Abstract
Objective
We examined whether having a psychiatric disorder among HIV-infected individuals is associated with differential rates of discontinuation of HAART and whether the number of mental health visits impact these rates.
Design
This longitudinal study (fiscal year: 2000–2005) used discrete time survival analysis to evaluate time to discontinuation of HAART. The predictor variable was presence of a psychiatric diagnosis (serious mental illness versus depressive disorders versus none).
Setting
Five United States outpatient HIV sites affiliated with the HIV Research Network. Patients: The sample consisted of 4989 patients. The majority was nonwhite (74.0%) and men (71.3%); 24.8% were diagnosed with a depressive disorder, and 9% were diagnosed with serious mental illness.
Main outcome measures
Time to discontinuation of HAART adjusting for demographic factors, injection drug use history, and nadir CD4 cell count.
Results
Relative to those with no psychiatric disorders, the hazard probability for discontinuation of HAART was significantly lower in the first and second years among those with SMI [adjusted odds ratio: first year, 0.57 (0.47–0.69); second year, 0.68 (0.52–0.89)] and in the first year among those with depressive disorders [adjusted odds ratio: first year, 0.61 (0.54–0.69)]. The hazard probabilities did not significantly differ among diagnostic groups in subsequent years. Among those with psychiatric diagnoses, those with six or more mental health visits in a year were significantly less likely to discontinue HAART compared with patients with no mental health visits.
Conclusion
Individuals with psychiatric disorders were significantly less likely to discontinue HAART in the first and second years of treatment. Mental health visits are associated with decreased risk of discontinuing HAART.
Keywords: HIV, psychiatric disorders, treatment outcomes
Background
Psychiatric disorders are common among those infected with the HIV [1,2]. Over the last decade, HAART has dramatically reduced HIV-related morbidity and mortality [3–6]. However, disparities exist. Some studies have found that individuals with HIV and depressive disorders, compared with those with HIV alone, are more likely to encounter greater delays in accessing antiretroviral therapy [7] and have worse adherence to taking antiretroviral medication [8–10]. Mental health treatment, though, has been reported to increase the probability that individuals with depression receive and utilize HAART [11,12].
Although those with serious mental illness (SMI) (i.e. those with schizophrenia spectrum disorders and bipolar disorders) are at unusually high risk of being infected with HIV [13–16], fewer studies have evaluated HAART treatment outcomes among this group of people. One study [17] using New Jersey Medicaid claims data from 1996 to 1998 found that patients with SMI were more likely to have initiated new antiretroviral therapy (defined as receiving a protease inhibitor or a nonnucleoside reuptake inhibitor) compared with those without SMI. Another study [18] using data from the University of Washington HIV Cohort found no difference in the time to initiation of HAART comparing those with SMI with those without mental illness. Whether those with SMI remain in treatment over time is largely unknown.
The goal of this study then is to longitudinally investigate, among a group of HIV-infected patients being prescribed HAART, whether or not a diagnosis of a psychiatric disorder is associated with the time to discontinuation of HAART. Specifically, we were interested in investigating whether there were differences in rates of discontinuation of HAART comparing those with depression, those with SMI, and those without any mental disorders. Finally, we were interested in determining among those with psychiatric disorders whether number of mental health visits impact the rates of discontinuation of HAART.
Methods
Design
This is a retrospective study of a sample of HIV-infected individuals who received HIV outpatient treatment during calendar years 2000–2005.
Site selection
The HIV Research Network (HIVRN) is a consortium of 21 sites that provides primary and subspecialty care to HIV-infected patients. To be included, a site had to have a minimum dataset available in electronic format or through paper abstraction. The minimum data required were the patients’ age, sex, race, HIV transmission risk factor, AIDS-defining illnesses, CD4 cell count level, HIV-1 RNA, and use of antiretroviral medication. Eleven sites also collected data on resource utilization, including hospital admissions, length of stay, and outpatient clinic and office visits. Five sites collected additional information regarding psychiatric diagnosis. Data from these five sites, located in coastal regions of the United States where HIV is most endemic, make up our final sample. This analysis was limited to adult patients (≥18-years-old) who were in ongoing HIV primary care, as defined by at least two visits to a primary care provider at one of these sites and one recorded CD4 cell count test.
Data collection
The data elements described above were abstracted from electronic or paper records at each site. Abstracted data were sent in electronic format to a data coordinating center after personal identifying information was removed. For this analysis, data were available for calendar years 2000 through 2005. The date of the encounter (not the date of billing or payment of claim) was used. Electronic data received by the coordinating center were reviewed to ensure that each data element was correctly formatted, and that all elements were captured. Data elements with incorrect formatting, unknown or incomplete information, or other inaccuracies were reviewed with the site and corrected. After this verification process, the data were combined across sites to achieve a uniformly constructed multisite database. A variable identifying the site was included in the database.
