Abstract
Mycobacterium tuberculosis, the causative agent of tuberculosis, is unique among bacterial pathogens in that it contains a wide array of complex lipids and lipoglycans on its cell wall. Among them, the sulfated glycolipid, termed sulfolipid is thought to mediate specific host pathogen interactions during infection. Sulfolipids (SLs) including sulfolipid I (SL-I) and sulfolipid II (SL-II) are 2,3,6,6'-tetraacyltrehalose 2'-sulfates. SL-I was identified as a family of homologous 2-palmitoyl(stearoyl)-3-phthioceranoyl, 6,6'-bis(hydroxyphthioceranoy1)-trehalose 2'-sulfate and was believed to be the principal sulfolipid of Mycobacterium tuberculosis strain H37Rv. We cultured and extracted sulfolipids using various conditions including those originally described and employed high-resolution multiple-stage linear ion-trap mass spectrometry with electrospray ionization to characterize the structure of the principal SL. We revealed that SL-II, a family of homologous 2-stearoyl(palmitoyl)-3,6,6'-tris(hydroxyphthioceranoy1)-trehalose2'-sulfates, rather than SL-I is the principal sulfolipid class. We identified a great number of isomers resulting from permutation of the various hydroxyphthioceranoyl substituents at 6- and 6'-position of the trehalose backbone for each of the SL-II species in the entire family. We redefined the structure of this important lipid family that was mis-assigned using the traditional methods 40 years ago.
Keywords: glycolipids, sulfolipids, sulfolipid I, sulfolipid II, hydroxyphthioceranoic acid, mycobacterium tuberculosis, high resolution mass spectrometry, multiple-stage mass spectrometry, ESI
Introduction
Mycobacterium tuberculosis contains a range of complex glycolipids with a trehalose backbone, including a family of sulfated acyl trehaloses that was first recognized by Middlebrook et al in the virulent Tubercle bacilli (1). This family of sulfated acyl trehaloses defined as sulfolipids (SLs) were characterized by Goren and coworkers in their early studies on M. tuberculosis H37Rv (2-5). The principal SLs were characterized as sulfolipid-I (SL-I), which is a homologous mixture of 2,3,6,6'-tetraacyl-α, α'-d-trehalose-2'-sulfate consisting of a pair of 2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoate (hydroxyphthioceranoic acid) located at 6, and 6'-position, and a nonhydroxylated multiple methyl-branched derivative (phthioceranoic acids) and a short saturated fatty acid (palmitic acid or stearic acid) located at the 3- and 2-position of the trehalose backbone, respectively. In addition to the major SL-I, minor species that were termed as SL-II (2-palmitoyl/stearoyl-3,6,6'-tris-hydroxyphthioceranoyl-2'-sulfate), SL-I’ (2-palmitoyl/stearoyl-3,6-bis-phthioceranoyl-6'-hydroxyphthioceranoyl-2'-sulfate) and SL-II’ (2-palmitoyl/stearoyl-4,6,6'-tris-hydroxyphthioceranoyl-2'-sulfate) were also reported. However, the structural studies on SLs have been focused on SL-I (4).
A wealth of in vivo and in vitro studies that point to the role of sulfolipids in the virulence of the tubercle bacillus have been documented. For example, Okamoto et al reported that SL could contribute to virulence at early stage of mycobacterial infection or stimulation with the glycolipids by counteracting the immunopotentiating effect of TDM (6). In vitro, SL-I induced swelling and disruption of mitochondrial membranes and strongly inhibited mitochondrial oxidative phosphorylation (7). M. tuberculosis sulfolipids are also capable of preventing phagosome-lysosome fusion in cultured macrophages (8). From in vitro studies, Zhang and coworkers reported the remarkable properties of sulfolipids, including their ability to modulate the oxidative response and the cytokine secretion of human monocytes and neutrophils (9, 10). It was also found that diacylated sulfoglycolipids are mycobacterial antigens that can stimulate CD1-restricted T cells during infection with M. tuberculosis (11-13). Studies addressing the aspects of the biosynthesis and the function of SL-1 in vivo have recently begun to emerge (14-17). However, its function in the life cycle of the M. tuberculosis remains largely unknown.
The structures of sulfolipids are complex, and studies toward the structural definition of SL-I conducted by Goren and coworkers 40 years ago is a showcase of the exceptional talent of scientists of their generation. They were able to deduce the complex structure of unknown compounds using the traditional methods that required many laborious steps to a large-scale separation and purification, followed by multiple chemical reaction steps and chromatographic separations, in conjunction with various spectroscopic techniques including IR, NMR and mass spectrometry for structure analysis (2-4). In this contribution, we describe a simple and direct electrospray ionization (ESI) mass spectrometric approach employing multiple-stage and high-resolution mass spectrometry for structural elucidation of the sulfolipids of M. tuberculosis H37Rv, the same strain previously described by Goren et al (2-5). When cultured and extracted using various conditions including those described by Goren, we found that SL-II not SL-I is always the principal sulfolipid class. Our data are similar to those reported by others who nevertheless referred it as SL-I (4, 16, 18-20). Our approaches using various mass spectrometric techniques permit us to reveal the detailed structures of SLs including the various isobaric isomers that had been reported but not confirmed by the traditional methods.
Experimental procedures
Materials
Sulfolipid-1 standard was supplied by the Mycobacteria Research Laboratories at Colorado State University (CSU) as part of the TB Research materials contract that is currently administered by BEI Resources (http://www.beiresources.org/TBVTRMResearchMaterials/tabid/1431/Default.aspx). According to standard operating protocols, the SL-I standard was harvested from M. tuberculosis H37Rv that had been grown in glycerol-alanine salts (GAS) broth, killed (γ-irradiation) and extracted using chloroform and methanol. All other chemicals used are spectroscopic grade and were purchased from Sigma Chemical Co.
Culture media
Modified Wong-Weinzirl broth was prepared and autoclaved as described by Goren (2). The broth was composed of malic acid (0.3%), NH4OH (0.12%), ammonium citrate (0.5%), KH2PO4 (0.6%), anhydrous Na2CO3 (0.2%), NaCl (0.2%), MgSO4 (0.1%), ammonium iron(III) citrate (0.005%), glycerol (0.05% v/v), and glucose (0.2%), supplemented with sodium pyruvate (0.05%), Cu2+ (0.0001%) and Zn2+(0.0001%). Middlebrook 7H9 broth and Middlebrook 7H10 plates (BD, Franklin Lakes, NJ) were prepared according to manufacturer instructions with supplemental OADC enrichment and Tween-80 (0.05%). Glycerol-alanine-salts (GAS) broth contained (per liter) 2 g of NH4Cl, 1 g of l-alanine, 0.3 g of Bacto casitone (Difco), 4 g of K2HPO4, 2 g of citric acid, 50 mg of ferric ammonium citrate, 1.2 g of MgCl2-6H2O, 0.6 g of K2SO4, 1.8 ml of 10 M NaOH, 10 ml of glycerol, and Tween 80 (0.05%).
Mycobacterial culture and harvest
Seed stocks of M. tuberculosis (H37Rv or CDC1551) were grown for 7-10 days (O.D 0.7–0.9) in 7H9-OAD at 37 °C. For broth-grown cultures, 250 ml of broth was inoculated (1:500) and incubated at 37 °C for 4-6 weeks to late-log phase. Pellicles formed in the modified Wong-Weinzirl broth, and flocculent cultures grew in the 7H9 broth. Broth cultures were harvested by centrifugation at 3000 rpm, and the pellets were washed successively in PBS/Tween (0.05%). For plate-grown cultures, seed cultures were diluted 1:10 in PBS/Tween and vortexed; and 4 ml was spread onto 7H10 agar (150 cm-dia. plates) and incubated at 37 °C for 6 weeks. Confluent growth with a dry, waxy appearance was scraped into collection tubes and weighed.
Lipid extraction
Pellets of live bacilli were extracted in hexane-decylamine or chloroform:methanol (C/M) (2:1; v:v). Plate-grown pellets were extracted three times (100 ml solvent per 100 g). Hexane-decylamine extractions were performed as described by Goren (2). Briefly, the first extraction was in 0.1% decylamine (4°C for 1-4 weeks), and the subsequent extractions were in 0.5% decylamine with sonication for 30 min at room temperature. For C/M extractions, washed pellets were extracted the first time at 4°C for 1-4 weeks and subsequently with sonication for 30 min at room temperature. Extracts were pooled, filtered (0.2 micron, PFTE filters) and dried on a rotary evaporator. To remove decylamine, residues were dissolved in hexane, and an equal volume of citric acid (1N) was added. The flask was shaken and allowed to separate. The top phase was percolated through a column (8cm length; 0.7 cm dia.) of anhydrous H2SO4 and chased with 1 column volume of hexane. The eluent was dried under nitrogen. All crude extracts were stored dried and under nitrogen at -70 °C.
Thin Layer Chromatography
Lipid extracts were dissolved in hexane. If a sample was insoluble, chloroform:methanol (2:1) was added until the sample dissolved completely. Lipids were spotted onto silica gel 60 TLC plates (EM Science) and developed in a 2-solvent system as previously reported by Goren et al. (2). Plates were developed first in chloroform:methanol:acetic acid:water (95:1:5:0.3; v:v) to 20 cm, dried, and then re-developed in chloroform:acetone:methanol:water:acetic acid (158:83:1:6:32; v:v) to 15 cm. Plates were sprayed with ethanolic sulfuric acid (50%) and charred with a heat gun. Samples that were fractionated by preparative TLC were loaded across a TLC plates and developed successively in the same solvent systems for 20 cm and 18 cm, respectively. A strip of the plate was charred to visualize lipid bands, and the corresponding areas were scraped into separate tubes and extracted from the silica with hexane followed by chloroform:methanol (2:1), each for 5 min at 45 °C with sonication. The two extracts were combined, dried under nitrogen and stored at -70 °C.
