SUMMARY
Recruitment of the K63-linkage specific deubiquitinating enzyme AMSH is an important step in ESCRT-dependent membrane protein sorting. In this issue of Structure, Solomons et al. now reveal an extraordinarily high affinity complex between “MIM4” region of one ESCRT-III subunit, CHMP3, and the MIT domain of AMSH.
There are times when a cell must decisively get rid of some receptor, transporter, or other plasma membrane proteins. The major means of disposal involves the ubiquitination of these proteins and their sorting into the multivesicular body (MVB) pathway (Hurley and Stenmark, 2011). MVBs are endosomes that contain intralumenal vesicles (ILVs). MVBs fuse with lysosomes leading to the hydrolysis of their contents. Ubiquitination is the main signal that directs membrane proteins into MVBs (Hurley and Stenmark, 2011; Shields and Piper, 2011). Often, ubiquitination is carried out by members of the Nedd4 family of ubiquitin ligases (Hurley and Stenmark, 2011). The ubiquitin (Ub) modifications most commonly occur in the form of short K63-linked chains (Hurley and Stenmark, 2011; Shields and Piper, 2011), though this type of linkage is not always obligate.
The Ub signal is first detected by the endosomal sorting complex required for transport (ESCRT)-0 complex, which contains five Ub binding sites and thus binds avidly to polyUb chains. ESCRT-0 is the most upstream of the ESCRT complexes, which are conserved from yeast to humans, and are involved in cell processes from receptor sorting to cytokinesis to viral budding (Hurley and Hanson, 2010). Inward budding of the endosomal membrane is the job of ESCRT-I and –II (Hurley and Hanson, 2010). ESCRT-I and –II also bind to Ub, although they contain fewer binding sites than ESCRT-0. ESCRT-II recruits and activates the ESCRT-III complex, which is responsible for cleavage of membrane buds into ILVs (Hurley and Hanson, 2010). Unlike the upstream complexes, ESCRT-III does not bind to Ub. Lastly, the Vps4-Vta1 complex, a dodecameric AAA ATPase, binds to ESCRT-III and disassembles it for reuse.
Deubiquitinating enzymes (DUBs) join the ligases and the Ub receptors as a third pillar of the system (Komander et al., 2009). One role of DUBs is to recycle Ub and so maintain free Ub pools. Different DUBs interact with both upstream and downstream ESCRT components, suggesting their roles go deeper than pool replenishment. Much evidence favors the idea that DUBs have such deeper roles, yet no individual study has proved this decisively (Hurley and Stenmark, 2011; Shields and Piper, 2011). Indeed, the putative DUB requirement can be overridden by fusing a single non-cleavable and non-extendable Ub moiety, at least in yeast (Shields and Piper, 2011).
In human cells, the DUBs ubiquitin specific protease 8 (USP8) and Associated molecule with the SH3 domain of STAM (AMSH) are targeted by their Pro-rich regions to the SH3 domain of the STAM subunit of ESCRT-0 (Hurley and Stenmark, 2011; Komander et al., 2009). USP8 and AMSH action might be important for disengaging avidly bound K63-linked Ub chains from ESCRT-0 in order for cargo to be handed off to ESCRT-I and –II. AMSH is a Zn2+ -dependent DUB (Hurley and Stenmark, 2011; Komander et al., 2009), whose exquisite specificity for K63-linked Ub chains is hardwired into its structure (Sato et al., 2008). Both AMSH and USP8 contain N-terminal MIT domains, which target them to ESCRT-III (Hurley and Stenmark, 2011; Komander et al., 2009). Thus these DUBs act at multiple points in the ESCRT pathway. In addition to releasing free Ub at the last possible moment before cargo is committed to the lysosome hydrolysis, DUB action at this late stage might disengage cargo from ESCRT-I and –II. Indeed, we still don’t know what is the driving force for cargo migration from the ESCRT-0 coat to the ESCRT-I-II assembly, and finally into the forming bud. It is tempting to speculate that DUBs could be involved.
