Abstract
MicroRNAs (miRNAs) are a large and growing class of small, non-coding, regulatory RNAs that control gene expression predominantly at the post-transcriptional level. The production of most functional miRNAs depends on the enzymatic activity of Dicer, an RNase III class enzyme. To address the potential action of Dicer-dependent miRNAs in mammalian kidney development, we conditionally ablated Dicer function within cells of nephron lineage and the ureteric bud-derived collecting duct system. Six2Cre-mediated removal of Dicer activity from the progenitors of the nephron epithelium led to elevated apoptosis and premature termination of nephrogenesis. Thus, Dicer action is important for maintaining the viability of this critical self-renewing progenitor pool and, consequently, development of a normal nephron complement. HoxB7Cre-mediated removal of Dicer function from the ureteric bud epithelium led to the development of renal cysts. This was preceded by excessive cell proliferation and apoptosis, and accompanied by disrupted ciliogenesis within the ureteric bud epithelium. Dicer removal also disrupted branching morphogenesis with the phenotype correlating with downregulation of Wnt11 and c-Ret expression at ureteric tips. Thus Dicer, and by inference Dicer-dependent miRNA activity, have distinct regulatory roles within different components of the developing mouse kidney. Furthermore, an understanding of miRNA action may provide new insights into the etiology and pathogenesis of renal cyst-based kidney disease.
Keywords: branching morphogenesis, Dicer, miRNA, nephron progenitors, primary cilium, renal cyst
MicroRNAs (miRNAs) are a large and growing class of small, non-coding, regulatory RNAs that control gene expression predominantly at the post-transcriptional level through direct binding to target mRNAs. In general, miRNAs function by inhibiting protein translation and/or degrading target mRNAs (for recent reviews, see Carthew and Sontheimer1; Behm-Ansmant et al.2; Pillai et al.3), although recent reports indicate that miRNAs may occasionally enhance expression from their target mRNAs in a tissue context- or cell cycle state-dependent fashion.4,5 A single miRNA may regulate hundreds of target genes, and a given gene can be regulated by multiple miRNAs. In this way, miRNA action underpins many fundamental biological processes, including developmental timing, apoptosis, cell proliferation, cell-fate choice and morphogenesis, as well as pathogenic cellular activities, notably oncogenesis.6
miRNAs are initially transcribed as long pri-miRNAs from miRNA-coding genes and need to be processed to function. Dicer, an RNase III class enzyme, is required for the processing of most miRNAs. pri-miRNAs are first processed in the nucleus by another RNase III, Drosha, in a complex with DGCR8, or the splicing machinery, to ~70 nt stem-loop pre-miRNAs. Dicer then processes pre-miRNAs in the cytoplasm to mature miRNAs, which recognize cognate mRNAs through an Argonaute-dependent process, modulating the stability or translation of mRNA targets (see Carthew and Sontheimer1 for a recent review). Genetic studies with conditionally removing Dicer function have demonstrated critical roles for Dicer-mediated miRNA regulation in the development and function of a variety of mammalian tissues and organs.7,8
Kidney development is driven by reciprocal interactions between the ureteric bud (UB) epithelium and the overlying metanephric mesenchyme (for a recent review, see Dressler9), with additional inputs from the renal interstitium and vascular tissues.10–16 The UB epithelium originates as an evagination of the Wolffian duct epithelium at the hind-limb level.17 UB-derived signals regulate maintenance and nephron commitment within a Six2+ mesenchymal progenitor compartment that caps the branching ureteric tips (nephron progenitors).18–20 Inductive UB signals induce a mesenchymal-to-epithelial transition within overlying progenitor pools, resulting in the emergence of the renal vesicle beneath the UB tip.19 The renal vesicle is the nephron precursor, and its morphogenesis, patterning, and differentiation establishes the mature renal tubular, visceral, and parietal epithelia of the nephron. Metanephric mesenchyme-secreted Glial cell line-derived neurotrophic factor (GDNF) stimulates branching morphogenesis of the subjacent UB tips, establishing the highly branched network of the renal collecting duct system (recently reviewed in Costantini17). Epithelial growth within both nephron and collecting duct epithelia is tightly controlled; deregulation leads to cystic dilation and renal cystic diseases. Through these intricate interactions, the UB branching morphogenesis regulates nephron numbers, a predisposing factor for renal diseases and hypertension.21,22
Evidence for the involvement of Dicer and miRNAs in kidney development and homeostasis is accumulating.23–25 miRNAs have been detected in embryonic and adult kidney tissues and changes in miRNA expression have been observed in various kidney diseases, including polycystic kidney disease, diabetic nephropathy, kidney and bladder cancer, and in autoimmune disease like lupus nephritis.23–25 Podocyte-specific removal of Dicer causes multiple abnormalities in postnatal renal corpuscle homeostasis and function, leading to proteinuria and a rapid progression to end-stage kidney diseases.26–28 Maintenance of Juxtaglomerular cells also requires Dicer input.29 Removal of Dicer within the collecting duct epithelium results in postnatal hydronephrosis and collecting duct cysts,30 demonstrating the functional importance of Dicer and miRNAs in kidney homeostasis and function. In addition, miRNAs regulate Xenopus pronephros patterning and differentiation.31
In this paper, we use nephron progenitor-specific Dicer removal to demonstrate the importance of Dicer in the maintenance of nephron progenitors and nephron epithelia. Conditional removal of Dicer within the adjacent ureteric bud epithelium extends a previous report30 of Dicer/miRNA-associated cystic dilation by identifying a requirement for Dicer in controlling cell proliferation and apoptosis in the collecting duct epithelium. Furthermore, we demonstrate that Dicer action is required for normal branching growth of the ureteric network.
RESULTS
Removal of Dicer activity from the nephron lineage leads to premature depletion of nephron progenitors
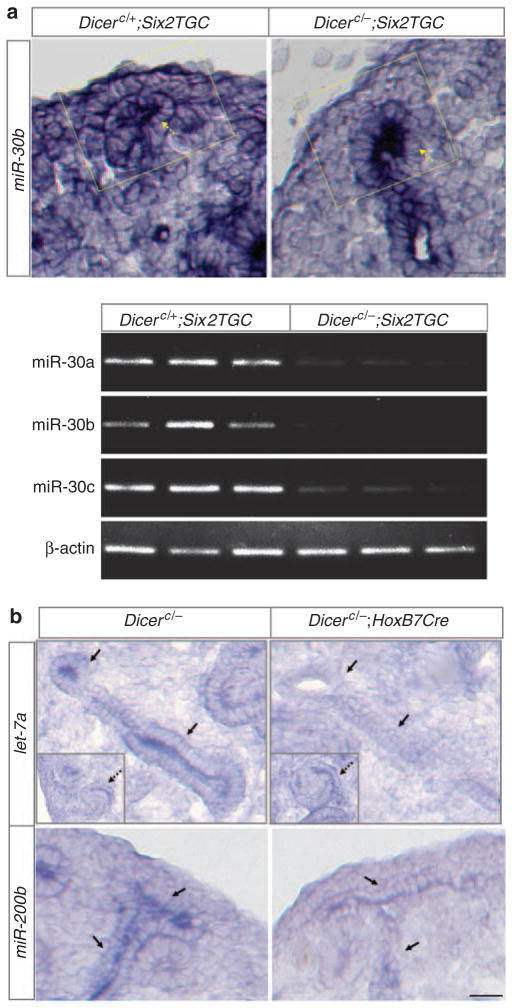
To examine the overall involvement of nephron lineage-expressed Dicer and miRNAs in kidney development, we ablated Dicer function from the entire nephron lineage using a Six2Cre (Six2TGC) transgenic mouse strain.18 The resulting mutant mice (Dicerc/−; Six2TGC, referred to as Dicer nephron mutants hereafter) died within 36h post-partum. Examination of expression of randomly selected mature miRNAs with miRNA in situ hybridization and semiquantitative reverse transcription-PCR analyses suggests that miRNA maturation was specifically disrupted in the nephron lineage in the mutant kidneys (Figure 1a).
