lethality induced by the Smith diffuse strain of S. aureus.
Mice immunized with polysaccharide antigens extracted from cells of Smith diffuse were not protected against challenge with 108 CFU of the homologous strain in saline (25). However, if the staphylococcal inoculum was mixed with 5% hog gastric mucin, the mouse-lethal dose of strain Smith diffuse was reduced from 108 to 105 CFU. Under these experimental conditions, immunization with polysaccharide antigens extracted from S. aureus cells (25) or heat-stable culture supernatants (31) was protective against lethality induced by the homologous strain. The protection could be passively transferred to naive animals by the injection of immune serum (25, 30). However, no protection against infection by the Smith diffuse strain was elicited by immunization with whole cells or culture extracts prepared from two heterologous strains of S. aureus. Likewise, mice immunized with the Smith diffuse strain were not protected against a lethal inoculum of a heterologous S. aureus isolate (strain Foggie; capsular phenotype unknown) (24).
Protection Afforded by Purified CP1
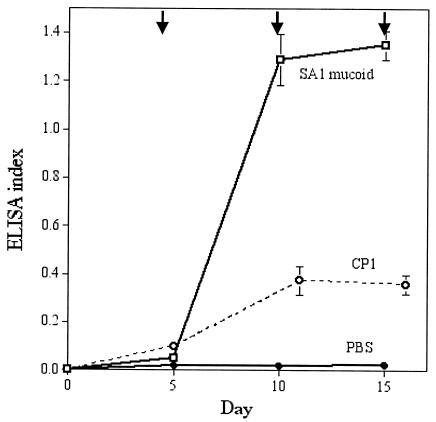
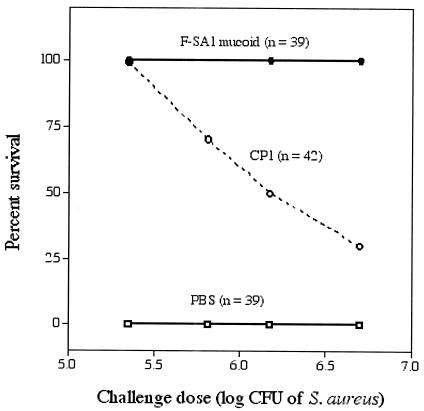
Our group purified and chemically characterized capsular polysaccharide from a mucoid serotype 1 strain, SA1 mucoid (65). The virulence of this S. aureus strain for mice is indicated by its LD5o of 2.1 × 104 CFU (challenge by the intraperitoneal route in saline) (62). To determine whether antibodies to purified CP1 were protective, we immunized mice with either phosphate-buffered saline, killed strain SA1 mucoid, or purified capsular polysaccharide. Killed S. aureus elicited high levels of antibodies to CP1, whereas the purified polysaccharide was only weakly immunogenic (Fig. 1) (65, 67). One week after the third immunization, groups of mice were challenged intraperitoneally with S. aureus. As shown in Fig. 2, all of the mice immunized with formalin-killed bacteria survived lethal doses ranging from 2 × 105 to 5 × 106 CFU, representing 10.5 to 119 times the LD50 of strain SA1 mucoid. Mice immunized with CP1 were protected in a dose-dependent fashion, with 50% of the animals surviving challenge with 1.5 × 106 CFU (71 times the LD50). Control mice immunized with phosphate-buffered saline all died (61).
FIG. 1.
Serum antibodies to CP1 in groups of mice immunized intraperitoneally with either phosphate-buffered saline (PBS), 108 CFU of formalin-killed S. aureus SA1 mucoid, or CP1. Arrows denote immunizations, and each point represents the mean ELISA index (± standard error) of serum diluted 1:100. The ELISA index was calculated by dividing the absorbance reading of the test serum by the absorbance reading of a pool of high-titered immune mouse serum (raised to killed SA1 mucoid). Data are from reference 61.
FIG. 2.
Active immunization with formalin-killed cells of S. aureus SA1 mucoid or CP1 protects mice against lethality induced by various challenge doses of strain SA1 mucoid. Data are from reference 61.
To determine whether antibodies alone were protective, animals were passively immunized with normal mouse serum or immune mouse serum raised either to formalin-killed cells of strain SA1 mucoid cells or CP1. All 10 mice injected with serum raised to killed S. aureus cells survived challenge with 1.5 × 106 CFU of SA1 mucoid. In contrast, none of 10 mice passively immunized with serum raised to CP1 survived a challenge inoculum 22 times the LD50 dose (6.5 × 105 CFU) for SA1 mucoid. All mice injected with normal serum died after either challenge dose. The results of these studies suggest that antibodies to CP1 are protective and that there is a correlation between capsular antibody levels and protection against lethality in mice. Active but not passive immunization with CP1 provided the mice with sufficient levels of CP1 antibodies to protect against S. aureus-induced lethality.
Capsular antibodies were also protective in a sublethal model of S. aureus bacteremia and renal abscess formation. Mice were immunized with killed strain SA1 mucoid or CP1, as described above, and then challenged intravenously with one of three S. aureus strains with various amounts of cell-associated capsule (67). Immunization with killed cells of the homologous strain protected mice against infection with each of the three S. aureus isolates (67). Mice immunized with CP1 were protected when challenged intravenously with either the highly encapsulated strain SA1 mucoid or the nonmucoid microencapsulated mutant, but not with the acapsular mutant. Protection against infection with the encapsulated S. aureus correlated with capsular antibody levels in the immunized animals. Immunization with a heterologous strain lacking CP1 was not protective against challenge with strain SA1 mucoid. Naïve mice passively immunized with antiserum raised to strain SA1 mucoid or CP1 had significantly fewer CFU of strain SA1 mucoid in their blood and kidney samples than did mice given preimmune serum (67). The results of these experiments indicate that capsular antibodies are protective against infection by the prototype serotype 1 strains. Moreover, these studies provided the foundation for subsequent investigations to test whether the more clinically relevant serotype 5 and 8 capsules could serve as the target of protective antibodies.
SEROTYPE 5 AND 8 S. AUREUS CAPSULES
Most clinical isolates of S. aureus produce either CP5 or CP8. The original serotyping scheme was developed by Karakawa and Vann (52), who reacted CP5- or CP8-specific antibodies with S. aureus capsular extracts by immunodiffusion. Alternative methods, such as enzyme-linked immunosorbent assay inhibition (1) or colony immunoblotting (64), allow multiple isolates to be serotyped in a shorter period of time. In either case, CP5- and CP8-specific antibodies are necessary reagents for these assays, which are not in widespread use because the necessary antisera are not commercially available. Colonies of serotype 5 and 8 strains of S. aureus are not mucoid, and their colony morphology is usually indistinguishable from that of an acapsular strain.
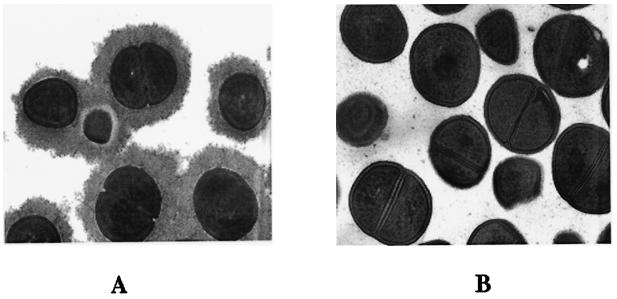
CP5- and CP8-producing staphylococci are often referred to as microencapsulated. This terminology is used to distinguish these isolates from mucoid serotype 1 or 2 strains and was based on visualization of scant capsular material produced by staphylococci harvested from poorly aerated or logarithmic-phase broth cultures (1). It is noteworthy that serotype 5 and 8 S. aureus cells cultivated on agar plates or in well-aerated overnight broth cultures produce a substantial capsule, as visualized by immunoelectron microscopy (Fig. 3).
FIG. 3.
Transmission electron micrographs of S. aureus cells cultured on agar plates. Prior to fixation, both strains were incubated with rabbit CP5-specific antibodies to stabilize and visualize the capsule. (A) CP5-producing strain Reynolds; (B) acapsular S. aureus mutant.
Capsule Production In Vitro
Expression of S. aureus CP5 and CP8 in vitro is highly sensitive to various environmental signals and is probably influenced by the in vivo environment as well (see below). Bacterial growth conditions, such as the culture medium, have been shown to dramatically influence capsular polysaccharide production (19, 90, 91, 107, 108). Growth of S. aureus under iron limitation and on solid medium both augmented production of CP8 (68). Capsule production in vitro is inhibited by yeast extract, alkaline growth conditions, and anaerobiasis (19, 48, 107) but enhanced by growth of the bacterium in milk (108) or in medium supplemented with up to 5% NaCl (88). Capsular polysaccharide production was not affected by changes in the phosphate content of the medium (34).
Poutrel et al. (91) analyzed CP5-specific monoclonal antibody binding to 30 different serotype 5 S. aureus strains by flow cytometry. Their results confirmed that the growth medium affects surface expression of CP5 and revealed intraisolate heterogeneity of cell-associated CP5 expression. Flow cytometry analysis provides information on the expression of capsular polysaccharide by thousands of individual cells, and the results revealed large variations in the amount of CP5 produced by individual bacteria in a population. There was also striking strain-to-strain variability in the response of S. aureus capsular polysaccharide production to environmental culture conditions (90, 91). The results of most studies, however, have indicated that little capsular polysaccharide is produced in the logarithmic phase of growth and that maximal capsule production occurs during the postexponential growth phase (15, 17, 88, 90). This observation is consistent with the fact that capsular polysaccharide expression has been shown to be positively controlled by the global regulator agr (18, 72, 88). The agr locus is a complex multigene operon in S. aureus that acts as a two-component quorum-sensing system. Genes under the control of agr include secreted virulence factors and adhesins that are regulated in a growth phase-dependent manner.
Herbert et al. (48) first reported that CO2 was an environmental signal that regulated CP5 expression both in vitro and in vivo. Although CP5 was expressed under normal air conditions (0.03% CO2), CP5 expression by three different serotype 5 strains was inhibited by cultivation in air supplemented with 1 to 5% CO2 (47, 48). CO2 was shown to affect cap5 gene expression at the transcriptional level (47). In contrast, quantitative detection of CP8 on different S. aureus strains cultivated in the presence of CO2 yielded conflicting results (47). Of nine strains tested, four showed decreased CP8 expression in the presence of 5% CO2, in four strains the effect of CO2 was marginal, and strain Becker demonstrated enhanced CP8 expression in the presence of CO2. The authors employed reporter gene fusion studies to determine whether specific differences in the sequence of the cap5 or cap8 promoter would influence the pattern of CO2 regulation among different S. aureus strains. Promoters amplified from five different strains were fused to the reporter gene xylE; each genetic construct was then transformed into two different S. aureus strains, the type 5 strain Newman and the type 8 strain Becker. XylE activity associated with each construct was evaluated in both genetic backgrounds. The results indicated that the genetic background of the individual S. aureus strain rather than the promoter sequence determined the regulation of capsular polysaccharide expression by CO2. XylE activity was always upregulated by CO2 in strain Becker and was always negatively regulated by CO2 in strain Newman. The authors postulated that trans-acting regulatory molecules such as transcriptional activators or sigma factors may be differentially expressed in different staphylococcal strains.