Definitions of variables
HAART was defined as use of: three or more nucleosides; use of any one or more protease inhibitors or a nonnucleoside reverse transcriptase inhibitor (RTI); or a protease inhibitor, nonnucleoside RTI, nucleoside RTI combination. Patients were considered to be on HAART if they received any of these combinations during the calendar year. The CD4 and HIV-1 RNA laboratory values used in this analysis were the nadir CD4 cell count.
SMI was defined using the following International Statistical Classification of Diseases and Related Health Problems (ICD-9) codes for schizophrenia (ICD-9 codes: 295.0–295.7, 295.9), other psychoses (ICD-9 codes: 290.8–290.9, 293, 297–298, 780.1), and bipolar disorder (ICD-9 codes: 296.0–296.1, 296.4–296.5). Depressive disorders were defined using the ICD-9 codes for depressive disorders (ICD-9 codes: 296.2–296.3, 296.82, 296.9, 300.4, 311). Those who did not receive a psychiatric diagnosis were categorized as having no mental illness. For the five sites listed above, these codes were generated by evaluations performed by psychiatrists.
A hierarchical algorithm was used to ensure that mental health diagnoses were mutually exclusive. This was done as cooccurring psychiatric disorders can occur (e.g. cooccurring schizophrenia and depressive disorders). Among those with multiple diagnoses, those who had at least one diagnosis of schizophrenia were categorized as having a diagnosis of schizophrenia. Among those with multiple diagnoses and who did not have a diagnosis of schizophrenia, those with at least one diagnosis of other psychoses were categorized as having other psychoses. Among those with multiple diagnoses and who did not have a diagnosis of schizophrenia nor other psychoses, those with at least one diagnosis of bipolar affective disorder were categorized as having a diagnosis of bipolar affective disorder. For those who had no diagnosis of schizophrenia, bipolar disorder, or other psychosis but had a diagnosis of a depressive disorder were categorized as having a diagnosis of a depressive disorder. Those who did not receive a psychiatric diagnosis were categorized as having no mental illness. Injection drug use (IDU) was defined as having any history of IDU. The study was approved by the institutional review boards of the Johns Hopkins School of Medicine as well as each of the participating sites.
Definition of discrete event times
The binary variable ‘HAART’ indicating whether a patient was on HAART in each year was constructed from chart abstraction and was available for as many as 6 calendar years from year 2000 to 2005 depending on when the individual first became active in treatment at the HIVRN clinic. A patient was considered active in treatment at a clinic in a given year if they had at least one outpatient visit and one CD4 cell count measurement in that year. Those not meeting this requirement were assumed to be not on HAART in that year.
Time until discontinuation of HAART was defined as number of consecutive years from the year HAART was first initiated until the last year HAART was received. If a patient was on HAART on the first day of the study, HAART start date was the date of entry into the study during the time frame of analysis. The event of discontinuation was defined to occur if the patient either went off HAART but remained active in care at the clinic or dropped out of active care at the clinic entirely.
All individuals in the analysis dataset were required to have a clear starting year (i.e. either a nonactive year followed by a year on HAART or an active year not on HAART followed by a year on HAART.) Anyone on HAART in the year 2000 was considered left censored and removed prior to analysis.
Patients were assumed to be on HAART for all of years j (where j░=░2001, 2002, 2003, or 2004) if they were observed to be on HAART in years j and j░+░1. Discontinuation of HAART in year j was defined to occur when a patient was observed to be on HAART in year j but was not observed to be on HAART in year j░+░1. Since 2005 was the last year of available data and there was no subsequent year, observations from 2005 were not included in the survival analyses and were only used for defining whether there was discontinuation in year 2004. Patients observed to be on HAART in years 2004 and 2005 were considered right censored after year 2004; however, these cases were included in the analysis as is standard survival analysis practice. Possible discrete times until discontinuation over the years 2001–2004 were therefore 1, 2, 3, or 4 years.
Data analysis
Sample characteristics
We first conducted descriptive analyses of demographic and baseline year clinical characteristics, including age, sex, race/ethnicity (white, black, and other), use of HAART, number of mental health visits, CD4 cell count (≤50, 51–200, 201–500, and >500░cells/μl), HIV-1 RNA (≤400 or >400░copies/ml), and diagnostic category.
Group comparisons of hazard probabilities for discontinuation of HAART
For each comparison group, we first computed the crude hazard probability of discontinuing HAART among those that were on HAART for each of 1, 2, 3, and 4 consecutive years. Discontinuing HAART in year 2, for example, was assumed to occur if a patient was observed to be on HAART for 2 consecutive years but was not on HAART in the third year. The hazard probability during year 2 is then the proportion of patients who discontinue sometime during the second year out all patients who were on HAART during years 1 and 2.