Figure 1. MicroRNA (miRNA) biogenesis is disrupted from Dicer depletion.
In situ hybridization for miRNAs with locked nucleic acid (LNA) probes on E14.5 kidney sections and semiquantitative miRNA reverse transcription-PCR (RT-PCR) analysis on the cap mesenchyme. (a) miR-30b expression in the nephron progenitors (the cap mesenchyme cells overlying the ureteric tip in the boxed area) is markedly reduced to background levels in Dicer nephron mutants. Its expression in the ureteric bud epithelium (dashed arrows) is unaffected. Semiquantitative RT-PCR for miR-30a, miR-30b, miR-30c, and β-actin (control) in the fluorescence-activated cell sorting (FACS)-sorted green fluorescent protein (GFP)-positive cap mesenchyme (nephron progenitors) from three controls and three mutants showing the reduction of mature miRNAs in the E13.5 Dicer nephron mutant cap mesenchyme. The trace amount of mature miRNAs in the mutants may be due to incomplete degradation of mature miRNAs previously produced in a subset of cap mesenchyme cells because of more mosaic expression of Six2TGC at earlier developmental stages (Kobayashi et al.18 and unpublished observations, JY). (b) let-7a and miR-200b expression is greatly reduced in the ureteric bud (UB) epithelium (solid arrows) in Dicer UB mutants but unaffected in non-UB cell types (S-shaped bodies, insets and dashed arrows). Scale bars = 20 μm.
The mutant newborn kidneys were smaller than the control littermates, with a striking 93.2% reduction in nephron numbers (control, 1755; mutant, 119; P<3.1E-12, n = 7) (Figure 2 and Supplementary Figure S1A online). Moreover, the nephron progenitors and developing nephron structures (renal vesicle, comma-shaped body, and S-shaped body) were absent from many regions of the renal cortex (Figure 2e and f). Consistent with the latter observation, Six2 expression in the nephron progenitors was lost in many areas of the mutant kidney cortex (Figure 3b). Notably, in regions where the nephrogenic zone was still present, there were much fewer nephron progenitor cells capping the UB tips (Pax2+ cap mesenchyme cells) (Figure 3a). Moreover, most of these mutant nephron progenitor cells expressed green fluorescent protein (GFP; as a fusion protein with Cre) at greatly reduced to undetectable levels, in contrast to the strong expression of GFP in all cells of their control counterparts (Figure 3a). The Six2TGC driver line was generated by BAC (bacterial artificial chromosome) transgenics in that a large fragment of the genomic sequence encoding the endogenous Six2 promoter drives the expression of the GFP/Cre fusion protein. The reduction in GFP levels in Dicer mutant nephron progenitors may reflect downregulation of the activity of the Six2 promoter driving GFP/Cre expression. If this is the case, the expression of endogenous Six2 gene should also be compromised in Dicer nephron mutants. Indeed, we observed a decrease in the levels of Six2 transcripts, where present, in mutant nephron progenitors (Figure 3b, insets). Taken together, these data suggest that Dicer and presumably miRNAs are critical for the maintenance of nephron progenitors and Six2 expression.
Figure 2. Disruption of Dicer functions from the nephron lineage leads to premature termination of nephrogenesis.
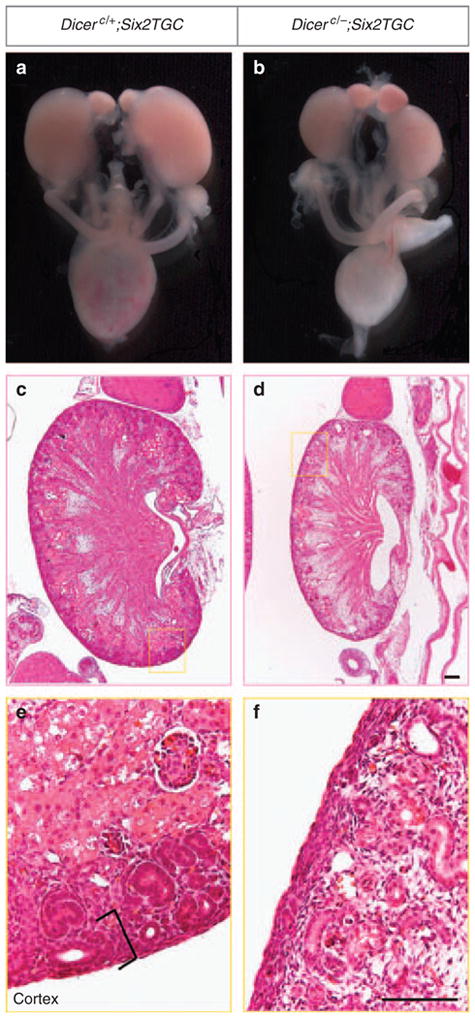
(a, b) The Dicer nephron mutant kidneys are smaller than their control littermates at P0. (c–f) Hematoxylin and eosin staining of P0 kidney sections showing regions with a marked absence of the nephrogenic zone (brackets in e) except for the nephrogenic zone intersitium in the mutant kidney. Scale bars = 200 μm.
Figure 3. Reduction in the number of nephron progenitor cells and Six2 expression resulted from Dicer abrogation from the nephron lineage.
(a) Immunofluorescent staining of Pax2 to label the nephron progenitors and of green fluorescent protein (GFP). Pax2 also labels the ureteric bud (UB) epithelium and the developing nephron epithelium. The UB epithelium is demarcated with pan-Cytokeratin immunostaining. The nephron progenitors are discerned from the nephron epithelium by their positions relative to the UB epithelium. Nephron progenitors overlie the UB epithelium, whereas the nephron epithelium lies underneath the UB epithelium. All nephron progenitor cells are GFP+ in control kidneys, but a subset of them exhibit reduced or undetectable levels of GFP expression in mutants starting at E13.5. Scale bars = 50 μm. (b) In situ hybridization and quantitative reverse transcription PCR (qRT-PCR) analyses for Six2 expression in the nephron progenitors on kidney sections. At E14.5, Six2 expression is weaker in mutants than in controls. At P0, Six2 transcripts are detected throughout the entire periphery of the control kidney, but are missing in most regions of the mutant kidney periphery and expressed at lower levels where detected. Arrows point to the regions enlarged in the insets. Scale bars = 100 μm. At E13.5, the Six2 mRNA levels are 66.4% of that of controls (n = 3). (c) The number of nephron progenitor cells (Pax2+ cap mesenchyme) per ureteric tip per kidney section is not statistically significantly different between Dicer mutants and controls at E14.5, but decreases statistically significantly in the mutants at E15.5. (d) The percentage of GFP+ nephron progenitor cells (GFP+/Pax2+) is significantly reduced in Dicer mutants at both E14.5 and E15.5. Controls in b–d, Dicerc/+; Six2TGC mice.
Quantitative analysis showed that the reduction in the number of nephron progenitor cells (Pax2+ cap mesenchyme cells) per ureteric tip was first evident at embryonic day (E)15.5 (Figure 3c). The reduction in GFP expression in nephron progenitors was observed in a subset of cells as early as E13.5 (Figure 3a), preceding the onset of the premature loss of nephron progenitors, and exacerbated later in development (Figure 3a and d, the percentage of GFP+ nephron progenitors: 82.3% at E14.5, 73.2% at E15.5). Likewise, Six2 transcript levels were reduced prior to the onset of the premature loss of nephron progenitors (Figure 3b).