Capsule Production In Vivo
Several research groups have demonstrated capsule production in vivo. Arbeit and Nelles (5) were able to detect CP8 in the serum of rats with experimental catheter-induced endocarditis provoked by a serotype 8 strain but not in serum from control animals infected with a serotype 5 strain. Similarly, Arbeit and Dunn (3) reported that CP8 could be detected in the serum of guinea pigs with persistent subcutaneous infections. Our group demonstrated CP8 production in vivo in the endocarditis model of infection. Catheterized rabbits were inoculated with broth-grown S. aureus expressing low levels of CP8. S. aureus harvested (without subculture) from endocardial vegetations of the infected rabbits 4 days later expressed a high level of CP8, similar to that of organisms cultivated in vitro on solid medium (68).
We also evaluated capsule production in a mouse model of S. aureus nasal colonization (54). Staphylococci recovered from the nares of the colonized mice (without subculture) expressed CP5 in vivo, and a capsule-defective mutant showed reduced persistence in the nares compared to the parent strain (54). Hensen et al. (45) demonstrated in vivo expression of S. aureus CP5 in experimental bovine mastitis by immunochemical staining of tissue sections obtained from cows with acute or chronic mastitis.
In contrast, CP5 expression was not detected in vivo in several other staphylococcal infections. Minimal expression of CP5 was observed in either lung tissue or nasal polyp tissue obtained from two cystic fibrosis patients infected with S. aureus (48, 75). Similarly, when rats were challenged with a serotype 5 S. aureus strain in the granuloma pouch model, ≤5% of the cells harvested from the pouch exudates were CP5 positive (48). In both of these tissues, the absence of CP5 expression correlated with elevated CO2 levels (≥4%). As discussed above, CO2 has been shown to be an environmental signal that downregulates CP5 expression in vitro (47, 48).
Phagocytic Killing of Serotype 5 and 8 S. aureus Strains
Early reports on the role of the capsule in the pathogenesis of S. aureus infections were conflicting. Karakawa et al. reported that the production of CP5 or CP8 protected the bacterium from phagocytic uptake and killing by human polymorphonuclear leukocytes (51). They showed that serotype 5 and 8 S. aureus strains were opsonized for in vitro phagocytic killing only in the presence of either heat-inactivated polyclonal rabbit serum or monoclonal antibodies as opsonic sources. Antibodies to teichoic acid or to heterologous capsule types lacked opsonic activity. The results of these experiments suggested an important role for capsular antibodies in phagocytic clearance of serotype 5 and 8 S. aureus strains.
In contrast, our laboratory performed similar experiments but obtained very different results. Serotype 5 and 8 isolates grown to logarithmic phase were opsonized for phagocytosis by either preimmune serum with complement activity or heat-inactivated serum raised to type 5, type 8, or nonencapsulated S. aureus strains (121). Our results indicated that antibodies to the capsule or to cell wall components other than the capsule were opsonic for serotype 5 and 8 staphylococci. However, because these experiments were performed with staphylococci harvested in the logarithmic phase of growth, little to no capsular polysaccharide was expressed (15, 88). When the experiments were repeated after growing the staphylococci under conditions of optimal capsule expression, the bacteria resisted opsonophagoctyic killing in the presence of either complement or antibodies. The serotype 5 strain Reynolds was efficiently killed by human polymorphonuclear leukocytes only after opsonization with both specific capsular antibodies and complement (109). These results confirmed the initial observations reported by Karakawa et al. (51) and underscore the influence of culture conditions on the biological properties of S. aureus serotype 5 and 8 isolates.
Interaction of S. aureus Capsules with Serum Complement Components
Opsonization of S. aureus is critical for uptake and killing of the bacterium by professional phagocytic cells. Complement and antibody are the principal serum opsonins and have been shown to play an important role in opsonophagocytic killing of the staphylococcus (69, 87, 116, 117). C3b and its degradation fragment iC3b are the principal complement opsonins and represent important components of the host innate immune system. C3b can potentially be generated by three activation pathways —the classical, alternative, and lectin pathways. The classical pathway is most commonly activated by the interaction of antibody (immunoglobulin G [IgG] or IgM) with an antigenic bacterial surface. The alternative pathway, activated by a repeating polysaccharide or similar polymeric structure, is probably most important in nonimmune serum lacking antibodies specific to the bacterium. The lectin pathway is mediated through a mannan-binding lectin found in serum.
Studies on the interaction between complement components and the more clinically relevant capsular types of S. aureus were only recently reported (15, 16). Cunnion et al. (15) challenged control and C3-depleted mice intravenously with 107 CFU of a serotype 5 S. aureus strain. Whereas only 8% of the control mice succumbed to the infection, 64% of the complement-depleted animals died. The investigators also evaluated the in vitro parameters of C3 binding to serotype 5 and 8 isolates of S. aureus. At a high serum concentration (20%, similar to whole blood), the alternative pathway was active, contributing 90% of the total C3 binding to S. aureus. At a lower serum concentration (2%) that might be present in tissues, the classical complement pathway predominated.
In a comparison of bacterial strains grown on solid medium, a CP5 strain bound 42% fewer C3 molecules than its isogenic capsular polysaccharide-negative mutant. Both C3b and iC3b fragments of C3 bound to S. aureus cells (15, 16), and about one third of the bound C3b was shed from the bacterial surface as iC3b regardless of the capsular polysaccharide phenotype of the strain. CP5 was shown to mask C3 fragments deposited on the organism from binding to complement receptor 1 (16). The observation that encapsulated S. aureus organisms interfere with opsonization by complement is consistent with the fact that these cells are poorly phagocytosed in normal human serum (109). Ongoing studies by our group are aimed at determining whether purified CP5 or CP8 activates complement in human serum.
Role of Serotype 5 and 8 Capsules in Virulence
The first capsule-deficient mutant from the serotype 5 prototype strain Reynolds was obtained by Tn918 mutagenesis (1). In addition, the type 5 strain was mutagenized with ethyl methanesulfonate to obtain a capsule-negative mutant. The initial virulence studies were performed with inocula composed of logarithmic-phase S. aureus cells. The results showed that the LD5o values for the wild-type and capsule-negative mutant strains were similar, between 4 × 108 and 9 × 108 CFU per mouse (1). Similarly, animals injected intraperitoneally with either wild-type or mutant strains had comparable levels of bacteremia at 3 and 24 h after challenge. Quantitative cultures of blood and kidneys from animals challenged intravenously with sublethal doses of the S. aureus strains showed no differences in bacterial clearance or renal abscess formation. The results of these experiments suggested that the type 5 and 8 capsules produced by most S. aureus isolates did not enhance bacterial virulence in the animal models tested.
The virulence of the serotype 5 strain Reynolds in a mouse bacteremia model of infection was reexamined by inoculating mice with organisms cultivated on solid medium, which supports optimal capsule expression (109). The wild-type strain showed an LD50 value that was 10-fold lower than that of the capsule-negative mutant. Likewise, strain Reynolds sustained a significantly higher level of bacteremia and was cleared from the bloodstream of the infected animals less readily than the CP5-deficient mutant strains. We attributed this to the antiphagocytic nature of CP5, because in vitro assays indicated that the parental strain was susceptible to phagocytic killing by human polymorphonuclear leukocytes only in the presence of specific capsular antibodies (109). Capsule-deficient mutant strains (or the parental strain grown in poorly aerated broth cultures, where little capsule is expressed on the bacterial surface) were opsonized for phagocytic killing by nonimmune serum with complement activity.
A serotype 5 S. aureus strain was shown to be more virulent than an isogenic, acapsular mutant in a mouse model of renal abscess formation and in a subcutaneous abscess model of infection (89). Likewise, the type 5 strain was more virulent than its capsule mutants when compared in a murine model of septic arthritis (83). Mice inoculated with the prototype strain Reynolds developed more frequent and severe arthritis than mice inoculated with the CP5 mutant strains. Furthermore, mice challenged with the wild-type strains had a higher mortality rate and more pronounced weight loss than mice inoculated with the mutants. In vitro assays indicated that mouse macrophages were not able to phagocytose the parental strain Reynolds as efficiently as a capsule-defective mutant. Once phagocytosed, the CP5-positive strain was less efficiently killed than the mutant strain (83).
As noted above in the studies related to the serotype 1 capsule, capsular polysaccharide production attenuates staphylococcal virulence in the rat model of catheter-induced endocarditis (82). In a comparative study, we evaluated the virulence of serotype 5 and 8 S. aureus strains and their capsule-deficient mutants in the endocarditis model (7). Catheterized animals were challenged with inocula ranging from 102 to 106 CFU/rat. Mutants derived from serotype 5 and 8 S. aureus strains that were deficient in capsule production had ID50 values ≈10-fold lower than those of the parental strains (7). These observations are consistent with the inverse relationship that has been demonstrated between encapsulation and bacterial adherence to or invasion of host tissues (36, 106, 105). The capsule may be masking adhesins that have been shown to be important virulence determinants in endocarditis (57, 79).
The influence of capsule production on in vitro adherence of the staphylococcus to endothelial cells was recently reported (88). The data from that study indicate that S. aureus adherence to endothelial cells is maximal during the logarithmic phase of growth, when CP5 production is minimal. In the stationary phase of the bacterial growth cycle, the organisms are poorly adherent, and staphylococcal capsule expression is maximal. A mutation in the regulatory agr locus diminished CP5 production and led to increased staphylococcal adherence to endothelial cells in culture. Likewise, induction of CP5 expression by addition of NaCl to the growth medium resulted in reduced staphylococcal adherence. An acapsular mutant showed significantly greater adherence to endothelial cells than did the parental serotype 5 strain. Complementation experiments restored capsule production and reduced adherence to a level similar to that of the parental strain. The results from these studies suggest that the binding domain of the S. aureus adhesin for endothelial cells is masked by CP5. Similarly, we have observed that expression of both CP5 and CP8 diminishes S. aureus clumping factor A-mediated binding to fibrinogen (J. C. Lee, unpublished observations).
IMMUNOMODULATORY PROPERTIES OF CAPSULAR POLYSACCHARIDE
Our group recently investigated the role of S. aureus CP5 and CP8 in modulating abscess formation in an experimental rat model of intraabdominal infection (112). S. aureus is a potent inducer of abscess formation in rats; the ID50 value for abscess induction was <100 CFU/rat. An acapsular mutant strain also induced abscesses, but its ID50 was 20-fold greater than that of strain Reynolds (P = 0.02). Purified CP5 and CP8 facilitated intraabdominal abscess formation in animals when given intraperitoneally with a sterile cecal contents adjuvant.
Structural studies of CP8 revealed that it has a zwitterionic charge motif conferred by the negatively charged carboxyl group of N-acetylmannosaminuronic acid and free amino groups available on partially N-acetylated fucosamine residues. Chemical modifications that neutralized the charged groups on CP8 also abrogated its ability to provoke abscesses in vivo, indicating that this charge motif was critical for CP8 biological activity. As shown in Table 1, animals treated subcutaneously with CP8 24 h before challenge with homologous or heterologous zwitterionic polysaccharides were protected against abscess formation (112). Likewise, treatment with CP8 protected against challenge with viable S. aureus strains that produced CP5 or CP8.
TABLE 1.
