Abstract
Flavopiridol is a cyclin-dependent kinase inhibitor that induces cell cycle arrest, apoptosis, and clinical responses in selected patients with acute myeloid leukemia (AML). A better understanding of the molecular pathways targeted by flavopiridol is needed to design optimal combinatorial therapy. Here, we report that in vivo administration of flavopiridol induced expression of the BCL-2 anti-apoptotic gene in leukemic blasts from adult patients with refractory AML. Moreover, flavopiridol repressed the expression of genes encoding oncogenic transcription factors (HMGA1, STAT3, E2F1) and the major subunit of RNA Polymerase II. Our results provide mechanistic insight into the cellular pathways targeted by flavopiridol and suggest that blocking anti-apoptotic pathways could enhance cytotoxicity and improve outcomes in patients treated with flavopiridol.
Keywords: Flavopiridol, acute myeloid leukemia, BCL-2, HMGA1, STAT3, E2F1
BACKGROUND
Acute myeloid leukemia (AML) in adults remains a formidable clinical challenge that demands further investigation to identify more rational therapy [1-5]. This year, almost 13,000 new cases will be diagnosed, and AML will claim over 9,000 lives in the U.S. alone [5]. AML is also a frequent, fatal complication of myelodysplastic syndrome (MDS), a clonal myeloid malignancy that affects over 40,000 individuals in this country [5]. In newly diagnosed AML without poor risk features, only 70% of adult patients under the age of 60 years will achieve complete remission (CR) after induction therapy and more than half of these CR patients will ultimately relapse and die from their disease [5]. Certain subgroups of AML patients have an even worse prognosis with cure rates less than 15%, including older patients (over 60 years), individuals with underlying MDS or other hematologic disorders, or those with AML linked to prior chemotherapy, environmental, or occupational exposures [5]. These abysmal epidemiologic data underscore the dire need for further research to investigate novel therapeutic targets and optimize therapy [1-5].
Flavopiridol is a small, semi synthetic, cytotoxic flavone derived from two Indian plants used for herbal therapy (Amoora rohiuka or Dysoxylum binectariferum) [6-10]. Previous studies show that flavopiridol induces apoptotic death in diverse hematologic malignancies [11-14]. Flavopiridol is thought to function through at least three distinct mechanisms [13-18]. First, it is a potent cyclin dependent kinase (cdk) inhibitor that blocks cell cycle progression [13,14]. In the setting of pan-cdk inhibition, E2F is released and drives apoptosis [12,13]. Second, by blocking cdk 7 and 9 function, flavopiridol prevents phosphorylation and activation of the RNA Polymerase II (Pol II) protein [9,12]. This is thought to result in the global down-regulation of gene expression, including genes that promote leukemic cell survival and proliferation [13]. Previous studies also show repression of Cyclin D1 or CCND1 [13-14], MCL-1 [16] and BCL-2 [16,18] at the RNA and protein level. Third, studies show that flavopiridol disrupts STAT3 function by blocking its binding to DNA in tumor cells [15]. Because STAT3 is constitutively active in many subtypes of leukemia and serves as a central regulator in multiple pro-survival and oncogenic signaling pathways [19-21], flavopiridol could block the tumor promoting pathways induced by STAT3. Given the pleiotropic effects of flavopiridol, it is likely that it functions through additional, unknown molecular pathways.
Previous in vitro studies demonstrated that flavopiridol causes apoptotic cell death in leukemic blasts from patients with poor-risk AML or acute lymphoblastic leukemia (ALL) [11]. A subsequent clinical trial with flavopiridol for 3 days, followed by ara-C and mitoxantrone, resulted in a response rate of 31% in adults with relapsed or refractory AML or ALL [22]. Correlative studies of leukemic bone marrow blasts obtained before and after flavopiridol showed decreases in the protein levels of at least one putative flavopiridol target, including phosphorylated RNA Pol II, phosphorylated STAT3, CCND1, BCL-2, or MCL-1 in some cases [22]. These observations were translated into a Phase II clinical trial and CRs were achieved in 75% (12/15) of newly diagnosed, secondary AML patients [23]. Notably, 10 (67%) of these secondary AML patients had MDS and CRs were achieved in 90% (9/10) of these high-risk patients [23].
The high mobility group A1 (HMGA1) gene is a member of the HMGA gene family [24-44] and encodes a potent oncogenic transcription factor that is highly overexpressed in diverse, high-grade malignancies, including ALL [29,33], AML [20,29,41], and Burkitt’s lymphoma [20,24-25]. HMGA1 induces a transformed phenotype in cultured, hematopoietic cells [20,24-25] and causes aggressive leukemia in transgenic mice [33-34]. Conversely, inhibiting HMGA1 expression blocks transformation phenotypes in diverse cancer cells, including those from hematopoietic malignancies and solid tumors [20,24,27,35,38,40]. In addition, recent gene expression profile analyses indicate that HMGA1 is a key transcription factor enriched in human embryonic stem cells [36], hematopoietic stem cells [41,44-45], and leukemic stem cells [46]. In hematopoietic malignancies, HMGA1 induces expression of STAT3 [20,]. More recent preliminary data suggests that HMGA1 up-regulates E2F1 expression (Resar, unpublished data). HMGA1 also enhances global gene expression by interfering with histone H1-mediated repression of transcription [40]. Because flavopiridol affects these HMGA1 pathways by down-regulating transcription through inhibition of Pol II phosphorylation [12], promoting apoptosis through E2F1 [12-13], and blocking STAT3 activity [15], we hypothesized that flavopiridol will be cytotoxic in tumors dependent upon HMGA1 overexpression.