Second, we utilized a discrete time survival analysis model [19] to compare hazard probabilities across groups after adjusting for covariates. The initial a priori selected covariates included age, sex, race, CD4 cell count nadir (time dependent), and history of intravenous drug use (IDU: yes/no). Other independent variables included site (to adjust for site differences) and year (to capture change in hazard probability over time). Continuous covariates (e.g. age, CD4 cell count) were transformed into ordinal categories to simplify interpretation of results.
To perform the discrete time survival analysis, we used logistic regression with time to discontinuation represented by the sequence of yearly HAART indicators as the response variable [20]. As recommended by Allison [21] when observed discrete times are assumed to reflect actual underlying continuous times, we utilized the complementary log–log link function. Wald chi-square tests were used for individual regression coefficients and global tests.
Pairwise comparisons of groups from the initial survival analysis are expressed as adjusted odds ratios (AORs) of hazard probabilities. We conducted a test of the proportional odds assumption (i.e. that group comparison AORs are constant over time) by conducting a global test of the group by year interaction.
Mental health visits and discontinuation of HAART
Following the above primary survival analysis model, we further explored the effect of frequency of clinic mental health visits on the hazard probability of discontinuing HAART. To do this, we re-fit the model using the subsample of only those with a mental disorder (depressive disorders or SMI) and replaced the group variable with yearly number of mental health visits grouped into frequency categories.
All analyses were conducted using SAS [(version 9) SAS Institute Inc., Cary, North Carolina, USA]. All reported P values are two-sided.
Results
Sample characteristics
Our sample consisted of 4989 HIV-infected patients in primary HIV care who began HAART sometime between year 2001 and 2005. As shown in Table 1 Table 1, the majority of the sample was men (71.4%), less than 50 years of age (83.5%), and of minority ethnicity (74.0%). Approximately 55% of the sample had a CD4 cell count nadir of less than 200░cells/μl. A little over a quarter of the sample reported a history of IDU (26.4%). Among the 450 patients with SMI, 56.4% had a diagnosis of bipolar affective disorder, 29.1% had a diagnosis of schizophrenia, and 14.4% had a diagnosis of other psychotic disorders.
Table 1.
Baseline characteristics of the sample stratified by diagnostic group (n░=░4989)a.
| Characteristic | Category | Total n (%) (n░=░4989) |
SMI n (%) (n░=░450) |
DD n (%) (n░=░1235) |
None n (%) (n░=░3304) |
Chi-square test |
DF |
|---|---|---|---|---|---|---|---|
| Age (years) | 21.34 | 4 | |||||
| ≤39 | 2214 (44.4) | 181 (40.2) | 535 (43.3) | 1498 (45.4) | |||
| 40–49 | 1961 (39.3) | 207 (46.0) | 523 (42.3) | 1231 (37.3) | |||
| ≥50 | 813 (16.3) | 62 (13.8) | 177 (14.3) | 574 (17.4) | |||
| Sex | 57.18 | 2 | |||||
| Male | 3538 (71.3) | 277 (62.1) | 804 (65.6) | 2457 (74.7) | |||
| Female | 1421 (28.7) | 169 (37.9) | 422 (34.4) | 830 (25.3) | |||
| Race | 26.50 | 4 | |||||
| Black | 2100 (42.1) | 160 (35.6) | 503 (40.7) | 1437 (43.5) | |||
| Hispanic/other | 1593 (31.9) | 180 (40.0) | 366 (29.6) | 1047 (31.7) | |||
| White | 1295 (26.0) | 110 (24.4) | 366 (29.6) | 819 (24.8) | |||
| Nadir CD4 cell count (cells/μl) |
12.32 | 6 | |||||
| <50 | 1168 (23.4) | 104 (23.1) | 253 (20.5) | 811 (24.5) | |||
| 50–200 | 1562 (31.3) | 135 (30.0) | 407 (33.0) | 1020 (30.9) | |||
| 201–500 | 1718 (34.4) | 161 (35.8) | 453 (36.7) | 1104 (33.4) | |||
| >500 | 541 (10.8) | 50 (11.1) | 122 (9.9) | 369 (11.2) | |||
| Prior history of IDU |
42.08 | 2 | |||||
| No | 3570 (72.2) | 288 (64.3) | 824 (67.3) | 2458 (75.1) | |||
| Yes | 1378 (27.8) | 160 (35.7) | 401 (32.7) | 817 (24.9) | |||
| No. of MH visits |
1424.13 | 8 | |||||
| 0 | 3629 (72.7) | 123 (27.3) | 577 (46.7) | 2929 (88.7) | |||
| 1 | 444 (8.9) | 63 (14.0) | 200 (16.2) | 181 (5.5) | |||
| 2–5 | 587 (11.8) | 159 (35.3) | 275 (22.3) | 153 (4.6) | |||
| 6–11 | 191 (3.8) | 61 (13.6) | 99 (8.0) | 31 (0.9) | |||
| ≥12 | 138 (2.8) | 44 (9.8) | 84 (6.8) | 10 (0.3) |
DD, depressive disorder; DF, degree of freedom; IDU, injection drug use; MH, mental health; SMI, serious mental illness.