Protection against abscess formation by S. aureus CP8a
| Challenge | Treatment (20 μg) | Challengeb | No. of rats with abscesses/total (%) | P vs. saline control |
|---|---|---|---|---|
| Heterologous | Saline | CP8 | 24/27 (89) | |
| polysaccharide | CP8 | CP8 | 7/19 (37) | 0.0003 |
| (20 μg) | Saline | PSA | 17/20 (85) | |
| CP8 | PSA | 3/20 (15) | <0.0001 | |
| Heterologous | Saline | PS80 | 17/20 (85) | |
| S. aureus | CP8 | PS80 | 6/18 (33) | 0.002 |
| (107 CFU) | Saline | COL | 12/14 (86) | |
| CP8 | COL | 4/15 (27) | 0.003 |
Data are adapted from Tzianabos et al. (112).
PSA, capsular polysaccharide A, a zwitterionic polysaccharide isolated from Bacteroides fragilis. PS80 and COL are serotype 8 and 5 S. aureus strains, respectively.
CP8 purified from S. aureus was a potent activator of rat and human CD4+ T cells in vitro (112). A biological role for CD4+ T cells activated by CP8 was demonstrated by transferring activated T cells with adjuvant to the peritoneal cavities of naïve rats. This transfer facilitated peritoneal abscess formation. Alternatively, if the activated T cells were administered by the intracardiac route 24 h before challenge with viable S. aureus, they conferred protection against abscess formation. These results offer a structure-function rationale for abscess formation by S. aureus and provide the groundwork for examining the role of the capsule in the induction of abscesses at other body sites.
CP5 AND CP8 AS PROTECTIVE ANTIGENS
Immunity to S. aureus infections remains poorly understood. Although there appears to be little resistance to colonization by S. aureus, healthy humans and animals have a high degree of innate resistance to invasive staphylococcal infections. In fact, experimental S. aureus infections are difficult to establish in human and animal hosts, requiring an inoculum containing millions of organisms and, in many cases, the concomitant introduction of a foreign body (21, 41, 100). Natural immunity in the host is attributed to epidermal and mucosal surface barriers and to intact cellular and humoral immune defenses. The phagocytic response of polymorphonuclear leukocytes is the body's first line of defense against invasion by S. aureus and a critical determinant in the outcome of staphylococcal infections. Factors that influence opsonophagocytic killing include the strain of S. aureus involved and the presence of opsonizing antibodies and complement. Once phagocytosed by leukocytes, most S. aureus cells are readily killed intracellularly.
Protection Afforded by Whole-Cell Vaccines
Normal humans have serum antibodies to S. aureus cell wall components, including peptidoglycan, teichoic acid, CP5, and CP8 (2, 12, 27, 115, 119, 120). However, levels of antibodies to CP5 and CP8 in normal human serum are low (27). In an in vitro opsonophagocytic killing assay, <50% of the inoculum of a serotype 5 strain was opsonized for phagocytic killing by human polymorphonuclear leukocytes in fresh normal human serum (109). However, if a pool of serum from humans immunized with a CP5-conjugate vaccine (see below) was added to the assay as an opsonic source, 95% of the bacterial inoculum was killed. These data suggest that the level of capsular antibodies in normal humans is not sufficient to promote efficient opsonophagocytic killing of an encapsulated S. aureus strain. Boosting capsular antibody levels by immunization may correlate with enhanced bacterial clearance in humans at risk for staphylococcal infections.
Several studies evaluated the protective efficacy of antibodies to CP5 and CP8 in experimental models of S. aureus infection. The first report evaluated the protective efficacy of capsular antibodies in a rat model of catheter-induced staphylococcal endocarditis (82). Rats were actively immunized with killed serotype 5 S. aureus cells or passively immunized intravenously with CP5 antiserum. Control animals were injected with saline or passively immunized with normal rabbit serum. Despite having elevated levels of capsular antibodies, the immunized animals were susceptible to staphylococcal endocarditis, and immunized and control animals had similar numbers of bacteria in the blood and vegetations. Likewise, antibodies to teichoic acid failed to protect the animals against staphylococcal endocarditis in this study (82) and in that reported by Greenberg et al. (42). These initial studies with whole killed S. aureus cells as immunogens did not offer promise that capsular antibodies alone would protect against staphylococcal infection.
Protection Afforded by CP5 and CP8 Antigens
Most purified capsular polysaccharides are poorly immunogenic in animals and humans. The immunogenicity of polysaccharides is classically T-cell independent, and no booster response is observed following multiple immunizations. Neither CP5 nor CP8 elicit serum antibodies when injected into mice (27, 28, 46) or cows (37, 111). However, if purified polysaccharides are covalently coupled to protein carrier molecules, they gain both increased immunogenicity and T-cell-dependent properties. Polysaccharide-protein conjugate vaccines elicit high levels of polysaccharide-specific antibodies, and antibody levels rise following booster doses of the vaccine. Several laboratories have synthesized conjugate vaccines consisting of S. aureus CP5 and CP8 covalently linked to protein (27, 37, 94). These conjugates are highly immunogenic in mice, cows, and humans, and they induce antibodies that opsonize serotype 5 and 8 S. aureus strains for phagocytosis (27, 28).
Fattom et al. (27) conjugated S. aureus CP5 and CP8 to nontoxic recombinant exotoxin A from Pseudomonas aeruginosa and administered this preparation to mice. The mice developed serum antibodies to the polysaccharides after two injections; the third injection stimulated a booster response. Differences in the carrier proteins and the chemical methods used to couple the proteins to the polysaccharide affected the magnitude of the immune response in mice, but these variables did not affect the distribution of IgG subclasses detected in immune serum (26). Use of monophosphoryl lipid A as an adjuvant enhanced the immunogenicity of the conjugate vaccines and induced a shift in the IgG subclass composition toward the more opsonic IgG2a and IgG2b subclasses in the immunized mice.
The protective efficacy of antibodies to the CP5-recombinant exotoxin A conjugate vaccine was tested in a mouse model of lethality and disseminated infection (29). Immunization with CP5-recombinant exotoxin A protected mice against lethality induced by a serotype 5 S. aureus strain. Ten days after bacterial challenge, 33 of 45 mice immunized with the conjugate survived, compared with 4 of 30 mice injected with phosphate-buffered saline. Similarly, passive immunization with immune IgG protected mice against S. aureus-induced lethality. Passive immunization of mice with immune IgG also resulted in a reduction in the level of bacteremia at 6 and 24 h after intraperitoneal inoculation of mice with a sublethal dose of serotype 5 S. aureus. In addition, fewer animals given immune IgG showed metastatic infection by S. aureus in their livers, kidneys, and peritoneal lavage fluids (Table 2).
TABLE 2.
CP5-specific antibodies given subcutaneously protect mice against systemic infection induced by 5 × 104 CFU of S. aureusa
| Sample | Normal IgG
|
Immune IgG
|
||
|---|---|---|---|---|
| Mean CFU | No. of positive mice/total | Mean CFU | No. of positive mice/total | |
| Peritoneal lavage fluid | 0.28 × 102 | 7/18 | 7 × 101 | 1/20 |
| Liver | 4.03 × 102 | 14/18 | 0.37 × 101 | 5/20 |
| Kidney | 7.20 × 102 | 17/18 | 0.28 × 101 | 3/20 |
Data are adapted from Fattom et al. (29).
Antibodies to the S. aureus capsular polysaccharide conjugate vaccine were then evaluated in a modified, catheter-induced model of staphylococcal endocarditis (66). In that study, rats were passively immunized intraperitoneally with IgG purified from nonimmunized rabbits or from rabbits immunized with the bivalent CP5- or CP8-recombinant exotoxin A conjugate vaccine. The following day the immunized rats were challenged by the intraperitoneal route, which resulted in a slow infusion of S. aureus into the blood of the catheterized rats. As shown in Table 3, rats given capsular antibodies showed a significantly (P < 0.05) lower prevalence of staphylococcal endocarditis (induced by each of three strains) than rats injected with nonimmune IgG. Similarly, quantitative cultures of the blood, kidneys, and aortic valve vegetations revealed that fewer S. aureus cells were recovered from rats given capsule-specific IgG than from rats administered nonimmune IgG. Human antibodies to the CP5 and CP8 conjugate vaccine also protected rats against endocarditis induced by two of three different serotype 8 S. aureus strains (J. C. Lee, unpublished data).
TABLE 3.
CP5-specific antibodies given intraperitoneally protect against endocarditis in rats challenged with serotype 5 S. aureusa
| Challenge strain and dose of S. aureus and IgG type | Log CFU/ml of blood at 24 h | No. of rats infected/total | Vegetation wt (g) | Log CFU/g of vegetation | Log CFU/g of kidney |
|---|---|---|---|---|---|
| Reynolds (4 × 107 CFU) | |||||
| Normal | 4.31 | 4/4 | 0.012 | 11.0 | 7.33 |
| CP-specific | 0.70 | 0/5 | 0 | 3.4 | 1.05 |
| Lowenstein (6 × 107 CFU) | |||||
| Normal | 1.30 | 8/11 | 0.011 | 11.0 | 7.84 |
| CP-specific | 0.70 | 4/13 | 0.003 | 3.4 | 4.85 |
| VP (7 × 107 CFU) | |||||
| Normal | 3.93 | 7/9 | 0.009 | 10.2 | 7.20 |
| CP-specific | 0.70 | 1/9 | 0 | 3.4 | 2.06 |
Data are adapted from Lee et al. (66) with permission.
The protection afforded by capsular antibodies elicited by the conjugate vaccine is in contrast to the lack of protection reported earlier by Nemeth and Lee (82). In that study, capsular antibodies were elicited by immunization with killed bacteria rather than by a capsular polysaccharide-conjugate vaccine. Another difference in experimental design between the two studies was that Nemeth and Lee (82) challenged the rats with an intravenous bolus dose of organisms, whereas in the more recent study, the animals were challenged by the intraperitoneal route. Capsular antibodies elicited by the conjugate vaccine did not protect against endocarditis when the bacterial inoculum was delivered as a bolus dose by the intravenous route to rabbits (A. S. Bayer, M. Ing, E. Kim, M. R. Yeaman, S. Shepherd, R. Naso, and A. Fattom, Abstr. 36th Int. Conf. Antimicrob. Agents Chemother., abstr. G-096) or rats (J. C. Lee, unpublished data). This finding suggests that the protective effect of capsular antibodies in the study reported by Lee et al. (66) may have been due primarily to local clearance of the bacterial cells in the peritoneal cavity, thereby preventing the staphylococci from gaining access to the bloodstream. The variable results of these vaccine studies underscore the importance of testing vaccine efficacy in multiple models of experimental S. aureus infection.
Active Immunization of Humans with CP5-CP8 Conjugate Vaccines
Fattom and colleagues at Nabi, Inc. (Boca Raton, Fla.), prepared conjugates of CP5 and CP8 linked to recombinant exotoxin A that are intended for commercial use. They combined the two polysaccharide vaccines into a bivalent vaccine called StaphVax that is intended for immunization of individuals at high risk for S. aureus infection.
Phase I and II clinical trials.
The CP8- and CP5-recombinant exotoxin A conjugate vaccines were evaluated for safety and immunogenicity in 70 healthy adult volunteers (27). Neither conjugate caused significant local or systemic reactions in the volunteers. The conjugate vaccine induced capsular polysaccharide-specific antibodies of both the IgM and IgG classes. A second injection 6 weeks later did not have a booster effect. The authors suggest that due to low levels of prevaccination capsular antibodies in these subjects, the initial vaccination behaved more like a booster than a primary dose.
