Abstract
The ventromedial nucleus of the hypothalamus (VMH) is a key cell group in the medial-basal hypothalamus that participates in the regulation of energy balance. Previous studies have shown that the cellular organization of the VMH is altered in mice with a disruption of the steroidogenic factor-1 (NR5a1) gene (SF-1 KO mice). The present study examined orexigenic/anorexigenic peptides (neuropeptide Y (NPY), agouti-related peptide (AgRP) and cocaine- and amphetamine-regulated transcript (CART)) and neural connections to and from the VMH in SF1 KO mice. NeuroVue tracing and Golgi staining were used to evaluate connections between the preoptic area (POA) and VMH and the orientation of dendrites in the VMH, respectively. Results of this study reveal changes in the cytoarchitecture of the region of the VMH with respect to the distribution of immunoreactive NPY, AgRP and CART. In WT mice projections from the POA normally surround the VMH while in SF-1 KO mice, projections from the POA stream through the region that would otherwise be VMH. Golgi impregnation of the region revealed fewer dendrites with ventrolateral orientations and in general, more variable dendritic orientations in SF-1 KO mice providing additional evidence that the connectivity of cells in the region is likely altered due to the cellular rearrangements consequent to disruption of the NR5a1 gene. In conclusion, this study greatly extends the data showing that the morphology of the regions containing the VMH is disrupted in SF-1 KO mice and suggests that changes in the location of cells or fibers containing NPY, AgRP and CART may, in part, account for changes in body weight homeostasis in these mice.
Keywords: Steroidogenic factor 1, ventromedial hypothalamus, cellular organization, immunocytochemistry
INTRODUCTION
The ventromedial nucleus of the hypothalamus (VMH or VMN) is a bilateral cell group in the medial basal hypothalamus. Neurons in the VMH are involved in control of energy homeostasis (Brobeck, 1946; Iwamoto et al., 1999; Majdic et al., 2002; Powley, 1977), sexual behavior (Flanagan-Cato et al., 2006; Matsumoto and Yamanouchi, 2000; Yahr and Greene, 1992), parental behavior (Harding and McGinnis, 2005), anxiety (Zhao et al., 2008) and defensive behavior (Staples et al., 2005), and other physiological processes (Hosoi et al., 1999; Iwamoto et al., 1999). Older studies suggested that the VMH acts as a satiety center as VMH lesions in rats caused hyperphagia and obesity (Brobeck, 1946). While this hypothesis lost ground as other more selective lesion studies indicated the involvement of other nearby cell cell groups (arcuate and paraventricular nuclei), it has returned with more recent studies (King, 2006), including genetic mouse models that suggest roles for the VMH in the regulation of energy balance (Butler et al., 2000; Majdic et al., 2002). The role of the VMH in the control of multiple processes and behaviors is complex due to its close interconnection with other sites, including the amygdala and the preoptic area (POA) (Choi and Dallman, 1999; Saper et al., 1976; Ter Horst and Luiten, 1987).
Steroidogenic factor 1 (NR5A1, SF-1) is a major regulator of expression of steroidogenic enzymes and other genes in steroidogenic and non steroidogenic tissues (reviewed in Parker and Schimmer, 1997). Outside the central nervous system, SF-1 is expressed in adrenal glands, gonads, pituitary, placenta and spleen (Ikeda et al., 1993; Ingraham et al., 1994; Katoh-Fukui et al., 2005). In the central nervous system, SF-1 transcripts were detected in the diencephalon of mice on E11.5 (Ikeda et al., 1994) 5-6 days before the VMH becomes distinguishable based on Nissl stains. As the nucleus develops, SF-1 is selectively expressed n dorsomedial (VMHdm) and central parts (VMHc) of the VMH. Disruption of the NR5a1 gene results in alterations of the cytoarchitecture of the VMH in SF-1 KO mice (Budefeld et al., 2008; Davis et al., 2004; Dellovade et al., 2000; Ikeda et al., 1995; McClellan et al., 2006) that also lead to changes in the terminal differentiation of the remaining VMH neurons (Tran et al., 2003). Changed cytoarchitecture of the VMH in SF-1 KO mice is likely dependent in part on alterations in the migration of neurons from the adjoining ventricular proliferative zone of the third ventricle (Dellovade et al., 2000, 2001; Tobet, 2002).
Several aspects of VMH morphology are sexually dimorphic and gonadal steroid hormones are thought to be a major factor contributing to the development of these differences (Dorner and Staudt, 1969; Matsumoto and Arai, 1986; Micevych et al., 1987; Mong et al., 1999). Sex differences in the number of calbindin D-28k immunoreactive (ir) cells were found in the VMH region in adult mice lacking SF-1 that are not exposed to endogenous gonadal steroid hormones, suggesting a genetic influence on the development of this sex difference (Budefeld et al., 2008).
SF-1 KO mice exhibit complete adrenal and gonadal agenesis (Ikeda et al., 1993) and develop obesity in adult life when adrenal transplants are used to keep them alive. Obesity is not a consequence of dysregulation of corticosteroid secretion since corticosterone levels in adult SF-1 KO mice are normal (Majdic et al., 2002). The present study was conducted to investigate the distributions of cells and fibers that were immunoreactive for the metabolic peptides agouti-related peptide (AgRP), neuropeptide Y (NPY) and cocaine- and amphetamine-regulated transcript (CART) in the region of the VMH in brains of adult WT nd SF-1 KO mice. These peptides were chosen for their importance as metabolic 1 peptides in the hypothalamus (Dietrich and Horvath, 2009). A fourth one, melanocortin or α-MSH, was not included in this study because it was examined in SF-1 KO mice previously (G.M.; unpublished data) and did not reveal obvious differences in the distribution of α-MSH. The aim of this study was to more fully characterize the alterations of the VMH region when NR5a1 is genetically disrupted with special emphasis on the distribution of orexigenic/anorexigenic peptides that might partially explain the obese phenotype observed in these mice. Furthermore, immunoreactive arginine vasopressin (AVP) fibers that normally surround the VMH, connections between the preoptic area and the VMH using carbocyanine dye tracing, and a Golgi impregnation of the dendritic organization of cells in the region of the VMH were all analyzed to further characterize potential alterations in the connectivity of the VMH region in adult SF-1 KO mice. Differences in the immunoreactive locations for all of these peptides in the VMH region between WT and SF-1 KO mice suggest that the lack of SF-1 function plays a major role in the organization of orexigenic/anorexigenic pathways in the VMH region.