Sample includes five sites. Patients were observed to go on HAART sometime during the years 2001–2005.
Baseline is the year patient started HAART.
Reported P values are from chi-square tests of equal categorical distributions across the three diagnostic groups.
Compared with those without SMI or depressive disorders, those with these disorders were significantly more likely to be women and have a history of IDU (Table 1). About 73 and 53% of those in the SMI and depressive disorders groups, respectively, had at least one mental health visit at the HIVRN clinic in their first year of HAART versus 11% of those with no mental illness.
Group comparisons of hazard probabilities for discontinuation of HAART
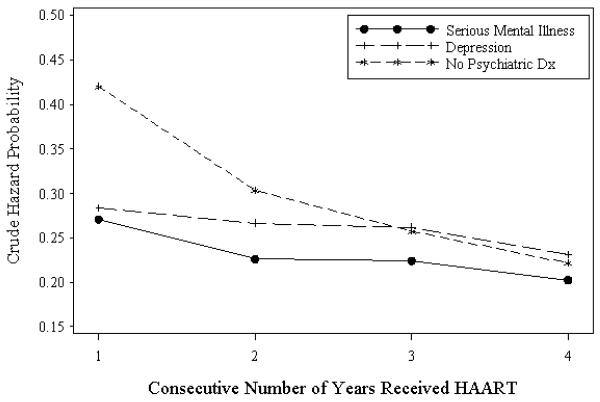
Crude hazard probabilities for those with 1, 2, 3, and 4 consecutive years on HAART for the SMI, depressive disorders, and no mental illness groups are plotted in Fig. 1 Fig. 1. In the first year on HAART, the hazard probability of discontinuing HAART is significantly lower for patients in the SMI group (0.271) versus the nonmental illness group (0.420) [crude odds ratio (OR), 0.51; 95% confidence interval (CI), 0.41–0.64]. Similarly, the hazard probability is significantly lower for the depressive disorders group versus the nonmental illness group (0.283 versus 0.420, crude OR, 0.55; 95% CI, 0.47–0.63) relative to the nonmental illness patients. In the second year on HAART, the hazard probability was again significantly lower for SMI patients versus the nonmental illness patients (0.226 versus 0.303, crude OR, 0.67; 95% CI, 0.50–0.91), but not significantly lower for depressive disorders patients versus nonmental illness patients (0.266 versus 0.303, crude OR, 0.83; 95% CI, 0.68–1.02). Among patients on HAART for third and fourth years, there were no differences in hazard probabilities among the SMI, depressive disorders, and nonmental illness patients.
Fig. 1.
Crude hazard probabilities for the serious mental illness, depressive disorders, and no mental illness groups. Dx, diagnosis. gr1
In the primary survival analysis model, the global test for a group by time interaction was significant; therefore, the group comparison ORs are reported by year. Similar to the crude analysis, the adjusted odds of discontinuing HAART in the first year for the SMI group was 57% of the odds of the nonmental illness group (AOR, 0.57; 95% CI, 0.47–0.69), and the adjusted odds of discontinuing HAART in the depressive disorders group was 61% of the odds in the nonmental illness group (AOR, 0.61; 95% CI, 0.54–0.69) (see Table 2 Table 2). Among those who were on HAART for 2 consecutive years, the odds of discontinuation in the second year for the SMI and depressive disorders groups were 68% (AOR, 0.68; 95% CI, 0.52–0.89) and 87% (AOR, 0.87; 95% CI, 0.73–1.03), respectively, of the odds of discontinuation in the nonmental illness group. After 3 and 4 years on HAART, odds of discontinuation did not significantly differ between the two mental illness groups and the nonmental illness group.
Table 2.
Discrete time survival analysis comparing the two mental illness groups with those without mental illness on risk of discontinuing HAART.
| Variablea | Category | Adjusted odds ratio | 95% CI | Pc |
|---|---|---|---|---|
| Age | ≤39 | 1.25 | (1.12–1.39) | <0.001 |
| 40–49 | 1.05 | (0.94–1.16) | 0.400 | |
| ≥50 | 1.00 | Referent | ||
| Sex | Male | 1.00 | Referent | |
| Female | 1.05 | (0.96–1.14) | 0.280 | |
| Race | White | 1.00 | Referent | |
| Hispanic/Other | 0.89 | (0.80–0.99) | 0.035 | |
| Black | 0.95 | (0.86–1.06) | 0.37 | |
| Nadir CD4 cell count (cells/μl) |
<50 | 0.87 | (0.76–1.00) | 0.046 |
| 50–200 | 0.78 | (0.68–0.89) | <0.001 | |
| 201–500 | 0.82 | (0.72–0.94) | 0.003 | |
| >500 | 1.00 | Referent | ||
| History of IDU | No | 1.00 | Referent | |
| Yes | 1.22 | (1.12–1.33) | <0.001 | |
| SMI versus NSMI | 1st yearb | 0.57 | (0.47–0.69) | <0.001 |
| 2nd year | 0.68 | (0.52–0.89) | 0.005 | |
| 3rd year | 0.77 | (0.53–1.11) | 0.161 | |
| 4th year | 0.85 | (0.48–1.49) | 0.57 | |
| DD versus Non-MI | 1st year | 0.61 | (0.54–0.69) | <0.001 |
| 2nd year | 0.87 | (0.73–1.03) | 0.110 | |
| 3rd year | 1.03 | (0.81–1.32) | 0.79 | |
| 4th year | 1.11 | (0.75–1.65) | 0.61 |
CI, confidence interval; DD, depressive disorder; DF, degree of freedom; IDU, injection drug use; MI, mental illness; NSMI, nonserious mental illness; SMI, serious mental illness.