Nabi conducted a phase II, double-blinded, placebo-controlled clinical study of StaphVax in ≈230 chronic ambulatory peritoneal dialysis patients, individuals who are at high risk of staphylococcal disease. The patients were actively immunized with StaphVax, and their antibody responses and infection rates were monitored. The vaccine was shown to elicit only mild local or systemic symptoms. The results of this trial indicated that the vaccine dose of 25 μg of each capsular polysaccharide in the conjugate formulation was suboptimal in these patients. Their antibody responses to the vaccine were weak, and they had infection rates similar to those of nonimmunized patients.
In subsequent phase II clinical trials, 32 volunteers with end-stage renal disease and 29 healthy controls were injected twice (6 weeks apart) with 25 μg of CP5-recombinant exotoxin A or the bivalent conjugate vaccine (25 μg each of CP5 and CP8 linked to recombinant exotoxin A). The vaccines elicited only mild local or systemic symptoms in both populations (119; G. Horwith, personal communication). Four weeks after the second dose of vaccine, 23 of 24 healthy volunteers and 14 of 17 patients in one study responded to the immunization with a ≥4-fold rise in preimmunization IgG and IgM antibody levels. However, the IgG and IgM levels of the patients were only ≈50% of those achieved by the healthy controls at all postimmunization intervals. The monovalent and bivalent vaccines did not contain any adjuvant. Data from animal studies indicate that enhanced immunogenicity may be achieved by incorporating an adjuvant such as monophosphoryl lipid A (26).
Phase III clinical trial.
Between 1998 and 2000, Nabi conducted a double-blind clinical trial to evaluate the safety, immunogenicity, and efficacy of StaphVax for prevention of bacteremia in 1,800 patients with end-stage renal disease receiving hemodialysis (99). These patients are at high risk for staphylococcal infection, with 3 to 4 of every 100 patients infected with S. aureus per year. Half of the patients in the trial were administered a placebo, and the other half were immunized with a single injection of Nabi's bivalent StaphVax (100 μg each of CP5 and CP8 conjugated to an equal weight of nontoxic recombinant exotoxin A). Efficacy was estimated by comparing the incidence of S. aureus bacteremia in the patients who received the vaccine with the incidence in control patients over weeks 3 to 54 following immunization.
During this time interval, the vaccine reduced the incidence of bacteremia in the study population by only 26% (not significant, P = 0.23) (99). However, when the data were analyzed to include only the time period between weeks 3 and 40, the vaccine efficacy was estimated to be 57% (P = 0.02). During this time period, S. aureus bacteremia developed in 11 of 892 patients in the vaccine group, compared with 26 of 906 patients in the control group. After 40 weeks the antibody levels in the vaccinated patients declined, mirroring the decline in efficacy of the vaccine. There were no significant differences in the number of deaths in the vaccine and control groups, and none of the deaths were considered related to the vaccine.
Many new questions were raised as a result of this clinical trial. Does immunization reduce S. aureus nasal colonization in these patients? Why was there no obvious correlation between capsular antibody levels induced by vaccination and susceptibility to staphylococcal bacteremia? Does impaired phagocyte function in these patients (about half are diabetic) explain why the estimated protective level of protective antibodies (>80 μg/ml) is so high? Can a vaccine that only targets the capsular polysaccharide protect patients against a bacterium with such a multitude of virulence determinants (adhesins, exoenzymes, and exotoxins)? Since CP5 and CP8 are not expressed in vitro during the logarithmic phase of bacterial growth or under all experimental conditions, does this contribute to the lack of efficacy of StaphVax in the clinical trial? Nabi plans another phase III clinical trial of their CP5-CP8 vaccine in hemodialysis patients. It is hoped that continued evaluation of StaphVax and other multicomponent S. aureus vaccines will lead to answers to some of these important questions.
PASSIVE IMMUNIZATION OF HUMANS WITH CAPSULAR ANTIBODIES
Another goal of Nabi, Inc., is to immunize healthy people with StaphVax to generate an immunoglobulin product with high levels of antibodies to S. aureus CP5 and CP8. Polyclonal antibodies have been purified from the plasma of healthy individuals who were immunized with StaphVax. The IgG antibody generated in this fashion, referred to as AltaStaph, would be administered in passive immunization studies to patients who are at immediate risk for staphylococcal infection. The circulating half-life of such antibodies is estimated to be 14 to 21 days (81). Twenty-nine infants with very low birth weight, at high risk for staphylococcal infection, were enrolled in a phase I-II safety and pharmacokinetics study conducted between 1998 and 1999 at the University of Texas. Subjects, stratified by weight (501 to 1,000 g and 1,001 to 1,500 g), were administered two injections of AltaStaph 2 weeks apart. Three doses (500, 750, and 1,000 μg kg−1) of AltaStaph were administered, assessing the safety of each dose prior to escalating to the next dose level. The results indicated that all of the doses were well tolerated. Additional trials with AltaStaph are planned to test its efficacy as a prophylactic agent to prevent bacteremia. In addition, Nabi hopes to evaluate the therapeutic effect of AltaStaph as an adjunct to antibiotics in patients with documented S. aureus infection (G. Horwith, personal communication).
GENETIC ANALYSIS OF S. AUREUS CAPSULE EXPRESSION
The earliest studies on the genetics of staphylococcal capsule expression were reported by Smith et al. (102), who showed that plasmid DNA did not play a role in capsule expression by the serotype 2 strain Smith diffuse. Ohtomo and Yoshida (84) subsequently provided evidence that chromosomal genes encode proteins involved in capsule biosynthesis by using DNA prepared from an encapsulated strain to transform a nonencapsulated S. aureus recipient.
Serotype 1 Capsule Gene Cluster
The capsule genes from the highly encapsulated S. aureus strain M (serotype 1) were cloned and sequenced in the laboratory of C. Y. Lee (60, 71, 74). Fifteen genes (cap1A through cap1O) clustered together on the bacterial chromosome constitute the capsule biosynthesis locus (cap1) in strain M. The cap1 operon was shown to be unique to serotype 1 isolates by Southern hybridization experiments performed under high-stringency conditions (58).
Molecular characterization, mutagenesis, and transcriptional analysis of the cap1 locus showed that the 15 cap1 genes were transcribed in the same orientation, resulting in a single ≈15.5-kb transcript (71, 85). Five internal promoters within the cap1 operon were identified but were much weaker than the primary promoter located upstream of the first gene, cap1A, as measured by gene fusion experiments with Pseudomonas xylE as the reporter (85). Nevertheless, the internal promoters were demonstrated to be biologically functional. Ouyang and Lee (85) proved that the internal promoters were sufficiently active for CP1 expression by creating a CP1-negative mutant strain in which the promoter upstream of cap1A was deleted. CP1 expression was restored to the mutant by integrating a single copy of a DNA fragment comprising the promoter upstream of cap1A together with cap1A through cap1E at the phage L54a attB site in the chromosome (remote from the cap1 locus). This construct physically separated the primary promoter together with the first five genes from the downstream cap1 genes. The recombinant strain was mucoid and produced a level of CP1 similar to that of the wild-type strain, indicating that transcription of the downstream cap1 genes by internal promoters was sufficient for capsule synthesis.
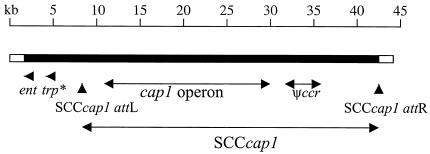
Recently, a 50-kb region comprising the cap1 gene locus and flanking region was sequenced (74). From this analysis, Luong et al. proposed that the cap1 operon is located within a staphylococcal cassette chromosome (SCC) element similar to the type III SCCmec island associated with methicillin resistance in S. aureus. The cap1 operon comprises more than half the size of the 27.4-kb SCCcap1 element (Fig. 4). The right boundary of SCCcap1, nearly identical to that of other SCCmec elements, had an attR site located approximately 10 kb downstream from the cap1 operon. The left boundary of SCCcap1, located 1 kb upstream of the cap1 cluster, contained direct and inverted repeats that matched the SCCmec consensus for att sites. The authors observed a high occurrence of mutations, deletions, and rearrangements within SCCcap1 and its left flanking region. Notably, only the cap1 genes and the enterotoxin gene located in the DNA region flanking SCCcap1 were intact and functional. The SCCcap1 element carries defective recombinase genes required for mobilization events (74), and this defect likely prevents the element from moving horizontally. This recombinase defect may at least partially explain why serotype 1 strains are so rare among clinical isolates of S. aureus.
FIG. 4.
SCCcap1 and flanking sequences from S. aureus strain M. Vertical arrowheads represent attachment sites of the SCCcap1 element. The cap1 operon is shown by double-headed arrows, as is the Ψccr gene complex of three open reading frames conserved among SCCmec elements. A novel enterotoxin gene (ent) and a truncated transposase gene (trp*) identified outside of the SCCcap1 are indicated by short arrows. The DNA region to the left of the ent gene is homologous to a gene region found in NCTC 8325, but many of the open reading frames within this region contain mutations. Data are adapted from Luong et al. (74).
A genetic analysis of the promoter upstream of cap1A in strain M (85) showed that deletions upstream of the −35 region had no effect on promoter activity, as measured by xylE reporter gene fusion experiments. The cap1 promoter required no upstream cis-acting element for activity, suggesting that the cap1 genes are constitutively expressed. Nonetheless, production of the type1 capsule has been shown to be unstable both in vitro and in vivo. Nonmucoid revertants of strain M arose at a frequency of 10−4 at 37°C and 10−2 to 3.8 × 10−1 at 43°C (60). Loss of the mucoid phenotype in strain M was due not to rearrangements but to random mutations within the genes in the cap1 locus (59).
Switching between the mucoid and nonmucoid phenotypes of a serotype 1 strain has been shown to occur upon animal passage. When nonmucoid mutants of strain M were injected intraperitoneally into mice, only mucoid colonies were recovered from the peritoneal washes of mice that succumbed to challenge (98). In contrast, loss of the mucoid phenotype was observed in a sublethal model of renal abscess formation. Organisms isolated from mouse kidneys early in the infection (prior to day 10) were all mucoid. However, by day 24, the majority of colonies cultured from the kidneys of infected animals were no longer mucoid, even though they were otherwise phenotypically identical to the mucoid challenge strain (J. C. Lee, unpublished observations). Thus, serotype 1 and 2 capsules, although not regulated at the transcriptional level, may be regulated by mutation and reversion in the structural genes required for capsule production.
Serotype 5 and 8 Capsule Gene Clusters
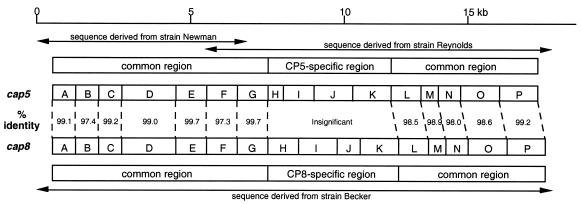
The nucleotide sequences of both the cap5 and cap8 gene clusters (GenBank accession numbers U81973 and U73374, respectively) have been determined (96). C. Y. Lee's laboratory sequenced the cap8 gene cluster, and the laboratories of T. J. Foster and J. C. Lee sequenced the cap5 gene cluster. The cap5 and cap8 loci are allelic and comprise a ≈17.5-kb region of the chromosome; each contains 16 closely linked genes, cap5A (cap8A) through cap5P (cap8P), transcribed in one orientation (Fig. 5). Both CP5 and CP8 are composed of the same three sugar residues, ManNAcA, l-FucNAc, and d-FucNAc. Therefore, it is not surprising that 12 of the 16 genes in the two gene clusters are nearly identical (96). The type-specific genes are located in the central region of the loci (comprising cap5H, cap5I, cap5J, and cap5K and the same genes for cap8), and they showed little homology between the two gene clusters. Wann and coworkers (118) transduced cap5HIJK into the type 8 strain P1; the genes were integrated into the chromosome by homologous recombination, with the reciprocal loss of cap8HIJK. The resultant strain produced CP5, indicating that cap5HIJK was responsible for CP5 serotype specificity.