The potential for DUBs to give directionality to cargo sorting highlights the importance of the timing of their recruitment. This brings us to MIT domains. Their best-characterized function is to bind to the C-termini of ESCRT-III subunits. These C-terminal tails are sequestered in soluble ESCRT-III monomers, and are exposed upon the polymerization of ESCRT-III into a complex. Both subunits of the Vps4-Vta1 disassembly machine contain MIT domains, which are their main points of contact with their ESCRT-III substrate. Canonical MIT domains are three-helix bundles. Vps4 binds to a C-terminal helical segment, now termed “MIM1” for MIT-interacting motif 1, found in the ESCRT-III subunits, CHMP1, 2, and 3. The MIM1 binds with 10s of μM affinity to the groove between the 2nd and 3rd α-helices of the MIT domain (Obita et al., 2007; Stuchell-Brereton et al., 2007). This interaction relies on hydrophobic interactions with exposed Leu residues along a spine of the MIM1, as well as charged interactions with MIM1 Arg residues. To complicate matters, the Vps4 MIT contains a binding site between its 1st and 3rd helices for the C-termini of CHMP4 (weak) and CHMP6, which binds in a β-conformation (Kieffer et al., 2008). Moreover, the microtubule severing enzyme spastin, which coordinates microtubule and membrane severing in cytokinesis, binds to an extended MIM1, but uses the α1/α3 groove to do so instead of the canonical α2/α3 groove (Yang et al., 2008). Spastin MIT has a tighter specificity than Vps4 in that it only binds CHMP1B and IST1, another ESCRT-III subunit. The restriction is achieved with additional steric constraints requiring small residues N-terminal to the canonical Leu residues of MIM1. Several other MIT structures have been determined, including that of USP8, but in the absence of complexation with CHMPs, they have been less informative.
The article in this issue by Weissenhorn and colleagues (Solomons et al., 2011) is an important addition to the field, as the first for a DUB in complex with a CHMP, and the highest affinity MIT-CHMP complex solved to date. Consisting of five helices, AMSH-MIT is bigger than the canonical MIT domains of Vps4 and spastin, but to the N-terminal domain of USP8. Helices α2, α3, and the N-terminal part of α5 correspond to helices 1–3 of the canonical MIT domains. The CHMP3 extended MIM1 (or “MIM4”) binds to the counterpart of the MIM1 binding site of Vps4. The affinity of AMSH-MIT for CHMP3 is extraordinarily high, in the tens of nM. Additional hydrogen bonds with two N-terminal Glu residues, unique to CHMP3, seem to contribute to the higher affinity. Various reports in the field have consistently found a strong interaction between CHMP3 and AMSH, but have diverged with respect to interactions with other CHMPs. This study confirms the reports that CHMP1 also binds to AMSH-MIT, but with a far lower affinity, explaining the less consistent findings on CHMP1 binding in previous studies.
The MIT-MIM recognition code now appears more complex than ever and Table 2 in the study by Solomons et al. provides a nice summary of the current stage of knowledge. These are details that have been discovered one MIT at a time, by careful structural analysis. The MIT family is so divergent in sequence and recognition space that it has so far not been possible to make useful predictions about specificity from homology modeling. At least one more set of MIT:CHMP complexes, for the Vta1 subunit of the disassembly complex, is urgently needed to complete a basic outline of the code. With such a code in hand, the next goal will be to have a more refined understanding the order of assembly and action of ESCRT-III subunits. Based on data coming mainly from yeast, it is currently thought that assembly occurs in the order CHMP6>CHMP4>CHMP3>CHMP2>CHMP1=CHMP5=IST1. This scheme is admittedly an oversimplification of the complex picture in mammalian cells. In vitro studies of MVB biogenesis by yeast ESCRTs suggest membrane scission could occur as early as the end of the CHMP3 stage. On the other hand, mammalian live cell imaging studies suggest scission might occur later that this in HIV budding and cytokinesis. As a rough outline though, it suggest that AMSH begins to be recruited before the assembly of ESCRT-III is complete, and certainly prior to membrane scission. This model fits well with the concept that AMSH could have a role in disengaging cargo from ESCRT-I and –II so as to facilitate its release into ILVs.