Here, we investigate expression of pro-oncogenic transcription factors and anti-apoptotic pathways in primary, AML blasts from adults with refractory or high-risk AML before and after in vivo flavopiridol administration. We found that flavopiridol induces expression of the gene encoding the anti-apoptotic protein, BCL-2, while it represses expression of the genes encoding the oncogenic transcription factors, HMGA1, STAT3, and E2F1. In addition, expression of the gene encoding the major subunit of RNA Polymerase II (POLR2A) was repressed. Our findings provide mechanistic insight into cellular pathways targeted by flavopiridol and potential combinatorial strategies to optimize therapy in AML.
MATERIALS AND METHODS
Patient selection, treatment schema and responses
Adult patients with relapsed or refractory AML received flavopiridol as part of an NCI-sponsored clinical trial (NCI 00470197) in accordance with the Johns Hopkins Medical Institutional Review Boards and guidelines. Flavopiridol was administered daily for three consecutive days by a bolus-infusion schedule developed by Byrd et al. and based on pharmacologic data [47]. Flavopiridol doses were escalated from a total flavopiridol dose of 50 mg/m2 (level 1) up to 100 mg/m2 (level 6) after treating 2-10 patients at each dose level (Table I). On day 6, patients received a 72 hour continuous infusion of ara-C (667 mg/m2/24 hours from days 6-9). Mitoxantrone (40 mg/m2) was given as a single intravenous bolus over 60-120 minutes on day 9 [23].
Table I.
Clinical characteristics of the AML cases
| Patient # | Age/Sex | Diagnosis | Dosage Level |
Karyotype | % Blasts (Absolute Blast Count) |
Response CR/PR/NR/NE |
|---|---|---|---|---|---|---|
| P1 | 61/M | 2° refractory | 3 | 45,XY,−7 | 50% (1,100) |
CR |
| P2 | 26/F | 2° refractory | 3 | 46,XX, −20q |
26% 1,118) |
CR |
| P3 | 36/M | 1° refractory | 4 | 46,XY | 12% (966) |
CR |
| P4 | 20/F | Multi-refractory | 5 | complex | 61% (10,181 |
CR |
| P5 | 47/F | 1° refractory | 5 | complex | 90% (540) |
CR |
| P6 | 62/M | 1° refractory* | 2 | Trisomy 8 | 10% (710) |
PR |
| P7 | 34/M | 1° refractory** | 2 | complex | 29% (1,189) |
PR |
| P8 | 60/F | 1° refractory | 2 | complex | 95% (31,873 |
NR |
| P9 | 63/F | 1° refractory | 2 | 46,XX | 20% (2,284) |
NR |
| P10 | 43/M | 1° refractory | 3 | 46,XY, t(11;19) |
75% (18,120 |
NR |
| P11 | 55/M | Multi-refractory | 4 | 46,XY | 75% (750) |
NR |
| P12 | 41/F | 1° refractory | 4 | 46,XX | 41% (2,530) |
NR |
| P13 | 59/M | 1° refractory | 5 | 46,XY | 55% (6,914) |
NR |
| P14 | 53/F | 1° refractory | 5 | complex | 41% (18,425 |
NR |
| P15 | 69/M | Multi-refractory | 6 | 47,XY,+8 | 32% (403) |
NR |
| P16 | 23/F | 1° refractory | 2 | Inv 8 t(8;8) |
83% (14,757 |
NE |
Patient characteristics are listed above.
denotes a death during treatment; 20 refers to AML that occurred in the setting of MDS or chemotherapy for a prior malignancy; M-male; F-female; CR-Complete remission; NR-No response; PR-Partial response; NE-Non-evaluable.
Patient response was designated as CR if the bone marrow aspirate performed after peripheral blood counts recovered showed no evidence for leukemia by morphologic examination and flow cytometry, and the absolute neutrophil count was ≥1000/mm3, the platelet count was ≥100,000/mm3, and there were no blasts in the peripheral blood by morphologic examination [48]. Clearance of cytogenetic abnormalities was not required for CR. No response (NR) was defined as persistent leukemia in the marrow and/or peripheral blood and no significant decrease in the % blasts from pretreatment levels. Partial remission (PR) was defined as the presence of trilineage hematopoiesis in the marrow with normalization of peripheral counts, but with persistent abnormal blasts (5-25%). In this study group, there were 5 CRs, 2 PRs, and 8 NRs. One patient had complete tumor clearance on day 14, but died of infection while neutropenic on day 14, and is therefore designated not evaluable (NE). Results of the full clinical trial showed similar response rates and are reported elsewhere [41].
RNA isolation and cDNA synthesis
Peripheral blood blasts were obtained on day 1 prior to flavopiridol therapy and within 2 hours after the end of the first infusion [22]. Patients were eligible if the pretreatment circulating, peripheral blood blasts prior to and following the initial flavopiridol infusion were >400/mm3 to ensure adequate RNA. All studies were conducted following blast enrichment by Ficoll-Paque density gradient separation (Pharmacia, Piscataway, New Jersey) with the blast percentage following enrichment estimated to be ≥70% based on prior experience [11]. Total RNA from the blasts was then isolated using RNeasy Mini Kit (QIAGEN, Valencia, CA) and quantified on a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). RNA (500 ng) was converted to cDNA using a high capacity cDNA reverse transcription kit (Applied Biosystems, CA, USA) in 100 μl reaction containing 10 μL 10X reverse transcription buffer, 4.0 uL 25X dNTP and 5 uL murine moloney reverse transcriptase as previously described [33,35]. A negative control reaction without reverse transcriptase amplification was included in all experiments. Although we initially planned to assess proteins by Western analysis in addition to gene expression, the limited cell number precluded protein analysis. The gene expression results from the peripheral blood blasts were compared to gene expression in CD34+ hematopoietic stem cells (HSCs); mRNA levels from the HSCs were arbitrarily assigned a value of 1.0. HSC RNA was obtained from a commercial source (ALLCELLS, CA, USA).