FIG. 5.
Comparison of S. aureus cap5 and cap8 gene clusters. The cap5 sequence was derived from strains Newman and Reynolds, and the cap8 sequence was derived from strain Becker as shown. Gene designations are shown in boxes. Percent identity indicates the amino acid identity of the deduced proteins of the two clusters. Both gene clusters are transcribed from left to right. Data are from reference 96.
Molecular characterization of the cap8 locus.
Sau et al. reported that 11 of the 16 cap8 open reading frames are required for CP8 synthesis (97). Chromosomal mutations in cap8A, cap8B, cap8C, cap8J, and cap8P had no effect on the level of CP8 expression, as determined by immunoblotting detection techniques with CP8 antiserum. The cap8A mutation, made by a five-codon in-frame insertion, may have resulted in a silent mutation that did not affect CP8 expression. CP8 produced by a cap8B mutant exhibited a lower molecular mass than that made by the wild-type strain Becker, so Cap8B is likely a regulator of CP8 chain length. Because a cap8J mutant did not react with a monoclonal antibody specific to O-acetylated CP8, Cap8J is probably an O-acetyltransferase, similar to Cap5H, discussed below. The function of Cap5C and Cap8C is unknown, and, as discussed below, Cap5P is not essential because it has a functional homologue elsewhere on the staphylococcal chromosome (51).
Fusion studies with xylE as the reporter gene indicated that the promoter upstream of cap8A was the principal promoter, although weak internal promoters were observed in front of most of the downstream cap8 genes. Analysis of the cap8 gene sequence revealed several inverted and direct repeats upstream of the primary promoter. Ouyang et al. (86) performed deletion analyses and site-directed mutagenesis to demonstrate that one of these repeats, a 10-bp inverted repeat located 14 bp upstream of the cap8 promoter, was essential for promoter activity. Mutations within the 10-bp repeat reduced CP8 production to an undetectable level. The inverted repeat could serve as a DNA binding site for an activator that regulates cap8 gene transcription. Efforts to identify such a DNA-binding activator are under way in the laboratory of C. Y. Lee.
Luong and Lee recently replaced the native cap8 reporter of strain Becker with the strong constitutive promoter of the cap1 operon from strain M (73). The resultant strain, CYL770, synthesized approximately sevenfold more cap8-specific mRNA than wild-type strain Becker, and the strain produced about 80-fold more CP8. The CP8-overproducing strain CYL770 was more resistant to in vitro opsonophagocytic killing by human polymorphonuclear leukocytes. In a mouse infection model of bacteremia, strain CYL770 persisted longer in the bloodstream, liver, and spleen than the parent strain Becker. These results were the first to show that CP8 promoted S. aureus virulence in an animal model of infection, since previous studies had employed serotype 5 strains. The results of these studies also confirm those performed with serotype 1 strains (62) that demonstrated a positive correlation between increased capsule production and bacterial virulence.
BIOSYNTHESIS OF TYPE 5 CAPSULAR POLYSACCHARIDE
Until recently, little was known about the biosynthesis or regulation of capsule expression by S. aureus. ManNAcA, l-FucNAc, and d-FucNAc residues are components of S. aureus CP5 and CP8, but some of these sugars are also present in other bacterial polymers, such as the enterobacterial common antigen, capsular polysaccharides produced by Streptococcus pneumoniae and Bacteroides fragilis, and O antigens expressed by Pseudomonas aeruginosa and Escherichia coli (53). Many of the genes involved in the biosynthesis of these sugars are similar among these disparate genera. Because these polysaccharides play a clearcut role in the host-parasite interaction, a better understanding of their biosynthesis can further our understanding of bacterial pathogenesis and may offer new targets for therapeutic development.
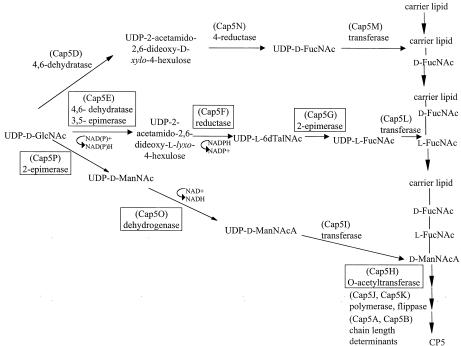
A comparison of the amino acid sequences of the putative cap5 and cap8 gene products with sequences found in the databases allowed us to predict functions for 15 of the 16 open reading frames and to propose a pathway for CP5 biosynthesis (Fig. 6). The laboratory of J. C. Lee has elucidated the function of six of the 16 cap5 genes.
FIG. 6.
Proposed pathway for the biosynthesis of S. aureus CP5. The gene products for which functions have been experimentally determined are shown in boxes. UDP-d-GlcNAc, UDP-2-acetamido-2-deoxy-d-glucose or UDP-N-acetyl-d-glucosamine; UDP-d-ManNAc, UDP-2-acetamido-2-deoxy-d-mannose or UDP-N-acetyl-d-mannosamine; UDP-l-FucNAc, UDP-2-acetamido-2,6-dideoxy-l-galactose or UDP-N-acetyl-l-fucosamine; UDP-d-ManNAcA, UDP-2-acetamido-2-deoxy-d-mannuronic acid or UDP-N-acetyl-d-mannosaminuronic acid; UDP-d-QuiNAc, UDP-2-acetamido-2,6-dideoxy-d-glucose or UDP-N-acetyl-d-quinovosamine; UDP-l-6dTalNAc, UDP-2-acetamido-2,6-dideoxy-l-talose or UDP-N-acetyl-l-pneumosamine.
cap5H
The predicted amino acid sequence of Cap5H encodes a protein of ≈26 kDa with a high degree of homology to a family of bacterial O-acetyltransferase genes. A mutant of S. aureus strain Reynolds containing a Tn918 insertion within cap5H produced wild-type levels of O-deacetylated CP5 (9). When provided in trans, cap5H complemented the gene defect in the O-deacetylated mutant. A type 5 strain with a cap5H mutation was less virulent than the parent strain in a mouse model of staphylococcal infection. In an in vitro assay, the parental strain was more resistant to opsonophagocytic killing than the cap5H mutant strain (9). These data suggest that the O-acetylated CP5 may be more proficient than the O-deacetylated polysaccharide in protecting S. aureus from host defenses.
cap5P and cap5O
The putative gene products of cap5P and cap5O bear homology to epimerase and dehydrogenase enzymes, respectively, that are involved in the biosynthesis of UDP-N-acetylmannosaminuronic acid (UDP-ManNAcA) from the precursor UDP-N-acetylglucosamine (UDP-GlcNAc) (96). The gene product of the cap5P gene was overexpressed in Escherichia coli, purified, and shown to have 2-epimerase activity, converting UDP-N-acetylglucosamine (UDP-GlcNAc) to UDP-N-acetylmannosamine (UDP-ManNAc) (53). Surprisingly, CP5 expression was not affected by insertional inactivation of cap5P. Kiser et al. demonstrated that this was due to the presence of a second UDP-GlcNAc-2-epimerase, mnaA, on the staphylococcal chromosome outside of the cap5 or cap8 locus (53).
The predicted amino acid sequence of Cap5O shows homology to dehydrogenase enzymes involved in the biosynthesis of ManNAcA (96). Portoles et al. overexpressed Cap5O in E. coli, purified the enzyme, and demonstrated that it oxidized UDP-ManNAc to UDP-ManNAcA in an NAD+-dependent reaction (89). A cap5O mutation created in S. aureus Reynolds rendered the bacterium negative for CP5; capsule expression was restored when cap5O was provided to the mutant in trans.
cap5E, cap5F, and cap5G
The putative gene products of cap5E, cap5F, and cap5G were predicted to be involved in the synthesis of UDP-N-acetyl-l-fucosamine (UDP-l-FucNAc) (63). Recently, S. aureus Cap5E, Cap5F, and Cap5G (and the homologues in Pseudomonas aeruginosa) were purified and shown to synthesize UDP-l-FucNAc in vitro from the precursor UDP-d-GlcNAc (55). Cap5E was shown to be a multifunctional enzyme that catalyzes the 4,6-dehydration and 3,5-epimerization of UDP-GlcNAc to yield a mixture of three ketodeoxy sugars. The third intermediate (UDP-2-acetamido-2,6-dideoxy-β-l-lyxo-4-hexulose) was subsequently reduced at C-4 by Cap5F to yield UDP-2-acetamido-2,6-dideoxy-l-talose (UDP-l-6dTalNAc). Incubation of UDP-l-6dTalNAc with Cap5G resulted in a new peak that was separable by capillary electrophoresis and presumed to be UDP-l-FucNAc. By mass spectroscopy, the new peak showed mass and fragmentation patterns identical to those of UDP-l-6dTalNAc. This result, consistent with 2-epimerization of UDP-l-6dTalNAc to UDP-l-FucNAc, was supported by the significant homology of Cap5G to other 2-epimerases (55). This report was the first to elucidate the biosynthetic pathway leading to UDP-l-FucNAc, a sugar residue common to several microbial capsules and lipopolysaccharide O antigens.
Previous reports by Wann and coworkers (118) indicated that the cap5E gene was essential for capsule production. A naturally occurring mutation in S. aureus NCTC 8325-4 could be mapped to cap5E, rendering the strain CP5 negative. If cap5E was provided to the strain in trans, capsule expression was restored. It has become apparent from sequence analysis of cap5E from this strain that a single amino acid change (M134 to R134) is responsible for its defect in capsule synthesis (55). This cap5E point mutation occurs in the SMK domain, a catalytic triad that has been determined to be critical for the activity of many functionally related enzymes.
Gene-specific mutations constructed in cap5F or cap5G of strain Newman abolished CP5 production (55). cap5G, provided in trans, restored CP5 production to the Newman cap5G mutant. However, cap5F did not appear to have a promoter to initiate its own transcription, since a plasmid carrying cap5F alone failed to complement the cap5F mutation. However, complementation of the cap5F deletion mutant could be achieved by introducing a plasmid carrying cap5ABCDEF into the mutant strain. A control plasmid carrying cap5ABCDE did not restore CP5 expression to the cap5F mutant (55).
Other cap5 and cap8 Genes
Although functions have been ascribed to other capsule genes based on sequence homology (59, 63), experimental evidence is lacking to support their actual activities. Cap5B and Cap8B could be a regulator of polysaccharide chain length, since CP8 produced by a cap8B mutant exhibited a lower molecular mass than that synthesized by the wild-type strain (63). Cap5D and Cap5N are likely involved in the synthesis of UDP-d-FucNAc. Based on its homology to other proteins that are involved in the biosynthesis of bacterial polysaccharides, such as P. aeruginosa WbpM (10, 14), we predict that Cap5D has 4,6 dehydratase activity, converting the precursor UDP-GlcNAc to UDP-2-acetamido-2,6 dideoxy-d-xylo-4-hexulose. This intermediate could then be stereospecifically reduced at C-4 to UDP-d-FucNAc by Cap5N. Cap5N shares considerable identity (40%) at the amino acid level with various UDP-Glc-4-epimerases. However, Cap5N also has at least two motifs that are characteristic of proteins with reductase activity: a GxxGxxG nucleotide binding motif and an SYK domain that is the signature for a family of enzymes that includes many reductases. Work is underway in our laboratory to experimentally verify this pathway. The functions of these genes and the cap5 and cap8 genes involved with putative transferase, polymerase, and flippase activities are still unconfirmed.