Mass spectrometry
Low-energy collision-induced dissociation (CID) tandem mass spectrometry experiments were conducted on a Thermo Scientific (San Jose, CA) LTQ Orbitrap Velos and a LIT-FT mass spectrometers (MS) with Xcalibur operating system. High-resolution mass spectrometry was also performed on a Bruker solariX (Bremen, Germany) 12 Tesla FTMS system, which provides baseline resolution among ions from SL-I and SL-II. Solution of sulfolipid in methanol was infused (1.5 μL/min) to the ESI source, where the skimmer was set at ground potential, the electrospray needle was set at 4.0 kV, and temperature of the heated capillary was 300°C. The automatic gain control of the ion trap was set to 5×104, with a maximum injection time of 100 ms. Helium was used as the buffer and collision gas at a pressure of 1×10-3 mbar (0.75 mTorr). The MSn experiments were carried out with an optimized relative collision energy ranging from 35-40% and with an activation q value at 0.25, and the activation time at 10 ms to leave a minimal residual abundance of precursor ion (around 20%). The mass selection window for the precursor ions was set at 4 Da wide to include the monoisotopic species to the ion-trap for collision-induced dissociation. For higher-energy collision-induced dissociation (HCD), precursor ions were selected in the linear ion trap and fragmentation in the multipole HCD collision cell with high resolution accurate mass detection in the Orbitrap mass analyzer. Mass spectra were accumulated in the profile mode, typically for 3-10 min for MSn-spectra (n=2,3,4).
Nomenclature
To facilitate data interpretation, the following abbreviations were adopted. The abbreviation of the nonhydroxylated multiple methyl-branched phthioceranoic acids, for example, the 2,4,6,8,10,12,14,16-Octamethyl-dotriacontanoic acid is designated as C40-acid to reflect the fact that the structure consists of 40 saturated hydrocarbon chain. For hydroxydotriacontanoic acids, for example, 2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoic acid is designated as hC40-acid to reflect that the compound consists of 40 hydrocarbon chain with one hydroxyl group attached at C-17. Therefore, the principal SL-II species (the position of the substituents on the trehalose backbone is adopted from the definition by Goren (4)), which is a 2-stearoyl-3,6,6'-tris-2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoyl-α,α'-d-trehalose-2'-sulfate is designated as (18:0, hC40, hC40, hC40 )-SL, signifying that the compound consists of one stearoyl and three 2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoyl groups located at 2-, 3-, 6-and 6'-position of the trehalose backbone, respectively; while SL-I molecule such as 2-palmitoyl-3-2,4,6,8,10-Pentamethyl-pentaeicosanoyl-6.6'-bis-2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoyl-α,α'-d-trehalose-2'-sulfate is designated as (16:0, C30, hC40, hC40 )-SL.
Results and Discussion
Crude sulfolipid extracts from different culture media and extraction methods
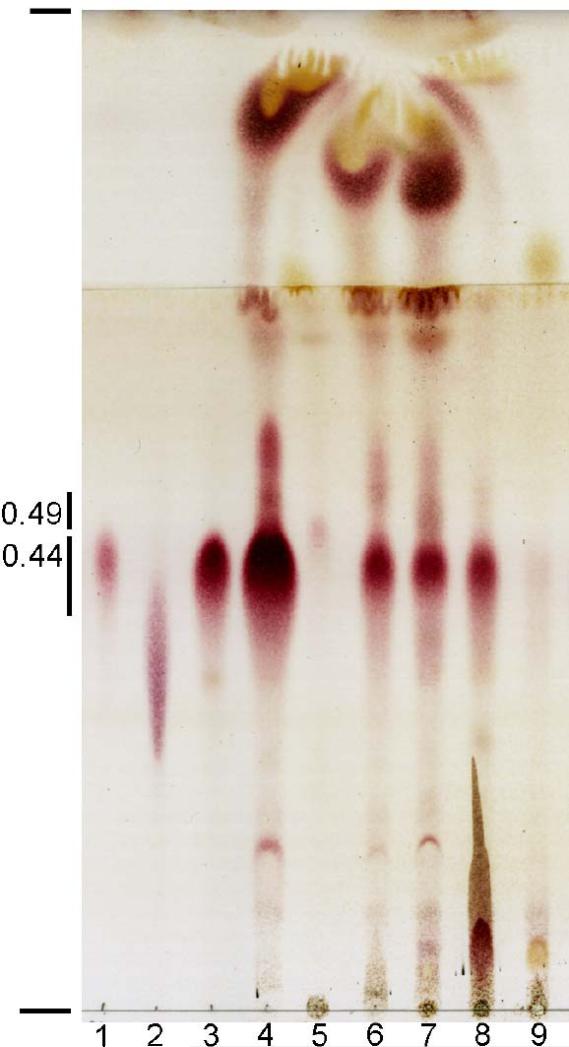
Various culture media and extraction methods have been used in reports characterizing the SLs from M. tuberculosis H37Rv (2, 11, 16, 19). To determine whether such variables could alter the relative abundance of the major SL families,H37Rv was cultured to late-log phase in four culture mediums, and two extraction methods were performed. H37Rv and a clinical isolate (CDC1551; data not shown) yielded relatively abundant SLs in crude lipid extracts under all conditions (Figure 1). Glycolipids appeared as prominent red spots or streaks TLC plates upon spraying with sulfuric acid and heating, and spots with approximate Rfs of 0.44 and 0.49 that were scraped from prepTLC plates were confirmed to be SLs by ESI mass spectrometry in the negative-ion mode. The most abundant SL exhibited an Rf of 0.44 with mobility identical to the SL-I family and a less abundant SL (Rf 0.49) which was apparent in hexane-decylamine extracts, corresponding to the SL family that was designated SL-II by Goren (2, 4).
Figure 1.
TLC comparison of crude lipid extracts from M. tuberculosis H37Rv. Purified standards or crude lipids that were obtained under various conditions were spotted and developed in two solvents in the same direction. Standards, samples and the conditions that they were obtained with (if known) are indicated. Lane 1-SL-I standard from the CSU TB materials research contract (GAS broth; chloroform/methanol extraction (C/M); lane 2-trehalose dimycolate standard from Sigma-Aldrich; lane 3-purified major SL (GAS broth; C/M); lane 4-crude lipids (7H10 plates, hexane-decylamine extract (HD); lanes 5 and 6-crude lipids from different cultures (7H9 broth, HD); lane 7-crude lipids (Wong-Weinzirl broth, HD); lane 8-crude lipids (7H10 plates, C/M), and lane 9-crude lipids (7H9 broth, C/M). The average Rfs of the SL families are indicated as well as the origin and the farthest solvent front.
Sulfolipids from plate-grown cultures were especially abundant. SLs were also readily extracted from broth-grown cultures despite some culture-to-culture variation in the amounts of crude lipid (Figure 1; lanes 5 and 9 vs. lane 6). The SLs that migrated at Rf 0.44 were always the most abundant SL family in all broth cultures. Hexane-decylamine or chloroform-methanol extraction methods isolated the major SL family; however, hexane-decylamine extraction was more efficient for isolating the SLs that migrated at Rf 0.49, as described by Goren (2). Another parameter that was explored briefly was whether live and dead bacterial pellets yielded the same relative abundance of SL families. Preliminary studies showed no significant differences in crude extracts from heat-killed (autoclaved) vs. live bacterial pellets. Taken together, these data indicate that the SL family with an Rf of 0.44 in our TLC system is the most abundant family, irrespective of differences in commonly used culture media and extraction methods.