Quantitative real-time PCR (qRT-PCR)
Quantitative RT-PCR (qRT-PCR) of cDNA was performed as previously described [33] with the following modifications. For most genes, we used Taqman PCR reagents (Applied Biosystems, NJ, USA) and primers (E2F1, BCL-2, VEGF-A, RNA polymerase II 2A or POLR2A, CCND1, or MCL-1) (Applied Biosystems, CA, USA). The gene of interest was compared to a housekeeping gene to control for sample loading and reaction conditions were optimized for maximal efficiency. Human phosphoprotein (PO) was used as the control for HMGA1, E2F1, and MCL-1 as previously described [33,35]; β-actin was used as the control for BCL-2, VEFG-A, and POLR2A. Because the Taqman primers do not distinguish STAT3 from STAT3β, we assessed STAT3 using SYBR green master mix (Applied Biosystems, UK) as we previously described [36] with β2 microglobulin as the control gene for sample loading. Reactions were performed in triplicate and repeated at least once if there were discordant results. Results indicate the mean from all results +/− the standard deviations. The qRT-PCR results were analyzed using the software provided by the manufacturer (Applied Biosystems, CA, USA) using the δδCT method (according to the manufacturer’s instructions).
Statistical analysis
GraphPad Prism version 5.0 for windows (GraphPad software, CA, USA) was used for statistical analysis and graph preparation. The Wilcoxon signed-rank test was used to compare the expression of each gene before and after flavopiridol therapy.
RESULTS
Patient characteristics, cytotoxicity following flavopiridol and clinical responses
Leukemic blasts were isolated from peripheral blood before and after flavopiridol in 36 patients enrolled in the Phase I part of the NCI 00470197 protocol [41]. Sufficient RNA was available for further analysis from 16 patients. The remaining 20 samples had either degraded RNA or blast counts that were too low to obtain adequate quantities of RNA. (Clinical characteristics of the 16 patients are summarized in Table I). Flavopiridol resulted in a 50% or greater decrease in the peripheral blood blast counts in 9/16 (56%) of cases after the first infusion and in 15/16 (94%) of cases after all 3 doses, indicating significant cytotoxicity from flavopiridol alone. Of the 16 patients studied in this cohort, there were 5 CRs (31%), 2 PRs (12.5%), and 8 NRs (50%); and; one patient (6%) died during aplasia and was therefore not evaluable (NE). The clinical responses of all 55 patients treated with this protocol are reported separately [42] and were similar to the results from this cohort of 16 (CRs in 40% of all cases with an overall and disease-free survival of ≥60% at over 2 years in patients achieving a CR).
Flavopiridol up-regulates BCL-2 in leukemic blasts
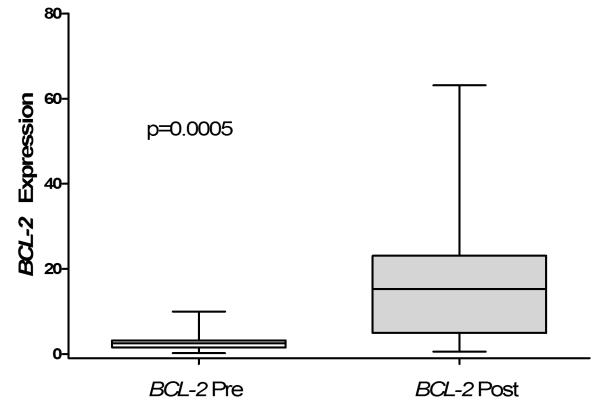
To elucidate molecular pathways disrupted by flavopiridol in AML, we investigated gene expression of in primary leukemic blasts obtained from patients with refractory or poor-risk AML before and after therapy with flavopiridol (Figure 1A). Previous studies have shown that expression of the anti-apoptotic gene, BCL-2, at the mRNA [18] and protein [16,22] level decrease in cultured HL60 cells and leukemic blasts after flavopiridol therapy. Strikingly, we found that the gene encoding BCL-2 was induced in the leukemic blasts from all patients (16/16) following flavopiridol (Figure 1A). Before therapy, the mean mRNA level of BCL-2 in the peripheral blood leukemic blasts and HSCs were similar (3.58 ± 3.32 for AML blasts versus 1.0 ± 0.87 for HSCs). After flavopiridol treatment, BCL-2 expression increased significantly in the blasts to 19.38 ± 18.40 (p=0.0005; Table II).
Figure 1. Changes in gene expression following in vivo flavopiridol therapy in leukemic blasts from adults with AML.
A.) Bcl-2 mRNA expression was significantly induced following in vivo treatment with flavopiridol. Gene expression in leukemic blasts was assessed by qRT-PCR. The relative gene expression is reported as Box and Whisker Plots for n=16 (in triplicate). The minimum and maximum values are shown as the top and bottom values of each whisker, and the median is shown as the center line of each box.