Dicer regulates nephron progenitor cell survival
To determine the cellular mechanisms underlying the premature depletion of nephron progenitor cells following Dicer removal, we examined cell proliferation, cell survival, and cell differentiation at E14.5, prior to the observed phenotype. Cell proliferation, as determined by 5-bromo-deoxyuridine (BrdU) incorporation, was unaffected (data not shown). The earliest markers of nephron induction, Wnt4 and Fgf8, were expressed in the normal pattern underneath the UB tips, and not in mutant nephron progenitors overlying the ureteric tips (Supplementary Figure S2A online), ruling out precocious differentiation of nephron progenitors. Moreover, cell-fate mapping with a Rosa26lacZ Cre reporter32 in the Dicer nephron mutant background showed that mutant nephron progenitors only contributed to the nephron lineage and did not trans-differentiate into other renal cell types (Supplementary Figure S1B online). In contrast, when apoptosis in the Pax2+ cap mesenchyme cells was examined with TUNEL (TdT-mediated dUTP nick end labeling) analysis, an increase in apoptosis in the mutant nephron progenitors was readily observed (Figure 4a). In the control kidneys, apoptotic cells were rarely seen in nephron progenitors (1.24±0.94%, n = 3), whereas in the mutants the apoptosis rate increased by almost six-fold to 7.27±2.59% (n = 4). Taken together, these data demonstrate that Dicer action is important for survival of nephron progenitors.
Figure 4. Elevated apoptosis in Dicer nephron mutant nephron progenitors (a) and nephron epithelium (b).
Immunofluorescent staining of Pax2 for nephron progenitors, the ureteric bud (UB) epithelium and part of the nephron epithelium, Laminin (basal lamina) for the nephron and UB epithelia, pan-Cytokeratin for the UB epithelium, WT1 (nuclear) for the proximal segment of the S-shaped body, and Cadherin 6 (Cdh6) for the loop of Henle. Scale bar = 20 μm.
Increased apoptosis in the nephron epithelium
Disruption of Dicer function did not appear to affect nephron patterning, as judged by the normal expression patterns of markers for different segments of the S-shaped bodies, Wt1 (proximal segment), Wnt4 (medial segment), Fgf8 (medial segment), and Brn1 (medial and distal segments) in Dicer nephron mutants (Supplementary Figure S2B). Moreover, nephron segmentation and terminal differentiation was also largely normal at E15.5, based on the expression of nephron segmentation and differentiation markers (Supplementary Figure S3 online).
Although most of segmentation and differentiation markers were expressed at normal levels at the early stage of nephron segmentation and terminal differentiation, the expression level of Gsh1, one of the earliest markers of the podocyte lineage, was greatly reduced in mutant kidneys (Supplementary Figure S3 online). To determine whether reduced Gsh1 expression reflected global disruption of podocyte differentiation, we examined the expression of two additional podocyte markers, WT1 and p57Kip2. The expression of neither gene in mutant podocytes was obviously altered (Supplementary Figure S3 online), suggesting that Gsh1 expression was specifically affected by Dicer removal.
Although cell differentiation was undisturbed in mutant nephrons, cell death was markedly elevated in the proximal segment of the S-shaped body and the Cadherin 6+ nephron tubules (Figure 4b), indicating an extended role for Dicer action in cell survival within the developing nephron epithelium.
Premature termination of branching morphogenesis in Dicer UB mutant kidneys
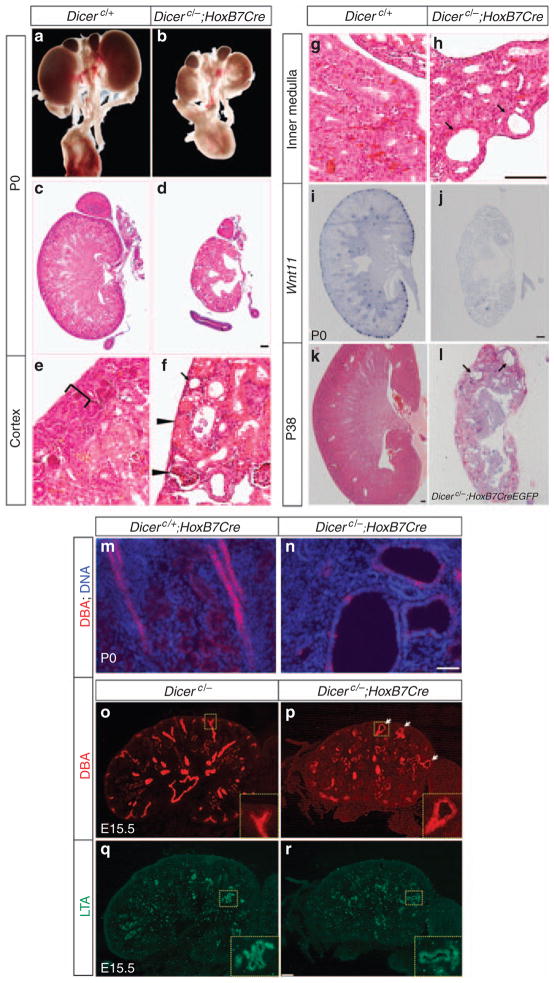
Dicer was specifically ablated from the UB epithelium with a HoxB7Cre deletor line; Cre recombinase driven by a Hoxb7 promoter is specifically expressed throughout the Wolffian duct from E9.5, prior to the outgrowth of the ureteric bud, and high levels of expression are maintained within all UB derivatives after kidney development initiates.33 The resultant mutants (Dicerc/−; HoxB7Cre) died by 24–48h post-partum, suggesting that Dicer in the UB epithelium is essential for kidney development. This is in contrast to viable HoxB7Cre/+; Dicer1flox/flox mice reported by Pastotelli et al.30 The discrepancy in viability probably reflects decreased efficiency in Cre-mediated deletion of Dicer, where both alleles are conditional null that may lead to higher frequency of mosaic removal, an observation that accounts for variability in Dicer mutant phenotypes in podocytes.27 In a second genetic cross, we removed Dicer activity with a HoxB7Cre-Ires-EGFP line (HoxB7CreEGFP for short).34 Here, analysis of enhanced GFP (EGFP) activity suggests variable levels of Cre expression (data not shown). Remarkably, most Dicerc/−; HoxB7-CreEGFP survived to adulthood (Figure 5j, and data not shown) and exhibited hydronephrosis and hydroureter as observed in HoxB7Cre/+; Dicer1flox/flox mice. In some hydronephrotic mutant kidneys, only a thin layer of the renal cortex remained, whereas some mutants with milder phenotypes retained most of the renal parenchyma but developed cysts in the collecting ducts (Figure 5j and data not shown), similar to HoxB7Cre/+; Dicer1flox/flox kidneys.30
Figure 5. Dicer ureteric bud (UB) mutant kidneys are hypoplastic, dysplastic, and cystic.
(a, b) The newborn (P0) Dicer UB mutant kidneys (b) are smaller than their wild-type littermate controls (a). (c–h) Hematoxylin and eosin staining of kidney sections from newborn control (c, e, g) and Dicer UB mutant (d, f, h) mice as indicated. The mutant renal cortex (f) is devoid of the nephrogenic zone (bracket in e) and mature nephron tubules and renal corpuscles (arrowheads) reach the kidney surface. Cysts were seen in cortical and medullary collecting ducts (arrows in f and h). (i, j) Wnt11 mRNA expression in newborn control (i) and Dicer UB mutant (j) kidneys. (k, l) Hematoxylin and eosin staining of kidney sections from Dicerc/−; HoxB7CreEGFP and control P38 kidneys. The mutant kidney is highly cystic (arrows). Scale bars = 200 μm. (m–r) Renal cysts of the UB epithelium origin in Dicer UB mutants. (m, n) Immunofluorescent staining of P0 kidney sections with Dolichos bifloris agglutinin (DBA) for the UB epithelium and Hoechst 33324 for DNA showing cystic dilations in the UB epithelium. Scale bar = 20 μm. (o–r) Immunofluorescent staining of E15.5 kidney sections with DBA (o, p) and Lotus tetragonolobus agglutinin (LTA) for the proximal tubules (q, r). Cystic dilations were observed in the UB epithelium but not the proximal tubules. Scale bar = 100 μm.