High-resolution mass spectrometry on the [M – H]- ions of sulfolipids
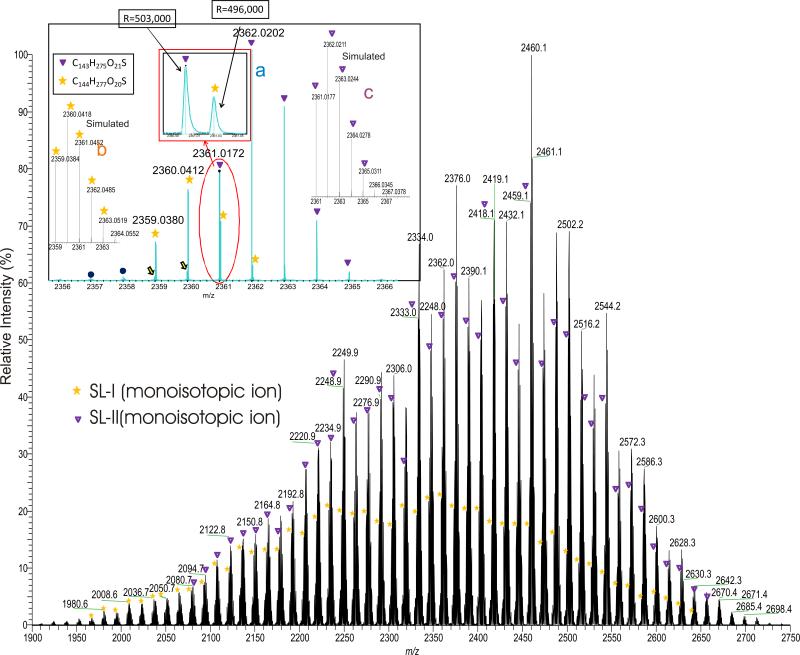
We examined the SLs isolated from M. tuberculosis strain H37Rv and strain CDC1551 grown in 7H9 broth, and in modified Wong-Weinzirl broth, together with the sulfolipid standard obtained from the Mycobacterial Research Laboratories at Colorado State University using ESI mass spectrometry in the negative-ion mode. An array of the [M – H]- ions in the range of 2000-2900 Da, with an intermittence of 14 Da were observed. The ESI/MS profiles of the SLs from H37Rv grown in 7H9 broth (Figure 2) and from the SL standard (not shown) are similar, and the profiles are also similar to those previously reported (16, 18-21); while the SL profiles from strain H37Rv grown in modified Wong-Weinzirl broth (See supplemental data, Figure S1) are also similar, and the ions representing SL-II are the dominant species, but the average M.W. is 56 Da higher than that obtained from 7H9 broth. The results indicate that the length of the hydroxyphthioceranoic acid substituents of SLs is dependent on the growth conditions. High-resolution mass spectrometric analysis on the ion series with an increment of 14 Da using an FT-ICR instrument (Resolution ~500,000) confirmed that the 14 Da increment corresponds to a CH2 residue and the homologous [M – H]- ions ranged from 1900 to 2750 Da all possess an elemental composition of C120H229(CH2)nO21S1 (n = 0, 1, 2, .. 60) (See supplemental data, Table S1), in which the nominal masses for the monoisotopic peaks at m/z 2333, 2375, 2417, 2459, ..., 2501 are among the most prominent (Figure 2, ions marked with “▼”). This ion series consists of palmitoyl/stearoyl, three hydroxyphthioceranoyl and one sulfate residues on the trehalose backbone and was previously defined as SL-II by Goren and coworkers (4). The spectrum also contained the ion series with nominal monoisotopic masses that are 2 Da lighter (see Figure 2, ions marked with “*”), similar to that recently reported by Layre et al (21). High resolution mass measurements on these ions (Table S1) indicate that these ions possesses an elemental composition of C120H229(CH2)nO20S1 (n = 0, 1, 2, .. 60), corresponding to the SL-I structures previously defined (4).
Figure 2.
The high resolution ESI-MS spectrum of the [M – H]- ions of sulfolipids of H37Rv grown in Middlebrook 7H9 broth. The spectrum was obtained with a FTICR instrument. The high resolution (R=500,000) readily separated the SL-I (monoisotopic ions marked with “*”) class from SL-II (monoisotopic ions marked with “▼”), which is the principal sulfolipid class from M. tuberculosis strain H37Rv (inset). The elemental compositions deduced from accurate mass measurements also define SL-I and SL-II.
The separation of the ion clusters of SL-I from those of SL-II (Figure 2, inset a) (Figure 2, inset a) also provides the accurate profile of the isotopic cluster ions for confirmation of elemental composition. For example, the monoisotopic ion observed at m/z 2359.0380 (Figure 2, inset a) corresponds to an elemental composition of C144H277O20S (calculated m/z: 2359.0384), which gave a simulated spectrum (Inset b, ions labeled with “*”) similar to the experimental (Inset a, ions labeled with “*”). The monoisotopic ion observed at m/z 2361.0172 and its isotopic ion cluster pattern (Inset a; ions labeled with “▼”) are also consistent with the elemental composition of C143H275O21S (calculated mass: 2361.0177; the simulated plot of the isotopic ion clusters is labeled with “▼” in Inset c), indicating the presence of SL-II. The mass spectrometric approaches toward structural characterization of the major sulfolipid species (i.e., SL-II) are described below.
Structural analysis of SL-II by multiple-stage mass spectrometry in conjunction with high-resolution accurate mass measurements
High-resolution mass measurement on the [M –H]- ion of the monoisotopic ion of m/z 2459 (base peak) and its isotope ion cluster profile led to an elemental composition of C150H289O21S1 (calculated: 2459.1272, measured: 2459.1272), corresponding to a SL-II molecule (Table S1; Figure 2).
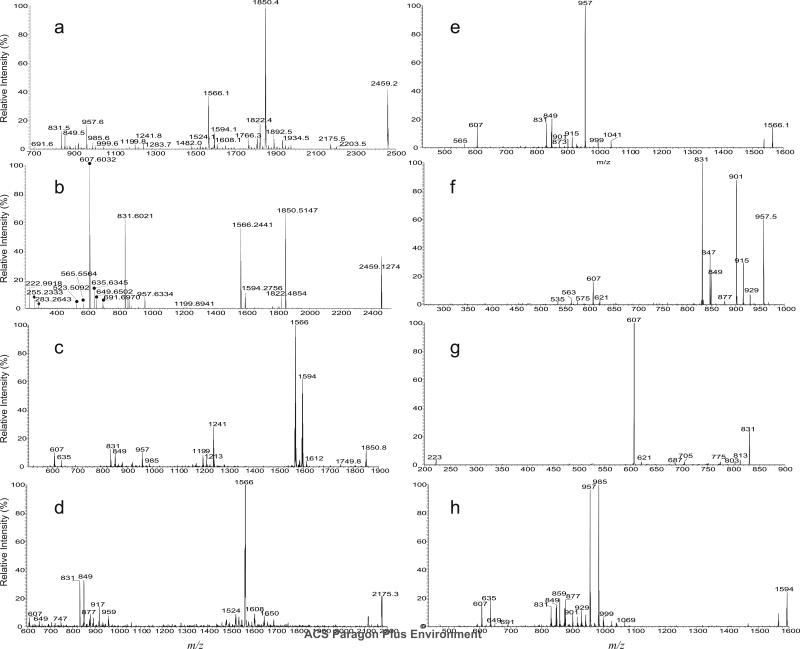
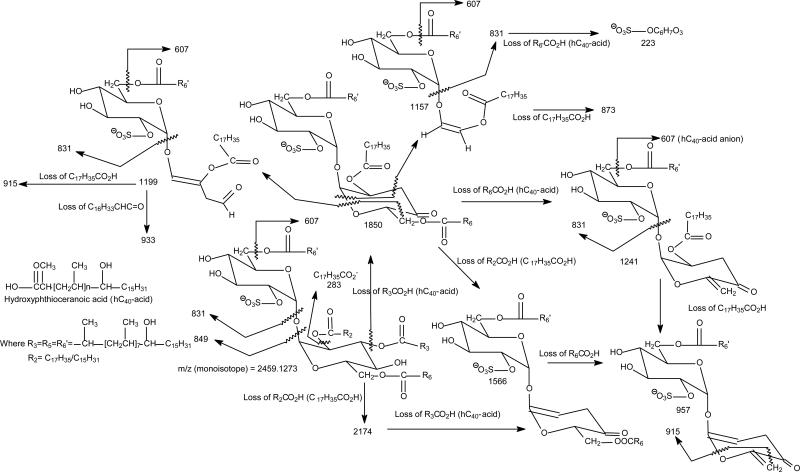
The MS2 spectra of the ions of m/z 2459.1 following CID (Figure 3a) and HCD (Figure 3b) yielded prominent ion at m/z 1850.6 (2459.1 – 608.6), corresponding to loss of a C40-hydroxyphthioceranoic acid residue probably residing at 3 position of the trehalose core of the SL. The spectra also contained the abundant ion at m/z 1566.3 from further loss of a stearic acid (18:0-acid) residue at the 2-position of the trehalose skeleton. This consecutive loss of the 18:0-fatty acid is supported by CID MS3 spectrum of the ion at m/z 1850 (2459.1 → 1850.6; Figure 3c), which is dominated by the ion at m/z 1566.3 (1850.6 -284.3). The ion of m/z 1566.3 can also arise from primary loss of the 18:0-fatty acid, followed by loss of a C40-hydroxyphthioceranoic acid. These fragmentation processes were supported by the observation of the ion at m/z 1566.3 in the MS3 spectrum of the ion at m/z 2174.8 (2459.1 → 2174.8; Figure 3d), and consistent with the presence of the ion at m/z 2174.8 (2459.1 – 284.3) in Figure 3a. The prominence of the ions of 1850 and 1566 (Figure 3a and 3b) may indicate the preferential losses of the 3-acyl and 2-acyl substituents as the free fatty acid over those located at 6 or 6', and are consistent with the notion of the greater stability of the substituents at 6 (or 6') towards acid or alkaline hydrolysis as compared with those located at 3- and 2-position (5). The MS4 spectrum of the ion of m/z 1566.3 (2459.1 → 1850.3 → 1566.3; Figure 3e) is identical to the MS3 spectrum of the m/z 1566.3 ion (2459.1 → 1566.3) (not shown), and the spectrum is dominated by the ion at m/z 957.7 (1566.3 – 608.6), arising from further loss of a C40-hydroxyphthioceranoic acid residue, residing at 6-, or 6’-position. The spectrum also contained the ions at m/z 849.5 and 831.5, corresponding to a 6'-C40-hydroxyphthioceranoyl-glucose-2'-sulfate and dehydrated 6'-C40-hydroxyphthioceranoyl-glucose-2'-sulfate anions, respectively. These two ions consist of the glucose moiety originally bearing the lone acyl group and the sulfate substituent, and were formed by further elimination of a C6H4O2 and a C6H6O3 residues from m/z 957, respectively, supporting that a C40-hydroxyphthioceranoyl is located at 6'. This latter fragmentation process is supported by the MS4 spectrum of the ion of m/z 957 (2459.1 → 1566.3 → 957.7; Figure 3f). The MS5 spectrum of the ion at m/z 831.5 (2459.1 → 1566.3 → 957.7 → 831.5; Figure 3g) is dominated by the ion at m/z 607.5 (831.5 – 224) representing a 6'-C40-hydroxyphthioceranoic anion and the ion at m/z 223, representing a dehydrated glucose-2'-sulfate anion (Scheme 1; Figure 3b), confirming that the ion at m/z 831 indeed contains the glucose moiety originally bearing the lone acyl residue and the sulfate substituent and represents a 6'-C40-hydroxyphthioceranoyl-glucose-2'-sulfate anion. The above results indicate that the major ion at m/z 2459.1 represents a 2-stearoyl-3,6,6'-tris-2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoyl-α,α'-d-trehalose-2'-sulfate ([18:0, hC40, hC40, hC40]-SL). The fragmentation processes were further supported by the elemental compositions of the fragment ions obtained from the high-resolution CID and HCD tandem mass spectra (Table 1). The HCD MS2 spectrum of the ion of m/z 2459.1 (Figure 3b) contained the tandem quadrupole-like spectrum, which is dominated by the hydroxyphthioceranoic acid anion of m/z 607 (measured: 607.6032; calculated:607.6034) with an elemental composition of C40H79O3-, consistent with the suggested structure of 2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoyl acid, along with the homologous ions at m/z 691, 649, 635, 565 and 523 (Table 1). The characterization of hydroxyphthioceranoic acid was further achieved by its MSn spectra, which is described later.