Table II.
Summary of Gene Expression Levels in Patients Pre and Post Flavopiridol Treatment (n=16)
| BCL-2 | HMGA1 | STAT3 | E2F1 | POLR2A | MCL-1 | VEGFA | CCND1 | |
|---|---|---|---|---|---|---|---|---|
| All (16) | ||||||||
| Mean Pre | 3.58 | 2.48 | 3.73 | 0.55 | 1.36 | 18.9 | 1.05 | 0.88 |
| Range Pre | 0.19-9.97 | 0.81-4.88 | 0.24-14.64 | 0.00-1.12 | 0.54-2.90 | 3.36-55.75 | 0.02-0.80 | 0.12-10.84 |
| Mean Post | 19.38 | 1.08 | 2.68 | 0.11 | 0.76 | 19.58 | 0.76 | 4.22 |
| Range Post | 0.54-63.17 | 0.32-2.28 | 0.07-13.43 | 0.00-0.45 | 0.23-1.54 | 1.67-39.42 | 0.00-4.92 | 0.00-29.24 |
| **p Value % of patients with p≤0.05 |
p=0.0005
(100%) |
p=0.0005
(81.2%) |
p=0.041
(68.7%) |
p=0.009
(56.3%) |
p=0.034
(83.3%) |
p=0.518 (93.7%) |
p=0.103 (75%) |
p=0.104 (37.5%) |
|
| ||||||||
| CR(5) | ||||||||
| Mean Pre | 6.00 | 2.36 | 4.31 | 0.68 | 1.06 | 18.49 | 1.61 | 0.13 |
| Range Pre | 1.35-9.48 | 0.75-6.42 | 0.23-11.25 | 0.05-2.46 | 0.39-2.90 | 6.07-27.84 | 0.51-2.86 | 0.01-0.33 |
| Mean Post | 33.13 | 0.84 | 3.26 | 0.14 | 0.59 | 29.87 | 1.11 | 5.76 |
| Range Post | 4.02-63.17 | 0.50-1.30 | 0.07-9.66 | 0.01-0.31 | 0.23-1.54 | 19.69-37.16 | 0.05-4.83 | 0.04-25.90 |
| **p Value % of patients with p≤0.05 |
p=0.0625 (100%) |
p=0.0625 (60%) |
p=0.0625 (80%) |
p=0.0625 (80%) |
p=0.0625 (60%) |
p=0.62 (80%) |
p=0.125 (100%) |
p=0.125 (60%) |
|
| ||||||||
| NR(8) | ||||||||
| Mean Pre | 2.79 | 2.96 | 2.34 | 0.52 | 1.58 | 12.51 | 0.45 | 1.65 |
| Range Pre | 0.19-9.97 | 0.60-5.90 | 0.07-6.31 | 0.00-1.84 | 0.44-2.70 | 2.67-38.74 | 0.02-1.94 | 0.01-10.84 |
| Mean Post | 15.89 | 1.34 | 1.36 | 0.073 | 0.87 | 10.51 | 0.73 | 4.32 |
| Range Post | 0.54-54.70 | 0.43-2.28 | 0.10-3.59 | 0.00-0.38 | 0.43-1.40 | 1.67-19.04 | 0.00-4.92 | 0.00-29.24 |
| **p Value % of patients with p≤0.05 |
p=0.039
(100%) |
p=0.008 (87.5%) |
p=0.023
(62.5%) |
p=0.078 (37.5%) |
p=0.015
(100%) |
p=0.039
(100%) |
p=0.19 (50%) |
p=0.47 (37.5%) |
|
| ||||||||
| PR(2) | ||||||||
| Mean Pre | 2.37 | 0.68 | 8.95 | 0.092 | NA | 35.03 | 1.18 | NA |
| Range Pre | 1.52-3.21 | 0.56-0.81 | 3.25-14.64 | 0.04-0.14 | NA | 14.33-55.75 | 0.41-1.94 | NA |
| Mean Post | 7.62 | 0.33 | 7.29 | 0.041 | NA | 22.95 | 0.11 | NA |
| Range Post | 1.78-13.46 | 0.32-0.35 | 1.15-13.43 | 0.00-0.08 | NA | 6.48-39.42 | 0.09-0.13 | NA |
| **p Value % of patients with p≤0.05 |
p=0.50 (100%) |
p=0.25 (100%) |
p=0.25 (50%) |
p=0.25 (50%) |
NA | p=0.25 (100%) |
p=0.25 (100%) |
NA |
This table summarizes the gene expression changes (increased or decreased expression) for the 12 AML cases. CR-Complete remission; PR-Partial Response; NR-No response; NE-Non-evaluable.
(W) p-value; statistical significance assigned by the Wilcoxon signed-rank test.
