Abstract
Alcohol is the most frequently abused substance in the world. Both acute and chronic alcohol consumption have diverse and well documented effects on the human immune system, leading to increased susceptibility to infections like bacterial pneumonia. S. pneumoniae is the most common bacterial etiology of community acquired pneumonia world-wide. The frequency and severity of pneumococcal infections in individuals with a history of alcohol abuse is much higher than the general population. Despite this obvious epidemiological relevance, very few experimental studies have focused on the interaction of pneumococci with the immune system of a host acutely or chronically exposed to alcohol. Understanding these host-pathogen interactions is imperative for designing effective prophylactic and therapeutic interventions for such populations. Recent advances in pneumococcal research have greatly improved our understanding of pneumococcal pathogenesis and virulence mechanisms. Additionally, a large body of data is available on the effect of alcohol on the physiology of the lungs and the innate and adaptive immune system of the host. The purpose of this review is to integrate the available knowledge in these diverse areas of for a better understanding of the how the compromised immune system derived from alcohol exposure responds to pneumococcal infections.
Keywords: Pneumococcus, S. pneumoniae, Alcohol, Innate immune response, Adaptive immune response, Pathogenesis
The pathogen: Streptococcus pneumoniae
Streptococcus pneumoniae (pneumococcus) is an encapsulated Gram positive bacterium which normally occurs as a commensal in the nasopharynx of humans. This harmless commensal turns into a major human pathogen when the immune response of the host is weakened as in the extremes of age or in people with underlying conditions like alcoholism (Burman et al., 1985; Samokhvalov et al., 2010). Pneumococcus can cause a wide range of infections like pneumonia, bacteremia, septicemia, meningitis, otitis media, sinusitis, endocarditis and peritonitis. Pneumococcal pneumonia is the most common manifestation of infection.
Pneumococcal virulence factors
Pneumococcus has a wide arsenal of virulence factors that are expressed as cell surface proteins or as toxins or secreted proteins (Fig.1). The most important of these virulence factors is the polysaccharide capsule. On the basis of the capsular structure, pneumococci are divided into more than 90 serotypes. While all the serotypes are able to establish a carriage, invasive disease is restricted to just a small subset of serotypes. In fact, only ten common serotypes account for 62% of invasive infections worldwide (http://www.cdc.gov/Features/Pneumonia/). The prevalence of different serotypes varies according to the geographical location, vaccine use and anatomic site of infection.
Fig. 1. Pneumococcal virulence factors.
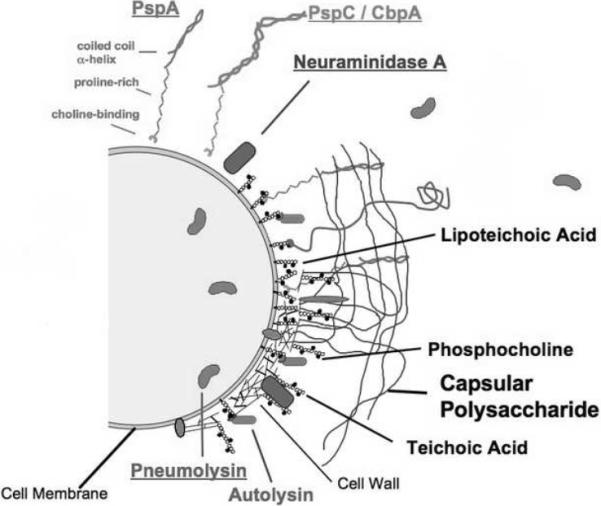
This figure was adapted from a previously published figure (Clin Microbiol Rev. 1998 11:645–57) and was provided by Dr. David E. Briles, University of Alabama at Birmingham, USA. Abbreviations: PspA, pneumococcal surface protein A; PspC, pneumococcal surface protein C; CbpA, choline binding protein A.
The role of all the virulence factors in the pathophysiology of the disease and/ or evasion of the host immune response is not completely understood. These virulence factors have been recently reviewed in detail (Mitchell and Mitchell, 2010). Table 1 summarizes the important pneumococcal virulence factors and their role in pathogenesis.
Table l.
Important pneumococcal virulence factors and their role in pathogenesis.
| Pneumococcal virulence factor | Role in pathogenesis | Reference |
|---|---|---|
| Capsule | Prevents opsonophagocytosis and mechanical removal by mucus | (Jonsson et al., 1985; Nelson et al., 2007) |
| Pneumolysin (Ply) | Cytolytic toxin, complement activating activity | (Mitchell et al., 1991) |
| Neuraminidase (NanA,B & C) | Cleaves N-acetyl neuraminic acid on host cell surfaces and exposes potential pathogen binding sites, Nan A plays an important role in biofilm formation. | (Camara et al., 1994) |
| Hyaluronidase | Helps in spread of infection | (Fitzgerald and Repesh, 1987) |
| Pneumococcal surface protein A(PspA) | Binds to lactoferrin and inhibits complement activation | (Hammerschmidt et al., 1999) |
| Pneumococcal surface protein C (PspC or CbpA or SpsA) | Binds to slgA and factor H; prevents C3b formation | (Dave et al., 2001; Hammerschmidt et al., 1997) |
| Pilus | Mediates binding of pneumococci to host cells. | (Barocchi et al., 2006) |
| Autolysin A(LytA) | Causes pneumococcal autolysis, proposed to release ply; prevents phagocytosis | (Martner et al., 2008; Martner et al., 2009) |
Epidemiology of pneumococcal pneumonia
S. pneumoniae is the most common bacterial etiology of community acquired pneumonia worldwide. An estimated 570,000 cases of pneumococcal pneumonia occur annually in the United States, leading to approximately 175,000 hospitalizations. Besides being a major global childhood pathogen (~10.6 million children less than 5 years affected each year), it also causes significant morbidity and mortality in the adult population. Pneumococci account for up to 36% of the cases of adult community-acquired pneumonia in the United States. Increasing resistance of pneumococcal strains to β-lactam and other antibiotics has complicated the treatment. The introduction of a 23-valent polysaccharide vaccine based on the most common serotypes has helped reduce the number of pneumococcal infections. However, the efficacy of the polysaccharide vaccine in preventing invasive disease is only about 60% in the groups for which it is indicated (Fine et al., 1994). Moreover, as polysaccharides are T cell independent antigens, the memory immune response evoked by the vaccine is poor and is not improved by booster doses. Conjugating the polysaccharide antigen to a protein carrier is known to improve induction of an anamnestic response and induce a more prolonged antibody response. In 2010, a polysaccharide vaccine containing 13 pneumococcal serotypes conjugated with a non toxic form of diphtheria toxin (CRM 197) was licensed in the United States, for use in children. This replaces of the previously available 7-valent conjugate vaccine (2010a). Since both the polysaccharide and conjugate vaccine are serotype dependent, the extensive use of the vaccine against selected serotypes can lead to a replacement in serotypes causing invasive pneumococcal disease, which will ultimately result in decreased vaccine efficacy. A protein-based serotype-independent vaccine seems like a promising alternative. Although many proteins and combinations of proteins have been rigorously evaluated in animal models as potential pneumococcal vaccine candidates, but a licensed protein based vaccine for human use still remains an elusive goal. Until effective means of controlling the transmission and spread of antibiotic resistance in pneumococcus are devised, infections caused by this organism will remain a major human health concern, especially in high risk groups like people with a history of alcohol abuse.