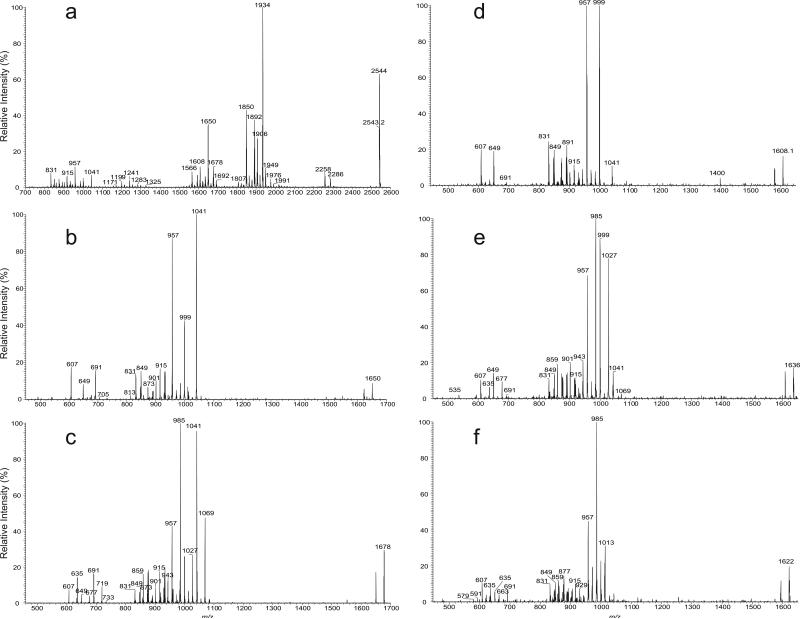
Figure 3.
The LIT CID MS2 spectrum (a), and high-resolution HCD MS2 spectrum (b) of the ion of m/z 2459. The low-mass fragment ions from HCD MS2 (b) readily identify the hydroxyphthioceranoic acid (●), long chain fatty acid (◆) and sulfate groups (seen at m/z 222.9918); while the LIT CID MS3 spectra of m/z 1850 (2459 → 1850) (c), 2178 (2459 → 2178) (d), 1566 (2459 → 1566) (e), and MS4 spectra of m/z 957 (2459 → 1566 → 957) (f), and of 831 (2459 → 1566 → 831) (g) together with the MS3 spectrum of m/z 1594 (2459 → 1594) (h) support the fragmentation processes as illustrated in Scheme 1.
Scheme 1.
The fragmenation processes proposed for 2,3,6,6'-Tetraacyl-α,α'-D-trehalose 2'-sulfate (Sulfolipid II). Example shown is the pathways for the most prominent [M -H]- ion of m/z 2459 (monoisotopic) mainly representing a [18:0, hC40, hC40, hC40]-SL. The structures of the indicated fragment ions are supported by the elemental compositions deduced from accurate mass measurements with high-resolution MSn mass spectrometry (See Table 1).
Table 1.
The elemental composition of the fragment ions obtained from high-resolution CID and HCD MS2 on [M – H]- ion of sulfolipid of m/z 2459
| nominal mass (m/z) | measured mass (Da) | calculated mass (Da) | elementary composition | nominal mass (m/z) | measured mass (Da) | calculated mass (Da) | elementary composition |
|---|---|---|---|---|---|---|---|
| 2459 | 2459.1274 | 2456.1273 | C150H289O21S | 1041 | 1041.7274 | 1041.7281 | C58H105O13S |
| 2202 | 2202.8860 | 2202.8870 | C134H257O19S | 999 | 999.6804 | 999.6812 | C55H99O13S |
| 2174 | 2174.8547 | 2174.8557 | C132H253O19S | 985 | 985.6645 | 985.6655 | C54H97O13S |
| 1934 | 1934.6082 | 1934.6104 | C116H221O18S | 957 | 957.6334 | 957.6342 | C52H93O13S |
| 1892 | 1892.5634 | 1892.5635 | C113H215O18S | 933 | 933.7065 | 933.7070 | C52H101O11S |
| 1864 | 1864.5292 | 1864.5322 | C111H211O18S | 917 | 917.6386 | 917.6393 | C50H93O12S |
| 1850 | 1850.5147 | 1850.5165 | C110H209O18S | 901 | 901.6438 | 901.6444 | C50H93O11S |
| 1836 | 1836.5026 | 1836.5009 | C109H207O18S | 891 | 891.6583 | 891.6601 | C49H95O11S |
| 1822 | 1822.4854 | 1822.4852 | C108H205O18S | 877 | 877.6436 | 873.6444 | C48H93O11S |
| 1808 | 1808.4676 | 1808.4695 | C107H203O18S | 873 | 873.6490 | 873.6495 | C49H93O10S |
| 1766 | 1766.4212 | 1766.4226 | C104H197O18S | 859 | 859.6331 | 859.6338 | C48H91O10S |
| 1692 | 1692.3864 | 1692.3858 | C101H191O16S | 849 | 849.6127 | 849.6131 | C46H89O11S |
| 1678 | 1678.3723 | 1678.3702 | C100H189O16S | 847 | 847.5968 | 847.5975 | C46H87O11S |
| 1650 | 1650.3378 | 1650.3389 | C98H185O16S | 831 | 831.6021 | 831.6025 | C46H87O10S |
| 1608 | 1608.2910 | 1608.2919 | C95H179O16S | 747 | 747.5088 | 747.5086 | C40H75O10S |
| 1594 | 1594.2756 | 1594.2763 | C94H177O16S | 691 | 691.6970 | 691.6974 | C46H91O3 |
| 1566 | 1566.2441 | 1566.2450 | C92H173O16S | 649 | 649.6502 | 649.6504 | C43H85O3 |
| 1538 | 1538.2130 | 1538.2137 | C90H169O16S | 635 | 635.6345 | 645.6348 | C42H83O3 |
| 1524 | 1524.1961 | 1524.1980 | C89H167O16S | 607 | 607.6032 | 607.6034 | C40H79O3 |
| 1510 | 1510.1821 | 1510.1824 | C88H165O16S | 565 | 565.5564 | 565.5565 | C37H73O3 |
| 1482 | 1482.1506 | 1482.1511 | C86H161O16S | 523 | 523.5092 | 523.5095 | C34H67O3 |
| 1241 | 1241.9047 | 1241.9058 | C70H129O15S | 283 | 283.2643 | 283.2643 | C18H35O2 |
| 1213 | 1213.8732 | 1213.8745 | C68H125O15S | 255 | 255.2333 | 255.2330 | C16H31O2 |
| 1199 | 1199.8941 | 1199.8952 | C68H127O14S | 223 | 222.9921 | 222.9918 | C6H7O7S |
| 1171 | 1171.8630 | 1171.8639 | C66H123O14S | 204 | 204.9820 | 204.9812 | C6H5O6S |
| 1157 | 1157.8445 | 1157.8453 | C65H121O14S |
In Figure 3c, the ion at m/z 1594.3 arising from further loss of 16:0-fatty acid residue is also present, consistent with the observation of the ion at m/z 2202.8 in Figure 3a. The results indicate the presence of the isomer bearing a 16:0-fatty acid substituent at the 2-position and a C40-hydroxyphthioceranoic acid residue mostly likely at the 3-position. The ion of m/z 1594.3 can also arise from the losses in a reversed manner (i.e., first loss of palmitoyl group followed by C40-hydroxyphthioceranoic acid residue), similar to the formation of the ion of m/z 1568 as described earlier. Further dissociation of the ion of m/z 1594.3 (2459.1 → 1594.3; Figure 3h) yielded ions of m/z 985 and 957, arising from losses of a C38-hydroxyphthioceranoic and a C40-hydroxyphthioceranoic acids respectively, along with ions at m/z 635 and 607, representing a C42-hydroxyphthioceranoic and C40-hydroxyphthioceranoic anions, respectively. The results indicate the presence of a C42-hydroxyphthioceranoic and a C40-hydroxyphthioceranoic residues at 6- or 6'-position. The spectrum also contained the ions at m/z 859 and 831, corresponding to a 6'-C42-hydroxyphthioceranoyl-glucose-2'-sulfate and 6'-C40-hydroxyphthioceranoyl-glucose-2'-sulfate anions, respectively. These results indicate that both the C42- and C40-hydroxyphthioceranoyl groups can reside at the 6'-position, suggesting the presence of 2-palmitoyl-3,6-bis-2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoyl-,6'-2,4,6,8,10,12,14,16-Octamethyl-17- hydroxytetratriacontanoyl-α,α'-D-trehalose-2'-sulfate ([16:0, hC40, hC40, hC42]-SL) and 2-palmitoyl-3,6'-bis-2,4,6,8,10,12,14,16-Octamethyl-17-hydroxytetratriacontanoyl 6-2,4,6,8,10,12,14,16-Octamethyl-17-hydroxytetratriacontanoyl-α,α'-D-trehalose-2'-sulfate ([16:0, hC40, hC42, hC40]-SL) isomers. The observation of these latter two isomers indicates that the 2-substituent appears to be restricted as previously reported (2-4), while the substituents at the 6 and 6’ positions are interchangeable. Again, the structural assignments of the fragment ions are consistent with the elemental composition deduced from high-resolution mass measurements (Table 1). Collectively, the ion at m/z 2459.1 represents a major [18:0, hC40, hC40, hC40]-SL isomer together with two minor isomers of [16:0, hC40, hC40, hC42]-SL and [16:0, hC40, hC42, hC40]-SL.