Flavopiridol results in the down-regulation of genes encoding oncogenic transcription factors (HMGA1, STAT3, E2F1) and the major subunit of RNA Pol II (POLR2A)
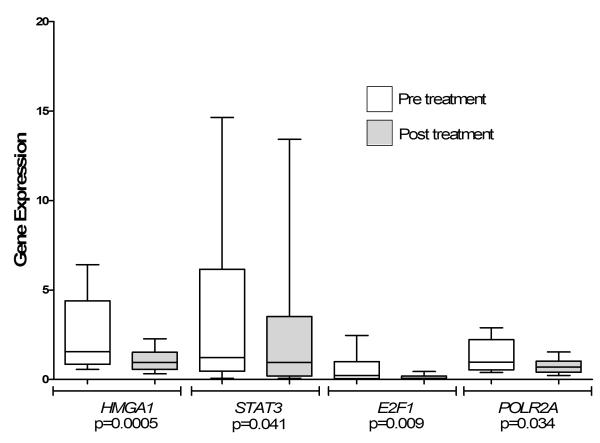
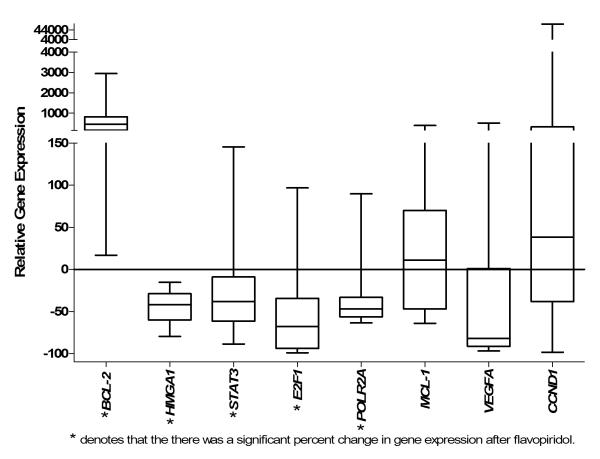
We investigated expression of oncogenic and cell cycle regulatory genes before and after therapy with flavopiridol. The HMGA1, STAT3, and E2F1 mRNA levels were similar in the leukemic blasts before therapy to those observed in the CD34+ HSCs. Strikingly, there was a statistically significant decrease in gene expression for HMGA1, STAT3, E2F1, and POLR2A in most patients (Figure 1B; Table II). Flavopiridol resulted in the down-regulation of HMGA1 expression in all cases (16/16 or 100%). The mean HMGA1 mRNA decreased from 2.48 before flavopiridol to 1.07 after flavopiridol (p=0.0005). In addition, STAT3 and E2F1 mRNA decreased in most patients studied (13/16 or 81% for STAT3 and 15/16 or 94% for E2F1) (Table II). The mean STAT3 mRNA decreased from 3.73 to 2.68 (p=0.041). The mean E2F1 mRNA levels also fell from 0.55 before therapy to 0.11 after therapy (p=0.009). POLR2A expression in pre-treatment blasts was also similar to expression in HSCs and decreased from 1.36 to 0.76 (p=0.034). These results indicate that flavopiridol significantly repressed expression of three oncogenic transcription factors (HMGA1, E2F1, and STAT3) involved in leukemogenesis. In addition, the gene encoding a major subunit of RNA Pol II (POLR2A) was also down-regulated in leukemic blasts by flavopiridol. For all genes studied, the mean changes in expression after flavopiridol were similar in the patients achieving CRs or NRs (Table II). The other genes investigated in this study did not change significantly after flavopiridol, including VEGF-A, CCND1, and MCL-1(Figure 2; Table II).
Figure 1B. Changes in gene expression following in vivo flavopiridol therapy in leukemic blasts from adults with AML.
HMGA1, STAT3, E2F1 and POLR2A were significantly down-regulated in leukemic blasts following treatment with flavopiridol. The relative gene expression is reported as Box and Whisker Plots n=16 (in triplicate). The minimum and maximum values are shown as the top and bottom values of each whisker, and the median is shown as the center line of each box.
Figure 2. Average percent change in gene expression following therapy with flavopiridol.
The relative change in gene expression for all patients was calculated as the difference of the pre-treatment level and post-treatment level divided by the pre-treatment level and multiplied by 100 as follows: Δ mRNA level pre-flavopiridol – mRNA levels post flavopiridol/pre mRNA level pre-flavopiridol*100. The relative gene expression is reported as Box and Whisker Plots n=16 (in triplicate). The minimum and maximum values are shown as the top and bottom values of each whisker, and the median is shown as the center line of each box.
DISCUSSION
To define the molecular pathways targeted by flavopiridol in AML, we investigated gene expression from primary human leukemic blasts before and after therapy with flavopiridol. Notably, BCL-2 expression was induced, which contrasts from previous studies demonstrating down-regulation of BCL-2 at the mRNA and protein level following exposure to flavopiridol [16,18,22]. Our results suggest that flavopiridol induces a BCL-2-mediated anti-apoptotic response, potentially as a protective mechanism in the leukemic blasts during cell cycle arrest driven by cdk inhibition.
We also found that flavopiridol significantly represses expression of HMGA1, STAT3, and E2F1, three oncogenes previously shown to be involved in hematopoietic malignancies [20-21,24-25,29,40-41]. These findings are important because recent studies also identified HMGA1 as a key transcription factor enriched in human embryonic stem cells [36], leukemic stem cells [46], and poorly differentiated solid tumors [40], suggesting that HMGA1 could drive a primitive, stem-like phenotype in AML. Like normal embryonic stem cells, leukemic stem cells appear to have long-term self-renewal and other stem-like properties that could confer drug resistance. Of note, the HMGA1 expression levels in the AML blasts before therapy were similar to levels in HSCs. STAT3 also appears to play a pivotal role in murine HSC self-renewal and could regulate similar pathways in leukemic cells [20,49]. In addition, E2F1 is a master regulator of cell cycle progression and a recent study found that E2F1 promotes a poorly differentiated state in AML by repressing the microRNA-223 [50]. Taken together, our results suggest that targeting these pathways with drugs like flavopiridol could be efficacious in leukemia and other malignancies with stem cell-like molecular signatures.