Alcohol use and pneumococcal pneumonia
Benjamin Rush recognized the association of alcohol with increased susceptibility to pneumonia as early as the 18th century. Subsequently, several studies have identified alcohol as an independent risk factor for pneumococcal pneumonia. There is a dose-response relationship between alcohol consumption and the incidence of community acquired pneumonia (Samokhvalov et al., 2010). An estimated 50% of adult patients with pneumonia have a history of alcohol abuse (Goss et al., 2003). In addition to the frequency, the severity of complications and associated mortality of pneumococcal pneumonia is much higher in these populations (Fernandez-Sola et al., 1995; Saitz et al., 1997). In an extensive recent study on invasive pneumococcal disease involving about 19,000 patients over the span of 10 years, it was found that the overall mortality attributable to pneumococcus in alcohol related disorders is 30% as compared to a mortality rate of 17% in those without alcohol related conditions (Harboe et al., 2009). The incidence and mortality of pneumococcal pneumonia is significantly increased in individuals abusing alcohol.
Laboratory models for evaluation of the effects of alcohol on the host immune response
Various in vitro and in vivo approaches are available for evaluation of the effects of alcohol on the host immune response. The methodology and the use of these approaches to simulate different alcohol abuse patterns in humans (acute and chronic) have been reviewed recently by Nagy (Nagy, 2008) and D'Souza El-Guindy et al., (D'Souza El-Guindy et al., 2010). In the in vitro approach, the relevant immune cells like monocytes, macrophages and dendritic cells are isolated and exposed to alcohol. Alcohol administration for up to 24 hours is considered acute exposure (Szabo and Mandrekar, 2008). The amount of alcohol in these studies ranges from 1 to 500 mM (D'Souza El-Guindy et al., 2010). Chronic exposure to ethanol in cell culture systems is less clearly defined and time of exposure varies with different cell types and ranges between 48 h to 10 days. These in vitro approaches have been reviewed recently by Szabo and Mandrekar (Szabo and Mandrekar, 2008).
Although the in vitro approaches are less cumbersome and generate consistent results, numerous studies including a study from our lab (Pruett et al., 2005) indicate that the immune responses observed in vitro and in vivo are profoundly different. Animal models serve as an indispensible tool for studying the in vivo effects of alcohol on different immune components. Mouse and rat are the two most commonly used animal models for alcohol research.
Although cirrhosis in humans in mostly caused by chronic alcohol abuse, because of the shorter life span of rodents, administration of alcohol alone does not lead to cirrhosis. Therefore, in most rodent models intragastric administration of carbon tetrachloride for 8 to 12 weeks is used for the induction of cirrhosis (Mellencamp and Preheim, 1991). Various models for chronic alcohol administration are available in both mice and rats; these models vary in the method of alcohol administration. Alcohol exposure for 4 to 32 weeks has been used in rodent models to mimic chronic alcohol abuse in humans. The Tsukamoto-French model involves continuous intragastric administration of alcohol (Tsukamoto and French, 1993). Desired levels of blood alcohol concentration can be maintained using this model for long durations of time but this model is very expensive and requires expertise and training to perform the catheter implantation surgery which hampers the common use of this model. Other methods like Lieber–DeCarli diet involve the administration of alcohol in a liquid diet as the only source of food and water to the experimental animals (DeCarli and Lieber, 1967). This model is much more affordable than the Tsukamoto-French model but it does not simulate human drinking as animals are forced to consume alcohol. Yet another method uses the addition of alcohol to drinking water (Coleman et al., 2008). Alcohol administration for up to 32 weeks which is difficult to attain with other models can be achieved using this model. The ease of administration, low cost and the various variations of this model make it attarctive for chronic administration of alcohol without inducing a stress response. This model does not cause substantial liver damage (Cook et al., 2007) present in advanced stages of alcohol abuse.
In acute ethanol exposure models, ethanol is generally administered either through intraperitoneal route (Plackett and Kovacs, 2008) or as an oral gavage (Carson and Pruett, 1996). The peak blood concentrations are achieved after about 30 minutes of alcohol administration depending upon the amount of alcohol, animal strain and the route of alcohol administration (faster in intraperitoneal route than oral gavage). The oral gavage method of alcohol administration mimics human drinking much better than the intraperitoneal method. Besides the models mentioned above there are several others which are available, for a detailed review of these models and the factors that should be taken into consideration for choosing an appropriate model for studies in alcohol research; the readers are referred to a recent review by D'Souza El-Guindy et al., (D'Souza El-Guindy et al., 2010).