MATERIAL AND METHODS
Animals and tissue recovery
C57BL/6J SF-1 heterozygous mice (SF-1+/− backcrossed to C57BL6/J for more than 10 generations and inbred for more than 10 generations) were bred to generate SF-1 KO and littermate wild type (WT) control mice. Mice were maintained on a 12:12 dark-light cycle with phytoestrogen free chow (Global 16% protein rodent diet (2016), Harlan Teklad, Bicester, Oxfordshire, UK) and water ad libitum. Normally, SF-1 KO mice die within 24h of birth due to adrenal insufficiency. Therefore, all newborn pups (WT and SF-1 KO) received daily subcutaneous (s.c.) injections of 50μl of corticosteroids in corn oil (400μg/ml hydrocortisone (Sigma, Steinheim, Germany), 40ng/ml dexamethasone (Sigma) and 25ng/ml fludrocortisone acetate (Sigma) until animals were genotyped on days 6-7 postnatally using a PCR assay. Adrenal glands were transplanted into SF-1 KO pups from WT female donors on postnatal days 7 or 8 as described previously (Majdic et al., 2002). After adrenal transplantation, pups received four more s.c. injections of corticosteroids until weaning at 21 days. Control WT male and female mice (+/+ genotype) were derived from the same litters as SF-1 KO mice or in rare cases (when no littermates were available) from other age-matched litters, received corticosteroid injections postnatally, and were gonadectomized after weaning before the onset of puberty between P21 and P25. Body weights were measured once weekly from three weeks until sacrifice. For gonadectomies, WT mice were anesthetized with a mixture of ketamine (Vetoquinol Biowet, Gorzowie, Poland; 100 μg/g BW), xylazine (Chanelle Pharmaceuticals Ltd., Loughrea, Ireland; 10 μg/g BW) and acepromazine (Fort Dodge Animal Health, Fort Dodge, IA, USA; 2 μg/g BW). On the day of sacrifice at 6 months of age, mice were anesthetized with a mixture of ketamine, xylazine and acepromazine and perfused with 4% paraformaldehyde (Sigma) in 0.1M phosphate buffer PB, pH = 7,3). After dissection from the skull, brains were post-fixed in the same fixative overnight at 4°C and then stored until immunocytochemical processing in 0.1M PB at 4°C. All animal experiments were done according to ethical principles and in accordance with EU directive (86/609/EEC). Animal experiments were approved by the Veterinary commission of Slovenia and the Animal Care and Use Committee at Colorado State University.
SF-1 genotyping and sex determination
Tissue from mice 6-7 days of age were used to determine SF-1 genotype and chromosomal sex. DNA samples were obtained by tail clipping and digested in a thermostatic shaker in 200 μl of PCR DNA buffer (Promega, Madison, WI, USA) containing 0.15 mg of Proteinase K (Sigma) at 55°C overnight. 3 μl of lysate was used for PCR reaction to determine the presence of WT or KO SF-1 allele and the presence or absence of Sry gene as described previously (Luo et al., 1994).
Immunocytochemistry on floating sections
Brains were embedded in 5% agarose (Sigma) and sectioned at 50 μm in cold 0.05M PBS using a vibrating microtome (Integraslice 7550 MM, Campden Instruments, UK). Sections were incubated in 0.1M glycine (Sigma) in 0.05M PBS for 30 min followed by incubation in 0.5% sodium borohydride (Sigma) for 15 min at 4°C. Glycine and sodium borohydride were washed out with 15 min and 20 min washes in 0.05M PBS. Sections were blocked in 5% normal goat serum (Chemicon, Temecula, CA, USA) containing 0.5% Triton X-100 (Sigma) and 1% H2O2 (Merck, Darmstadt, Germany) for 30 min at 4°C. Rabbit primary antibodies gainst arginine vasopressin (AVP, 1:15000, ImmunoStar, Hudson, 1 WI, USA), AgRP (1:4000, Phoenix Pharmaceuticals, Belmont, CA, USA), CART (1:10000, Phoenix Pharmaceuticals) and NPY (1:10000, Dia Sorin, Stillwater, MN, USA) were diluted in 0.05M PBS containing 1% bovine serum albumin (Sigma) and 0.5% Triton X-100. Sections were incubated with primary antibodies over 3 nights at 4°C with shaking. Sections were then washed in 0.05M PBS containing 1% normal goat serum and 0.02% Triton X-100 four times 15 minutes at room temperature. Biotinylated secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA) against primary rabbit antibodies were diluted 1:500 in 0.05M PBS containing 1% normal goat serum and 0.5% Triton X-100. Sections were incubated with secondary antibodies for two hours, followed by 4 washes (15 minutes each) in 0.05M PBS buffer containing 0.02% Triton X-100. Streptavidin – HRP complex (Jackson Immunoresearch) was diluted 1:2000 in 0.05M PBS solution containing 0.5% TritonX-100. Sections were incubated with Streptavidin – HRP for 1 hour at room temperature and then washed in Tris-buffered saline (0.05M Tris-HCl/0.9% NaCl; pH 7.5; Sigma) for 1 hour at room temperature. Antigen-antibody complexes were visualized as a black reaction product by incubating sections in 0.025% 3′3′-Diaminobenzidine/ammonium nickel (II) sulfate substrate (Sigma) in Tris-buffered saline (pH 7.5) containing 0.02% H2O2 for 5 min at room temperature. After mounting, sections were dried and coverslipped using hydrophobic medium (Pertex, Burgdorf, Germany). Immunocytochemical controls included omission of the primary antiserum and validation of immunoreactivity with patterns of distribution from prior publications.
Brain labeling
Micro-strips of lipophilic carbocyanine dye NeuroVue (PTI Research, Exton, 1 PA, USA) were inserted into POA of intact brains (5 WT and 5 SF-1 KO mice) from the direction of the base of the brain. Labeled brains were stored in 0.1% paraformaldehyde in 0.1M PBS for 8 weeks at 30°C and then sectioned at 100 μm in cold 0.05M PBS using a vibrating microtome (Integraslice 7550 MM). Sections were immediately mounted and coverslipped using Crystal mount medium (Sigma).
Golgi impregnation
Golgi staining was carried out according to manufacturer’s instructions with some modifications using FD Rapid GolgiStain™ Kit (FD NeuroTechnologies, Ellicott City, MD, USA). After removal from the skull, fresh brains were washed in MilliQ water to remove extra blood and then transferred into the impregnation solution containing mercuric chloride, potassium dichromate and potassium chromate for 11 days at room temperature. After that, brains were transferred into the solution C from FD Neurotechnologies kit for softening for 3 days and stored at 4°C. Impregnated brains were embedded in 5% agarose and sectioned at 100 μm in 30% sucrose (Sigma) at room temperature using vibrating microtome (Integraslice 7550 MM). Sections were washed in MilliQ water two times for 2 min to remove sucrose before incubation in the developing solution. Dark gray to black reaction product was allowed to form 10 min at room temperature in the dark. To stop the formation of a silver precipitant, sections were incubated four times for 5 min in MiliQ water and two times for 5 min in Tris-buffered saline at room temperature. Sections were immediately mounted, allowed to dry for 6 hours at room temperature and coverslipped using hydrophobic medium (Pertex).