Lastly, we found that flavopiridol represses the gene encoding the major subunit (2A) of RNA Pol II, which could lead to a global down-regulation in gene expression. Although this gene is integral to Pol II function and transcription in general, not all genes were repressed, indicating some level of specificity in the effects of flavopiridol.
In summary, our studies demonstrate that flavopiridol induces BCL-2 expression while it represses expression of the HMGA1, STAT2, E2F1, and POLR2A in primary AML blasts from adult patients. This finding suggests that targeting anti-apoptotic pathways in combination with flavopiridol could potentiate flavopiridol’s cytotoxic effects and improve patient outcomes, a hypothesis that could be tested in the clinic and laboratory. Because HMGA1 is an oncogenic chromatin remodeling protein enriched in stem cells and high-grade, refractory tumors from diverse tissues, flavopiridol could be useful adjunctive therapy in other malignancies with poor prognostic features.
Acknowledgements
The authors wish to thank the Sidney Kimmel Cancer Center specimen accession core staff for collecting and banking the samples used in our study. We also thank the following funding agencies: NIH RO1 CA092339; The Leukemia & Lymphoma Scholar Award 1694-06 (L.M. S. Resar); Alex’s Lemonade Stand Foundation Award (L.M. S. Resar and J. Hillion); The Maryland Stem Cell Research Fund (L. M. S. Resar, J. Hillion, and B. Joseph); J.P. McCarthy Foundation (L.M.S. Resar and J.E. Karp); NCI Co-operative Agreement U01 CA70095 (J.E. Karp); NCI Cancer Center Support Grant P30 069773-45 (J.E. Karp), philanthropic funds from Dr. Robert E. Fischell (In memory of his wife, Mariam (J.E. Karp), and the Prevent Cancer Foundation (J. Hillion).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- [1].Karp JE, Smith MA. The molecular pathogenesis of treatment-induced (secondary) leukemias: foundations for treatment and prevention. Semin Oncol. 1997;24:103–13. [PubMed] [Google Scholar]
- [2].Lowenberg B, Downing RJ, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–62. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- [3].Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–63. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- [4].Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, Edwards BK, List AF. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- [5].Fathi AT, Karp JE. New Agents in AML: Beyond Cytarabine and Anthracyclines. Curr Oncol Rep. 2009;11:346–52. doi: 10.1007/s11912-009-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Naik RG, Kattige SL, Bhat SV, Alreja B, de Souza NJ, Rupp RH. An anti-inflammatory cum immunomodulatory piperidinylbenzopyranone from Dysoxylum binectariferum: isolation, structure, and total synthesis. Tetrahedron. 1998;44:2081–86. [Google Scholar]
- [7].Melillo G, Sausville EA, Cloud K, Lahusen T, Varesio L, Senderowicz AM. Flavopiridol, a protein kinase inhibitor, down-regulates hypoxic induction of vascular endothelial growth factor expression in human monocytes. Cancer Res. 1999;59:5433–37. [PubMed] [Google Scholar]
- [8].Senderowicz AM, Sausville EA. Preclinical and clinical development of cyclin-dependent kinase modulators. J Natl Cancer Inst. 2000;92:376–87. doi: 10.1093/jnci/92.5.376. [DOI] [PubMed] [Google Scholar]
- [9].Chao S-H, Price D-H. Flavopiridol inactivates p-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem. 2001;76:31793–99. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- [10].Klasa RJ, List AF, Cheson BD. Rational approaches to design of therapeutics targeting molecular markers. Hematology. 2001;1:443–62. doi: 10.1182/asheducation-2001.1.443. [DOI] [PubMed] [Google Scholar]
- [11].Karp JE, Ross DD, Yang W, Tidwell ML, Wei Y, Greer J, Mann DL, Nakanishi T, Wright JJ, Colevas AD. Timed sequential therapy of acute leukemia with flavopiridol: in vitro model for a phase I clinical trial. Clin Cancer Res. 2003;9:307–15. [PubMed] [Google Scholar]
- [12].Sausville EA. Complexities in the development of cyclin-dependent kinase inhibitor drugs. Trends in Mol Med. 2002;8:S32–S37. doi: 10.1016/s1471-4914(02)02308-0. [DOI] [PubMed] [Google Scholar]
- [13].Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–83. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- [14].Sedlacek HH. Mechanisms of action of flavopiridol. Crit Rev Oncol Hematol. 2001;38:139–70. doi: 10.1016/s1040-8428(00)00124-4. [DOI] [PubMed] [Google Scholar]
- [15].Lee YK, Isham CR, Kaufman SH, Bible KC. Flavopiridol disrupts STAT3/DNA interactions, attenuates STAT3-directed transcription, and combines with the Jak kinase inhibitor AG490 to achieve cytotoxic synergy. Mol Cancer Ther. 2006;5:138–48. doi: 10.1158/1535-7163.MCT-05-0235. [DOI] [PubMed] [Google Scholar]