As Dicerc/−; HoxB7Cre mice exhibited a more consistent, severe phenotype distinct from that reported earlier, subsequent studies focused on this genotype (referred to as Dicer UB mutants hereafter). As the kidneys of Dicer heterozygotes including Dicerc/+; HoxB7Cre littermates retaining one active Dicer allele appeared normal, these samples were pooled with wild-type littermates for controls.
Examination of randomly selected miRNAs showed that expression of mature miRNAs in the UB epithelium of Dicer UB mutants was greatly reduced to near-undetectable levels, whereas their expression in the non-UB epithelial components of the kidneys was unaffected (Figure 1), suggesting that ablation of Dicer from the UB epithelium disrupted mature miRNA expression specifically in this cell population.
A gross examination of Dicer UB mutant kidneys at P0 indicated that kidneys were markedly smaller than those of wild-type littermates (Figure 5). In contrast to the wild-type kidney at P0, where branching ureteric tips uniformly distributed about the periphery of the renal cortex, the renal cortex was (mostly) devoid of branching tips in Dicer UB mutants (Figure 5). Consistent with the histological observation, in situ hybridization to P0 Dicer UB mutants revealed a complete absence of expression of Wnt11 (Figure 5i and j) or c-Ret (Supplementary Figure S4 online), the two critical regulators of normal UB branching, in the periphery of most mutant kidneys. Furthermore, pan-Cytokeratin staining of whole-mount E15.5 kidneys showed a drastic reduction of the ureteric bud network (Supplementary Figure S4). Thus, Dicer activity is essential for protracted branching of the UB epithelium, with loss of Dicer resulting in a premature termination of branching morphogenesis.
Reduced expression of Wnt11 and c-Ret, but not GDNF, at the onset of branching defects in Dicer UB mutants
Quantitative measurements showed that Dicer UB mutant kidneys were of similar size to the controls at E13.5 (P<0.43, n = 3), but were 21.8% smaller than their controls at E14.5 (P<0.04, n = 3, and Supplementary Figure S5 online), suggesting that defects in branching morphogenesis occurred shortly before E14.5. We thus examined branching morphogenesis at E13.5 to investigate the molecular causes for the branching defect. In situ analysis on whole-mount E13.5 kidneys revealed a dramatic reduction in the number of Wnt11-positive tips and in the levels of Wnt11 expression in Dicer UB mutants (Figure 6a and b). To differentiate whether the decreased number of Wnt11-positive tips is because of a reduced number of ureteric tips or the lack of Wnt11 expression in a subset of ureteric tips, we performed double in situ hybridization analysis on E13.5 kidney sections (Figure 6) to simultaneously detect Wnt11 at the UB tips (brown) and mark the UB trunks with Wnt7b expression (purple). Examination of all ureteric branches in tissue sections spanning entire kidneys clearly demonstrated that all mutant tips expressed Wnt11, although at reduced levels.
Figure 6. Disrupted branching morphogenesis of the Dicer ureteric bud (UB) mutant kidneys at E13.5.
(a, b) Whole-mount in situ hybridization analysis demonstrating a significant loss of Wnt11 expression in Dicer UB mutant kidneys (b) compared with their control littermates (a). (c–f) Representative images of double in situ hybridization on kidney sections showing that in mutants (d, f), all the Wnt7b-positive UB trunks bear Wnt11-positive branching tips, but the expression levels of Wnt11 are significantly reduced compared with their control littermates (c, e). (g–l) In situ hybridization on kidney sections reveals a significant reduction in the levels of expression of Wnt11 and c-Ret in the branching UB tips of mutants compared with their control littermates. No detectable reduction in Gdnf signals in the mesenchyme was observed in the mutants compared with the control littermates. Scale bars = 50 μm.
GDNF is the major stimulatory factor for UB branching morphogenesis and Gdnf/Ret signaling is itself required for Wnt11 expression at ureteric tips.35 To determine whether reduced Wnt11 expression at E13.5 resulted from decreased Gdnf expression, we examined Gdnf mRNA expression at E13.5 (Figure 6). Gdnf expression appeared normal in Dicer UB mutants. The results indicate that the primary deficiency in Wnt11 expression appears to lie within the UB epithelium and not indirectly from reduced GDNF levels in the mesenchyme. Consistent with a role for Dicer in controlling the epithelial branching circuitry, expression of c-Ret, the Gdnf receptor, was also markedly reduced within the ureteric tips, and most likely as a result, reception of the branching signal was decreased (Figure 6j).
Defects in cell proliferation and apoptosis underlie cyst formation in Dicer UB mutants
Consistent with and extended from the study by Pastorelli et al.,30 which reported cortical collecting duct cysts in mutant mice at ≥3 weeks of age, our histological examination revealed the presence of cysts throughout the UB epithelium in Dicer UB mutant kidneys at P0 (Figure 5), which were first apparent at around E15.5 (Supplementary Figure S5 online) in the nascent trunk region adjacent to the ureteric tips (Figure 5, Supplementary Figure S6A online). More enlarged cysts were detected in Dicerc/−; HoxB7CreEGFP mice (Figure 5l) (cyst area, 20.2±14.0 × 103 μm2 in Dicerc/−; HoxB7CreEGFP p38 kidneys; 2.4±1.3 × 103 μm2 in Dicerc/−; HoxB7Cre P0 kidneys, n = 25, P<1.4E-06), probably because the longer survival time allows for more extended cyst progression.
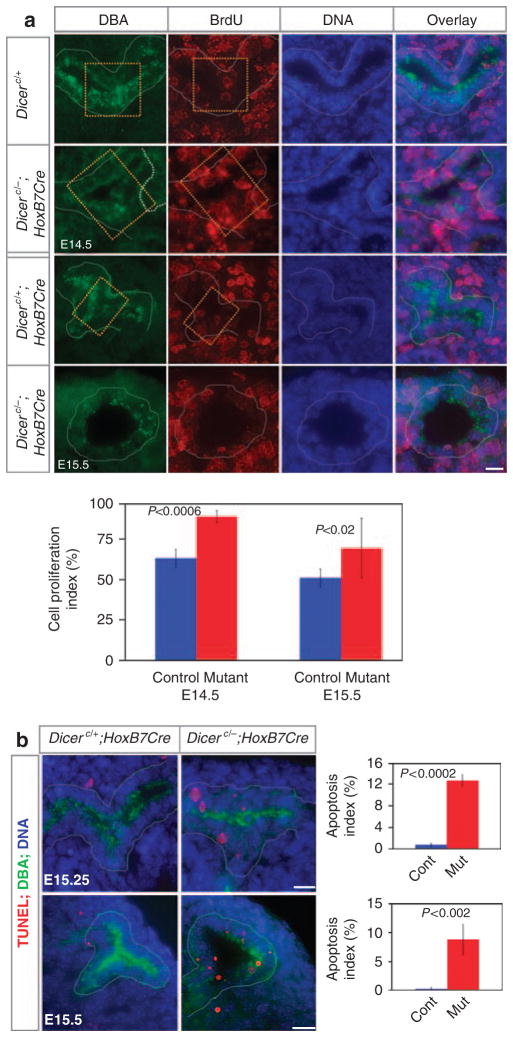
Overproliferation precedes and accompanies renal cyst initiation and progression in most polycystic kidney disease models.36–39 To dissect for the cellular causes for cyst formation in Dicer UB mutants, we quantified the cell proliferation rate in the nascent ureteric trunk region at E14.5 and cystic UBs at E15.5 by BrdU incorporation. A significantly elevated rate of cell proliferation was observed in mutant UB epithelia both before and after the initiation of cystic dilation when compared with their control counterparts (Figure 7a). Thus, excessive cell proliferation is probably a major contributing factor for both the onset and acceleration of cystic phenotypes in Dicer-deficient UB epithelium.