Data collection and analyses
Digital images of brain regions of interest for immunocytochemical analysis were obtained using a Nikon Eclipse 80i microscope with Nikon DS-Fi1 camera. Images were enhanced for contrast using an Adobe Photoshop software package (Version 8.0). Immunoreactive AgRP, NPY and CART were analyzed in coronal sections containing medial aspects of VMH and its surrounding area 1.7 mm caudal from bregma according to stereotaxic coordinates (Paxinos and Franklin, 2001) in WT gonadectomized mice and the corresponding ventromedial hypothalamic region in SF-1 KO mice. Digital images were taken using 10x objective lens and the third ventricle and base of the brain were considered as reference boundaries. Immunoreactive AVP in the hypothalamus was studied in fibers in coronal sections 0.9 mm to bregma using 10x objective lens. Due to the possibility of asymmetry in antigen detection between the left and right sides of the brain, and to be conservative, the side with more immunoreactive cells or fibers was always chosen for analysis. As the sections were run free15 oating and the brains were not notched ahead of time, the actual left or right side of the brain was unknown. For all markers, only one coronal section was analyzed quantitatively.
The number of immunoreactive cells in the VMH was evaluated using Image J software (NIH, Bethesda, MD, USA). All analyzed regions were divided into grid squares as described in previous analyses (Budefeld et al., 2008; Davis et al., 2004; Wolfe et al., 2005) to provide a mechanism to discern objective changes in the positions of cells and fibers. For the CART immunoreactive cells in the VMH we used a grid of 7×9 squares, measuring 104×104 μm each when taken with 10x objective lens. The number of immunoreactive cells was counted in each box and all boxes in the grid. AgRP and NPY immunoreactivity in fibers in the VMH in figures taken under 100x magnification was quantified using custom software (Surfkvad; ade by Dr. Marko Kreft, Institute of pathophysiology, Faculty of Medicine, Ljubljana); that divides an image into 6×8 squares and calculates a percentage of dark area for each square. To standardize the immunoreactive area data, all images were taken under the same illumination and converted to grayscale. Grayscale images were first standardized for illumination and were then subjected to threshold conversion to selectively identify immunoreactive elements using Photoshop software package Adobe Version 8.0 with threshold limit set at 50%. Black and white images were than analyzed with Surfkvad software.
NeuroVue labeled brain sections were inspected under excitation wavelength at 560 nm and digital images were obtained using a Nikon Eclipse 80i microscope with Nikon DS-Fi1 camera using 10x objective lens. Efferent projections from the POA were analyzed in coronal sections through the VMH region. Brains were only used if the NeuroVue placement was below the anterior commissure and restricted to the area of medial preoptic nucleus approximately 0.1 mm from bregma. 3D reconstructions of NeuroVue data were created using Biovis3D software package (Biovis, Montevideo, Uruguay).
Golgi impregnated sections from 4 WT and 4 SF-1 KO mice were taken on Nikon Eclipse 80i microscope with 20x objective lens from coronal sections 1.7 mm caudally from bregma. For each animal, only the side of the brain with more stained neurons was included into analysis. Angle of primary dendrites was measured for neurons in the ventrolateral part of the VMH and corresponding area in SF-1 KO mice using Image J software.
Data were analyzed either by using two- factor analysis of variance 1 (ANOVA; sex x genotype) to detect sex or genotype differences in total number of cells or immunoreactivity in a given area or using a three- factor ANOVA with the inclusion of a location as a repeated measure in addition to the independent variables of sex and genotype. Variation in angles of primary dendrites was analyzed by Bartlett’s test for homogeneity of variances, and as an additional measure genotype differences in the angles of primary dendrites in Golgi impregnated sections was determined by calculating the percentage of dendrites with angles between 200o – 225o in WT and SF-1 KO mice. All statistical analyses were performed with NCSS software package for PC (NCSS Inc., Kaysville, UT, USA) with statistical significance considered at p < 0.05. All data are presented as mean ± S.E.M.
RESULTS
Body weight
At 26 weeks of age, when mice were killed for brain studies, the average body weights were 30.1 ± 1.4 g in WT OVX females, 30.8 ± 1.6 g in WT CAS males. In SF-1 KO mice, obesity was confirmed by an approximately 50% increase in body weights (43.5 ± 1.8 in females and 45.6 ± 2.5 in SF-1 KO males). There was a statistically significant effect of genotype (F(1,46) = 2275.1; p < 0.001), but no effect of sex.