- [16].Kitada S, Zapata JM, Andreeff M, Reed JC. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate anti apoptosis proteins in B-cell chronic lymphocytic leukemia. Blood. 2000;96:393–97. [PubMed] [Google Scholar]
- [17].Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin Cancer Res. 2002;8:3527–38. [PubMed] [Google Scholar]
- [18].König A, Schwartz GK, Mohammad RM, Al-Katib A, Gabrilove JL. The novel cyclin-dependent kinase inhibitor flavopiridol downregulates Bcl-2 and induces growth arrest and apoptosis in chronic B cell leukemia lines. Blood. 1997;90:4307–12. [PubMed] [Google Scholar]
- [19].Schuringa JJ, Wierenga AT, Kruijer W, Vellenga E. Constitutive Stat3, Tyr705, and Ser727 phosphorylation in acute myeloid leukemia cells caused by the autocrine secretion of interleukin-6. Blood. 2000;95:3765–70. [PubMed] [Google Scholar]
- [20].Hillion J, Dhara S, Sumter TF, Mukherjee M, Di Cello F, Belton A, Turkson J, Jaganathan S, Cheng L, Ye Z, Jove R, Aplan P, Lin YW, Wertzler K, Reeves R, Elbahlouh O, Kowalski J, Bhattacharya R, Resar LM. The HMGA1a-STAT3 axis: an “Achilles heel” for acute leukemia? Cancer Res. 2008;68:10121–27. doi: 10.1158/0008-5472.CAN-08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Benekli M, Baumann H, Wetzler M. Targeting signal transducer and activator of transcription signaling pathway in leukemias. J Clin Oncol. 2009;27:4422–32. doi: 10.1200/JCO.2008.21.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Karp JE, Passaniti A, Gojo I, Kaufmann S, Bible K, Garimella TS, Greer J, Briel J, Smith BD, Gore SD, Tidwell ML, Ross DD, Wright JJ, Colevas AD, Bauer KS. Phase I and pharmacokinetic study of flavopiridol followed by 1-B-D arabinofuranosylcytosine and mitoxantrone in relapsed and refractory acute leukemias. Clin Cancer Res. 2005;11:8403–12. doi: 10.1158/1078-0432.CCR-05-1201. [DOI] [PubMed] [Google Scholar]
- [23].Karp JE, Smith BD, Levis MJ, Gore SD, Greer J, Hattenburg C, Briel J, Jones RJ, Wright JJ, Colevas AD. Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: a phase II trial in adults with poor-risk acute myelogenous leukemia. Clin Cancer Res. 2007;13:4467–73. doi: 10.1158/1078-0432.CCR-07-0381. [DOI] [PubMed] [Google Scholar]
- [24].Wood LJ, Mukherjee M, Dolde CE, Xu Y, Maher JF, Bunton TE, Williams JB, Resar LMS. HMG-I/Y: A new c-Myc target gene and potential human oncogene. Mol Cell Biol. 2000;20:5490–502. doi: 10.1128/mcb.20.15.5490-5502.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wood LJ, Maher J, Bunton TE, Resar LMS. The oncogenic properties of the HMG-I gene family. Cancer Res. 2000;60:4256–61. [PubMed] [Google Scholar]
- [26].Pedulla ML, Treff NR, Resar LMS, Reeves R. Cloning and comparative sequence analysis of the murine Hmgiy (Hmga1) gene. Gene. 2001;271:51–58. doi: 10.1016/s0378-1119(01)00500-5. [DOI] [PubMed] [Google Scholar]
- [27].Dolde CE, Mukherjee M, Cho C, Resar LM. The role of HMG-A1/Y in the human breast cancer. Breast Cancer Res Treat. 2002;71:181–91. doi: 10.1023/a:1014444114804. [DOI] [PubMed] [Google Scholar]
- [28].Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla LM, Angelo M, McLaughlin ME, Kim JY, Goumnerova LC, Black PM, Lau C, Allen JC, Zagzag D, Olson JM, Curran T, Wetmore C, Biegel JA, Poggio T, Mukherjee S, Rifkin R, Califano A, Stolovitzky G, Louis DN, Mesirov JP, Lander ES, Golub TR. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–42. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- [29].Pierantoni GM, Agosti V, Fedele M, Bond H, Caliendo I, Chiappetta G, Lo Coco F, Pane F, Turco MC, Morrone G, Venuta S, Fusco A. High-mobility group A1 proteins are overexpressed in human leukemias. Biochem J. 2003;372:145–50. doi: 10.1042/BJ20021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Takaha N, Resar LM, Vindivich D, Coffey DS. High mobility protein HMGI(Y) enhances tumor cell growth, invasion, and matrix metalloproteinase-2 expression in prostate cancer cells. The Prostate. 2004;60:160–67. doi: 10.1002/pros.20049. [DOI] [PubMed] [Google Scholar]
- [31].Dhar A, Hu J, Reeves R, Resar L, Colburn N. Dominant negative c-Jun (TAM67) target genes: HMGA1 is required for tumor promoter-induced transformation. Oncogene. 2004;23:4466–76. doi: 10.1038/sj.onc.1207581. [DOI] [PubMed] [Google Scholar]
- [32].Hommura F, Katabami M, Leaner VD, Donninger H, Felder T Sumer, Resar LMS, Birrer MJ. HMG-I/Y is a cJun/AP-1 responsive gene and is necessary for cJun induced anchorage-independent growth. Mol Cancer Res. 2004;2:303–14. [PubMed] [Google Scholar]
- [33].Xu Y, Sumter TF, Bhattacharya R, Tesfaye A, Fuchs EJ, Wood LJ, Huso DL, Resar LM. HMG-I transgenic mice develop highly penetrant aggressive lymphoid malignancy in transgenic mice and is overexpressed in human leukemia. Cancer Res. 2004;64:3371–75. doi: 10.1158/0008-5472.CAN-04-0044. [DOI] [PubMed] [Google Scholar]