Alcohol consumption patterns
The amount and the pattern of alcohol consumption influence its effects on the immune system. Most studies indicate that acute intoxication is associated with attenuation of the inflammatory response while chronic exposure has a pro-inflammatory effect. These exposure dependent effects of alcohol have been recently reviewed by Goral et al., (Goral et al., 2008). Parallel studies evaluating the effects of different drinking patterns on pneumococcal infections are not available. The few studies reported in literature that have evaluated the effect of either acute or chronic alcohol in pneumococcal infections will be discussed in the following sections. In this review we will focus on correlating the current understanding innate and adaptive immune response to pneumococci with the information available on the effect of alcohol on these responses (Fig. 2).
Fig. 2. Effect of alcohol on innate and adaptive immune responses important for defense against pneumococcal infections.
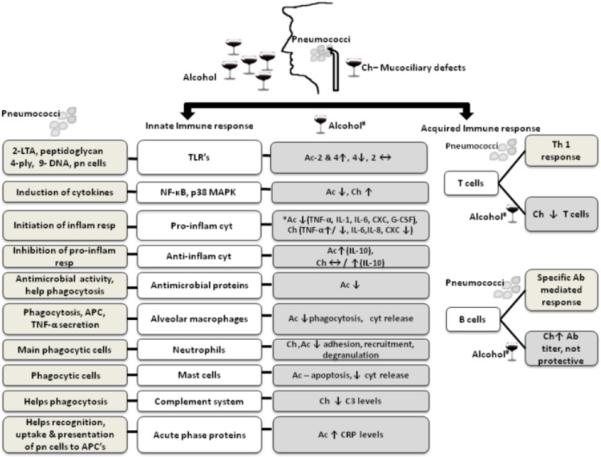
Ch- chronic alcohol, Ac- acute alcohol, LTA- lipotechoic acid, ply- pneumolysin, pn-pneumococcal, inflam- inflammatory, resp-response, APC- antigen presenting cell, cyt-cytokines, ↑- increase, ↓- decrease, ↔ - No change, *In most studies, #Effects of alcohol on stimulated cells
Innate immunity and inflammation
The innate immune system is the first line of host defense against any pathogen. The organisms that are able to evade the innate defenses are subsequently targeted by the adaptive immune system which generates antigen specific responses. Both these arms of the host immune response play an important role in defense against pneumococcal infections.
Alcohol induced suppression of host defenses provides an ideal opportunity for normally commensal pneumococci to invade and cause infections. Invasion usually begins by the aspiration of pneumococci from the nasopharynx to the lower respiratory tract. This transit is facilitated by the decreased cough and epiglottis reflex commonly observed in people with a history of alcohol abuse. As pneumococci descend from the nasopharynx to the lungs the first structural barrier encountered is the mucociliary apparatus. In a normal host, the mucous present in this region traps the invading microorganism and the coordinated ciliary movement pushes it back to the upper respiratory tract. Chronic ethanol ingestion in a rat model has shown that ethanol causes defects in the mucociliary apparatus by decreasing the ciliary beat frequency. This aids the transition of pneumococci into the rat lungs in a dose dependent fashion (Vander Top et al., 2005). This defect is one reason chronic alcohol abusers are more prone to pneumococcal infections. In contrast, acute ethanol exposure increases the ciliary beat frequency in vitro in bovine bronchial epithelial cells (Sisson, 1995; Sisson et al., 1999; Wyatt et al., 2003) and a cirrhotic rat model shows no mucociliary defects (Propst-Graham et al., 2007). Upon reaching the alveoli pneumococci encounter host immune cells and soluble effector molecules.
The Toll like receptors (TLR's) belong to a family of molecules called pathogen associated molecular pattern receptors that play a key role in the initial activation of the innate immune response. The stimulation of cells by a TLR ligand results in intracellular signaling cascades that lead to production of pro-inflammatory cytokines and chemokines. These, in turn, synchronize the local and the systemic inflammatory responses (Akira and Takeda, 2004). TLR's 2, 4 and 9 are considered to be important for the recognition of pneumococcal components. TLR2, expressed on the alveolar macrophages and alveolar epithelial type II cells (Droemann et al., 2003) recognizes lipotechoic acid and peptidogylcan present in the pneumococcal cell wall (Yoshimura et al., 1999). Since pneumococcus is a Gram positive pathogen it lacks LPS (lipopolysaccharide) which is the normal ligand for TLR4. Instead, TLR4 interacts with pneumolysin which is an important cytolytic toxin produced by almost all clinical isolates of pneumococcus (Malley et al., 2003). Although pneumolysin is recognized by TLR4 both TLR2 and 4 are important for pneumolysin induced inflammatory responses (Dessing et al., 2009). Besides this cytolytic toxin, pneumococcus also produces autolysins which cause the bacterial cell to rupture and in the process release DNA and other cellular products (Moscoso and Claverys, 2004). The pneumococcal DNA released by autolysin induced cell lysis is recognized by TLR9 (Moscoso and Claverys, 2004). It is now known that even viable, intact pneumococcal cells are able to stimulate TLR9 (Mogensen et al., 2006). The crucial roles of TLR's 4 and 9 in pneumococcal pathogenesis are demonstrated in mouse models (Albiger et al., 2007; Malley et al., 2003). However, some studies in mouse and human lung tissue models indicate that absence or blocking of TLR's 2 and 4 did not have a significant impact on cytokine release, pneumococcal clearance and morbidity (Knapp et al., 2004). Although the exact role of each of these TLR's in pneumococcal infection remains controversial, it seems that some TLR's may be independently dispensable but might have significant additive impact. A recent study demonstrated a significant synergy between TLR2 and both TLR4 and TLR9 for the induction of MyD88 dependent splenic cytokine and chemokine response to pneumococcus (Lee et al., 2007). The combined effects of TLR2 and 4 in response to acute ethanol exposure have been evaluated in human monocytes. It was found that the presence of both TLR2 and 4 ligands resulted in a synergistic inflammatory response. TLR4 ligand resulted in an attenuated response while there was no change in response with TLR2 ligand alone (Oak et al., 2006). Thus, it seems important to study the collective TLR responses. Although specific effects of alcohol on TLR's induced by pneumococci are not known, previous studies from our laboratory and others suggest that acute ethanol suppresses cytokine and chemokine responses induced by most TLR ligands in mice (Boe et al., 2001; Pruett et al., 2004b) including ligands for TLR4, 2 and 9 (Dai and Pruett, 2006a, b; Goral and Kovacs, 2005; Mandrekar et al., 1999; Mason et al., 2000). Besides chemokine levels ethanol exposure also affects the functioning of alveolar macrophage and neutrophil function, (discussed in detail in the subsequent sections).