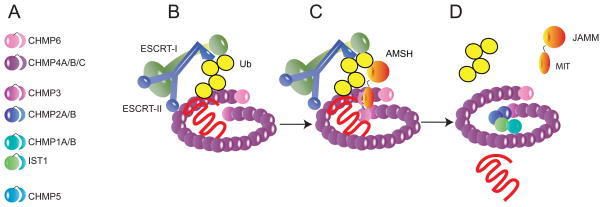
Figure 1.
A speculative model for the role of the AMSH-CHMP interaction in ubiquitinated cargo sorting. A. Key to human ESCRT-III subunits. Subunits are shown according to the order of assembly demonstrated for their yeast orthologs. The precise order of assembly in human cells is not known. B. At an early stage in ESCRT-III assembly, ESCRT-II is shown promoting the polymerization of CHMP6, followed by CHMP4A/B/C. At this stage, ubiquitinated cargo (here, a heptahelical receptor, in red) might be trapped at the neck of the nascent bud by interactions between the attached K63-Ub chain and the Ub binding domains of ESCRT-I and -II. C. CHMP3 is thought to be the third subunit to assemble into the complex, by analogy with its yeast ortholog. CHMP3 binding to the membrane and to other subunits releases its C-terminal MIM (shown as a crescent shape). This binds to the MIT domain of AMSH and recruits it to the growing assembly. It is possible (though not shown here) that additional molecules of AMSH could be recruited by CHMP1 (or even by CHMP1 instead of CHMP3) using a lower affinity site on the MIT domain, or that CHMP3 and CHMP1 could cooperate in recruiting AMSH. D. The catalytic JAMM domain of AMSH cleaves the K63-Ub chain and allows the cargo protein to disengage from ESCRT-I and -II and be released into the nascent bud. ESCRT-I and -II are free to disassociate at this stage. With cargo now removed from the bud neck, ESCRT-III assembly can finish and close the neck of the bud, leading to membrane scission and ILV release into the lumen of the MVB.
Acknowledgments
Research in the Hurley laboratory in supported by the Intramural Program of the NIH, NIDDK and the Intramural AIDS Targeted Anti-viral Program of the Office of the Director.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Hurley JH, Hanson PI. Nat Rev Mol Cell Biol. 2010;11:556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH, Stenmark H. Ann Rev Biophys. 2011;40:119–142. doi: 10.1146/annurev-biophys-042910-155404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer C, Skalicky JJ, Morita E, De Domenico I, Ward DM, Kaplan J, Sundquist WI. Dev Cell. 2008;15:62–73. doi: 10.1016/j.devcel.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Komander D, Clague MJ, Urbe S. Nat Rev Mol Cell Biol. 2009;10:550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- Obita T, Saksena S, Ghazi-Tabatabai S, Gill DJ, Perisic O, Emr SD, Williams RL. Nature. 2007;449:735–739. doi: 10.1038/nature06171. [DOI] [PubMed] [Google Scholar]
- Sato Y, Yoshikawa A, Yamagata A, Mimura H, Yamashita M, Ookata K, Nureki O, Iwai K, Komada M, Fukai S. Nature. 2008;455:358–U319. doi: 10.1038/nature07254. [DOI] [PubMed] [Google Scholar]
- Shields SB, Piper RC. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01242.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomons J, Sabin C, Poudevigne E, Usami Y, Hulsik DL, Macheboeuf P, Hartlieb B, Gottlinger H, Weissenhorn W. Structure. 2011 doi: 10.1016/j.str.2011.05.011. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchell-Brereton M, Skalicky J, Kieffer C, Karren MA, Ghaffarian S, Sundquist WI. ESCRT-III recognition by VPS4 ATPases. Nature. 2007;449:740–744. doi: 10.1038/nature06172. [DOI] [PubMed] [Google Scholar]
- Yang D, Rismanchi N, Renvoisé B, Lippincott-Schwartz J, Blackstone C, Hurley JH. Nat Struct Mol Biol. 2008;15:1278–1286. doi: 10.1038/nsmb.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]