In Figure 3b, minor C46-, C37-, C34-hydroxyphthioceranoic anions at m/z 691, 565, 523 were also observed; and the minor homologous ions at m/z 1766, 1892, and 1934 arising from losses of the C46-, C37-, C34-hydroxyphthioceranoic acid were also present in Figure 3a. These results indicate the presence of the minor isomers possessing the C46-, C37-, and C34-hydroxyphthioceranoic acid substituents. Further dissociation of the ion of 1524 (2459.1 → 1524.2; data not shown) yielded the ion pairs at m/z 957/915 and 999/873 arising from losses of and a C37/C40- and C34/C43-hydroxyphthioceranoic acids, together with the C37-, C40-, C34- and C43-hydroxyphthioceranoic anions at m/z 565, 607, 523, and 649. The results support the presence of [18:0, hC46, hC40, hC37]-SL and [18:0, hC46, hC43, hC34]-SL isomers. The presence of these minor isomers is consistent with the observation of the minor ion at m/z 1524 in Figure 3d, arising from consecutive losses of 18:0-fatty acid and C46-hydroxyphthioceranoic acid. The structural assignments were also supported by accurate mass measurements (Table 1).
Similarly, the MS2 spectrum of the ion at m/z 2543.2 (Figure 4a) contained the ions at m/z 2286 and 2258, arising from loss of a 16:0-, and 18:0-fatty acid residues, respectively. The ions at m/z 1934, 1906, 1892, and 1850 arise from losses of the C40-, C42-, C43-, and C46-hydroxyphthioceranoic residues at 3-position, respectively. The sequential losses of the 18:0-fatty acid at 2-position gave rise to ions at m/z 1650, 1622, 1608, and 1566, respectively; while the ion at m/z 1678 mainly arise from further loss of a 16:0-fatty acid residue from m/z 1934. The profile of the ion set of m/z 1934, 1906, 1892 and 1850 is similar to that of the ion set of m/z 1650, 1622, 1608, and 1566 (Figure 4a), consistent with the fragmentation mechanisms that first eliminate the 3-hydroxyphthioceranoic acid residue followed by loss of the 18:0-fatty acid at 2-position (or vice verse) as described earlier. The results indicate that the ion at m/z 2543 consists of the isomers in which the 3- and 2-position having hC40/18:0-, hC42/18:0-, hC43/18:0-, and hC46/18:0- and hC40/16:0-fatty acid substituents. The prominence of the these ion pairs (e.g., ions of m/z 1934 and 1650), again, indicate the preferential losses of the substituents at 2 and 3 over those located at 6 or 6', which have the greater stability towards acid or alkaline hydrolysis as compared with the substituents at 3- and 2-position (5).
Figure 4.
The LIT CID MS2 spectrum of the ion of m/z 2543 (a), the sequential MS3 spectra of the ions of m/z 1650 (2543 → 1650) (b), 1678 (2543 → 1678) (c), 1608 (2543 → 1608) (d), m/z 1636 (2543 → 1636) (e), and of 1622 (2543 → 1622) (f).
Further dissociation of the ion of m/z 1650 (2543 → 1650; Figure 4b) yielded the major ions at m/z 1041 and 999, together with m/z 957, arising from loss of C40-, C43-, and C46-hydroxyphthioceranoic acids, respectively. The results are consistent with the observation of the major C40-, and C46-hydroxyphthioceranoic acid anions at m/z 607 and 691, as well as the presence of the C43-hydroxyphthioceranoic acid anion at m/z 649. The spectrum also contained the 6'-C40-, 6'-C43-, and 6'-C46-hydroxyphthioceranoyl-glucose-2'-sulfate anions at m/z 831, 873, and 915, indicating that the ion at m/z 2543 consists of major [18:0, hC40, hC40, hC46]-SL and [18:0, hC40, hC46, hC40]-SL isomers together with minor isomer of [18:0, hC40, hC43, hC43]-SL..
MS3 on the ion of m/z 1678 (2543 → 1678; Figure 4c) yielded ions at m/z 1041 (loss of 636), 985 (loss of 692), 1069 (loss of 608), 957 (loss of 720), 999 (loss of 678), 1027 (loss of 650), consistent with the observation of the hydroxyphthioceranoic anions of m/z 635, 691, 607, 719, 677, and 649 that represent C42-, C46-, C40-, C48-, C45-, or C43-hydroxyphthioceranoic acid, respectively. The spectrum also contained the ions at m/z 859, 915, 831, 943, 901, and 873, indicating the presence of 6'-C42-, 6'-C46-, 6'-C40-, 6'-C48-, 6'-C45-, or 6'-C43-hydroxyphthioceranoyl-glucose-2'-sulfate anions as seen earlier. The observation of the abundant ion pairs at m/z 1041 (loss of hC42) and 985 (loss of hC46) signifies the presence of the major [16:0, hC40, hC42, hC46]-SL and [16:0, hC40, hC46, hC42]-SL isomers; while the 1069/957 pairs lead to the assignment of [16:0, hC40, hC40, hC48]-SL and [16:0, hC40, hC48, hC40]-SL isomers; and the minor 999/1027 ion pairs give assignment of isomers of [16:0, hC40, hC43, hC45]-SL and [16:0, hC40, hC45, hC43]-SL.
As described earlier, the ions at m/z 1566 and 1594 arose from the consecutive losses of the C46- and C44-hydroxyphthioceranoic acid, respectively, followed by loss of the 18:0-fatty acid residue at C-2. Further dissociation of the ion at m/z 1566 (2543 → 1566) yielded the spectrum identical to that shown in Figure 4c, indicating the presence of C40-hydroxyphthioceranoic acid residues at 6 and 6'. The results lead to the assignment of [18:0, hC46, hC40, hC40]-SL isomer, which is a positional isomer to [18:0, hC40, hC40, hC46]-SL and [18:0, hC40, hC46, hC40]-SL as seen earlier. The profile of the MS3 spectrum of the ion at m/z 1594 (2543 → 1594; not shown) is also similar to that shown in Figure 4f, and is dominated by the ions at m/z 985 (1594 – 608) and 957 (1594 – 636), indicating the presence of [18:0, hC44, hC40, hC42]-SL. Similarly, the MS3 spectrum of the ion at m/z 1608 (2543 → 1608; Figure 4d) is dominated by the ions at m/z 999 and 957, arising from losses of the C40-, and C43-hydroxyphthioceranoic acids, respectively; consistent with the observation of the C40-, and C43-hydroxyphthioceranoic anions at m/z 607 and 649. These results further support the presence of the [18:0, hC40, hC43, hC43]-SL isomer.
More minor isomeric structures were revealed by MS3 on the ions at m/z 1636 (2543 → 1636; Figure 4e), and at m/z 1622 (2543 → 1622; Figure 4f). The former spectrum contained the m/z 985/999 ion pair from loss of C43-/C42-hydroxyphthioceranoic acids, as well as the m/z 1027/957 ion pair from loss of the C40-/ C44-hydroxyphthioceranoic acids. These results give assignment of [18:0, hC41, hC43, hC42]-SL and [18:0, hC41, hC40, hC45]-SL isomers. The latter spectrum (Figure 4f) is dominated by m/z 985/985, and 957/1013 ion pairs, arising from losses of C42-/C42-hydroxyphthioceranoic acids and C43-/C41-hydroxyphthioceranoic acids, respectively, leading to assignment of isomers of [18:0, hC42, hC42, hC42]-SL and [18:0, hC42, hC43, hC41]-SL. Collectively, the ion of m/z 2543 consists of more than 12 isomers in which [18:0, hC40, hC40, hC46]-SL and [18:0, hC40, hC43, hC43]-SL predominate. This structural complexity in a single species of SLs was recently reported with less detail by Layre et al (21).