AgRP and NPY immunoreactivity in the VMH
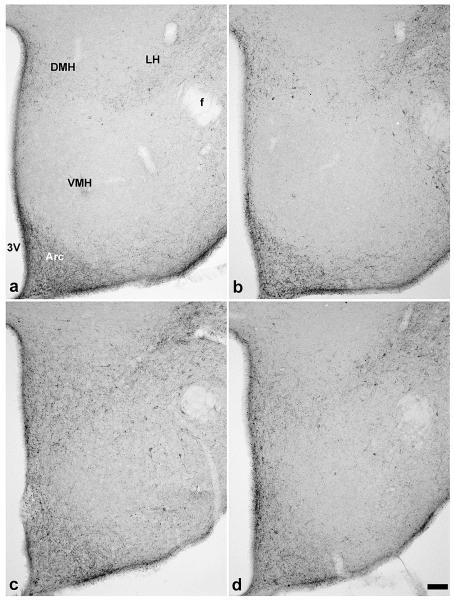
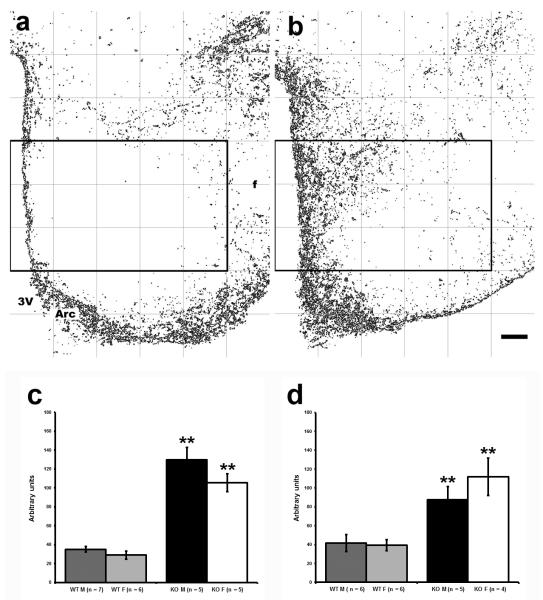
NPY- ir fibers in coronal brain sections 1.7 mm caudal to bregma were found in the arcuate and caudal part of paraventricular nucleus (PVH), VMH and its surrounding area, orsomedial nucleus of the hypothalamus (DMH) and in the dorsal lateral hypothalamus (Fig. 1). This pattern of immunoreactivity is similar to that previously described (Broberger et al., 1999; Kesterson, et al., 1997). AgRP- ir fibers (not shown) were found in the same areas and showed a similar distribution as NPY (Broberger et al., 1998). Immunoreactive AgRP and NPY were analyzed in the VMH region in the 5×3 grid of boxes as shown in figure 2a and b. Two- way ANOVA (sex x genotype) revealed statistically significant differences between genotypes for AgRP- ir area (F(1, 20) = 32.0, p < 0.001, Fig. 2c) and NPY-ir area (F(1, 22) = 162.9, p < 0.001, Fig. 2d). In WT mice, AgRP and NPY- ir fibers were mostly excluded from the area of the VMH while in SF-1 KO mice AgRP and NPY- ir fibers were dispersed throughout the corresponding dorsal VMH region. Immunoreactive fibers in the arcuate nucleus were spread beyond its normal boundary as seen in WT mice into the cell poor zone and the VMH area in SF-1 KO mice as shown in figures 1 and 2 for NPY and AgRP, respectively. As shown in figure 1e, the rearrangement of fibers was observed throughout the entire area and was not specific to one section or plane of section.
Figure 1.
Digital images show NPY immunoreactivity in the medial hypothalamus in sections 1.7 mm caudal to bregma was limited to the arcuate (Arc) and dorsomedial nuclei (DMH) in WT male (a) and female (b) mice. In contrast, in SF-1 KO mice (male; c and female; d), NPY immunoreactivity was detected in the area of ventromedial nucleus (VMH), which was devoid of these fibers in WT mice of both sexes. 3V- the third ventricle, Arc– arcuate nucleus, f- fornix, LH- lateral hypothalamus, DMH– dorsomedial nucleus of the hypothalamus; scale bar in d represents 100 μm for all panels.
Figure 2.
Digital images showing AgRP- ir fibers in WT male mouse (a) and in corresponding sections in SF-1 KO male mouse (b) in the box 5×3 covering VMH nucleus as shown after processing for intensity thresholding (a, b). Graphs (c, d) show the quantification of AgRP and NPY- ir area (mean ± S.E.M.) that indicate significantly greater immunoreactive area in the designated VMH region in SF-1 KO mice (c- AgRP, p < 0.001; **) and (d- NPY, p < 0.001; **). 3V- the third ventricle, Arc- arcuate nucleus, f- fornix, scale bar in b represents 100μm for both panels a and b.
CART immunoreactivity in VMH
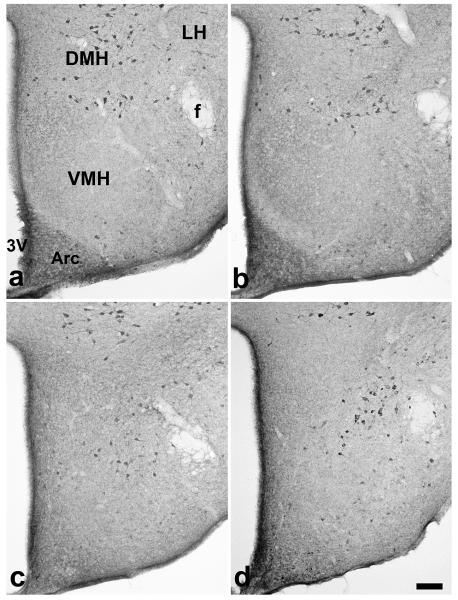
Immunoreactive CART peptide was detected in neuronal cell bodies and in fibers in the arcuate nucleus, at the dorsal and ventral boundaries of the VMH and in dorsomedial nucleus and dorsal lateral hypothalamus in coronal brain sections 1.7 mm caudal from bregma in WT mice (Fig. 3). This pattern of immunoreactivity is similar to that previously described (Broberger, 1999). Immunoreactive neurons of the arcuate nucleus, dorsomedial nucleus and in the lateral hypothalamus were excluded from the analysis. Three-way ANOVA (sex x genotype) x rows as a repetitive factor showed significant interaction between location and genotype (F(8, 207) = 14.5, p < 0.001). The position of CART- ir cells was changed in SF-1 O mice (Fig. 3, 4), with many CART- ir cell bodies present in a region corresponding to the dorsomedial part of the VMH (Fig. 4; 416-728 μm from base). Total number of CART- ir cells did not differ between groups (43 ± 6 in WT males, 39 ± 5 in WT females, 31 ± 5 in SF- 1 KO males, 37 ± 4 in SF-1 KO females), suggesting only an altered location of CART- ir cells in the VMH region in SF-1 KO mice.
Figure 3.
Digital images show CART- ir cells in the medial hypothalamus in sections 1.7 mm caudal to bregma in WT male (a), WT female (b), SF-1 KO male (c) and SF-1 KO female (d) mice. Location of CART- ir cells is altered in SF-1 KO mice, where CART -ir cells are shifted ventromedially into the corresponding dorsomedial part of VMH area. Number of CART- ir cells did not differ between all four groups. 3V- the third ventricle, Arc- arcuate ucleus, DMH- dorsomedial nucleus of the hypothalamus, f- fornix, LH- lateral hypothalamus, VMH- ventromedial hypothalamus, bar: 100 μm 4
Figure 4.
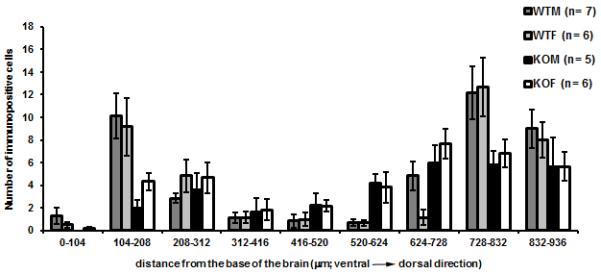
The graph shows the number (mean ± S.E.M.) of CART- ir cells in VMH region in WT mice and SF-1 KO mice as a function of distance from the base of the brain. Three-way ANOVA (age x sex) with the distance from the base of the brain (in 104μm bands or rows) as repetitive factor showed statistically significant differences between genotypes (* p< 0.001).