- [34].Fedele M, Pentimalli F, Baldassarre G, Battista S, Klein-Szanto AJ, Kenyon L, Visone R, De Martino I, Ciarmiello A, Arra C, Viglietto G, Croce CM, Fusco A. Transgenic mice overexpressing the wild-type form of the HMGA1 gene develop mixed growth hormone/prolactin cell pituitary adenomas and natural killer cell lymphomas. Oncogene. 2005;24:3427–35. doi: 10.1038/sj.onc.1208501. [DOI] [PubMed] [Google Scholar]
- [35].Tesfaye A, Di Cello F, Hillion J, Ronnett BM, Elbahloul O, Ashfaq R, Dhara S, Prochownik E, Tworkoski K, Reeves R, Roden R, Ellenson LH, Huso DL, Resar LM. The high-mobility group A1 gene up-regulates cyclooxygenase 2 expression in uterine tumorigenesis. Cancer Res. 2007;67:3998–4004. doi: 10.1158/0008-5472.CAN-05-1684. [DOI] [PubMed] [Google Scholar]
- [36].Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell–like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Di Cello F, Hillion J, Aderinto A, Ronnett B, Huso D, Kowlaski J, Resar LMS. COX-2 inhibitors block uterine tumorigenesis in HMGA1a transgenic mice and human uterine cancer xenografts. Mol Cancer Ther. 2008;7:2090–95. doi: 10.1158/1535-7163.MCT-07-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hillion J, Wood LJ, Mukherjee M, Bhattacharya R, Di Cello F, Kowalski J, Elbahloul O, Segal J, Poirier J, Rudin CM, Dhara S, Belton A, Joseph B, Zucker S, Resar LM. Upregulation of MMP-2 by HMGA1 promotes transformation in undifferentiated, large-cell lung cancer. Mol Cancer Res. 2009;7:1803–12. doi: 10.1158/1541-7786.MCR-08-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hristov AC, Cope L, Di Cello F, Reyes MD, Singh M, Hillion J, Belton A, Joseph B, Schuldenfrei A, Iacobuzio-Donahue CA, Maitra A, Resar LM. HMGA1 correlates with advanced tumor grade and decreased survival in pancreatic ductal adenocarcinoma. Mod Pathol. 2010;23:98–104. doi: 10.1038/modpathol.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Resar LMS. The High Mobility Group A1 gene: Transforming inflammatory signals into cancer? Cancer Res. 2010;70:436–39. doi: 10.1158/0008-5472.CAN-09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Karp JE, Smith BD, Resar LS, et al. Phase I and pharmacokinetic study of bolus-infusion flavopiridol followed by cytosine arabinoside and mitoxantrone for acute leukemias. Blood. 2011;117:3302–10. doi: 10.1182/blood-2010-09-310862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Di Cello F, Hillion J, Hristov A, Wood LJ, Mukherjee M, Schuldenfrei A, Bhattacharya R, Kowalski J, Resar LMS. HMGA2 participates in neoplastic transformation in human lung cancer. Mol Cancer Res. 2008;6:743–50. doi: 10.1158/1541-7786.MCR-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hristov A, Reyes M Delos, Singh M, Cope L, Iacobuzio-Donahue C, Maitra A, Resar LMS. HMGA2 protein expression correlates with lymph node metastasis and increased tumor grade in pancreatic ductal adenocarcinoma. Mod Pathol. 2009;22:43–9. doi: 10.1038/modpathol.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhou G, Chen J, Lee S, Clark T, Rowley JD, Wang SM. The pattern of gene expression in human CD34+ stem/progenitor cells. Proc Natl Acad Sci USA. 2001;98:13966–71. doi: 10.1073/pnas.241526198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chou B-K, Mali P, Huang X, Ye Z, Dowey SN, Resar LMS, Tong J, Cheng L. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signantures. Cell Res. 2011;21:518–29. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Somervaille TC, Matheny CJ, Spencer GJ, Iwasaki M, Rinn JL, Witten DM, Chang HY, Shurtleff SA, Downing JR, Cleary ML. Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells. Cell Stem Cell. 2009;4:129–40. doi: 10.1016/j.stem.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, Moran M, Blum KA, Rovin B, Brooker-McEldowney M, Broering S, Schaaf LJ, Johnson AJ, Lucas DM, Heerema NA, Lozanski G, Young DC, Suarez JR, Colevas AD, Grever MR. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Karp JE, Blackford A, Smith BD, Alino K, Seung AH, Bolaños-Meade J, Greer JM, Carraway HE, Gore SD, Jones RJ, Levis MJ, McDevitt MA, Doyle LA, Wright JJ. Clinical activity of sequential flavopiridol, cytosine arabinoside, and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Leuk Res. 2010;34:877–82. doi: 10.1016/j.leukres.2009.11.007. l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chung Y-J, Kim T-M, Eaves C, Oh I-H. Role of Stat3 for hematopoietic stem cells. Int J Hematology. 2002;S1:152. [Google Scholar]
- [50].Pulikkan JA, Dengler V, Peramangalam PS, Zada AA Peer, Muller-Tidow C, Bohlander SK, Tenen DG, Behre G. Cell-cycle regulator E2F1 and microRNA-223 comprise an autoregulatory negative feedback loop in acute myeloid leukemia. Blood. 2009;115:1768–78. doi: 10.1182/blood-2009-08-240101. [DOI] [PMC free article] [PubMed] [Google Scholar]