The signaling pathways triggered by most TLR's and members of the interleukin-1 receptor (IL-1R) family culminate in the activation of NF-κB which leads to the synthesis of pro-inflammatory cytokines and co-stimulatory molecules (Akira and Takeda, 2004). IL-1R associated kinases (IRAKs) are a group of molecules that play an important role in the MyD88 dependent pathways. Inherited IRAK-4 deficiency is associated with severe and recurrent invasive pneumococcal disease in humans (Picard et al., 2003). In the absence of IRAK-4, TLR ligands are unable to induce critical inflammatory cytokines which are important for prevention of pneumococcal disease. Although ethanol's effect on IRAK-4 has not been evaluated, studies from our laboratory and others with IRAK-1, which acts downstream of IRAK-4 indicate that acute ethanol exposure causes an inhibition of IRAK-1 (induced by bacterial lipopolysaccharide, a ligand for TLR4) in a mouse model (Pruett et al., 2004a) and in human monocytes (Oak et al., 2006).
Many of the studies discussed in this review have been conducted in human monocytes. However, this is still relevant for pneumococcal pneumonia, as monocytes are the precursors of alveolar macrophages which are vital in inflammatory response against pneumococcus. Also, recent evidence indicates that like neutrophils, monocytes are also recruited to the lung in response a pneumococcal infection (Goto et al., 2004).
The MyD88 signaling pathways release NF-κB from its inhibitor IκB complex, NF-κB then migrates to the nucleus and induces the expression of inflammatory cytokines (Akira and Takeda, 2004). This cytokine expression leads to recruitment of neutrophils to the lungs and plays a vital role in the killing of pneumococci. Polymorphisms in IκB genes are associated with increased susceptibility to invasive pneumococcal disease in humans (Chapman et al., 2007). It has been recently reported that PspA, an important surface exposed pneumococcal virulence factor, is involved in the activation of human monocytes via NF-κB and p38 MAPK signaling cascades in invasive pneumococcal disease (Cao et al., 2010). An increased activation of NF-κB has been observed in monocytes from patients with alcoholic hepatitis (which is caused by chronic alcohol abuse) as compared to controls (Hill et al., 2000). While, acute ethanol exposure decreases nuclear translocation and activation of NF-κB p65/p50 heterodimer, it increases NF-κB p50 homodimer. binding to DNA in human monocytic cells in response to LPS (Mandrekar et al., 1997). The NF-κB p50 homodimer is implicated in inhibition of NF-κB driven gene transcription. Upstream from NF-kB in the TLR and other signaling cascades in the innate immune system are the mitogen activated protein (MAP) kinases. One of these is p38 MAPK, which plays a significant role in inflammatory cytokine release from a human lung tissue model of pneumococcal infection (Xu et al., 2008). Previous work from our laboratory indicates that p38 phosphorylation in peritoneal macrophages induced by TLR ligands are suppressed by acute ethanol ingestion (Pruett et al., 2004a). Thus, acute ethanol intoxication may have an effect on p38 signaling induced in response to pneumococcal stimuli.
Cytokines are responsible for communication among different cellular components of the immune system. The pro-inflammatory cytokines are important to initiate an inflammatory reaction while the anti-inflammatory cytokines inhibit the pro-inflammatory response once the infection is cleared to protect against an exaggerated immune response which can damage the host cells. Alcohol intoxication has profound effects on both these responses and these effects depend on the amount and the duration of alcohol intake. While acute ethanol intoxication inhibits pro-inflammatory cell activation, chronic alcohol use is associated with increased pro-inflammatory cytokine activation. The effect of alcohol on cytokine expression has been recently reviewed in detail by Crews et al. (Crews et al., 2006). We will briefly review the cytokines important in pneumococcal defense.
On activation by pneumococcal stimuli, alveolar macrophages produce large amounts of tumor necrosis factor- alpha (TNF-α). This early response cytokine, along with IL-1 plays a crucial role in inducing the nuclear translocation of NF-κB transcription factors (Li and Verma, 2002) which in turn leads to the production of cytokines necessary for neutrophil recruitment to lungs. In a mouse model of pneumococcal infection, deficiency of signaling receptors for TNF-α and IL-1 leads to decreased bacterial clearance as it impairs neutrophil recruitment and expression of neutrophil chemokines. The diminished response from this combined deficiency is because of the indispensible role of TNF-α and IL-1 in chemokine expression through NF-κB (Jones et al., 2005). TNF-α antibodies worsen the outcome of pneumococcal pneumonia in a mouse model (Takashima et al., 1997). Monoclonal TNF-α antibodies which are used to treat certain chronic conditions are associated with increased risk of invasive pneumococcal disease in humans (Baghai et al., 2001). TNF-α can also cause upregulation of receptors implicated in tissue invasion of pneumococci, such as platelet activating factor receptor, increasing the risk of bacteremia (Cundell et al., 1995). Because of its pro-inflammatory effects, polymorphisms in humans leading to secretion of high amounts of TNF-α are associated with increased rick of septic shock and respiratory failure in community acquired pneumonia (Waterer et al., 2001; Wunderink et al., 2002). Thus, an optimal level of TNF-α is important for a productive outcome of pneumococcal pneumonia. Acute ethanol intoxication impairs pulmonary TNF-α response to LPS challenge (Karavitis et al., 2008; Kolls et al., 1995; Nelson et al., 1989). Acute ethanol also causes a change in cell membrane fluidity which prevents the cleavage of TNF-α from the cell surface (Zhao et al., 2003). Moderate acute ethanol consumption significantly reduces LPS induced TNF-α and IL-β production by human monocytes (Mandrekar et al., 2006). This might be an added factor in the impaired immune response against pneumococcal infection in individuals with a history of alcohol abuse. The effects of chronic ethanol exposure on TNF-α are contradictory. While some studies show that chronic exposure increases TNF-α production (Zhang et al., 2001), others suggest an opposite effect (D'Souza et al., 1996; Omidvari et al., 1998; Standiford and Danforth, 1997).