Model adjusts for site – not shown.
Diagnostic group comparisons presented by consecutive year on HAART due to interaction between diagnostic group and consecutive year on HAART. Because interaction is in the model, main effects for diagnostic group and year are not presented.
P values are from Wald chi-square tests of regression coefficients with 1 DF.
Among the covariate predictors of discontinuing HAART in the survival analysis model, those with a history of IDU and patients less than 40-years-old were more likely to discontinue HAART relative to non-IDU (AOR, 1.22; 95% CI, 1.12–1.33) and patients 50 years or older, respectively (AOR, 1.25; 95% CI, 1.12–1.39). Patients with nadir CD4 cell counts less than 50, 50–200, and 201–500░cells/μl were all significantly less likely to discontinue HAART compared with those with nadir CD4 cell counts greater than 500░cells/μl (AORs, 0.87, 0.78, and 0.82, respectively).
Mental health visits and discontinuation of HAART
In the survival analysis model examining the relationship of mental health visits to the discontinuation of HAART among those with mental illness, those with one mental health visit in a year had a 36% greater odds of discontinuing HAART relative to patients with zero mental health visits in a year (see Table 3 Table 3). There was no significant difference in odds of discontinuing HAART between those with two to five mental health visits and those with zero mental health visits in a year. In contrast, patients with six to 11 and 12 or more mental health visits in a year had 78 and 60% of the odds of discontinuing HAART, respectively, as compared with patients with no mental health visits.
Table 3.
Discrete time survival analysis among the mental illness groups only, assessing affect of number of mental health visits in a year on odds of discontinuing HAART in that year.
| Variablea | Category | Adjusted odds ratio | 95% CI | Pb |
|---|---|---|---|---|
| Number of mental health visits | 0 | 1.00 | Referent | |
| 1 | 1.36 | (1.13–1.65) | 0.0013 | |
| 2–5 | 0.93 | (0.78–1.11) | 0.43 | |
| 6–11 | 0.78 | (0.61–1.00) | 0.052 | |
| ≥12 | 0.60 | (0.45–0.80) | <0.001 |
CI, confidence interval; DF, degree of freedom; IDU, injection drug use.
Covariates age, sex, race, nadir CD4 cell count, history of IDU, year, and site are not displayed. Results for these variables did not significantly change from the primary analysis displayed in Table 2.
P values are from Wald chi-square tests of regression coefficients with 1 DF.
Discussion
Among those receiving HAART, our study found that relative to those with no mental illness, the adjusted hazard probability for discontinuation of HAART was significantly lower during the first and second years following initiation of HAART among those with SMI and significantly lower during the first year among those with depressive disorders. The probabilities did not significantly differ in years 3 and 4 among any of the diagnostic groups. These findings suggest that among those receiving HAART, those with mental illness are significantly more likely to stay in clinic care in the early years of HAART treatment, a critical time when adherence to HAART may have the most clinical impact.
In order to further evaluate why those with mental illness may be more likely to remain on HAART, we evaluated whether the number of mental health visits impacted the rates of discontinuation of HAART. We found that among those with a mental illness, patients with six to 11 mental health visits in a year were 22% less likely to discontinue HAART, whereas those with 12 or more mental health visits in a year were 40% less likely to discontinue HAART compared with patients with no mental health visits. The frequency of visits compares well with other studies that have found the median number of treatment visits for mental health problems in general to be 7.4 visits per year [22] and for depression in particular the mean number of treatment visits to be 8.7 visits per year [23]. Our findings suggest that a dose-response relationship may exist, such that patients with ongoing mental health treatment with consistent and frequent follow-up benefited the most. In contrast, those patients who were seen less consistently, those receiving five or fewer mental health visits in a year were no more likely to discontinue HAART compared with those without a mental illness. Those receiving only one mental health visit in a year in fact were significantly more likely to discontinue HAART compared with those with no mental health visits. This latter group may represent patients who had the most difficulty engaging in mental healthcare and may require more careful follow-up from their HIV providers. Finally, our results raise the interesting possibility that the increased contact and support associated with reduced HAART discontinuation that we found in our study may be found among patients without significant psychiatric conditions as well.