AVP immunoreactivity in the medial hypothalamus
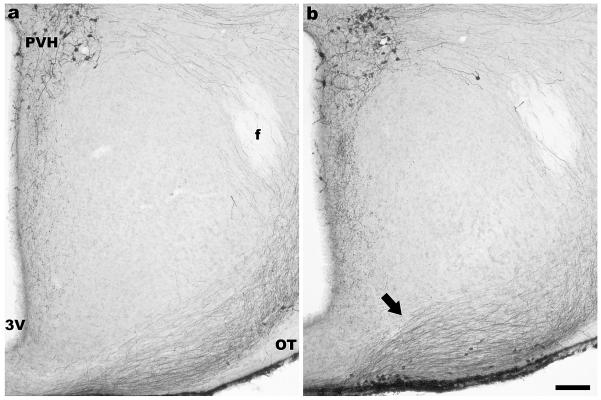
The rostral part of the VMH was surrounded by AVP- ir fibers as noted by others (Johren et al., 1997). Comparison of fibers in matched coronal sections between WT and SF-1 KO mice showed alterations in the positions of fibers proximal to the optic tract oriented basomedially toward the median eminence. These fibers, passing the VMH in the ventrolateral region, were positioned more dorsomedially in relation to the third ventricle in SF-1 KO mice (Fig. 5b; arrow) than in WT mice (Fig. 5a).
Figure 5.
Digital images show AVP immunoreactivity in the medial basal hypothalamus in the coronal sections approximately 0.9 mm caudal to bregma in representative figures for each genotype (WT - a, and SF-1 KO - b). The position of immunoreactive fibers is altered in the ventrolateral part of the region in SF-1 KO (b) in comparison to WT (a) mice regardless of sex. AVP- ir fibers that project toward the median eminence are positioned more medially toward the third ventricle and more perpendicularly to the base of a brain in SF-1 KO mice (arrow). 3V- the third ventricle, f- fornix, OT- optic tract, PVH- paraventricular nucleus of hypothalamus, scale bar in b represents 100μm for both a and b.
NeuroVue labeling of efferent projections from the POA
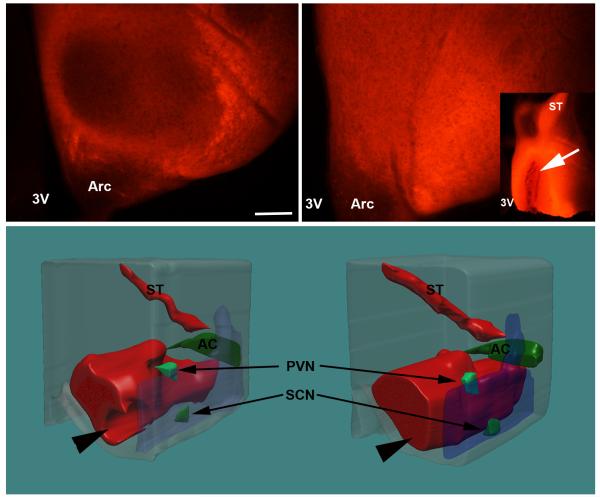
Analysis of coronal sections of brain with NeuroVue microstripes placed in the POA showed that in WT mice, labeled fibers from the POA surround the nucleus (Fig. 6a) likely targeting VMH neuronal dendrites in the shell of the nucleus, but leaving the central region of the VMH label free. In the corresponding VMH region in SF-1 KO mice, efferent projections from the POA reached and clearly filled the VMH region (Fig. 6b), further suggesting altered structure and/or connectivity for cells in the region of the VMH. 3D reconstructions of Neurovue labeled fibers further emphasized that the VMH area was filled with fibers in SF-1 KO mice (Fig. 6d) but not in WT mice (Fig. 6c). Neurovue labeling was performed in at least hree mice for each genotype and each sex and was consistent among the animals in each group.
Figure 6.
Digital images show representative coronal brain sections through the VMH area with NeuroVue labeled fibers originating in the POA. In WT mice (a) central part of the VMH nucleus remained unlabeled (seen as darker oval shape) while in SF-1 KO mice (b) labeled neuronal fibers from POA filled the entire the region. This is further illustrated by 3D reconstruction of serial sections from POA to the caudal VMH region (c and d); 3V- the third entricle, Arc- arcuate nucleus, PVH- 1 paraventricular nucleus, SCN– suprachiasmatic nucleus, AC– anterior commissure, ST– stria terminalis; scale bar: 100μm).
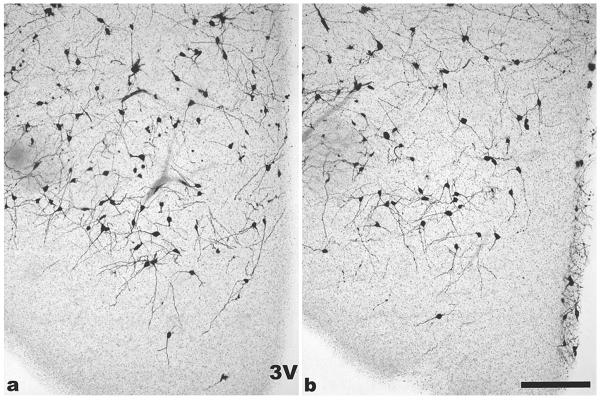
Golgi impregnation of VMH neurons
Using Golgi impregnation, VMH neurons were labeled dark grey to black and cell bodies and dendrites were clearly seen. Analysis of angles from primary dendrites in the ventrolateral VMH revealed that in WT mice, dendrites mostly extended in ventrolateral directions with a mean angle of 207°. Although the mean angle of dendrites was similar in SF-1 KO mice (214°), the variation was significantly larger (S.D. in WT mice was 25.9, but 49.2 in SF-1 KO mice) because dendrites projected in many different directions (Fig. 7). This was confirmed by Bartlett’s test for homogeneity of variance that showed a statistically reliable difference in variance between WT and SF-1 KO mice (p < 0.001). As an alternative analysis, the average percentage of dendrites with angles between 200o – 225o was 38.3 % ± 4.8 in WT mice and 15 % ± 10.1 in SF-1 KO mice (F(1, 8) = 5.76, p = 0.05).
Figure 7.
Digital images show representative coronal brain sections through the VMH area with Golgi impregnated neurons. In WT mice (a) most primary dendrites in the ventrolateral part of the VMH were oriented ventrolaterally while in the VMH from SF-1 KO mice (b), primary dendrites were oriented in numerous directions (3V- the third ventricle, bar: 100 μm).