To reveal the shortest hydroxyphthioceranoic chain that constitutes the SL-II molecules, MSn was conducted on the low mass end ion of m/z 2122.8. The MS2 spectrum (see supplemental data; Figure S2a) is dominated by the ion at m/z 1640, arising from loss of C31-hydroxyphthioceranoic acid, together with ions at m/z 1356 from further loss of 18:0-fatty acid, indicating the presence of 18:0-, and hC31-acids at 2- and 3-position. Further dissociation of the ion of m/z 1356 (2122.8 → 1356; Figure S2b) gave rise to 957/747 and 873/831 ion pairs by further elimination of C25-/C40-hydroxyphthioceranoic and C31-/C34-hydroxyphthioceranoic acids, respectively. These results led to the assignment of [18:0, hC31, hC25, hC40]-SL and [18:0, hC31, hC31, hC34]-SL structures. The loss of the hC25/hC40-acids is consistent with the observation of the hC25/hC40 anion at m/z 397 and 607 (Figure S2b). The ion at m/z 397 corresponds to a 2,4,6-Trimethyl-7-hydroxydodecanoric acid anion possessing 2 repeating isopropyl (-CH2CH(CH3)-) unit. This short-chain hydroxyphthioceranoic acid chain was recently reported by Layre et al (21) but not previously reported by Goren (4). The structural assignment is consistent with the observation of the ion pairs of m/z 1724/1514 and 1640/1598 in Figure S2a, arising from losses of hC25/hC40 (398/608), and hC31/hC34 (482/524). The observation of the ion of m/z 1384 from the consecutive losses of C31-hydroxyphthioceranoic acid and 16:0-fatty acid (Figure S2b), together with the ion pairs of m/z 859/873, 775/957, and 747/985 (2122.8 → 1384; Figure S2c) arising from loss of hC34/hC33, hC40/hC27, and hC42/hC25, respectively, readily give assignments of isomers of [16:0, hC31, hC33, hC34]-SL, [16:0, hC31, hC27, hC40]-SL, and [16:0, hC31, hC25, hC42]-SL.
For further insight into the long hydroxyphthioceranoic chain that constitutes SL-II, the high mass end ion of m/z 2795.5 was subjected to CID. Again, the MS2 spectrum of m/z 2795.5 (Figure S3a) contained the homologous ions at m/z 2102.8 and 2060.7, arising from losses of C46- and C49-hydroxyphthioceranoic acids, respectively, together with the ions at m/z 1776 and 1818 arising from further loss of 18:0-fatty acid. MS3 on the ion of m/z 1776 (2795.5 → 1776; Figure S3b) gave rise to the ion-pair at m/z 1041/1083 by loss of C49-/C46-hydroxyphthioceranoic acid, consistent with the observation of the C49- and C46-hydroxyphthioceranoic acid anions at m/z 733 and 691. The spectrum also contained the 6'-C49-, or 6'-C46-hydroxyphthioceranoyl-glucose-2'-sulfate anions at m/z 957 and 915, indicating that the ion represents the major [18:0, hC49, hC49, hC46]-SL and [18:0, hC49, hC46, hC49]-SL isomers. The MS3 spectrum of the ion of m/z 1818 (2795.5 → 1818; Figure S3c) is dominated by the ion-pair of m/z 1083/1083 arising from elimination of 6-C49/6’-C49-hydroxyphthioceranoic acid, indicating the presence of [18:0, hC46, hC49, hC49]-SL isomer. The spectrum (Figure S3c) also contained the 1125/1041 ion-pair arising from further losses of C46/C52-hydroxyphthioceranoic acid, indicating the presence of the minor [18:0, hC46, hC46, hC52]-SL isomer. The presence of this minor isomer is consistent with the observation of the ion at m/z 775 and 691 (Figure S3c). The ion of m/z 775 represents a 2,4,6,8,10,12,14,16,18,20,22,24-Dodecamethyl-22-hydroxytetracontanoic anion possessing 11 isopropyl repeat units, which is 1 isopropyl units longer than the that previously reported by Goren (4).
Characterization of the multiple methyl-branched hydroxyphthioceranoic acid
Upon subjected to ESI with skimmer CID (100 V), the SL extract yielded a whole array of anions in the mass range from m/z 383 to 775, corresponding to the [M – H]- ions of hydroxyphthioceranoic and of phthioceranoic acids (Table 2). Elemental compositions deduced from high resolution mass measurements confirmed the presence of the major hydroxyphthioceranoic acid ion series at m/z 383, 397, ...., 607, ..., and 775, together with the minor phthioceranoic acid ion series at m/z 409, 423, .., 591, ... and 703 (Table 2). The summed % of the ion abundance (Σ %) of the hydroxyphthioceranoic acid ion series is 88%; and that of the phthioceranoic acid ion series is 12%. These data gave the molar ratio of SL-II to SL-I close to 2/1, based on that SL-II consists of 3 hydroxyphthioceranoic acid residues and SL-I consists of 2 hydroxyphthioceranoic acid and one phthioceranoic acid residues, and both the hydroxyphthioceranoic and phthioceranoic acid residues were cleaved by skimmer CID in the same manner. These results further support the notion that the principal sulfolipid from H37Rv is SL-II; and SL-I is the minor component (Figure 2 and Table S1). The ion at m/z 607 is predominant (100%; Table 3), consistent with the earlier report that the major hydroxyphthioceranoic acid in SLs is a 2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoic acid (4). The characterization of the structure of hydroxydotriacontanoic acids using MSn and high-resolution mass spectrometry is described below.
Table 2.
High-resolution ESI mass spectrum of the [M - H]- ions of hydroxyphthioceranoic (*) and phthioceranoic (#) acids generated by skimmer CAD on the SLs.
| measured mass (Da) | calculated mass (Da) | Elmental composition | Relative intensity (%) | structure (class) |
|---|---|---|---|---|
| 383.3528 | 383.3531 | C24 H47 O3 | 1.33 | * |
| 397.3685 | 397.3687 | C25 H49 O3 | 0.66 | * |
| 409.4048 | 409.4051 | C27 H53 O2 | 1.88 | # |
| 411.3842 | 411.3844 | C26 H51 O3 | 0.20 | * |
| 423.4205 | 423.4208 | C28 H55 O2 | 3.08 | # |
| 425.3997 | 425.4000 | C27 H53 O3 | 1.59 | * |
| 437.4362 | 437.4364 | C29 H57 O2 | 4.29 | # |
| 439.4154 | 439.4157 | C28 H55 O3 | 0.12 | * |
| 451.4517 | 451.4521 | C30 H59 O2 | 2.74 | # |
| 453.4311 | 453.4513 | C29 H57 O3 | 0.02 | * |
| 465.4674 | 465.4677 | C31 H61 O2 | 0.83 | # |
| 467.4467 | 467.4470 | C30 H59 O3 | 0.22 | * |
| 479.4831 | 479.4834 | C32 H63 O2 | 4.68 | # |
| 481.4623 | 481.4626 | C31 H61 O3 | 1.95 | * |
| 493.4987 | 493.4990 | C33 H65 O2 | 1.91 | # |
| 495.4780 | 495.4783 | C32 H63 O3 | 0.69 | * |
| 507.5145 | 507.5147 | C34 H67 O2 | 2.87 | # |
| 509.4938 | 509.4939 | C33 H65 O3 | 4.19 | * |
| 521.5301 | 521.5303 | C35 H69 O2 | 0.74 | # |
| 523.5094 | 523.5096 | C34 H67 O3 | 9.64 | * |
| 535.5458 | 535.5460 | C36 H71 O2 | 1.25 | # |
| 537.5250 | 537.5252 | C35 H69 O3 | 1.04 | * |
| 549.5613 | 549.5616 | C37 H73 O2 | 2.04 | # |
| 551.5407 | 551.5409 | C36 H71 O3 | 2.22 | * |
| 563.5767 | 563.5773 | C38 H75 O2 | 0.25 | # |
| 565.5562 | 565.5565 | C37 H73 O3 | 9.00 | * |