Figure 7. Cell proliferation and cell death prior to and following the onset of cyst formation.
(a) Increased cell proliferation precedes and follows the onset of cyst formation in the nascent ureteric bud (UB) trunk region of Dicer mutants. Cell proliferation was analyzed in the yellow boxed areas and the entire cysts (outlined with white dashed lines) at E14.5 and E15.5 with 5-bromodeoxyuridine (BrdU) labeling. BrdU incorporation into dividing cells was visualized with anti-BrdU antibodies (red). Dolichos biflorus agglutinin (DBA) staining (green) delineates the UB epithelium. A significant increase in the cell proliferation rates was observed in the Dicer UB mutants (n = 4) in the cystic UB epithelium at E15.5 (P<0.02) as well as in the nascent trunk region at E14.5 (P<0.0006) when compared with their control littermates (n = 4). (b) Increased apoptosis in UBs of Dicer UB mutants. The cell death rate was measured with TdT-mediated dUTP nick end labeling (TUNEL) analysis (red) in the UB epithelium stained with DBA (green). A significant increase in cell death was observed in both non-cystic (n = 3) and cystic UBs (n = 4) relative to their control littermates (n = 4) at E15.25 and E15.5, respectively. Cont, control; Mut, mutant. Scale bars = 15 μm.
Another common feature associated with renal cysts is increased apoptotic cell death.40–42 We examined the rate of apoptosis in Dicer mutants with TUNEL analysis. No statistically significant differences were observed in the rate of cell death between the mutants and the controls at E14.5 (data not shown). In contrast, apoptosis was markedly increased in the non-dilated UB epithelium shortly before the onset of cyst formation (E15.25) and in cystic UB epithelium at E15.5 (Figure 7b). Thus, enhanced apoptosis correlates with both the initiation and progression of cyst formation.
Disruption of primary cilial ciliogenesis at the onset of cyst formation in the Dicer UB mutant UB epithelium
The primary cilium is a central organelle in cyst formation.43 Disruption of ciliogenesis (the absence of or runted primary cilia) results in cystic kidneys. Strikingly, we observed a significant decrease in the length of the primary cilium in ureteric trunks just initiating cystic dilation (dilating UBs) as well as in well-expanded cysts (cystic UBs) at E15.5 (Figure 8). In all, 42% of primary cilia were >4 μm in length and only 4.8% of them were <2 μm in control UBs. In contrast, only 2.5 and 6.8% of primary cilia were >4 μm, and 41.3 and 37.1% of them <2 μm in dilating and cystic mutant UBs, respectively. The defect in primary cilia length was specific to the UB epithelium, as no significant differences were observed between mutant and control littermates in other renal cell types (Supplementary Figure S6B online).
Figure 8. Reduction in the length of the primary cilium from deletion of Dicer from the ureteric bud (UB) epithelium.
(a–f) Scanning electron microscopic images of primary cilia (arrows in b, d, and f) in the UB epithelium at E15.5. Panels b, d, and f are higher magnification images of the regions indicated by arrows in panels a, c, and e, respectively. Mutant cilia were shorter than controls and most of them were reduced to short stubs. Scale bar = 10 μm for panels a, c, and e and 1 μm for panels b, d, and f. (g) Control primary cilia averaged 4.01±0.66 μm (n = 124) in length, whereas mutant cilia averaged 1.60±0.40 μm in the dilating UBs (n = 100; P<0.01) and 2.21±0.31 μm in the cystic UBs (n = 189; P<0.04). No statistically significant differences were observed in cilia length between dilating and cystic UBs (P<0.29). (h) Distribution of primary cilia length in control and dilating and cystic mutant UB epithelia. (i, j) Scanning electron micrographs showing short primary cilia at E15.25 (arrows in i and j) in the non-dilating UB epithelium and no significant differences in the length of the primary cilium between the controls (i) and mutants (j). Scale bar = 1 μm.
In contrast, at E14.5 and in non-dilating UBs at E15.25 (right before the cyst onset), there was no detectable difference in the primary cilia length between the wild-type and mutant ureteric trunks (Figure 8, and data not shown). Interestingly, we noticed that even in the wild type, the primary cilia in the E14.5 and E15.25 nascent ureteric trunk region were shorter than those at E15.5 (Figure 8), suggesting that the primary cilium normally elongates between E15.25 and E15.5. Therefore, the primary cilia defects in dilating and cystic UBs of Dicer UB mutants either reflect a consequence of cystic dilation, or a disruption of this developmental switch of primary cilia elongation. We also cannot rule out the possibility of precocious activation or acceleration of primary cilium disassembly during each cell cycle starting at E15.5 due to Dicer deficiency.
Terminal differentiation of the ureteric bud epithelium appears defective in Dicer UB mutants
To investigate terminal differentiation of the UB epithelium in Dicer UB mutants at P0, we examined the expression of Foxi1 and Aqp6, the markers for early and late differentiation of intercalated cells, and Nos1, a marker of the inner medullary collecting duct cells. Aqp6 and Nos1 were expressed at lower levels and/or at a reduced frequency in the mutant epithelium (Figure 9), whereas the Foxi1 expression pattern was unaltered. The disrupted expression of Aqp6 and Nos1 was not because of apoptosis of the collecting duct cells (Supplementary Figure S7 online). These results suggest that collecting duct differentiation was initiated normally but terminal differentiation was disturbed on removal of Dicer activity from the UB epithelium.
Figure 9. Terminal differentiation of the collecting duct is affected in Dicer ureteric bud (UB) mutants.
In situ hybridization analysis of expression of markers for terminal differentiation of collecting duct cells on P0 kidney sections. Foxi1 expression is similar in Dicer mutants and controls. Aqp6 and Nos1 are expressed at reduced levels and less frequently in the mutant collecting ducts. Scale bars = 200 μm for panels a, c, e, g, i, and k, and 50 μm for panels b, d, f, h, j, and l.
DISCUSSION
We have addressed the requirement for Dicer activity within the nephron lineage (nephron progenitors and nephron epithelia) and the epithelial network of the developing renal collecting duct system, the major functional components of the kidney. The study reveals a critical role for Dicer in survival of the nephron lineage, including the stem-like nephron progenitors and the nephron epithelium, and normal branching, radial growth, and terminal differentiation of the ureteric bud epithelium. Our findings confirmed a previous report of cyst formation in the absence of Dicer functions and pointed to increased cell proliferation and apoptosis and disruption of ciliogenesis in the etiology and pathogenesis of cyst formation in the Dicer UB knockout model. Given Dicer’s critical function in the maturation of most miRNAs in mammalian cells, our work implicates Dicer-dependent miRNA-mediated regulation in the control of nephron number and epithelial growth and survival, properties that are linked to a variety of renal diseases.44
Maintenance of nephron progenitors by Dicer
Dicer-deficient nephron progenitors underwent premature apoptosis. How Dicer and miRNAs are involved in the regulation of the survival of nephron progenitors is unclear at present. The transforming growth factor-β superfamily signaling has been shown to be involved in the maintenance of nephron progenitors.45 However, this superfamily signaling appears to regulate the organization/recruitment of nephron progenitors. In the absence of this signaling, these cells failed to condense, and are instead loosely associated and embedded in the renal interstitium.45 This phenotype is distinct from ablation of Dicer from nephron progenitors, in which nephron progenitors were tightly condensed over the ureteric tips and not intermingled with interstitial cells. Furthermore, recent studies showed that Bmp7 acts to promote proliferation of nephron progenitors.46 Taken together, this suggests that Dicer and miRNAs do not modulate nephron progenitor survival through transforming growth factor-β superfamily signaling.
Six2 mRNA levels were reduced in Dicer mutant nephron progenitor cells. Notably, it was previously reported that Six2-null nephron progenitors exhibited increased apoptosis,20 and decreased expression of Six2 was associated with low nephron numbers and small embryonic and adult kidney size in Brachyrrhine (Br/+) mice.47,48 It is possible that reduced levels of expression of Six2 contribute to the increase in apoptosis of nephron progenitors resulting from Dicer ablation.