DISCUSSION
In the present study, structure in relation to the function of the VMH was studied in SF-1 KO mice. In the mouse brain, SF-1 is specifically expressed in neurons of the VMH (Ikeda et al., 1995; Zhao et al., 2004) and the cytoarchitecture of the VMH is altered in SF-1 KO mice (Budefeld et al., 2008; Davis et al., 2004; Dellovade et al., 2000; Ikeda et al., 1995; Shinoda et al., 1995). SF-1 KO mice are a useful model animal for studying VMH development and function as they develop obesity (late onset as confirmed in the current study) and have diminished running-wheel activity (Majdic et al., 2002). The present study provides a detailed analysis of alterations of connectivity and immunoreactive peptides that may help explain a KO phenotype involving alterations in energy balance.
The neurons of the VMH may act as part of a satiety center and metabolic peptides AgRP and NPY, secreted from neurons of the arcuate nucleus, may be responsible for inactivation of some VMH neurons during a state of energy depletion (Li and Davidowa, 2004; Nishimura et al., 1996). AgRP and NPY are expressed in cell bodies in the arcuate nucleus together with proopiomelanocortin (POMC) with significant projections going toward paraventricular and dorsomedial hypothalamic nuclei (Elias et al. 1999; Elmquist, 2001). In the current study, colchicine was not used and the bulk of immunoreactivity was found within fibers of likely arcuate cell body origins. WT mice show an expected pattern of immunoreactive fibers oriented around the VMH in adjacent hypothalamic regions of PVH and DMH. However, in SF-1 KO mice, there was a notable increase in the immunoreactive AgRP and NPY fibers invading the VMH region, suggesting a displacement of fibers from their normal locations. The immunoreactive area for AgRP and NPY peptides in PVH (not shown) and DMH did not differ between WT and SF-1 KO mice, suggesting that fibers were not only displaced in SF-1 KO mice, but that the total density of immunoreactive fibers may have increased in the area xamined. A major increase in immunoreactive fibers in SF-1 KO mice 1 was found in the periventricular zone adjacent to the VMH. Increased numbers of fibers containing orexigenic peptides AgRP and NPY in the VMH could potentially affect the activity of VMH neurons in SF-1 KO mice that are involved in the regulation of energy homeostasis (e.g. physical activity, food consumption and thermogenesis). An increased orexigenic signal in the region of the reorganized VMH could cause long term hyperpolarization of VMH neurons that could be responsible for diminished running-wheel activity (Majdic et al., 2002) and spontaneous home cage activity (T.B. and G.M., unpublished results) in adult SF-1 KO mice, that presumably leads to obesity observed in adult SF-1 KO mice. Previously, POMC neurons were examined by in situ hybridization and cell bodies of these neurons were found in the same arcuate nucleus locations in WT and SF-1 KO (G. M., unpublished results), suggesting that fiber, but not cell body positions, are altered in SF-1 KO mice.
CART is an anorexigenic peptide whose expression is decreased in food deprived animals (Kristensen et al., 1998) and central administration of CART peptide inhibits food intake (Vrang et al., 2000). Similar to NPY and AgRP, CART is mostly found in neuronal cell bodies in the arcuate nucleus with projections to paraventricular and dorsomedial hypothalamic nuclei (Elmquist, 2001). In the present study, there were alterations in the position of CART- ir cells in the VMH region in obese adult SF-1 KO mice. In WT mice, CART- ir cells were largely excluded from the VMH. In SF-1 KO mice, however, the number of immunoreactive cells in the VMH area was increased in comparison to WT mice, although the total number in the mediobasal hypothalamus did not differ between WT and SF- 1 KO mice. This is consistent with altered positions of neurons in SF-1 KO mice and not in their total number of CART positive cells. A similar ventral shift in the position of cells into the VMH region was found for cells containing immunoreactive galanin in neonatal SF-1 KO ice (Dellovade et al., 2000). In that study galanin containing cells were 1 excluded from the VMH region in neonatal WT mice. In agreement with previous studies it is likely that CART3 cells in SF-1 KO mice were altered in their migration at some point in early development (Davis et al., 2002; Dellovade et al., 2001).
Several experiments in the current study suggest that fiber trajectories and potential connections are altered in the region of the VMH when SF-1 is absent in development and adulthood. Trajectories of phenotypically identified fibers containing immunoreactive NPY, AgRP, CART, and AVP in the medial basal hypothalamus were aberrant in SF-1 KO mice. In addition, fibers from the POA headed toward the medial basal hypothalamus labeled using NeuroVue microstripes were rearranged. Finally, the orientation of dendrites in the region of the VMH was altered, losing their ventrolateral alignment and becoming more random.
Connections between the POA and mediobasal hypothalamus were examined using the carbocyanine dye tracer Neurovue, which provides an improved filter fiber based delivery method for DiI. Neurovue fibers can be inserted with greater mechanical precision providing for stronger and more selective signals that can be traced further away from the insertion site than DiI (Fritzsch et al. (2005). In the current study using Neurovue the altered connections between POA and VMH regions were particularly striking. Differences in labeled fibers between POA and VMH were consistent with altered positions of neural elements in the medial basal hypothalamus in SF-1 KO mice. In WT mice, fibers from the POA appeared to target the dendrites of the VMH region that are oriented externally to the core of the VMH. However, in SF-1 KO mice, fibers from the POA lost their targeting specificity and filled the the region of the VMH. Similar changes in cytoarchitecture have been found in the VMH region of CNS specific SF-1 KO mice for fibers expressing urocortin III. In WT mice they target positions surrounding the VMH, but were shifted into the central VMH region in SF-1 O mice (Zhao et al., 2008). However, the source of the fibers expressing 1 urocortin III surrounding the VMH is probably not neurons in the POA (Li et al., 2002). Observed differences might result in changed connectivity between the neurons of the POA and VMH target neurons, or indicate rerouting of fiber trajectories from POA to other hypothalamic nuclei (e.g., arcuate, periventricular, dorsomedial nucleus), lateral hypothalamic areas, or even other areas further caudally.