GENETIC REGULATION OF CAPSULE EXPRESSION
The regulatory loci agr and sarA control the expression of many S. aureus adhesins and exoproteins. The agr locus is a complex multigene system that responds to bacterial cell density. At high cell densities, a secreted octapeptide binds to the membrane receptor AgrC, activating the AgrA regulator by a phosphorylation mechanism. Phosphorylated AgrA upregulates promoters within the agr locus to produce the regulatory effector molecule RNA III. The staphylococcal accessory regulator (sarA) gene product activates the agr promoter as well as the promoters of certain other virulence genes, independently of agr. SarA binds to a consensus motif upstream of the −35 sequences of those promoters that it regulates.
Dassy et al. (18) first demonstrated that agr positively regulated CP5 production by S. aureus, and this observation was confirmed by Polhlmann-Dietze et al. (88). Subsequently, Luong et al. (72) constructed agr and sarA single mutants and an agr-sarA double mutant from the serotype 8 strain Becker. The agr mutant showed minimal CP8 production and minimal cap8 gene transcription, confirming that agr is a major regulator of CP8 expression. The results of gene fusion studies indicated that regulation by agr was exerted at the transcriptional level. The sarA gene was also shown to activate cap8 gene expression at the transcriptional level, but the effect was only minor compared to that of agr (72). Likewise, van Wamel et al. reported that agr positively regulated cap5 expression both in vitro and in vivo in a rabbit endocarditis model (114). In comparison, sarA exerted a lesser positive impact on cap5 expression. Recently, Luong et al. (74a) described a novel global regulator called mgr that bears homology to the MarR family of transcriptional regulators. mgr upregulated CP8 and nuclease expression but repressed alpha toxin, coagulase, protease, and protein A expression. The specific role of other global regulators in capsule expression is still unknown.
CONCLUSIONS
The capsular polysaccharides expressed by S. aureus are clearly important in the pathogenesis of staphylococcal infections. They enhance staphylococcal virulence by impeding phagocytosis, resulting in bacterial persistence in the bloodstream of infected hosts. S. aureus capsules also promote abscess formation. Although CP5 and CP8 have been shown to modulate S. aureus adherence to endothelial surfaces in vitro, animal studies suggest that the capsule promotes bacterial colonization and persistence on mucosal surfaces. S. aureus capsular antigens are surface associated, limited in antigenic specificity, and highly conserved among clinical isolates.
With the emergence of vancomycin-resistant S. aureus in the United States in 2002, new strategies are needed to combat staphylococcal infections. CP5 and CP8 offer promise as target antigens for a vaccine to prevent staphylococcal infections, although the inclusion of other antigens is likely to be essential in the development of an effective S. aureus vaccine. The genetics and mechanisms of capsule biosynthesis are complex, and much work remains to enhance our understanding of capsule biosynthesis and its regulation.
Acknowledgments
This work was supported by NIH grants AI-23244, AI-29040, and AI-44136 and by previous support from Nabi, Inc.
The many contributions of the personnel that have worked with our group over the years are acknowledged.
REFERENCES
- 1.Albus, A., R. D. Arbeit, and J. C. Lee. 1991. Virulence of Staphylococcus aureus mutants altered in type 5 capsule production. Infect. Immun. 59:1008-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albus, A., J. M. Fournier, C. Wolz, A. Boutonnier, M. Ranke, N. Hoiby, H. Hochkeppel, and G. Doring. 1988. Staphylococcus aureus capsular types and antibody response to lung infection in patients with cystic fibrosis. J. Clin. Microbiol. 26:2505-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbeit, R. D., and R. M. Dunn. 1987. Expression of capsular polysaccharide during experimental focal infection with Staphylococcus aureus. J. Infect. Dis. 156:947-952. [DOI] [PubMed] [Google Scholar]
- 4.Arbeit, R. D., W. W. Karakawa, W. F. Vann, and J. B. Robbins. 1984. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2:85-91. [DOI] [PubMed] [Google Scholar]
- 5.Arbeit, R. D., and M. J. Nelles. 1987. Capsular polysaccharide antigenemia in rats with experimental endocarditis due to Staphylococcus aureus. J. Infect. Dis. 155:242-246. [DOI] [PubMed] [Google Scholar]
- 6.Arizono, T., A. Umeda, and K. Amako. 1991. Distribution of capsular materials on the cell wall surface of strain Smith diffuse of Staphylococcus-aureus. J. Bacteriol. 173:4333-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baddour, L. M., C. Lowrance, A. Albus, J. H. Lowrance, S. K. Anderson, and J. C. Lee. 1992. Staphylococcus aureus microcapsule expression attenuates bacterial virulence in a rat model of experimental endocarditis. J. Infect. Dis. 165:749-753. [DOI] [PubMed] [Google Scholar]
- 8.Reference deleted.
- 9.Bhasin, N., A. Albus, F. Michon, P. J. Livolsi, J.-S. Park, and J. C. Lee. 1998. Identification of a gene essential for O-acetylation of the Staphylococcus aureus type 5 capsular polysaccharide. Mol. Microbiol. 27:9-21. [DOI] [PubMed] [Google Scholar]
- 10.Burrows, L. L., R. V. Urbanic, and J. S. Lam. 2000. Functional conservation of the polysaccharide biosynthetic protein WbpM and its homologues in Pseudomonas aeruginosa and other medically significant bacteria. Infect. Immun. 68:931-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers, H. F. 2001. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 7:178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensson, B., A. Boutonnier, U. Ryding, and J. M. Fournier. 1991. Diagnosing Staphylococcus aureus endocarditis by detecting antibodies against S. aureus capsular polysaccharide type 5 and 8. J. Infect. Dis. 163:530-533. [DOI] [PubMed] [Google Scholar]
- 13.Cohn, Z. 1962. Determinants of infection in the peritoneal cavity. 1. Response to and fate of Staphyococcus aureus and Staphylococcus albus in the mouse. Yale J. Biol. Med. 35:12-28. [PMC free article] [PubMed] [Google Scholar]
- 14.Creuzenet, C., and J. S. Lam. 2001. Topological and functional characterization of WbpM, an inner membrane UDP-GlcNAc C6 dehydratase essential for lipopolysaccharide biosynthesis in Pseudomonas aeruginosa. Mol. Microbiol. 41:1295-1310. [DOI] [PubMed] [Google Scholar]
- 15.Cunnion, K. M., J. C. Lee, and M. M. Frank. 2001. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect. Immun. 69:6796-6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunnion, K. M., H. M. Zhang, and M. M. Frank. 2003. Availability of complement bound to Staphylococcus aureus to interact with membrane complement receptors influences efficiency of phagocytosis. Infect. Immun. 71:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dassy, B., and J. M. Fournier. 1996. Respiratory activity is essential for post-exponential-phase production of type 5 capsular polysaccharide by Staphylococcus aureus. Infect. Immun. 64:2408-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dassy, B., T. Hogan, T. J. Foster, and J. M. Fournier. 1993. Involvement of the accessory gene regulator (agr) in expression of type-5 capsular polysaccharide by Staphylococcus aureus. J. Gen. Microbiol. 139:1301-1306. [DOI] [PubMed] [Google Scholar]
- 19.Dassy, B., W. T. Stringfellow, M. Lieb, and J. M. Fournier. 1991. Production of type 5 capsular polysaccharide by Staphylococcus aureus grown in a semisynthetic medium. J. Gen. Microbiol. 137:1155-1162. [DOI] [PubMed] [Google Scholar]
- 20.Daum, R. S., A. Fattom, S. Freese, and W. Karakawa. 1994. Capsular polysaccharide serotypes of coagulase-positive staphylococci associated with tenosynovitis, osteomyelitis, and other invasive infections in chickens and turkeys: evidence for new capsular types. Avian Dis. 38:762-771. [PubMed] [Google Scholar]
- 21.Donnelly, T. M., and D. M. Stark. 1985. Susceptibility of laboratory rats, hamsters, and mice to wound infection with Staphylococcus aureus. Am. J. Vet. Res. 46:2634-2638. [PubMed] [Google Scholar]
- 22.Dufour, P., Y. Gillet, M. Bes, G. Lina, F. Vandenesch, D. Floret, J. Etienne, and H. Richet. 2002. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin. Infect. Dis. 35:819-824. [DOI] [PubMed] [Google Scholar]
- 23.Ekstedt, R. 1963. Studies on immunity to staphylococcal infection in mice. I. Effect of dosage, viability, and interval between immunization and challenge on resistance to infection following injection of whole cell vaccines. J. Infect. Dis. 112:143-151. [Google Scholar]
- 24.Ekstedt, R. 1966. Studies on immunity to staphylococcal infection in mice. intravenously. The role of specific and nonspecific immunity. J. Infect. Dis. 116:514-522. [DOI] [PubMed] [Google Scholar]
- 25.Ekstedt, R. D. 1963. Studies on immunity to staphylococcal infection in mice. II. Effect of immunization with fractions of Staphylococcus aureus prepared by physical and chemical methods. J. Infect. Dis. 112:152-157. [Google Scholar]
- 26.Fattom, A., X. Li, Y. H. Cho, A. Burns, A. Hawwari, S. E. Shepherd, R. Coughlin, S. Winston, and R. Naso. 1995. Effect of conjugation methodology, carrier protein, and adjuvants on the immune response to Staphylococcus aureus capsular polysaccharides. Vaccine 13:1288-1293. [DOI] [PubMed] [Google Scholar]
- 27.Fattom, A., R. Schneerson, D. C. Watson, W. W. Karakawa, D. Fitzgerald, I. Pastan, X. Li, J. Shiloach, D. A. Bryla, and J. B. Robbins. 1993. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect Immun. 61:1023-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fattom, A., R. Schneerson, S. C. Szu, W. F. Vann, J. Shiloach, W. W. Karakawa, and J. B. Robbins. 1990. Synthesis and immunologic properties in mice of vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides conjugated to Pseudomonas aeruginosa exotoxin A. Infect. Immun. 58:2367-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fattom, A. I., J. Sarwar, A. Ortiz, and R. Naso. 1996. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect. Immun. 64:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher, M., H. Devlin, and A. Erlandson. 1963. A new staphylococcal antigen. Its preparation and immunizing activity against experimental infections. Nature 199:1074-1075. [DOI] [PubMed] [Google Scholar]
- 31.Fisher, S. 1960. A heat stable protective staphylococcal antigen. Aust. J. Exp. Biol. 38:479-486. [DOI] [PubMed] [Google Scholar]
- 32.Fournier, J. M., K. Hannon, M. Moreau, W. W. Karakawa, and W. F. Vann. 1987. Isolation of type 5 capsular polysaccharide from Staphylococcus aureus. Ann. Inst. Pasteur Microbiol. 138:561-567. [DOI] [PubMed] [Google Scholar]
- 33.Fournier, J. M., W. F. Vann, and W. W. Karakawa. 1984. Purification and characterization of Staphylococcus aureus type 8 capsular polysaccharide. Infect. Immun. 45:87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox, K. F., G. C. Stewart, and A. Fox. 1998. Synthesis of microcapsule by Staphylococcus aureus is not responsive to environmental phosphate concentrations. Infect. Immun. 66:4004-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frank, A. L., J. F. Marcinak, P. D. Mangat, and P. C. Schreckenberger. 1999. Increase in community-acquired methicillin-resistant Staphylococcus aureus in children. Clin. Infect. Dis. 29:935-936. [DOI] [PubMed] [Google Scholar]
- 36.Gibson, R. L., M. K. Lee, C. Soderland, E. Y. Chi, and C. E. Rubens. 1993. Group B streptococci invade endothelial cells: type III capsular polysaccharide attenuates invasion. Infect. Immun. 61:478-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilbert, F. B., B. Poutrel, and L. Sutra. 1994. Immunogenicity in cows of Staphylococcus aureus type 5 capsular polysaccharide-ovalbumin conjugate. Vaccine 12:369-374. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert, I. 1931. Dissociation in an encapsulated staphylococcus. J. Bacteriol. 21:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillet, Y., B. Issartel, P. Vanhems, J. C. Fournet, G. Lina, M. Bes, F. Vandenesch, Y. Piemont, N. Brousse, D. Floret, and J. Etienne. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753-759. [DOI] [PubMed] [Google Scholar]
- 40.Gorak, E. J., S. M. Yamada, and J. D. Brown. 1999. Community-acquired methicillin-resistant Staphylococcus aureus in hospitalized adults and children without known risk factors. Clin. Infect. Dis. 29:797-800. [DOI] [PubMed] [Google Scholar]