Branching defects in Dicer UB mutants
Elimination of Dicer from the UB epithelium causes UB branching defects. Interestingly, ablation of Dicer from lungs also produced branching deficiencies,49 suggesting a principal function of Dicer and presumably of miRNAs in regulating epithelial branching morphogenesis, one of the most common developmental processes driving epithelial organ formation. On the other hand, the mechanisms whereby Dicer and miRNAs regulate branching morphogenesis appear organ specific. In lungs, epithelial miRNAs appear to modulate branching morphogenesis in a paracrine fashion by regulating the production of the lung branching signal, Fgf10, in the mesenchyme. In contrast, in the kidney, we found that at an early developmental time point when the branching defect begins to manifest and the expression of Wnt11 and c-ret, the two molecules in ureteric tips required for proper UB branching, was greatly reduced, the expression of the major UB branching signal in the mesenchyme, Gdnf, was not obviously affected. Therefore, in the kidney, unlike the lungs, Dicer in the UB epithelium appears to regulate the reception of branching signals and the execution of branching morphogenesis in the UB epithelium, instead of the production of the branching signal in the mesenchyme.
Wnt11 and Gdnf/c-Ret signaling form a positive feedback loop.35 It is possible that reduced expression of Wnt11 resulted from reduced Gdnf/c-Ret signaling because of decreased expression of c-Ret.
Molecular and cellular mechanisms underlying the cystic defects in Dicer UB mutants
Cysts were observed starting from E15.5 in the collecting duct system in our Dicer UB mutant mouse model. A recent study from Pastorelli et al.30 also reported the cystic phenotype at postnatal stages from removal of Dicer function with HoxB7Cre. These studies demonstrated the importance of Dicer and presumably miRNAs in UB epithelium tube size control. Our analysis showed that UB-specific Dicer and miRNAs are involved in the regulation of cell proliferation and apoptosis in UB cells. Elevated cell proliferation and apoptosis rates precede and accompany cyst onset and progression, arguing strongly for their causal link with the UB cystic defect.
Cystic renal disease is a ciliopathy.43 Runted primary cilia were observed in cystic renal epithelial cells of orpk (oak ridge polycystic kidney) mice,50 which bear a hypomorphic insertional mutation in Ift88 (Polaris),51,52 an intraflagellar transporter gene that is required for ciliogenesis.50,53 Notably, the primary cilia were severely shortened in the UB epithelium just initiating cystic dilation (dilating UBs) and in cystic UBs in Dicer UB mutants, but not in the UB epithelium before the cyst onset. However, the primary cilium of control kidneys is equally short as those of the mutants before the cyst onset, but increases dramatically in length at the developmental time point when cysts start to form in Dicer UB mutants. Taken together, this suggests that defects in ciliogenesis in Dicer UB mutants could result from either a disruption of this developmental switch of primary cilia elongation, or cystic dilation.
In proliferating cells, the primary cilium disassembles to release the centrioles (basal body) at each cell cycle for cell division.54 Therefore, the distribution of the primary cilium length within a population of proliferating cells may be affected by their proliferation rate. However, it seems less likely that the primary cilia phenotype observed in E15.5 Dicer UB mutant cells is secondary to their cell proliferation defect, as the primary cilium length in E14.5 Dicer mutant UB cells, which are also hyperproliferative, is unaffected.
Our analysis of global functions of UB and nephron lineage-derived Dicer identified key kidney developmental events that require the inputs from Dicer and presumably miRNAs. This defines the scope of involvements of nephron lineage- and UB-specific Dicer and presumably miRNAs in kidney organogenesis; moreover, it provides functional readouts for future analysis of individual miRNAs and their targets implicated in these processes. Experimental manipulation of individual miRNA expression has proved that a single miRNA regulates the expression of hundreds of targets, many of them only to a modest degree.55–57 Likewise, individual developmental defect resulted from Dicer ablation is likely to be a collective effect from disruption of multiple miRNAs and a large number of their target genes. A genomic approach is therefore necessary and is under way to identify these miRNAs and their target genes/pathways for future functional analysis of individual miRNAs and targets toward full appreciation of the molecular mechanisms underlying each of the renal developmental processes in the nephron lineages and the UB epithelium mediated by Dicer and miRNA functions.
MATERIALS AND METHODS
Mice
The conditional Dicer allele (Dicerc) was previously described.58 The Dicer− allele was generated from the Dicerc allele with a Sox2Cre.59 The Six2TGC driver mouse, a BAC transgenic line, was described in Kobayashi et al.18 The HoxB7Cre and HoxB7CreEGFP mice were previously described.33,34 Animal experiments were performed in accordance with the policies of the institutional animal care and use committee at the University of Virginia.
Tissue preparation
Tissue preparation for histological staining on paraffin sections, immunofluorescent staining, and in situ hybridization on cryosections were done as described in Yu et al.60 For scanning electron microscopy, freshly dissected mouse embryonic kidneys were fixed with 2.5% glutaraldehyde in phosphate-buffered saline (PBS) at 4 °C overnight and embedded in optimal cutting temperature. For BrdU labeling, pregnant female mice were injected intraperitoneally with 0.5 mg BrdU per 10 g body weight at 2h before the harvest of embryonic kidneys. For whole-mount in situ hybridization, kidneys were fixed with 4% paraformaldehyde in PBS at 4 °C for 24h, washed with PBS, and dehydrated with a graded series of methanol/0.85% NaCl solutions and stored in 100% methanol at −20 °C.
Histology, immunostaining, and quantification
Paraffin blocks were sectioned at 5 to 6 μm for hematoxylin and eosin staining as previously described.33 Nephron numbers were quantified by counting renal corpuscles in hematoxylin and eosin-stained sections. The kidney size (the pole-to-pole length) was measured from histological sections at the central-most plane. Frozen blocks were sectioned at 12 μm for immunostaining using a standard protocol.60 For co-staining with two primary antibodies made from the same host species, one of the antibodies was labeled with Zenon labeling technology (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. TUNEL staining was performed with the ApopTag Red In situ Apoptosis Detection Kit (Chemicon International, Billerica, MA), following the manufacturer’s instruction. Immunostained images were visualized using a Personal Deltavision microscope (Applied Precision, Issaquah, WA) and collected with a digital camera (Photometrix, CoolSNAP HQ2, Tucson, AZ). For quantification of cell proliferation and apoptosis rates, 200–400 cells were counted in 2 to 3 kidney sections per embryo, from 3 to 4 embryos. Primary antibodies used in this study were as follows: anti-Pax2 (Covance, Princeton, NJ; 1:500); anti-Laminin (Sigma, St Louis, MO; 1:2000); anti-GFP (Aves Labs, Tigard, OR; 1:500); anti-β-galactosidase (Abcam, Cambridge, MA; 1:1500); anti-β-galactosidase (MP Biomedicals, Solon, OH; 1:10,000); anti-pan-Cytokeratin (Sigma; 1:200); Dolichos bifloris agglutinin (DBA)-Biotin (Sigma; 1:500); anti-BrdU (BD Pharmingen, San Diego, CA; 1:100); anti-WT1 (Santa Cruz Biotechnology, Santa Cruz, CA; 1:50); and anti-Cdh6 (gifts of Dr Dressler; 1:100).
lacZ staining
Freshly dissected kidneys from the mice were fixed in 2% paraformaldehyde at 4 °C for 1h and embedded in optimal cutting temperature. Frozen blocks were sectioned at 12 μm and stained in lacZ staining solution at 4 °C overnight.33
In situ hybridization
In situ hybridization analysis was performed on whole-mount kidneys (whole-mount in situ hybridization) as well as on sections from frozen blocks (section in situ hybridization). Frozen blocks were sectioned at a thickness of 16 μm for single and double in situ hybridizations and 30 μm for some of the double in situ hybridizations. Section in situ hybridization was performed essentially the same as in Yu et al.33,60 For double in situ hybridization, tissue sections were hybridized with a mixture of digoxigenin- and fluorescein-labeled riboprobes, each at the concentration of 500 ng/ml. After detection of digoxigenin-labeled probe, sections were fixed and then incubated with anti-fluorescein-alkaline phosphatase antibody (1:2000; Roche, Indianapolis, IN) at 4 °C overnight. Signals were developed using INT/BCIP dissolved in 10% polyvinyl alcohol.