Our findings build on previous findings from other studies. For example, results from the Women’s Interagency HIV Study (WIHS) cohort found that after controlling for depression those who used mental health services had 20% increase in the adjusted odds of utilizing HAART as compared with those who did not use mental health services [12]. Data from HIV Cost and Services Utilization Study (HCSUS) found that among HIV-infected individuals with a psychiatric disorder, the presence of care from a psychiatric health provider was significantly associated with a 50% increase in the odds of receiving HAART [24]. It also builds on evidence that suggests that receipt of mental health treatment may increase the probability that individuals with psychiatric disorders receive and adhere to HAART [18,25,26].
Our study is also among the first to demonstrate that those with SMI were less likely to discontinue HAART compared with those without mental illness. This finding builds on earlier cross-sectional evidence that suggests that those with SMI are as likely to initiate or access HAART compared with those without SMI [17,18,27]. It is also consistent with evidence that providers are willing to consider prescribing HAART to individuals with schizophrenia who are receiving psychiatric care [28]. It is possible that those with SMI may benefit from receiving colocated HIV and mental healthcare (as was the case in this study) that may result in improved treatment coordination and adherence to HAART [29]. This association may be supported by the mental health visit data presented above as well as some [30,31] but not all [32] previous studies evaluating use of non-HIV somatic healthcare by those with SMI.
Our finding that those with depressive disorders who receive mental health treatment are less likely to discontinue HAART is consistent with other studies. One retrospective study in an urban clinic with integrated mental healthcare found that compared with HAART-naïve individuals with AIDS without a mental disorder, those HAART-naïve individuals with AIDS with a mental disorder who were receiving mental health treatment were 50% more likely to receive HAART, had over twice the odds of remaining on HAART for at least 6 months, and were 40% more likely to survive through the study period [11]. Another study [33] found among a sample of HIV-infected patients receiving HIV medical care in one of eight clinics in five American southern states that having depressive symptoms was not associated with time to discontinuation of antiretroviral therapy. Finally, a study [18] of patients in the University of Washington HIV Cohort found that there was no delay in HAART initiation among those patients who received treatment for depression/anxiety compared with those with no mental disorders. However, it is important to note that in the absence of mental health treatment, symptoms of depression have been reported to be associated with poor adherence to HAART [8–10].
Our study has several important limitations. First, the sample is not nationally representative and does not generalize to all HIV care sites. The sites in the sample do encompass a geographic distribution that is in keeping with the HIV epidemic, and multisite studies afford greater generalizeability than single-site studies. Moreover, the sites in the HIVRN were all highly experienced in the treatment of HIV with high rates of HAART [34] and opportunistic infection prophylaxis rates [35]; results may differ at sites with less provider experience with HIV or a smaller caseload of patients with HIV. Also, not all of the sites in the HIVRN collect comprehensive mental health and utilization data; therefore, we were only able to include five of the 17 adult sites in the Network. However, these five sites represent nearly 40% of the total patients captured by the HIVRN. As these sites have the capacity to provide mental health data, they may be more likely to provide a higher level of care for individuals with cooccurring mental health and substance abuse disorders. Third, as our definition of SMA was based on ICD-9 codes and not based on patient clinical interviews, we were unable to confirm the validity of the diagnoses. Furthermore, the ICD-9 codes reflect use of psychiatric services provided within the clinic and does not capture use of mental health services provided outside of the clinic. As all the clinics have onsite psychiatric services, it is unlikely that many patients seek mental healthcare outside the clinic. Fourth, in this analysis, we assumed that the majority of patients who dropped out of care at the HIVRN clinic also discontinued HAART. Although this assumption has considerable face validity, we are not aware of any published data that fully support this assumption.
Among those who initiate HAART, individuals with mental illness were significantly less likely to discontinue HAART or clinical care in the first and second years relative to those without mental illness. Mental health visits were associated with decreased risk of discontinuing HAART, suggesting the importance of ongoing and consistent mental health treatment among HIV-positive people with cooccurring mental health conditions.
Acknowledgements
Dr Himelhoch was supported by grants from the National Institute of Drug Abuse (NIDA) (K23-DA019820) as well as the National Institute of Mental Health (NIMH) (R34-MH080630-02). Dr Korthuis was supported by a NIDA grant (K23-DA019809), and Dr Chander was supported by the National Institute of Alcoholism and Alcohol Abuse grant (K23-DA019809). Dr Gebo was supported by grants from the Agency for Healthcare Research and Quality (AHRQ) (290-01-012) and National Institute on Aging (R01 AG026250) as well as support from the Johns Hopkins University Richard S. Ross Clinician Scientist Award. Dr Walkup was supported by a NIMH grant (RO1 MH60831) as well as AHRQ through a cooperative agreement from the Center for Research and Education on Mental Health Therapeutics at Rutgers (HS016097).