- 41.Gorrill, R. H. 1958. The establishment of staphylococcal abscesses in the mouse kidney. Br. J. Exp. Pathol. 39:203-212. [PMC free article] [PubMed] [Google Scholar]
- 42.Greenberg, D. P., J. I. Ward, and A. S. Bayer. 1987. Influence of Staphylococcus aureus antibody on experimental endocarditis in rabbits. Infect. Immun. 55:3030-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guidry, A., A. Fattom, A. Patel, and C. O'Brien. 1997. Prevalence of capsular serotypes among Staphylococcus aureus isolates from cows with mastitis in the United States. Vet. Microbiol. 59:53-58. [DOI] [PubMed] [Google Scholar]
- 44.Hanessian, S., and T. Haskell. 1964. Structural studies on staphylococcal polysaccharide antigen. J. Biol. Chem. 239:2758-2764. [PubMed] [Google Scholar]
- 45.Hensen, S. M., M. J. Pavicic, J. A. Lohuis, J. A. de Hoog, and B. Poutrel. 2000. Location of Staphylococcus aureus within the experimentally infected bovine udder and the expression of capsular polysaccharide type 5 in situ. J. Dairy Sci. 83:1966-1975. [DOI] [PubMed] [Google Scholar]
- 46.Herbelin, C., and B. Poutrel. 1996. Immunogenicity in mice of multivalent conjugates composed of Staphylococcus aureus type 5 and 8 capsular polysaccharides and α- and β-toxins. Vaccine Res. 5:15-28. [Google Scholar]
- 47.Herbert, S., S. W. Newell, C. Lee, K. P. Wieland, B. Dassy, J. M. Fournier, C. Wolz, and G. Doring. 2001. Regulation of Staphylococcus aureus type 5 and type 8 capsular polysaccharides by CO2. J. Bacteriol. 183:4609-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herbert, S., D. Worlitzsch, B. Dassy, A. Boutonnier, J.-M. Fournier, G. Bellon, A. Dalhoff, and G. Doring. 1997. Regulation of Staphylococcus aureus capsular polysaccharide type 5: CO2 inhibition in vitro and in vivo. J. Infect. Dis. 176:431-438. [DOI] [PubMed] [Google Scholar]
- 49.Hochkeppel, H. K., D. G. Braun, W. Vischer, A. Imm, S. Sutter, U. Staeubli, R. Guggenheim, E. L. Kaplan, A. Boutonnier, and J. M. Fournier. 1987. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J. Clin. Microbiol. 25:526-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hussain, F. M., S. Boyle-Vavra, C. D. Bethel, and R. S. Daum. 2000. Current trends in community-acquired methicillin-resistant Staphylococcus aureus at a tertiary care pediatric facility. Pediatr. Infect. Dis. J. 19:1163-1166. [DOI] [PubMed] [Google Scholar]
- 51.Karakawa, W. W., A. Sutton, R. Schneerson, A. Karpas, and W. F. Vann. 1988. Capsular antibodies induce type-specific phagocytosis of capsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect. Immun. 56:1090-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karakawa, W. W., and W. F. Vann. 1982. Capsular polysaccharides of Staphylococcus aureus. Semin. Infect. Dis. 4:285-293. [Google Scholar]
- 53.Kiser, K. B., N. Bhasin, L. Deng, and J. C. Lee. 1999. Staphylococcus aureus cap5P encodes a UDP-N-acetylglucosamine 2-epimerase with functional redundancy. J. Bacteriol. 181:4818-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kiser, K. B., J. M. Cantey-Kiser, and J. C. Lee. 1999. Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect. Immun. 67:5001-5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kneidinger, B., K. O'Riordan, J. Li, J.-R. Brisson, J. C. Lee, and J. S. Lam. 2003. Three highly conserved proteins catalyze the conversion of UDP-N-acetyl-d-glucosamine to precursors for the biosynthesis of O antigen in Pseudomonas aeruginosa O11 and capsule in Staphylococcus aureus type 5 — implications for the UDP-N-acetyl-l-fucosamine biosynthetic pathway. J. Biol. Chem. 278:3615-3627. [DOI] [PubMed] [Google Scholar]
- 56.Koenig, M. G. 1962. Factors relating to the virulence of staphylococci. I. Comparative studies on two colonial variants. Yale J. Biol. Med. 34:537-559. [PMC free article] [PubMed] [Google Scholar]
- 57.Kuypers, J. M., and R. A. Proctor. 1989. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect. Immun. 57:2306-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee, C. Y. 1995. Association of staphylococcal type-1 capsule-encoding genes with a discrete genetic element. Gene 167:115-119. [DOI] [PubMed] [Google Scholar]
- 59.Lee, C. Y. 2001. Capsule production, p. 49-66. In A. L. Honeyman, H. Friedman, and M. Bendinelli (ed.), Staphylococcus aureus infection and disease. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 60.Lee, C. Y. 1992. Cloning of genes affecting capsule expression in Staphylococcus aureus strain M. Mol. Microbiol. 6:1515-1522. [DOI] [PubMed] [Google Scholar]
- 61.Lee, J. C. 2001. Capsule and vaccine development, p. 39-47. In A. L. Honeyman, H. Friedman, and M. Bendinelli (ed.), Staphylococcus aureus infection and disease. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 62.Lee, J. C., M. J. Betley, C. A. Hopkins, N. E. Perez, and G. B. Pier. 1987. Virulence studies, in mice, of transposon-induced mutants of Staphylococcus aureus differing in capsule size. J. Infect. Dis. 156:741-750. [DOI] [PubMed] [Google Scholar]
- 63.Lee, J. C., and C. Y. Lee. 1999. Capsular polysaccharides of Staphylococcus aureus, p. 185-205. In J. B. Goldberg (ed.), Genetics of bacterial polysaccharides. CRC Press, Boca Raton, Fla.
- 64.Lee, J. C., M. J. Liu, J. Parsonnet, and R. D. Arbeit. 1990. Expression of type-8 capsular polysaccharide and production of toxic shock syndrome toxin-1 are associated among vaginal isolates of Staphylococcus aureus. J. Clin. Microbiol. 28:2612-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee, J. C., F. Michon, N. E. Perez, C. A. Hopkins, and G. B. Pier. 1987. Chemical characterization and immunogenicity of capsular polysaccharide isolated from mucoid Staphylococcus aureus. Infect. Immun. 55:2191-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee, J. C., J.-S. Park, S. E. Shepherd, V. Carey, and A. Fattom. 1997. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect. Immun. 65:4146-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee, J. C., N. E. Perez, C. A. Hopkins, and G. B. Pier. 1988. Purified capsular polysaccharide-induced immunity to Staphylococcus aureus infection. J. Infect. Dis. 157:723-730. [DOI] [PubMed] [Google Scholar]
- 68.Lee, J. C., S. Takeda, P. J. Livolsi, and L. C. Paoletti. 1993. Effects of in vitro and in vivo growth conditions on expression of type-8 capsular polysaccharide by Staphylococcus aureus. Infect. Immun. 61:1853-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leijh, P. C., M. T. van den Barselaar, M. R. Daha, and R. van Furth. 1981. Participation of immunoglobulins and complement components in the intracellular killing of Staphylococcus aureus and Escherichia coli by human granulocytes. Infect. Immun. 33:714-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liau, D. F., and J. H. Hash. 1977. Structural analysis of the surface polysaccharide of Staphylococcus aureus M. J. Bacteriol. 131:194-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin, W. S., T. Cunneen, and C. Y. Lee. 1994. Sequence analysis and molecular characterization of genes required for the biosynthesis of type 1 capsular polysaccharide in Staphylococcus aureus. J. Bacteriol. 176:7005-7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luong, T., S. Sau, M. Gomez, J. C. Lee, and C. Y. Lee. 2002. Regulation of Staphylococcus aureus capsular polysaccharide expression by agr and sarA. Infect. Immun. 70:444-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luong, T. T., and C. Y. Lee. 2002. Overproduction of type 8 capsular polysaccharide augments Staphylococcus aureus virulence. Infect. Immun. 70:3389-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luong, T. T., S. Ouyang, K. Bush, and C. Y. Lee. 2002. Type 1 capsule genes of Staphylococcus aureus are carried in a staphylococcal cassette chromosome genetic element. J. Bacteriol. 184:3623-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74a.Luong, T. T., S. W. Newell, and C. Y. Lee. 2003. mgr, a novel global regulator in Staphylococcus aureus. J. Bacteriol. 185:3703-3710. [DOI] [PMC free article] [PubMed]
- 75.McKenney, D., K. L. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Doring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523-1527. [DOI] [PubMed] [Google Scholar]
- 76.Melly, M., L. Duke, D.-F. Liau, and J. Hash. 1974. Biological properties of the encapsulated Staphylococcus aureus M. Infect. Immun. 10:389-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moran, G. J., and J. Mount. 2003. Staphylococcus aureus resistant to vancomycin—United States, 2002. Ann. Emerg. Med. 41:148-151.12514697 [Google Scholar]
- 78.Moreau, M., J. C. Richards, J. M. Fournier, R. A. Byrd, W. W. Karakawa, and W. F. Vann. 1990. Structure of the type-5 capsular polysaccharide of Staphylococcus aureus. Carbohydr. Res. 201:285-297. [DOI] [PubMed] [Google Scholar]
- 79.Moreillon, P., J. M. Entenza, P. Francioli, D. McDevitt, T. J. Foster, P. Francois, and P. Vaudaux. 1995. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect. Immun. 63:4738-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murthy, S. V., M. A. Melly, T. M. Harris, C. G. Hellerqvist, and J. H. Hash. 1983. The repeating sequence of the capsular polysaccharide of Staphylococcus aureus M. Carbohydr. Res. 117:113-123. [DOI] [PubMed] [Google Scholar]
- 81.Naso, R., and A. Fattom. 1996. Polysaccharide conjugate vaccines for the prevention of gram-positive bacterial infections, p. 133-140. In S. Cohen and A. Shafferman (ed.), Novel strategies in design and production of vaccines. Plenum Press, New York, N.Y. [DOI] [PubMed]
- 82.Nemeth, J., and J. C. Lee. 1995. Antibodies to capsular polysaccharides are not protective against experimental Staphylococcus aureus endocarditis. Infect. Immun. 63:375-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nilsson, I.-M., J. C. Lee, T. Bremell, C. Ryden, and A. Tarkowski. 1997. The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis. Infect. Immun. 65:4216-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohtomo, T., and K. Yoshida. 1978. Encapsulation by transformation of strains of Staphylococcus aureus determined by the serum-soft agar technique. Zentralbl. Bakteriol. Hyg. Abt. Orig. A 242:436-445. [PubMed] [Google Scholar]
- 85.Ouyang, S., and C. Y. Lee. 1997. Transcriptional analysis of type 1 capsule genes in Staphylococcus aureus. Mol. Microbiol. 23:473-482. [DOI] [PubMed] [Google Scholar]
- 86.Ouyang, S., S. Sau, and C. Y. Lee. 1999. Promoter analysis of the cap8 operon, involved in type 8 capsular polysaccharide production in Staphylococcus aureus. J. Bacteriol. 181:2492-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peterson, P., B. Wilkinson, Y. Kim, Schmeling, and P. Quie. 1978. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect. Immun. 19:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pohlmann-Dietze, P., M. Ulrich, K. B. Kiser, G. Doring, J. C. Lee, J. M. Fournier, K. Botzenhart, and C. Wolz. 2000. Adherence of Staphylococcus aureus to endothelial cells: influence of the capsular polysaccharide, the global regulator agr, and the bacterial growth phase. Infect. Immun. 68:4865-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Portoles, M., K. B. Kiser, N. Bhasin, K. H. N. Chan, and J. C. Lee. 2001. Staphylococcus aureus Cap5O has UDP-ManNAc dehydrogenase activity and is essential for capsule expression. Infect. Immun. 69:917-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poutrel, B., F. B. Gilbert, and M. Lebrun. 1995. Effects of culture conditions on production of type 5 capsular polysaccharide by human and bovine Staphylococcus aureus strains. Clin. Diagn. Lab. Immunol. 2:166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Poutrel, B., P. Rainard, and P. Sarradin. 1997. Heterogeneity of cell-associated CP5 expression on Staphylococcus aureus strains demonstrated by flow cytometry. Clin. Diagn. Lab. Immunol. 4:275-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Poutrel, B., and L. Sutra. 1993. Type 5 and type 8 capsular polysaccharides are expressed by Staphylococcus aureus isolates from rabbits, poultry, pigs, and horses. J. Clin. Microbiol. 31:467-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Projan, S. J., and R. P. Novick. 1997. The molecular basis of pathogenicity, p. 55-81. In K. B. Crossley and G. L. Archer (ed.), The staphyloccci in human disease. Churchill Livingstone, New York, N.Y.