Detection of miRNAs with in situ hybridization on kidney frozen sections was performed similarly except that tissue sections were hybridized with 25 nM digoxigenin-labeled locked nucleic acid probes. Kidney sections were not treated with RNase A after hybridization. Hybridization and post-hybridization washes were performed at 60 °C.
For whole-mount in situ hybridization, kidney samples were rehydrated with a graded series of methanol/0.85% NaCl, treated with 10 μg/ml Proteinase K for 10 min, and prehybridized in pre-hybridization buffer (50% formamide, 5 × SSC, pH 4.5, 50 μg/ml yeast tRNA, 1% SDS, and 50 μg/ml heparin) before hybridization at 70 °C. Post-hybridization washes were performed at 65 °C before treatment with 100 μg/ml RNase at 37 °C for 1h. Samples were then incubated with anti-digoxigenin-alkaline phosphatase at 4 °C overnight. Signals were detected with BM purple. After being post-fixed with 4% paraformaldehyde/0.1% glutaraldehyde, samples were cleared with a graded series of glycerol/PBS and stored in 80% glycerol/PBS at 4 °C. Images were collected with a Leica MZ16F stereoscope equipped with a DFC300 FX camera (Leica Micro Systems, Bannockburn, IL).
Electron microscopy
Sections were cut at 150 μm thickness and fixed further for 20 min in 2.5% gluteraldehyde. Sections were processed following a standard protocol.61 Samples were sputter coated with 30 nm of gold in sputter coater (BAL-TEC SCD005; Leica Micro Systems, Bannock-burn, IL) and observed on a scanning electron microscope (JEOL 6400; JEOL, Peabody, MA). The primary cilia length was measured using the ImageJ software (1.37b; National Institutes of Health, Bethesda, MD) from three to four mutant as well as control embryos.
RNA purification, reverse transcription, and quantitative or semiquantitative PCR
Total RNA was purified from E13.5 kidneys with RNeasy mini kit (Qiagen, Valencia, CA), and reverse transcribed with random hexamers with a Tetro cDNA synthesis kit (Bioline, Taunton, MA). Quantitative PCR on Six2 and β-actin was performed with SYBR Green JumpStart Taq ReadyMix (Sigma) on DNA Engine Chromo 4 (Bio-Rad, Hercules, CA). Six2 transcript levels were normalized with that of β-actin. Oligonucleotide primers used for PCR were: Six2RTs: 5′-GAAAGGGAGAACAGCGAGAA-3′, Six2R-Tas: 5′-CTTCTCATCCTCGGAACTGC-3′, β-actin fwd: 5′-GATC TGGCACCACACCTTCT-3′, β-actin rev: 5′-GGGGTGTTGAAGGTCTCAAA-3′.
GFP-positive cap mesenchyme cells (nephron progenitors) were sorted from E13.5 Dicerc/−; Six2TGC and Dicerc/+; Six2TGC (control) kidneys with a Becton Dickinson FACSVantage SE Turbo Sorter with DIVA Option (BD Biosciences, San Jose, CA). Total RNAs including miRNAs and mRNAs were purified from sorted cells with the miRNeasy mini kit (Qiagen), and reverse transcribed with the miScript PCR system (Qiagen), which reverse transcribes miRNAs and mRNAs in the same reaction. Semiquantitative PCRs for mature mmu-miR-30a, mmu-miR-30b, and mmu-miR-30c were performed with the miScript PCR system (Qiagen), using the miScript Primer Assays (Qiagen) for each mature miRNAs, for 29, 31, and 29 cycles, respectively. Semiquantitative PCR for β-actin was performed with SYBR Green JumpStart Taq ReadyMix (Sigma) for 25 cycles.
Statistical analysis
Statistical significance was determined using Student’s t-test.
Supplementary Material
Figure S1. Reduced nephron number in Dicer nephron mutants and cell fate mapping of Dicer mutant nephron progenitors.
Figure S2. Induction of the nephron progenitors and nephron patterning in the S-shaped bodies of Dicer nephron mutants are unaffected.
Figure S3. Nephron segmentation and terminal differentiation is largely unaltered in Dicer nephron mutants.
Figure S4. (A, B) Absence of c-Ret expression in newborn Dicer UB mutant kidneys.
Figure S5. The onset of hypoplastic and cystic phenotypes in Dicer UB mutants at E14.5 and E15.5.
Figure S6. (A) Cystic dilation was first observed in the nascent ureteric trunk region of Dicer UB mutants.
Figure S7. The apoptosis rate is normal in P0 Dicer UB mutant non-cystic medullary collecting ducts.
Acknowledgments
We thank Michael McManus, Brian Harfe, and Cliff Tabin for providing the Dicerc/c mice, Calton Bates for the HoxB7CreEGFP mice, Gregory Dressler for anti-Cadherin 6 antibodies, and Jan Redick at the University of Virginia Advanced Microscopy Facility for technical assistance in scanning electron microscopy. Work in the laboratory of APM was supported by a grant from the NIH (DK054364). Work in the laboratory of JY was supported by a UVA Fund for Excellence in Science and Technology (FEST) Award, and research grant 5-FY09-102 from the March of Dimes Foundation.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
References
- 1.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behm-Ansmant I, Rehwinkel J, Izaurralde E. MicroRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Cold Spring Harb Symp Quant Biol. 2006;71:523–530. doi: 10.1101/sqb.2006.71.013. [DOI] [PubMed] [Google Scholar]
- 3.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Kohane IS. Tissue and process specific microRNA-mRNA co-expression in mammalian development and malignancy. PLoS ONE. 2009;4:e5436. doi: 10.1371/journal.pone.0005436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 7.O’Rourke JR, Swanson MS, Harfe BD. MicroRNAs in mammalian development and tumorigenesis. Birth Defects Res C Embryo Today. 2006;78:172–179. doi: 10.1002/bdrc.20071. [DOI] [PubMed] [Google Scholar]
- 8.Meola N, Gennarino VA, Banfi S. microRNAs and genetic diseases. Pathogenetics. 2009;2:7. doi: 10.1186/1755-8417-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 10.Gao X, Chen X, Taglienti M, et al. Angioblast-mesenchyme induction of early kidney development is mediated by Wt1 and Vegfa. Development. 2005;132:5437–5449. doi: 10.1242/dev.02095. [DOI] [PubMed] [Google Scholar]
- 11.Levinson RS, Batourina E, Choi C, et al. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132:529–539. doi: 10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- 12.Mendelsohn C, Batourina E, Fung S, et al. Stromal cells mediate retinoid-dependent functions essential for renal development. Development. 1999;126:1139–1148. doi: 10.1242/dev.126.6.1139. [DOI] [PubMed] [Google Scholar]
- 13.Batourina E, Gim S, Bello N, et al. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet. 2001;27:74–78. doi: 10.1038/83792. [DOI] [PubMed] [Google Scholar]
- 14.Hatini V, Huh SO, Herzlinger D, et al. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10:1467–1478. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- 15.Quaggin SE, Schwartz L, Cui S, et al. The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development. 1999;126:5771–5783. doi: 10.1242/dev.126.24.5771. [DOI] [PubMed] [Google Scholar]
- 16.Cui S, Schwartz L, Quaggin SE. Pod1 is required in stromal cells for glomerulogenesis. Dev Dyn. 2003;226:512–522. doi: 10.1002/dvdy.10244. [DOI] [PubMed] [Google Scholar]
- 17.Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation. 2006;74:402–421. doi: 10.1111/j.1432-0436.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi A, Valerius MT, Mugford JW, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll TJ, Park JS, Hayashi S, et al. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Self M, Lagutin OV, Bowling B, et al. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBOJ. 2006;25:5214–5228. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller G, Zimmer G, Mall G, et al. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 22.Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int Suppl. 2005;97:S68–S77. doi: 10.1111/j.1523-1755.2005.09712.x. [DOI] [PubMed] [Google Scholar]