| 577.5926 | 577.5929 | C39 H77 O2 | 0.82 | # |
| 579.5719 | 579.5722 | C38 H75 O3 | 0.64 | * |
| 591.6082 | 591.6086 | C40 H79 O2 | 4.69 | # |
| 593.5875 | 593.5878 | C39 H77 O3 | 3.32 | * |
| 605.6239 | 605.6242 | C41 H81 O2 | 0.86 | # |
| 607.6031 | 607.6034 | C40 H79 O3 | 100.00 | * |
| 619.6394 | 619.6399 | C42 H83 O2 | 2.64 | # |
| 621.6187 | 621.6191 | C41 H81 O3 | 13.72 | * |
| 633.6551 | 633.6555 | C43 H85 O2 | 2.65 | # |
| 635.6343 | 635.6348 | C42 H83 O3 | 40.78 | * |
| 647.6707 | 647.6712 | C44 H87 O2 | 1.18 | # |
| 649.6499 | 649.6504 | C43 H85 O3 | 36.36 | * |
| 661.6863 | 661.6868 | C45 H89 O2 | 2.53 | # |
| 663.6655 | 663.6661 | C44 H87 O3 | 6.07 | * |
| 675.7019 | 675.7026 | C46 H91 O2 | 1.01 | # |
| 677.6811 | 677.6817 | C45 H89 O3 | 12.44 | * |
| 689.7172 | 689.7181 | C47 H93 O2 | 0.22 | # |
| 691.6968 | 691.6974 | C46 H91 O3 | 44.16 | * |
| 703.7331 | 703.7338 | C48 H95 O2 | 0.48 | # |
| 705.7124 | 705.7130 | C47 H93 O3 | 5.18 | * |
| 719.7280 | 719.7287 | C48 H95 O3 | 8.40 | * |
| 731.7641 | 731.7651 | C50 H99 O2 | 0.01 | # |
| 733.7437 | 733.7443 | C49 H97 O3 | 11.24 | * |
| 745.7802 | 745.7807 | C51 H101 O2 | 0.03 | # |
| 747.7592 | 747.7600 | C50 H99 O3 | 1.23 | * |
| 759.7960 | 759.7964 | C52 H103 O2 | 0.03 | # |
| 761.7749 | 761.7756 | C51 H101 O3 | 0.60 | * |
| 775.7905 | 775.7913 | C52 H103 O3 | 0.68 | * |
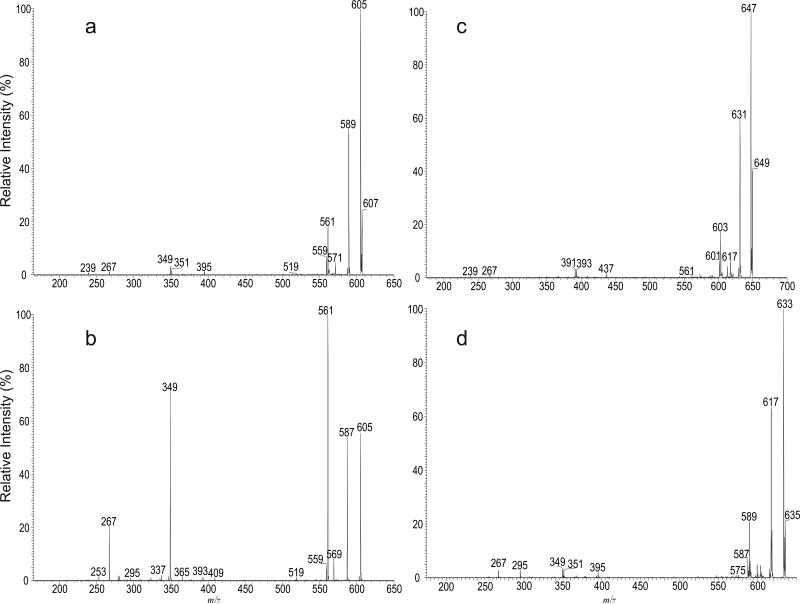
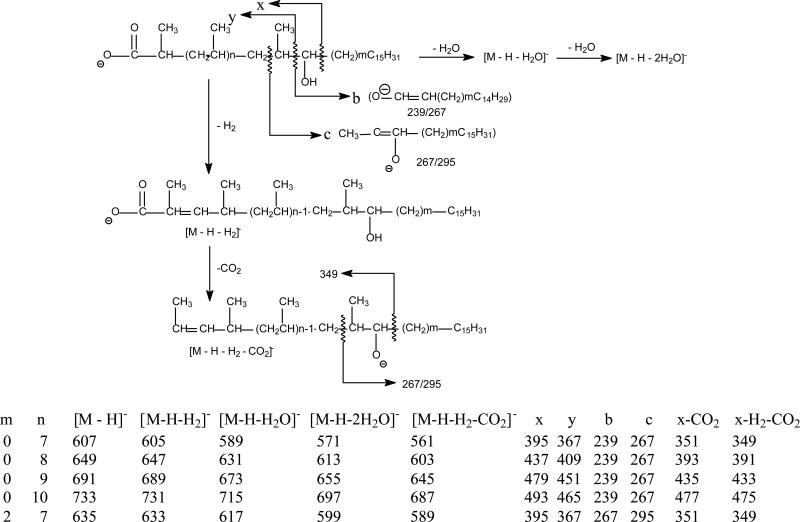
The MS2 spectrum of the ion of m/z 607 generated by skimmer CID on the sulfolipid mixture (Figure 5a) is identical to the MS3 spectrum of the ion of m/z 607 (2459 → 607; not shown) arising from dissociation of m/z 2459 (in this acquisition, the Q value of the ion-trap for MS2 experiment was set to 0.2 to trap m/z 607 ion for further dissociation using MS3). The spectrum (Figure 5a) contained the ion at m/z 395, arising from elimination of the C15H32 residue by cleavage of the C-C bond next to the hydroxyl group distal to the carboxylate terminal, together with the ion at m/z 367 by loss of a C15H31CHO residue via cleavage of the C-C bond flank to the hydroxyl group vicinity to the carboxylate group. The cleavage of this latter bond is consistent with the observation of the ion of m/z 239, representing the deprotonated ion of C15H31CHO (i.e., C14H29CH=CH-O- ion) (Scheme 2). The spectrum also contained the ion at m/z 267, arising from cleavage of the C-C bond bearing the methyl branch foremost distal to the carboxylate terminal. The above structural information suggests the presence of a hydroxyl group at C-17. The predominant ions at m/z 605, 589 arose from losses of H2, and of H2O, respectively; while the ion at m/z 561 arose from further loss of CO2 from m/z 605 and gave rise to the ion of m/z 349 by further elimination of a C15H32 residue. This fragmentation process is further supported by the MS4 spectrum of the ions of m/z 605 (Figure 5b) and 561 (data not shown). The ion at m/z 351 (607 - C15H32 - CO2) may arise from primary loss of a C15H32 residue followed by further losses of CO2 (Scheme 2). The assignments of the fragment ions were further supported by the elemental compositions deduced from high-resolution mass measurements (data not shown). The results are consistent with structural assignment of 2,4,6,8,10,12,14,16-Octamethyl-17-hydroxydotriacontanoic acid previously defined by Goren et al (4).
Figure 5.
The CID MS2 spectrum of the [M – H]- ion of C40-hydroxyphthioceranoic acid at m/z 607 generated by skimmer CID (a), its MS3 spectrum at m/z 605 (607 → 605) (b), and the MS3 spectra of the C43-hydroxyphthioceranoic ion at m/z 649 (2459 → 649) (c), and of the C42-hydroxyphthioceranoic ion at m/z 635 (2543 → 635) (d).
Scheme 2.
The fragment ions observed in the MSn spectra of the major hydroxyphthioceranoic acid substituents in SL-II and the proposed fragmentation pathways leading to the ion formation and structure identification. The structures of the indicated fragment ions are supported by high-resolution LIT MSn experiments (data not shown).
The MS2 spectrum of m/z 649 (also generated from skimmer CID) and MS3 spectrum of the ion at m/z 649 (2459 → 649; Figure 5c) are identical and contained the ions at m/z 437, 391, and 393, which are 42 Da (C3H6) heavier than the analogous ions at m/z 395, 349 and 351 seen in Figure 5a, indicating the presence of an additional isopropyl group along the fatty acyl chain. The spectrum also contained the ions at m/z 239 and 267, indicating the presence of the same C15H31 terminal group, together with the ions at m/z 647 (649 –H2), 631 (649 – H2O), and 603 (647 –CO2) that are analogous to ions at m/z 605, 589, 561 as seen in Figure 5a. The results indicate that the ion at m/z 649 represents a deprotonated 2,4,6,8,10,12,14,16,18-Nonamethyl-19-hydroxytetratriacontanoic acid anion. Similar mass shifts of the analogous ions were also seen in the MS3 spectra of the ion of m/z 691 (2543 → 691) and of m/z 733 (2795 → 733) (Scheme 2), suggesting the presence of the structures of 2,4,6,8,10,12,14,16,18, 20-Decamethyl-21-hydroxyhexatriacontanoic acid and 2,4,6,8,10,12,14,16,18, 20, 22-Undecamethyl-23-hydroxyoctatriacontanoic acid, respectively. The observation of the ion series of m/z 397, 439, 481, 523, 565, 607, 649, 691, 735, and 775 (Table 2) is in agreement with the suggested structures of -OOC-CH(CH3)-(CH2CHCH3-)nCH(OH)C15H31 (n = 2-11) for hydroxyphthioceranoic acids. The presence of the C25-hydroxyphthioceranoic acid at m/z 397 (n=2), and the C52-hydroxyphthioceranoic acid at m/z 775 (n=11) are consistent with the observation of the these two ions in the earlier structural assignment for the SL-II species of m/z 2122 (Figure S2) and 2795 (Figure S3), respectively.
Again, MS2 and MS3 on the ion of m/z 635 (2543 → 635; Figure 5d) yielded ions analogous to those seen in Figure 5a-5c. The spectrum contained the ions at m/z 395, 351 and 349, identical to those seen in Figure 5a. However, the ions at m/z 267 and 295 are 28 Da heavier than the analogous ions at m/z 239 and 267 (Scheme 2) seen in Figure 5a. The results indicate the presence of the structure of 2,4,6,8,10,12,14,16-Octamethyl-17-hydroxyditriacontanoic acid, in which a terminal C17H35 rather than a C15H31 residue was attached to the same carbon that possesses the hydroxyl group. The homologous ions were seen at m/z 425, 467, 509, 551, 593, 635, 677, and 719, representing the ion series possessing the -OOC-CH(CH3)-(CH2CHCH3-)nCH(OH)C17H35 structure, in which the homologous hydroxyphthioceranoic acids (n= 3-9) differed by a repeat C3 (isopropyl) unit are present, in agreement with the previous findings (4).