- 94.Reynaud-Rondier, L., A. Voiland, and G. Michel. 1991. Conjugation of capsular polysaccharide to α-hemolysin from Staphylococcus aureus as a glycoprotein antigen. FEMS Microbiol. Lett. 76:193-200. [DOI] [PubMed] [Google Scholar]
- 95.Robbins, J. B., S. R., W. B. Egan, W. Vann, and D. Liu. 1980. Virulence properties of bacterial capsular polysaccharides-unanswered questions, p. 115-132. In H. Smith, J. Skehel, and M. Turner (ed.), The molecular basis of microbial pathogenicity. Verlag Chemie GmbH, Weinheim, Germany.
- 96.Sau, S., N. Bhasin, E. R. Wann, J. C. Lee, T. J. Foster, and C. Y. Lee. 1997. The Staphylococcus aureus allelic genetic loci for serotype 5 and 8 capsule expression contain the type-specific genes flanked by common genes. Microbiology 143:2395-2405. [DOI] [PubMed] [Google Scholar]
- 97.Sau, S., J. Sun, and C. Y. Lee. 1997. Molecular characterization and transcriptional analysis of type 8 capsule genes in Staphylococcus aureus. J. Bacteriol. 179:1614-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scott, A. 1969. A capsulate Staphylococcus aureus. J. Med. Microbiol. 2:253-260. [DOI] [PubMed] [Google Scholar]
- 99.Shinefield, H., S. Black, A. Fattom, G. Horwith, S. Rasgon, J. Ordonez, H. Yeoh, D. Law, J. B. Robbins, R. Schneerson, L. Muenz, S. Fuller, J. Johnson, B. Fireman, H. Alcorn, and R. Naso. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346:491-496. [DOI] [PubMed] [Google Scholar]
- 100.Singh, G., R. R. Marples, and A. M. Kligman. 1971. Experimental Staphylococcus aureus infections in humans. J. Investig. Dermatol. 57:149-162. [DOI] [PubMed] [Google Scholar]
- 101.Smith, I. M., A. P. Wilson, E. Hazard, H. W. K., and M. E. Dewey. 1960. Death from staphylococci in mice. J. Infect. Dis. 107:369-378. [Google Scholar]
- 102.Smith, R. M., J. T. Parisi, L. Vidal, and J. N. Baldwin. 1977. Nature of the genetic determinant controlling encapsulation in Staphylococcus aureus Smith. Infect. Immun. 17:231-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sompolinsky, D., Z. Samra, W. W. Karakawa, W. F. Vann, R. Schneerson, and Z. Malik. 1985. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J. Clin. Microbiol. 22:828-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sordelli, D. O., F. R. Buzzola, M. I. Gomez, L. Steele-Moore, D. Berg, E. Gentilini, M. Catalano, A. J. Reitz, T. Tollersrud, G. Denamiel, P. Jeric, and J. C. Lee. 2000. Capsule expression by bovine isolates of Staphylococcus aureus from Argentina: genetic and epidemiologic analyses. J. Clin. Microbiol. 38:846-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stephens, D. S., P. A. Spellman, and J. S. Swartley. 1993. Effect of the (alpha 2→8)-linked polysialic acid capsule on adherence of Neisseria meningitidis to human mucosal cells. J. Infect. Dis. 167:475-479. [DOI] [PubMed] [Google Scholar]
- 106.St. Geme, J. W., and S. Falkow. 1991. Loss of capsule expression by Haemophilus influenzae type b results in enhanced adherence to and invasion of human cells. Infect. Immun. 59:1325-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stringfellow, W. T., B. Dassy, M. Lieb, and J. M. Fournier. 1991. Staphylococcus aureus growth and type 5 capsular polysaccharide production in synthetic media. Appl. Environ. Microbiol. 57:618-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sutra, L., P. Rainard, and B. Poutrel. 1990. Phagocytosis of mastitis isolates of Staphylococcus aureus and expression of type 5 capsular polysaccharide are influenced by growth in the presence of milk. J. Clin. Microbiol. 28:2253-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thakker, M., J.-S. Park, V. Carey, and J. C. Lee. 1998. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect. Immun. 66:5183-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tollersrud, T., K. Kenny, A. J. Reitz, and J. C. Lee. 2000. Genetic and serologic evaluation of capsule production by bovine mammary isolates of Staphylococcus aureus and other Staphylococcus spp. from Europe and the United States. J. Clin. Microbiol. 38:2998-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tollersrud, T., L. Zernichow, S. R. Andersen, K. Kenny, and A. Lund. 2001. Staphylococcus aureus capsular polysaccharide type 5 conjugate and whole cell vaccines stimulate antibody responses in cattle. Vaccine 19:3896-3903. [DOI] [PubMed] [Google Scholar]
- 112.Tzianabos, A. O., J. Y. Wang, and J. C. Lee. 2001. Structural rationale for the modulation of abscess formation by Staphylococcus aureus capsular polysaccharides. Proc. Natl. Acad. Sci. USA 98:9365-9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vann, W. F., M. Moreau, A. Sutton, R. A. Byrd, and W. W. Karakawa. 1988. Structure and immunochemistry of Staphylococcus aureus capsular polysaccharides, p. 187-198. In M. A. Horwitz (ed.), Bacterial-host cell interaction, vol. 64. UCLA Symposia on Molecular and Cellular Biology. Alan R. Liss, Inc., New York, N.Y.
- 114.van Wamel, W., Y. Q. Xiong, A. S. Bayer, M. R. Yeaman, C. C. Nast, and A. L. Cheung. 2002. Regulation of Staphylococcus aureus type 5 capsular polysaccharides by agr and sarA in vitro and in an experimental endocarditis model. Microb. Pathog. 33:73-79. [DOI] [PubMed] [Google Scholar]
- 115.Verbrugh, H., R. Peters, M. Rozenberg-Arska, P. Peterson, and J. Verhoef. 1981. Antibodies to cell wall peptidoglycan of Staphylococcus aureus in patients with serious staphylococcal infections. J. Infect. Dis. 144:1-9. [DOI] [PubMed] [Google Scholar]
- 116.Verbrugh, H. A., P. K. Peterson, B. Y. Nguyen, S. P. Sisson, and Y. Kim. 1982. Opsonization of encapsulated Staphylococcus aureus: the role of specific antibody and complement. J. Immunol. 129:1681-1687. [PubMed] [Google Scholar]
- 117.Verhoef, J., P. Peterson, Y. Kim, L. D. Sabath, and P. G. Quie. 1977. Opsonic requirements for staphylococcal phagocytosis. Heterogeneity among strains. Immunology 33:191-197. [PMC free article] [PubMed] [Google Scholar]
- 118.Wann, E. R., B. Dassy, J. M. Fournier, and T. J. Foster. 1999. Genetic analysis of the cap5 locus of Staphylococcus aureus. FEMS Microbiol. Lett. 170:97-103. [DOI] [PubMed] [Google Scholar]
- 119.Welch, P. G., A. Fattom, J. Moore, R. Schneerson, J. Shiloach, D. A. Bryla, X. R. Li, and J. B. Robbins. 1996. Safety and immunogenicity of Staphylococcus aureus type 5 capsular polysaccharide-Pseudomonas aeruginosa recombinant exoprotein A conjugate vaccine in patients on hemodialysis. J. Am. Soc. Nephrol. 7:247-253. [DOI] [PubMed] [Google Scholar]
- 120.Wergeland, H. I., L. R. Haaheim, O. B. Natas, F. Wesenberg, and P. Oeding. 1989. Antibodies to staphylococcal peptidoglycan and its peptide epitopes, teichoic acid, and lipoteichoic acid in sera from blood donors and patients with staphylococcal infections. J. Clin. Microbiol. 27:1286-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wiley, B., and N. Maverakis. 1974. Capsule production and virulence among strains of Staphylococcus aureus. Ann. N.Y. Acad. Sci. 236:221-232. [DOI] [PubMed] [Google Scholar]
- 122.Wilkinson, B. J. 1983. Staphylococcal capsules and slime, p. 481-523. In C. Easmon and C. Adlam (ed.), Staphylococci and staphylococcal infections, vol. 2. Academic Press, London, England.
- 123.Wu, T., and J. Park. 1971. Chemical characterization of a new surface antigenic polysaccharide from a mutant of Staphylococcus aureus. J. Bacteriol. 108:874-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu, S., R. D. Arbeit, and J. C. Lee. 1992. Phagocytic killing of encapsulated and microencapsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect. Immun. 60:1358-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]