The results show that the fibers surrounding the VMH, the site where many VMH neurons receive synaptic contacts from incoming fibers is altered significantly. The Golgi data suggests that this could be secondary to changes in the dendritic arbor organization of VMH neurons. There was more variability in the orientation of primary dendrites in the ventrolateral VMH in SF-1 KO mice in comparison to WT mice. Similar dendritic orientations to the current study in the ventrolateral part of the VMH in WT mice has been described for long primary dendrites in the ventrolateral part of the VMH in rats (Griffin and Flanagan-Cato, 2008, 2009). Interestingly, dendritic organization (number and length of dendrites) in the VMH can be sex specific and sensitive to gonadal hormones (Griffin and Flanagan-Cato, 2008, 2009). In rats, males had higher total number of dendrites per neuron and longer dendrites than females and testosterone converted to estradiol is likely to be responsible for the masculinization of dendritic spines in VMH during embryonic development (Schwarz et al., 2008). Additionally, dietary factors could also influence dendritic arbors in VMH neurons (Flanagan-Cato et al., 2008). Therefore, it is possible that the altered orientation of dendrites observed in the current study was due to the absence of SF-1, or a secondary effect due to missing sex steroid hormones, or even, since adult SF-1 KO mice are obese, due to differences in the energy homeostasis system. Whether the altered rganization of dendrites is tied to aberrant function for neurons in the VMH region in SF-1 KO mice is an open question.
In conclusion, this study characterizes novel alterations in the cytoarchitecture of the VMH and its surrounding areas in adult SF-1 KO mice from the perspective of peptides particularly important for regulating energy balance. Differences were found in the location and total amount of immunoreactive area for two orexigenic peptides, NPY and AgRP, and in the location of CART- ir cell bodies, and these differences could potentially contribute to the obese phenotype of adult SF-1 KO mice. Furthermore, NeuroVue tract tracing, Golgi impregnation, and the position of AVP fibers further indicate aberrant circuitries in the VMH region of SF-1 KO mice, although specific functional aspects of these changes remain to be elucidated.
Acknowledgement
This study was supported by NIH grant MH61376 (S.A.T. and G.M.), ICGEB grant CRP-SLO 06-02 (G.M.) and ARRS (Slovenian research agency) grants P4-0053 and J7-2093 (G.M.).
LITERATURE
- Brobeck JR. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol. Rev. 1946;26:541–559. doi: 10.1152/physrev.1946.26.4.541. [DOI] [PubMed] [Google Scholar]
- Broberger C. Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: histochemical relationship to thyrotropin-releasing hormone, melanin concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res. 1999;848:101–113. doi: 10.1016/s0006-8993(99)01977-0. [DOI] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, Visser TJ, Kuhar MJ, Hokfelt T. Neuropeptide Y innervation and neuropeptide-Y-Y1-receptor-expressing neurons in the paraventricular hypothalamic nucleus of the mouse. Neuroendocrinology. 1999;70:295–305. doi: 10.1159/000054490. [DOI] [PubMed] [Google Scholar]
- Budefeld T, Grgurevic N, Tobet SA, Majdic G. Sex differences in brain developing in the presence or absence of gonads. Dev Neurobiol. 2008;68:981–995. doi: 10.1002/dneu.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- Choi S, Dallman MF. Hypothalamic obesity: multiple routes mediated by loss of function in medial cell groups. Endocrinology. 1999;140:4081–4088. doi: 10.1210/endo.140.9.6964. [DOI] [PubMed] [Google Scholar]
- Davis AM, Henion TR, Tobet SA. Gamma-aminobutyric acidB receptors and the development of the ventromedial nucleus of the hypothalamus. J. Comp. Neurol. 2002;449:270–280. doi: 10.1002/cne.10293. [DOI] [PubMed] [Google Scholar]
- Davis AM, Seney ML, Stallings NR, Zhao L, Parker KL, Tobet SA. Loss of steroidogenic factor 1 alters cellular topography in the mouse ventromedial nucleus of the hypothalamus. J. Neurobiol. 2004;60:424–436. doi: 10.1002/neu.20030. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Davis AM, Ferguson C, Sieghart W, Homanics GE, Tobet SA. GABA influences the development of the ventromedial nucleus of the hypothalamus. J. Neurobiol. 2001;49:264–276. doi: 10.1002/neu.10011. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Young M, Ross EP, Henderson R, Caron K, Parker K, Tobet SA. Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J. Comp. Neurol. 2000;423:579–589. doi: 10.1002/1096-9861(20000807)423:4<579::aid-cne4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Horvath TL. Feeding signals and brain circuitry. Eur J Neurosci. 2009;30(9):1688–96. doi: 10.1111/j.1460-9568.2009.06963.x. [DOI] [PubMed] [Google Scholar]
- Dorner G, Staudt J. Structural changes in the hypothalamic ventromedial nucleus of the male rat, following neonatal castration and androgen treatment. Neuroendocrinology. 1969;4:278–281. doi: 10.1159/000121758. [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–86. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Physiol. Behav. 2001;74:703–708. doi: 10.1016/s0031-9384(01)00613-8. [DOI] [PubMed] [Google Scholar]
- Flanagan-Cato LM, Fluharty SJ, Weinreb EB, LaBelle DR. Food restriction alters neuronal morphology in the hypothalamic ventromedial nucleus of male rats. Endocrinology. 2008;149:93–99. doi: 10.1210/en.2007-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan-Cato LM, Lee BJ, Calizo LH. Co-localization of midbrain projections, progestin receptors, and mating-induced fos in the hypothalamic ventromedial nucleus of the female rat. Horm Behav. 2006;50:52–60. doi: 10.1016/j.yhbeh.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Muirhead KA, Feng F, Gray BD, Ohlsson-Wilhelm BM. Diffusion and imaging properties of three new lipophilic tracers, NeuroVue Maroon, NeuroVue Red and NeuroVue Green and their use for double and triple labeling of neuronal profile. Brain Res Bull. 2005;66:249–58. doi: 10.1016/j.brainresbull.2005.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin GD, Flanagan-Cato LM. Estradiol and progesterone differentially regulate the dendritic arbor of neurons in the hypothalamic ventromedial nucleus of the female rat (Rattus norvegicus) J. Comp. Neurol. 2008;510:631–640. doi: 10.1002/cne.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin GD, Flanagan-Cato LM. Sex differences in the dendritic arbor of hypothalamic ventromedial nucleus neurons. Physiol. Behav. 2009;97:151–156. doi: 10.1016/j.physbeh.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SM, McGinnis MY. Microlesions of the ventromedial nucleus of the hypothalamus: effects on sociosexual behaviors in male rats. Behav. Neurosci. 2005;119:1227–1234. doi: 10.1037/0735-7044.119.5.1227. [DOI] [PubMed] [Google Scholar]