Drs Himelhoch, Brown, Walkup, and Gebo did the study concept and design; Drs Himelhoch, Brown, Walkup, Gebo, Chander, Korthius, and Mr Afful are responsible for analysis and interpretation; Drs Himelhoch and Brown did the drafting of the manuscript; Drs Walkup, Gebo, Chander, and Korthius were responsible for critical revision of the manuscript for important intellectual content; and Drs Brown, Himelhoch, and Mr Afful did the statistical analysis.
The views expressed in this study are those of the authors. No official endorsement by Department of Health and Human Services, the National Institutes of Health, or the AHRQ is intended or should be inferred.
References
- 1.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 2.Justice AC, McGinnis KA, Atkinson JH, Heaton RK, Young C, Sadek J, et al. Psychiatric and neurocognitive disorders among HIV-positive and negative veterans in care: Veterans Aging Cohort Five-Site Study. AIDS. 2004;18(Suppl 1):S49–S59. [PubMed] [Google Scholar]
- 3.Palella FJ, Delaney KM, Moorman AC. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Mocroft A, Monforte A, Kirk O, Johnson MA, Friis-Moller N, Banhegyi D, et al. Changes in hospital admissions across Europe: 1995–2003. Results from the EuroSIDA study. HIV Med. 2004;5:437–447. doi: 10.1111/j.1468-1293.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- 5.Beck EJ, Mandalia S, Williams I, Power A, Newson R, Molesworth A, et al. Decreased morbidity and use of hospital services in English HIV-infected individuals with increased uptake of antiretroviral therapy 1996–1997. National Prospective Monitoring System Steering Group. AIDS. 1999;13:2157–2164. doi: 10.1097/00002030-199910220-00020. [DOI] [PubMed] [Google Scholar]
- 6.Baum SE, Morris JT, Gibbons RV, Cooper R. Reduction in human immunodeficiency virus patient hospitalizations and nontraumatic mortality after adoption of highly active antiretroviral therapy. Mil Med. 1999;164:609–612. [PubMed] [Google Scholar]
- 7.Fairfield KM, Libman H, Davis RB, Eisenberg DM. Delays in protease inhibitor use in clinical practice. J Gen Intern Med. 1999;14:395–401. doi: 10.1046/j.1525-1497.1999.08198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordillo V, del Amo J, Soriano V, Gonzalez-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13:1763–1769. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- 9.Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21:1175–1183. doi: 10.1097/QAD.0b013e32811ebf57. [DOI] [PubMed] [Google Scholar]
- 10.Pence BW, Miller WC, Gaynes BN, Eron JJ., Jr Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44:159–166. doi: 10.1097/QAI.0b013e31802c2f51. [DOI] [PubMed] [Google Scholar]
- 11.Himelhoch S, Treisman G, Moore RA, Gebo K. Does presence of a mental disorder in AIDS patients affect the initiation of anitretroviral treatment and duration of therapy? J Acquir Immune Defic Syndr. 2004;37:1457–1463. doi: 10.1097/01.qai.0000136739.01219.6d. [DOI] [PubMed] [Google Scholar]
- 12.Cook JA, Cohen MH, Burke J, Grey D, Anastos K, Kirstein L, et al. Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. J Acquir Immune Defic Syndr. 2002;30:401–409. doi: 10.1097/00042560-200208010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Cournos F, Empfield M, Horwath E, McKinnon K, Meyer I, Schrage H, et al. HIV seroprevalence among patients admitted to two psychiatric hospitals. Am J Psychiatry. 1991;148:1225–1230. doi: 10.1176/ajp.148.9.1225. [DOI] [PubMed] [Google Scholar]
- 14.Himelhoch S, McCarthy JF, Ganoczy D, Medoff D, Dixon LB, Blow FC. Understanding associations between serious mental illness and HIV among patients in the VA Health System. Psychiatr Serv. 2007;58:1165–1172. doi: 10.1176/ps.2007.58.9.1165. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg SD, Goodman LA, Osher FC, Swartz MS, Essock SM, Butterfield MI, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91:31–37. doi: 10.2105/ajph.91.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blank MB, Mandell DS, Aiken L, Hadley TR. Co-occurrence of HIV and serious mental illness among Medicaid recipients. Psychiatr Serv. 2002;53:868–873. doi: 10.1176/appi.ps.53.7.868. [DOI] [PubMed] [Google Scholar]
- 17.Walkup JT, Sambamoorthi U, Crystal S. Use of newer antiretroviral treatments among HIV-infected medicaid beneficiaries with serious mental illness. J Clin Psychiatry. 2004;65:1180–1189. doi: 10.4088/jcp.v65n0905. [DOI] [PubMed] [Google Scholar]