Interleukin-6 (IL-6) delays neutrophil apoptosis and enhances neutrophil cytotoxic function including oxygen radical production (Biffl et al., 1996), so it is often classified as a pro-inflammatory cytokine. However, it can also have anti-inflammatory properties under some circumstances (Tilg et al., 1994). Adaptive immune mechanisms such as T-cell proliferation, development of antigen-specific cytotoxic T-lymphocytes and B-cell stimulation are also dependent upon IL-6 (Barton, 1997). Stimulation of human monocytes with pneumococcal surface protein A (PspA) induces the release of significant amounts of IL-6 (Cao et al., 2010). IL-6 is the major inducer of acute phase proteins like C-reactive protein (CRP), which is particularly important in resistance to pneumococcus. In community-acquired pneumonia, there is a positive correlation between IL-6 and CRP levels (Ortqvist et al., 1995). A number of studies indicate that high IL-6 (Kellum et al., 2007; Ortqvist et al., 1995) and IL-8 lavels (Bonten et al., 1997) are the best predictors of increased severity and mortality of pneumococcal infection. Previous studies from our lab in a binge drinking model in mice indicate that ethanol exposure decreased the levels of IL-6 in peritoneal macrophages and serum (Pruett et al., 2004a; Pruett et al., 2004b). Other studies have also shown that acute ethanol exposure suppresses IL-6 production by alveolar macrophages in response to LPS in an ex vivo model (Karavitis et al., 2008). Chronic alcohol exposure in early stages of septic shock leads to significantly lower levels of IL-6 and IL-8 as compared to non alcoholics (von Dossow et al., 2004). It is intriguing that alcohol suppresses IL-6 and IL-8 levels which are associated with a poor prognosis in pneumococcal pneumonia, especially considering that individuals abusing alcohol have increased severity and mortality of pneumococcal infections.
Peripheral blood neutrophils express receptors for chemokines of the α (CXC) family which contain the glutamic acid-leucine-arginine (ELR) motif preceding the first conserved cysteine residue. Neutrophil recruitment to the alveolar space during bacterial pneumonia is mediated by the rapid expression of these ELR+CXC chemokines (Mizgerd, 2002). Acute alcohol intoxication suppresses ELR+ response in lungs (Boe et al., 2001; Boe et al., 2003; Happel et al., 2007). Cytokine-induced neutrophil chemoattractant (CINC) and macrophage inflammatory protein-2 (MIP-2) are the major ELR+CXC cytokines involved in neutrophil recruitment in rat lungs; the concentration of these chemokines increases upon pneumococcal challenge. Experimental evidence indicates that acute ethanol administration suppresses the mRNA and protein expression of these chemokines in bronchoalveolar lavage (BAL) fluid obtained from rats following pneumococcal challenge (Boe et al., 2001). The impaired chemokine production led to decreased chemotactic activity of neutrophils in the BAL fluid which could be reversed by the administration of these CXC chemokines (Boe et al., 2003). Chronic ethanol consumption also decreases MIP-2 mRNA and protein levels in alveolar macrophages in response to LPS. It has similar effects on another important CC chemokine, macrophage inflammatory protein 1α (MIP-1α) (Standiford and Danforth, 1997).
In order to maintain the levels of neutrophils in the blood stream and supply an increased influx to the lungs in response to pneumococcal infection, the host needs to increase the production of neutrophils. Granulocyte colony stimulating factor (G-CSF) is a lineage-specific hematopoietic growth factor that induces neutrophil production and also enhances their release from the bone marrow into the systemic circulation (Semerad et al., 2002). During pneumococcal pneumonia, it serves as an important signaling link between the lung and the bone marrow. Alcohol has long been related to defects in hematopoiesis (Heermans, 1998). Chronic alcohol abuse is frequently associated with leukopenia particularly granulocytopenia (Seppa et al., 1993). Reduction in the number of mature granulocytes with vacuolization of myeloid progenitor cells is observed in the bone marrow analysis of chronic alcohol abusers (Heidemann et al., 1981). The suppression of G-CSF in response to alcohol exposure has also been validated in animal models. Acute ethanol exposure suppresses the plasma G-CSF response to E. coli in a rat model of infection (Bagby et al., 1998). The hematopoietic precursor cell response to pneumococcal pneumonia is impaired by acute ethanol administration in a mouse model (Raasch et al., 2010). Administration of G-CSF prior to infection significantly attenuates the adverse effects of acute ethanol exposure on the expression of adhesion molecules and lung recruitment of neutrophils and also enhances its bactericidal activity against E. coli and K. pneumoniae (Nelson et al., 1991; Zhang et al., 1998). In a chronic ethanol exposure and a cirrhosis rat model, G-CSF administration did not provide any protection against pneumococcal pneumonia despite causing an increase in the number of circulating neutrophils (Lister et al., 1993b; Preheim et al., 1996).