- Hosoi M, Oka T, Abe M, Hori T, Yamamoto H, Mine K, Kubo C. Prostaglandin E(2) has antinociceptive effect through EP(1) receptor in the ventromedial hypothalamus in rats. Pain. 1999;83:221–227. doi: 10.1016/s0304-3959(99)00105-0. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP, Parker KL. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol. Endocrinol. 1993;7:852–860. doi: 10.1210/mend.7.7.8413309. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol. Endocrinol. 1995;9:478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol. Endocrinol. 1994;8:654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen WH, Nachtigal MW, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8:2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Nishihara M, Takahashi M. VMH lesions reduce excessive running under the activity-stress paradigm in the rat. Physiol. Behav. 1999;66:803–808. doi: 10.1016/s0031-9384(99)00017-7. [DOI] [PubMed] [Google Scholar]
- Johren O, Imboden H, Hauser W, Maye I, Sanvitto GL, Saavedra JM. Localization of angiotensin-converting enzyme, angiotensin II, angiotensin II receptor subtypes, and vasopressin in the mouse hypothalamus. Brain Res. 1997;757:218–227. doi: 10.1016/s0006-8993(97)00220-5. [DOI] [PubMed] [Google Scholar]
- Katoh-Fukui Y, Owaki A, Toyama Y, Kusaka M, Shinohara Y, Maekawa M, Toshimori K, Morohashi K. Mouse Polycomb M33 is required for splenic vascular and adrenal gland formation through regulating Ad4BP/SF1 expression. Blood. 2005;106:1612–1620. doi: 10.1182/blood-2004-08-3367. [DOI] [PubMed] [Google Scholar]
- Kesterson RA, Huszar D, Lynch CA, Simerly RB, Cone RD. Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the agouti obesity syndrome. Mol. Endocrinol. 1997;11:630–637. doi: 10.1210/mend.11.5.9921. [DOI] [PubMed] [Google Scholar]
- King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol. Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J. Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YZ, Davidowa H. Food deprivation decreases responsiveness 1 of ventromedial hypothalamic neurons to melanocortins. J. Neurosci. Res. 2004;77:596–602. doi: 10.1002/jnr.20192. [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Development of sexual dimorphism in synaptic organization in the ventromedial nucleus of the hypothalamus in rats. Neurosci. Lett. 1986;68:165–168. doi: 10.1016/0304-3940(86)90135-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yamanouchi K. Acceleration of mounting behaviors in female rats by ibotenic acid lesions in the ventromedial hypothalamic nucleus. Neurosci. Lett. 2000;291:143–146. doi: 10.1016/s0304-3940(00)01388-4. [DOI] [PubMed] [Google Scholar]
- McClellan KM, Parker KL, Tobet S. Development of the ventromedial nucleus of the hypothalamus. Front Neuroendocrinol. 2006;27:193–209. doi: 10.1016/j.yfrne.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Park SS, Akesson TR, Elde R. Distribution of cholecystokinin immunoreactive cell bodies in the male and female rat: I. Hypothalamus. J. Comp. Neurol. 1987;255:124–136. doi: 10.1002/cne.902550110. [DOI] [PubMed] [Google Scholar]
- Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J. Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura F, Nishihara M, Torii K, Takahashi M. Changes in responsiveness to serotonin on rat ventromedial hypothalamic neurons after food deprivation. Physiol. Behav. 1996;60:7–12. doi: 10.1016/0031-9384(95)02232-5. [DOI] [PubMed] [Google Scholar]
- Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr. Rev. 1997;18:361–377. doi: 10.1210/edrv.18.3.0301. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. second ed. Academic press; San Diego, San Francisco, New York, Boston, London, Sydney, Tokyo: 2001. [Google Scholar]
- Powley TL. The ventromedial hypothalamic syndrome, satiety, and a cephalic phase hypothesis. Psychol Rev. 1977;84:89–126. [PubMed] [Google Scholar]
- Saper CB, Swanson LW, Cowan WM. The efferent connections of the ventromedial nucleus of the hypothalamus of the rat. J. Comp. Neurol. 1976;169:409–442. doi: 10.1002/cne.901690403. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Liang SL, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, Shiba H, Sasaki H, Osawa Y, Ninomiya Y, Niwa O, Morohashi K, Li E. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev. Dyn. 1995;204:22–29. doi: 10.1002/aja.1002040104. [DOI] [PubMed] [Google Scholar]
- Staples LG, Hunt GE, Cornish JL, McGregor IS. Neural activation during cat odor-induced conditioned fear and ‘trial 2’ fear in rats. Neurosci Biobehav Rev. 2005;29:1265–1277. doi: 10.1016/j.neubiorev.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, Luiten PG. Phaseolus vulgaris leuco-agglutinin tracing of intrahypothalamic connections of the lateral, ventromedial, dorsomedial and paraventricular hypothalamic nuclei in the rat. Brain Res. Bull. 1987;18:191–203. doi: 10.1016/0361-9230(87)90190-0. [DOI] [PubMed] [Google Scholar]
- Tobet SA. Genes controlling hypothalamic development and sexual differentiation. Eur. J. Neurosci. 2002;16:373–376. doi: 10.1046/j.1460-9568.2002.02105.x. [DOI] [PubMed] [Google Scholar]
- Tran PV, Lee MB, Marin O, Xu B, Jones KR, Reichardt LF, Rubenstein JR, Ingraham HA. Requirement of the orphan nuclear receptor SF-1 in terminal differentiation of ventromedial hypothalamic neurons. Mol. Cell. Neurosci. 2003;22:441–453. doi: 10.1016/S1044-7431(03)00027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Kristensen P, Tang-Christensen M. Central administration of cocaine-amphetamine-regulated transcript activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 2000;141:794–801. doi: 10.1210/endo.141.2.7295. [DOI] [PubMed] [Google Scholar]
- Wolfe CA, Van Doren M, Walker HJ, Seney ML, McClellan KM, Tobet SA. Sex differences in the location of immunochemically defined cell populations in the mouse preoptic area/anterior hypothalamus. Brain Res. Dev. Brain Res. 2005;157:34–41. doi: 10.1016/j.devbrainres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Yahr P, Greene SB. Effects of unilateral hypothalamic manipulations on the sexual behaviors of rats. Behav. Neurosci. 1992;106:698–709. doi: 10.1037//0735-7044.106.4.698. [DOI] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Hanley NA, Majdic G, Stallings NR, Jeyasuria P, Parker KL. Tissue-specific knockouts of steroidogenic factor 1. Mol. Cell. Endocrinol. 2004;215:89–94. doi: 10.1016/j.mce.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Zhao L, Kim KW, Ikeda Y, Anderson KK, Beck L, Chase S, Tobet SA, Parker KL. Central nervous system-specific knockout of steroidogenic factor 1 results in increased anxiety-like behavior. Mol. Endocrinol. 2008;22:1403–1415. doi: 10.1210/me.2008-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]