Conclusions
Using high-resolution LIT MSn, we made assignment of the complex structures of sulfolipids of M. tuberculosis H37Rv and confirm that SL-II not SL-I predominates the sulfolipid family, similar to the recent report by Layre et al who used a different approach (21). Thus, care should be taken when referring to the molecular structures of the major SL families as not to mis-assign the structures, if Goren's original designations of the SL families are to be followed. We verified that differences in culture media or extraction methods would not account for differences in the relative abundance of SL-I and SL-II. In all culture conditions tested, SL-II is always more abundant than SL-I; and extraction with decylamine in hexane was more efficient for extracting the SL families. We also revealed the detailed structures of each of the SL-II species, of which many isomers arising not only from the presence of the stearoyl or palmitoyl at 2-position, but also from the various combinations of different chain lengths of the hydroxyphthioceranoic acid substituents located at 6- and 6’-position of the trehalose backbone. Thus, hundreds of structures are present for the entire SL-II family. This immense structure diversity has not been reported previously; and was seen in other important lipid families such as mycolic acids, phosphatidylinositol mannosides and trehalose dimycolates in our recent studies (22-24).
Supplementary Material
Acknowledgments
We thank Dr. Weidong Cui of Chemistry Department, Washington University for obtaining the FT-ICR mass spectrum. A SL standard was received as part of NIH, NIAID Contract No. HHSN266200400091C, entitled “Tuberculosis Vaccine Testing and Research Materials,” which was awarded to Colorado State University.
Funding information: United States Public Health Service Grants: P41-RR00954 (J.T.); P60-DK20579 (J.T.); P30-DK56341 (J.T.).
Abbreviations
- CID
collision induced dissociation
- ESI
electrospray ionization
- MS
mass spectrometry
- MSn
multiple-stage tandem mass spectrometry
- LIT
linear ion-trap
- FT
Fourier transform
Footnotes
Supporting Information Available
This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Middlebrook G, Coleman CM, Schaefer WB. Sulfolipid from Virulent Tubercle bacilli. Proc. Natl. Acad. Sci. USA. 1959;45:1801–1804. doi: 10.1073/pnas.45.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goren MB. Sulfolipid I of Mycobacterium tuberculosis, strain H37Rv. I. Purification and properties. Biochim Biophys Acta. 1970;210:116–126. doi: 10.1016/0005-2760(70)90067-6. [DOI] [PubMed] [Google Scholar]
- 3.Goren MB. Sulfolipid I of Mycobacterium tuberculosis, strain H37Rv. II. Structural studies. Biochim Biophys Acta. 1970;210:127–138. doi: 10.1016/0005-2760(70)90068-8. [DOI] [PubMed] [Google Scholar]
- 4.Goren MB, Brokl O, Das BC. Sulfatides of Mycobacterium tuberculosis: the structure of the principal sulfatide (SL-I). Biochemistry. 1976;15:2728–2735. doi: 10.1021/bi00658a003. [DOI] [PubMed] [Google Scholar]
- 5.Goren MB, Brokl O, Das BC, Lederer E. Sulfolipid I of Mycobacterium tuberculosis. Strain H37RV. Nature of the Acyl Substituents. Biochemistry. 1971;10:72–81. doi: 10.1021/bi00777a012. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto Y, Fujita Y, Naka T, Hirai M, Tomiyasu I, Yano I. Mycobacterial sulfolipid shows a virulence by inhibiting cord factor induced granuloma formation and TNF-alpha release. Microb Pathog. 2006;40:245–253. doi: 10.1016/j.micpath.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Kato M, Goren MB. Synergistic action of cord factor and mycobacterial sulfatides on mitochondria. Infect. Immun. 1974;10:733–741. doi: 10.1128/iai.10.4.733-741.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goren MB, D'Arcy Hart P, Young MR, Armstrong JA. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976;73:2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, English D, Andersen BR. Activation of human neutrophils by Mycobacterium tuberculosis-derived sulfolipid I. J. Immunol. 1991;146:2730–2736. [PubMed] [Google Scholar]
- 10.Zhang L, Goren MB, Holzer TJ, Andersen BR. Effect of Mycobacterium tuberculosis-derived sulfolipid I on human phagocytic cells. Infect. Immun. 1988;56:2876–2883. doi: 10.1128/iai.56.11.2876-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilleron M, Stenger S, Mazorra Z, Wittke F, Mariotti S, Böhmer G, Prandi J, Mori L, Puzo G, De Libero G. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J. Exp. Med. 2004;199:649–659. doi: 10.1084/jem.20031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mougous JD, Leavell MD, Senaratne RH, Leigh CD, Williams SJ, Riley LW, Leary JA, Bertozzi CR. Discovery of sulfated metabolites in mycobacteria with a genetic and mass spectrometric approach. Proc. Natl. Acad. Sci. U. S. A. 2002;99:17037–17042. doi: 10.1073/pnas.252514899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan PJ, Goren MB. Mycobacterial glycolipid as bacterial antigens. Biochem. Soc. Trans. 1977;5:1687–1693. doi: 10.1042/bst0051687. [DOI] [PubMed] [Google Scholar]
- 14.Converse SE, Mougous JD, Leavell MD, Leary JA, Bertozzi CR, Cox JS. MmpL8 is required for sulfolipid-1 biosynthesis and Mycobacterium tuberculosis virulence. Proc. Natl. Acad. Sci. USA. 2003;100:6121–6126. doi: 10.1073/pnas.1030024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domenech P, Reed MB, Dowd CS, Manca C, Kaplan G, Barry CE., III The Role of MmpL8 in Sulfatide Biogenesis and Virulence of Mycobacterium tuberculosis. J. Biol. Chem. 2004;279:21257–21265. doi: 10.1074/jbc.M400324200. [DOI] [PubMed] [Google Scholar]
- 16.Mougous JD, Petzold CJ, Senaratne RH, Lee DH, Akey DL, Lin FL, Munchel SE, Pratt MR, Riley LW, Leary JA, Berger JM, Bertozzi CR. Identification, function and structure of the mycobacterial sulfotransferase that initiates sulfolipid-1 biosynthesis. Nat. Struct. Mol. Biol. 2004;11:721–729. doi: 10.1038/nsmb802. [DOI] [PubMed] [Google Scholar]
- 17.Sirakova TD, Thirumala AK, Dubey VS, Sprecher H, Kolattukudy PE. The Mycobacterium tuberculosis pks2 Gene Encodes the Synthase for the Hepta- and Octamethyl-branched Fatty Acids Required for Sulfolipid Synthesis. J. Biol. Chem. 2001;276:16833–16839. doi: 10.1074/jbc.M011468200. [DOI] [PubMed] [Google Scholar]
- 18.Mougous JD, Senaratne RH, Petzold CJ, Jain M, Lee DH, Schelle MW, Leavell MD, Cox JS, Leary JA, Riley LW, Bertozzi CR. A sulfated metabolite produced by stf3 negatively regulates the virulence of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U S A. 2006;103:4258–4263. doi: 10.1073/pnas.0510861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar P, Schelle MW, Jain M, Lin FL, Petzold CJ, Leavell MD, Leary JA, Cox JS, Bertozzi CR. PapA1 and PapA2 are acyltransferases essential for the biosynthesis of the Mycobacterium tuberculosis virulence factor sulfolipid-1. Proc. Natl. Acad. Sci. USA. 2007;104:11221–11226. doi: 10.1073/pnas.0611649104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marjanovic O, Iavarone AT, Riley LW. Sulfolipid accumulation in Mycobacterium tuberculosis disrupted in the mce2 operon. J. Microbiol. 2011;49:441–447. doi: 10.1007/s12275-011-0435-4. [DOI] [PubMed] [Google Scholar]
- 21.Layre E, Cala-De Paepe D, Larrouy-Maumus G, Vaubourgeix J, Mundayoor S, Lindner B, Puzo G, Gilleron M. Deciphering sulfoglycolipids of Mycobacterium tuberculosis. J. Lipid Res. 2011;52:1098–1110. doi: 10.1194/jlr.M013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu FF, Soehl K, Turk J, Haas A. Characterization of mycolic acids from the pathogen Rhodococcus equi by tandem mass spectrometry with electrospray ionization. Anal. Biochem. 2011;409:112–122. doi: 10.1016/j.ab.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu FF, Turk J, Owens RM, Rhoades ER, Russell DG. Structural Characterization of Phosphatidyl-myo-inositol Mannosides from Mycobacterium bovis Bacillus Calmette Guerin by Multiple-Stage Quadrupole Ion-Trap Mass Spectrometry with Electrospray Ionization. I. PIMs and Lyso-PIMs. J. Am. Soc. Mass Spectrom. 2007;18:466–478. doi: 10.1016/j.jasms.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu FF, Turk J, Owens RM, Rhoades ER, Russell DG. Structural Characterization of Phosphatidyl-myo-Inositol Mannosides from Mycobacterium bovis Bacillus Calmette Guerin by Multiple-Stage Quadrupole Ion-Trap Mass Spectrometry with Electrospray Ionization. II. Monoacyl- and Diacyl-PIMs. J. Am. Soc. Mass Spectrom. 2007;18:479–492. doi: 10.1016/j.jasms.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.