Granulocyte monocyte colony stimulating factor (GM-CSF) is also a hematopoietic growth factor but unlike G-CSF, it has a wider spectrum of stimulation that includes leukocytes, erythrocytes and megakarocytes. GM-CSF plays a crucial role in stimulating the terminal differentiation of alveolar macrophages and accumulation of these cells in the lungs. Conjugation of GM-CSF with PspA provides increased protection against fatal pneumococcal challenge in a mouse model (Wortham et al., 1998). The effects of chronic ethanol ingestion on GM-CSF have been examined in a rat model. Chronic ethanol exposure had no effect on GM-CSF expression within the alveolar space but it significantly decreased membrane expression of the GM-CSF receptor on alveolar macrophages. Chronic ethanol ingestion also decreased the cellular expression and nuclear binding of PU.1, a master transcription factor for GM-CSF (Joshi et al., 2005).These effects could be reversed by treatment with rGM-CSF or glutathione precursors like S-adenosylmethionine (SAM) (Brown et al., 2009; Joshi et al., 2005). GM-CSF treatment also promotes the alveolar epithelial barrier integrity that is severely affected by chronic alcohol abuse (Pelaez et al., 2004).
Interleukin 12 (IL-12) is a crucial regulatory cytokine that contributes to both innate and acquired immunity. It preferentially activates Th1 and NK cells to induce the production of IFN-γ (Gately et al., 1998). Although some animal studies indicate that the role of IL-12 and IFN-γ are dispensable in pneumococcal infection (Kuranaga et al., 2006; Lauw et al., 2002; Rijneveld et al., 2002). However, most studies suggest a crucial role of IL-12 in host resistance to pneumococcal infection. IL-12 receptor deficiency is associated with recurrent pneumococcal sepsis in humans (Gruenberg et al., 2010). Mice deficient in IL-12, IFN-γ, or IL-6 show a reduced IgG anti-PspA response of all IgG isotypes (Khan et al., 2002). In a mouse model, intranasal administration of IL-12 alone or in combination with pneumococcal polysaccharide or PspA protects against carriage, pulmonary and systemic pneumococcal infection (Arulanandam et al., 2001; Lynch et al., 2003; Sun et al., 2007). Acute ethanol exposure suppresses IL-12 secretion by alveolar macrophages in response to LPS in an ex vivo model and in lungs and blood in a murine model (Karavitis et al., 2008; Mason et al., 2000). Production of IL-12 in response to other TLR's (including some that should be activated by pneumococci) is also inhibited in a binge drinking mouse model (Pruett et al., 2004b).
Interleukin-10 (IL-10) is an anti-inflammatory cytokine that inhibits the production of most of the pro- inflammatory cytokines discussed earlier. High levels of IL-10 hamper effective pneumococcal clearance and decrease survival in a mouse model. (van der Poll et al., 1996). In humans, high IL-10 levels are associated with poor prognosis of sepsis (van der Poll et al., 1997). A polymorphism in the IL-10 gene promoter is associated with increased IL-10 release and correlates with greater severity of illness in community acquired pneumonia (Gallagher et al., 2003; Schaaf et al., 2003). In a mouse model, IL-10 deficiency is associated with early onset and higher mortality from septic shock as a result of uninhibited pro-inflammatory reactions (Latifi et al., 2002). Thus in a normal host, this cytokine plays a crucial role in maintaining the balance between an effective immune response needed to confine the infection and an exaggerated inflammatory reaction. Acute ethanol exposure increases the production of IL-10 in vitro in human monocytes and monocyte derived macrophages (Mandrekar et al., 1996; Mandrekar et al., 2006; Szabo et al., 1996). The effect of chronic ethanol exposure is controversial, as one study indicates that there is no difference in IL-10 levels in individuals with a history of chronic alcohol abuse as compared to controls (von Dossow et al., 2004), while another study in postoperative patients indicates that chronic alcohol abusers have a four-fold higher levels of IL-10 after surgery as compared to non- alcoholics (Sander et al., 2002).
Many human cell types produce a number of antimicrobial proteins such as lysozyme, lactoferrin, complement, transferrins, and cathelicidins in response to pneumococcal infection. Some of these have direct antimicrobial activity while others facilitate opsonophagocytosis. Recent evidence indicates that antimicrobial peptides not only play an important role in the innate immune response but also act as a link between innate and adaptive immune responses and have immunomodulatory roles (Diamond et al., 2009). Lysozyme and lactoferrin are the most abundant antimicrobial proteins in the lung (Travis et al., 2001). These are produced both by myeloid cells such as alveolar macrophages and neutrophils, and by the alveolar epithelial cells. A recent study evaluated the effects of alcohol use on these two antimicrobial proteins in the epithelial lining fluid of humans. BAL fluid of alcohol abusing subjects showed impaired in vitro killing of pneumococcus associated with a decreased concentration of both lysozyme and lactoferrin (Burnham et al., 2010). The metabolic end product of alcohol, acetaldehyde also decreases the activity of lysozyme (Brecher et al., 1995). The modulation of the antimicrobial protein concentration and activity by alcohol could contribute to the increased severity of pneumococcal infection in individuals with a history of alcohol abuse.
Amongst the cell types most important in resistance to pneumococci are the alveolar macrophages; these are the resident phagocytes in the lung which constitute the first line of phagocytic defense at this site. Alveolar macrophages are not critical for bacterial clearance in pneumococcal pneumonia (Knapp et al., 2003). However, alveolar macrophages play an important role in the inflammatory response as they are the major source of TNF-α that stimulates the release of chemokines required for neutrophil recruitment (Kirby et al., 2005). They also play an indispensible role in modulation of the inflammatory response in pneumococcal pneumonia by phagocytosis of apoptotic neutrophils (Knapp et al., 2003). Recent evidence indicates that besides innate immune response to pneumococcal infections, alveolar macrophages also play a crucial role in antigen presentation to secondary lymphoid organs. Following exposure to pneumococcus murine alveolar macrophages rapidly transport bacteria to the draining lymph nodes. In case of pneumococcal infection, it is the alveolar macrophages and not the dendritic cells that are responsible for the earliest delivery of these bacteria to the secondary lymphoid tissues (Kirby et al., 2009). Chronic ethanol ingestion severely affects the production of pro-inflammatory cytokines (Standiford and Danforth, 1997), cell viability and phagocytic capability of alveolar macrophages despite the increased surface expression of C3b and Fc receptors on these cells (Bagasra et al., 1988; Brown et al., 2004). Some of these effects are attributed to decreased glutathione availability (Brown et al., 2004). Chronic alcohol consumption also severely affects oxidant release by alveolar macrophages in vitro and in vivo (Antony et al., 1993). Both acute and chronic ethanol administration inhibit nitric oxide secretion from rat alveolar macrophages in vitro but have differential effects on superoxide secretion (D'Souza et al., 1996). An interesting recent study evaluated the mechanisms by which chronic ethanol ingestion impairs alveolar macrophage function in a guinea pig model. The investigators found that chronic exposure not only decreases alveolar macrophage maturation but also severely impairs the phagocytic capability of the mature macrophages. This defect could be reversed by oral administration of glutathione precursor SAM (Brown et al., 2009).