- 23.Saal S, Harvey SJ. MicroRNAs and the kidney: coming of age. Curr Opin Nephrol Hypertens. 2009;18:317–323. doi: 10.1097/MNH.0b013e32832c9da2. [DOI] [PubMed] [Google Scholar]
- 24.Kato M, Arce L, Natarajan R. MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol. 2009;4:1255–1266. doi: 10.2215/CJN.00520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wessely O, Agrawal R, Tran U. microRNAs in kidney development: lessons from the frog. RNA Biol. 2010;7:296–299. doi: 10.4161/rna.7.3.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho J, Ng KH, Rosen S, et al. Podocyte-specific loss of functional microRNAs leads to rapid glomerular and tubular injury. J Am Soc Nephrol. 2008;19:2069–2075. doi: 10.1681/ASN.2008020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harvey SJ, Jarad G, Cunningham J, et al. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol. 2008;19:2150–2158. doi: 10.1681/ASN.2008020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi S, Yu L, Chiu C, et al. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J Am Soc Nephrol. 2008;19:2159–2169. doi: 10.1681/ASN.2008030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sequeira-Lopez ML, Weatherford ET, Borges GR, et al. The MicroRNA-processing enzyme Dicer maintains juxtaglomerular cells. J Am Soc Nephrol. 2010;21:460–467. doi: 10.1681/ASN.2009090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pastorelli LM, Wells S, Fray M, et al. Genetic analyses reveal a requirement for Dicer1 in the mouse urogenital tract. Mamm Genome. 2009;20:140–151. doi: 10.1007/s00335-008-9169-y. [DOI] [PubMed] [Google Scholar]
- 31.Agrawal R, Tran U, Wessely O. The miR-30 miRNA family regulates Xenopus pronephros development and targets the transcription factor Xlim1/Lhx1. Development. 2009;136:3927–3936. doi: 10.1242/dev.037432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zambrowicz BP, Imamoto A, Fiering S, et al. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–5312. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]
- 34.Zhao H, Kegg H, Grady S, et al. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol. 2004;276:403–415. doi: 10.1016/j.ydbio.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majumdar A, Vainio S, Kispert A, et al. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130:3175–3185. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- 36.Patel V, Li L, Cobo-Stark P, et al. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet. 2008;17:1578–1590. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nadasdy T, Laszik Z, Lajoie G, et al. Proliferative activity of cyst epithelium in human renal cystic diseases. J Am Soc Nephrol. 1995;5:1462–1468. doi: 10.1681/ASN.V571462. [DOI] [PubMed] [Google Scholar]
- 38.Masyuk T, Masyuk A, LaRusso N. Cholangiociliopathies: genetics, molecular mechanisms and potential therapies. Curr Opin Gastroenterol. 2009;25:265–271. doi: 10.1097/MOG.0b013e328328f4ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- 40.Edelstein CL. What is the role of tubular epithelial cell apoptosis in polycystic kidney disease (PKD)? Cell Cycle. 2005;4:1550–1554. doi: 10.4161/cc.4.11.2185. [DOI] [PubMed] [Google Scholar]
- 41.Zhou XJ, Kukes G. Pathogenesis of autosomal dominant polycystic kidney disease: role of apoptosis. Diagn Mol Pathol. 1998;7:65–68. doi: 10.1097/00019606-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Park EY, Sung YH, Yang MH, et al. Cyst formation in kidney via B-Raf signaling in the PKD2 transgenic mice. J Biol Chem. 2009;284:7214–7222. doi: 10.1074/jbc.M805890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinlan RJ, Tobin JL, Beales PL. Modeling ciliopathies: primary cilia in development and disease. Curr Top Dev Biol. 2008;84:249–310. doi: 10.1016/S0070-2153(08)00605-4. [DOI] [PubMed] [Google Scholar]
- 44.Hoy WE, Bertram JF, Denton RD, et al. Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens. 2008;17:258–265. doi: 10.1097/MNH.0b013e3282f9b1a5. [DOI] [PubMed] [Google Scholar]
- 45.Oxburgh L, Chu GC, Michael SK, et al. TGFbeta superfamily signals are required for morphogenesis of the kidney mesenchyme progenitor population. Development. 2004;131:4593–4605. doi: 10.1242/dev.01324. [DOI] [PubMed] [Google Scholar]
- 46.Blank U, Brown A, Adams DC, et al. BMP7 promotes proliferation of nephron progenitor cells via a JNK-dependent mechanism. Development. 2009;136:3557–3566. doi: 10.1242/dev.036335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fogelgren B, Yang S, Sharp IC, et al. Deficiency in Six2 during prenatal development is associated with reduced nephron number, chronic renal failure, and hypertension in Br/+ adult mice. Am J Physiol Renal Physiol. 2009;296:F1166–F1178. doi: 10.1152/ajprenal.90550.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma W, Lozanoff S. External craniofacial features, body size, and renal morphology in prenatal brachyrrhine mice. Teratology. 1993;47:321–332. doi: 10.1002/tera.1420470409. [DOI] [PubMed] [Google Scholar]
- 49.Harris KS, Zhang Z, McManus MT, et al. Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci USA. 2006;103:2208–2213. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pazour GJ, Dickert BL, Vucica Y, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moyer JH, Lee-Tischler MJ, Kwon HY, et al. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science. 1994;264:1329–1333. doi: 10.1126/science.8191288. [DOI] [PubMed] [Google Scholar]
- 52.Murcia NS, Richards WG, Yoder BK, et al. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–2355. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- 53.Yoder BK, Tousson A, Millican L, et al. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol. 2002;282:F541–F552. doi: 10.1152/ajprenal.00273.2001. [DOI] [PubMed] [Google Scholar]
- 54.Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell. 2007;129:1255–1257. doi: 10.1016/j.cell.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 55.Baek D, Villen J, Shin C, et al. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Selbach M, Schwanhausser B, Thierfelder N, et al. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 57.Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 58.Harfe BD, McManus MT, Mansfield JH, et al. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci USA. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayashi S, Lewis P, Pevny L, et al. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Gene Expr Patterns. 2002;2:93–97. doi: 10.1016/s0925-4773(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 60.Yu J, Carroll TJ, Rajagopal J, et al. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–171. doi: 10.1242/dev.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mokrzan EM, Lewis JS, Mykytyn K. Differences in renal tubule primary cilia length in a mouse model of Bardet-Biedl syndrome. Nephron Exp Nephrol. 2007;106:e88–e96. doi: 10.1159/000103021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Reduced nephron number in Dicer nephron mutants and cell fate mapping of Dicer mutant nephron progenitors.
Figure S2. Induction of the nephron progenitors and nephron patterning in the S-shaped bodies of Dicer nephron mutants are unaffected.
Figure S3. Nephron segmentation and terminal differentiation is largely unaltered in Dicer nephron mutants.
Figure S4. (A, B) Absence of c-Ret expression in newborn Dicer UB mutant kidneys.
Figure S5. The onset of hypoplastic and cystic phenotypes in Dicer UB mutants at E14.5 and E15.5.
Figure S6. (A) Cystic dilation was first observed in the nascent ureteric trunk region of Dicer UB mutants.
Figure S7. The apoptosis rate is normal in P0 Dicer UB mutant non-cystic medullary collecting ducts.