- 18.Tegger MK, Crane HM, Tapia KA, Uldall KK, Holte SE, Kitahata MM. The effect of mental illness, substance use, and treatment for depression on the initiation of highly active antiretroviral therapy among HIV-infected individuals. AIDS Patient Care STDS. 2008;22:233–243. doi: 10.1089/apc.2007.0092. [DOI] [PubMed] [Google Scholar]
- 19.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–202. [Google Scholar]
- 20.Singer JD, Willet JB. It’s about time: using discrete-time survival analysis to study duration and the timing of events. J Educ Stat. 1993;18:155–195. [Google Scholar]
- 21.Allison PD. Survival analysis using SAS: a practical guide. SAS Institute Inc.; Cary, NC: 1995. [Google Scholar]
- 22.Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, Kessler RC. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629–640. doi: 10.1001/archpsyc.62.6.629. [DOI] [PubMed] [Google Scholar]
- 23.Olfson M, Marcus SC, Druss B, Elinson L, Tanielian T, Pincus HA. National trends in the outpatient treatment of depression. JAMA. 2002;287:203–209. doi: 10.1001/jama.287.2.203. [DOI] [PubMed] [Google Scholar]
- 24.Turner BJ, Fleishman JA, Wenger N, London AS, Burnam MA, Shapiro MF, et al. Effects of drug abuse and mental disorders on use and type of antiretroviral therapy in HIV-infected persons. J Gen Intern Med. 2001;16:625–633. doi: 10.1046/j.1525-1497.2001.016009625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun LWH, Maravi M, Koayashi JS, Barton PL, Davidson AJ. Antidepressant treatment improves adhernce to antiretorviral therapy among depressed HIV-infected patients. J Acquir Immune Defic Syndr. 2005;38:432–438. doi: 10.1097/01.qai.0000147524.19122.fd. [DOI] [PubMed] [Google Scholar]
- 26.Cook JA, Grey D, Burke-Miller J, Cohen MH, Anastos K, Gandhi M, et al. Effects of treated and untreated depressive symptoms on highly active antiretroviral therapy use in US multisite cohort of HIV-positive women. AIDS Care. 2006;18:93–100. doi: 10.1080/09540120500159284. [DOI] [PubMed] [Google Scholar]
- 27.Himelhoch S, Chander G, Fleishman JA, Hellinger J, Gaist P, Gebo KA. Access to HAART and utilization of inpatient medical hospital services among HIV-infected patients with co-occurring serious mental illness and injection drug use. Gen Hosp Psychiatry. 2007;29:518–525. doi: 10.1016/j.genhosppsych.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Himelhoch S, Powe N, Breaky W, Gebo K. Schizophrenia, AIDS and the decision to prescribe HAART: results of a national survey of HIV clinicians. J Prev Interv Community. 2006;33:109–120. doi: 10.1300/J005v33n01_09. [DOI] [PubMed] [Google Scholar]
- 29.Ohl ME, Landon BE, Cleary PD, LeMaster J. Medical clinic characteristics and access to behavioral health services for persons with HIV. Psychiatr Serv. 2008;59:400–407. doi: 10.1176/ps.2008.59.4.400. [DOI] [PubMed] [Google Scholar]
- 30.Dickerson FB, McNary SW, Brown CH, Kreyenbuhl J, Goldberg RW, Dixon LB. Somatic healthcare utilization among adults with serious mental illness who are receiving community psychiatric services. Med Care. 2003;41:560–570. doi: 10.1097/01.MLR.0000053440.18761.F0. [DOI] [PubMed] [Google Scholar]
- 31.Druss BG, Rosenheck RA. Locus of mental health treatment in an integrated service system. Psychiatr Serv. 2000;51:890–892. doi: 10.1176/appi.ps.51.7.890. [DOI] [PubMed] [Google Scholar]
- 32.Chwastiak LA, Rosenheck RA, Kazis LE. Utilization of primary care by veterans with psychiatric illness in the National Department of Veterans Affairs Healthcare System. J Gen Intern Med. 2008;23:1835–1840. doi: 10.1007/s11606-008-0786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pence BW, Ostermann J, Kumar V, Whetten K, Thielman N, Mugavero MJ. The influence of psychosocial characteristics and race/ethnicity on the use, duration, and success of antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:194–201. doi: 10.1097/QAI.0b013e31815ace7e. [DOI] [PubMed] [Google Scholar]
- 34.Gebo KA, Fleishman JA, Moore RD. Hospitalizations for metabolic conditions, opportunistic infections, and injection drug use among HIV patients: trends between 1996 and 2000 in 12 states. J Acquir Immune Defic Syndr. 2005;40:609–616. doi: 10.1097/01.qai.0000171727.55553.78. [DOI] [PubMed] [Google Scholar]
- 35.Gebo KA, Fleishman JA, Reilly ED, Moore RD. High rates of primary Mycobacterium avium complex and Pneumocystis jiroveci prophylaxis in the United States. Med Care. 2005;43:III23–III30. doi: 10.1097/01.mlr.0000175631.34438.1e. [DOI] [PubMed] [Google Scholar]