Neutrophils or polymorphonuclear leukocytes (PMNL) are the main phagocytic cells responsible for bacterial clearance in pneumococcal pneumonia (Kolling et al., 2001). The number of PMNL's in the alveolar space is very low but in response to infectious stimuli, a large number of these cells are recruited from the circulation to the alveolar space. Impairment of neutrophil recruitment and function is the most commonly cited defect caused by alcohol that makes the host more susceptible to pneumococcal infection. Alcohol affects almost all aspects ranging from adhesion and migration to phagocytosis; involved in neutrophil dependent killing of pneumococci. Acute ethanol intoxication causes a dose dependent inhibition of neutrophil hyper adherence to activated endothelial surfaces (MacGregor et al., 1988). Studies in a rat model have shown that the expression of surface adhesion molecules CD11b/c and CD18 by neutrophils in response to LPS is suppressed by acute alcohol. This defect can be reversed by the administration of G-CSF (Zhang et al., 1998). The S100 proteins enhance the adhesion of phagocytes to the vascular endothelium, acute alcohol exposure inhibits the S100A8 and S100A9 response to LPS in the lungs in a rat model of infection (Zhang et al., 2007). In rodent models of pneumococcal infection acute ethanol intoxication profoundly suppresses the expression of lung chemokines which induce the recruitment of neutrophils to the lungs resulting in delayed neutrophil delivery, increased bacterial burden and mortality (Boe et al., 2001). In addition to these defects, acute ethanol exposure also leads to a dose dependent inhibition of the release of primary and secondary granule contents on activation (MacGregor et al., 1988). Hydrogen peroxide production by lung recruited PMNL's is also suppressed by acute ethanol administration (Zhang et al., 1997).
Chronic ethanol exposure significantly compromises the anti-pneumococcal activity of PMNL's in a rat model as it reduces both the oxidative burst and lysozyme release by PMNL's (Jareo et al., 1996). In a rat model, chronic ethanol ingestion shows a serotype-dependent effect on the bactericidal activity of the neutrophils in vitro and in vivo. In this study, the serotypes 10 A, 14 and 19F showed significant alcohol induced defect, while the serotypes 6B and 37 were not associated with any alcohol induced defect (Jareo et al., 1995). Chronic ethanol exposure does not have any effect on the adhesion of neutrophils in vitro and their recruitment in a rat model of pneumococcal pneumonia. In vitro studies indicate that neutrophil chemotaxis is impaired by ethanol (Lister et al., 1993a). In a rat model of cirrhosis, there is no defect in the adherence, chemotaxis and pulmonary recruitment (Preheim et al., 1991) while phagocytosis in vivo (Gentry et al., 1995; Propst-Graham et al., 2007) and degranulation, as determined by the level of lysozyme and complement component C3 in the BAL fluid in a rat model, are severely affected (Propst-Graham et al., 2007). Both chronic and acute ethanol exposure induces defects at multiple levels which adversely affect the neutrophil response to pneumococcal infection.
Mast cells are present throughout the respiratory tract. The main role of these cells is the release of pro-inflammatory cytokines including TNF-α, and the recruitment of neutrophils cells although they can also phagocytose and kill opsonized bacteria (Feger et al., 2002). The interaction of human mast cells with pneumococci has recently been demonstrated (Cruse et al., 2010). In a murine model of pneumococcal pneumonia, an increase in the number of mast cells is associated with pneumococcal clearance (Kerr et al., 2002). Subsequently, it has been established that human lung mast cells mediate pneumococcal killing in response to pneumolysin. Pneumococcal exposure causes the release of the pro-inflammatory mediator leukotriene C4 from human mast cells, which has the potential to recruit professional phagocytes to sites of infection (Cruse et al., 2010). A study in human mast cell line and mouse bone marrow derived mast cells indicates that ethanol exposure causes a dose dependent apoptosis of mast cells (Nurmi et al., 2009).
The classical complement system plays an important role in promoting the phagocytosis of pneumococci (Brown et al., 2002). Liver is the most important source of complement proteins; chronic alcohol abuse is associated with liver damage and cirrhosis and thus it adversely affects the production of complement components. Significantly decreased levels of serum C3 concentrations are observed in patients with alcoholic cirrhosis and in a cirrhotic rat model following pneumococcal infection (Homann et al., 1997; Propst-Graham et al., 2007). A study in a rat model indicates that the complement activating activity of pneumolysin is particularly detrimental in a cirrhotic host as it further reduces the inherently low complement levels. This ultimately prevents effective phagocytosis of pneumococcal cells (Alcantara et al., 1999).
Acute phase proteins are surrogate markers of inflammation. Two acute phase proteins, C- reactive protein (CRP) (Horowitz et al., 1987)and mannose binding lectin (MBL) (Eisen et al., 2008)are implicated in defense against pneumococcal infection. The role of MBL in pneumococcal defense is less clearly defined as one study indicates homozygotes of an MBL variant are at increased risk of invasive pneumococcal disease (Roy et al., 2002b) while another study indicates that MBL is not an acute phase reactant in pneumococcal pneumonia and its levels do not correlate with severity of the disease (Perez-Castellano et al., 2006) Passive administration of human CRP in mouse, protects against lethal pneumococcal infection (Yother et al., 1982) and in humans, CRP gene polymorphisms are associated with an increased risk of invasive pneumococcal disease (Roy et al., 2002a).. The inflammatory response is suppressed in acute ethanol exposure, but paradoxically binge drinking is associated with high levels of CRP (Imhof et al., 2001; Pruett and Pruett, 2006). This effect seems to be mediated by increased gut permeability caused by acute alcohol exposure, which leads to increased amounts of bacterial components in the circulation and induction of an acute phase response (Pruett and Pruett 2006). Since CRP plays an important role in recognition and uptake of pneumococcal cells and their presentation to the dendritic cells (Thomas-Rudolph et al., 2007), an elevated CRP levels should confer an advantage to the host. But it is clear that this does not fully counteract other anti-inflammatory and immunosuppressive effects of alcohol.
Acquired Immunity
The major cell type involved in initiating an acquired immune response is the dendritic cell, which are specialized phagocytic cells, easily detectable in the lungs (Segura and Villadangos, 2009). The main role of dendritic cells is to sample the airway and when they encounter an antigen, capture it, process it, move to lymph nodes that drain the lungs, and function as efficient antigen presenting cells to activate Th cells required for both humoral and cell mediated response. As previously mentioned, in pneumococcal infections, this immune surveillance is carried out both by the dendritic cells and the alveolar macrophages (Kirby et al., 2009). On induction with pneumolysin and choline- binding protein A (CbpA), human dendritic cells produce CC and CXC chemokines (Bernatoniene et al., 2008). These chemokines have pro-inflammatory activities early in the infection before antigen presentation has occurred to a substantial degree. Pneumococcus has elaborate mechanisms to evade human dendritic cell surveillance which are mediated through the production of pneumolysin and pneumococcal adherence and virulence factor A (PavA) (Littmann et al., 2009; Noske et al., 2009). This immune evasion is facilitated by the alcohol induced impairment of the dendritic cell function. Patients with alcoholic liver cirrhosis, both with active ethanol intake and on withdrawal (1 year) have decreased numbers of circulating dendritic cells (Laso et al., 2007). Chronic ethanol exposure in vitro impairs cytokine-driven differentiation and function of dendritic cells (Lau et al., 2006). Acute, moderate alcohol exposure both in vitro and in vivo affects the differentiation of dendritic cells and accessory cell function resulting in impairment of Th1 responses (Mandrekar et al., 2004; Szabo et al., 2004). Alcohol treated dendritic cells also show reduced IL-12, increased IL-10 production, and a decrease in expression of the co-stimulatory molecules CD80 and CD86 (Mandrekar et al., 2004).
Dendritic cells and the alveolar macrophages carry pneumococcal antigens to regional lymph nodes and invoke a specific immune response with cytotoxic T lymphocytes and antibody producing B lymphocytes (Jakubzick et al., 2006; Kirby et al., 2009). As described previously, alcohol abuse affects the functioning of both dendritic cells and alveolar macrophages. Decreased number of thymus cells and thymic weight is observed in animal models of chronic alcohol exposure (Grossman et al., 1988). Studies in a mouse model show an early rapid increase in T cell proliferation to areas with increased pneumococcal invasion. Also, CD4+ T cell deficient mice are unable to survive a lethal pneumococcal challenge indicating a crucial role of cells in pneumococcal infections (Malley et al., 2005). Lymphopenia is commonly observed in patients with alcohol related liver diseases. The number of circulating T cells is significantly reduced in individuals with a history of chronic alcohol abuse (Roselle et al., 1988). Besides affecting CD4+ T cells, chronic alcohol abuse also inhibits IL-17 production in vitro and in vivo in response to inflammatory stimuli (Shellito et al., 2001). IL-17A is important for immunity to pneumococcal colonization (Malley et al., 2006).
Interestingly, chronic alcohol abuse with liver disease leads to an increase in the specific plasma antibody titers against the pneumococcal serotype used for immunization, but these immunoglobulins are not protective against a subsequent pneumococcal challenge with the same serotype (Preheim et al., 1992). This review will focus primarily on antibody responses to pneumococcal vaccine polysaccharides rather than attempt an exhaustive survey of adaptive immune responses. These studies will be further discussed in the following section in the context of the effectiveness of pneumococcal vaccination in individuals with a history of alcohol abuse.
Efficacy of antibiotics, immunomodulation and vaccines for treatment of pneumococcal infections in individual's with alcohol exposure
Despite the increased severity and mortality of pneumococcal infection in people abusing alcohol, there are very few studies available that have specifically evaluated the efficacy of various therapeutic and prophylactic options for this select population. The recent guidelines for treatment of community-acquired pneumonia in patients with co-morbidities like alcoholism, recommend the use of a β-lactam plus macrolide combination or a respiratory fluoroquinolone alone as an empirical therapy (Mandell et al., 2007). Parenteral administration of macrolides (azithromycin), fluoroquinolones (trovafloxacin) and β-lactams (ceftriaxone) show similar efficacies against pneumococcal pneumonia in a cirrhotic rat model (Preheim et al., 2005). The efficacy of three different fluroquinolones (levofloxacin, moxifloxacin and trovafloxacin) at two different doses in a rat model of chronic alcohol intoxication was also found to be similar (Olsen et al., 2006). Based on the limited literature available, alcohol abuse does not seem to affect the efficacy of the antibiotics, but thorough and more extensive studies are needed to formulate specific guidelines.
Some immunomodulation therapies are being tried as an adjunct to antibiotics for treatment of pneumococcal pneumonia. As previously described, animal studies have shown that oral administration of SAM, a precursor of glutathione, decreases alcohol induced defects in alveolar macrophages (Brown et al., 2009). Also the administration of SAM or rGM-CSF reverses the decreased membrane expression of GM-CSF caused by chronic ethanol administration (Brown et al., 2009; Joshi et al., 2005). Intrapulmonary administration of IFN-γ enhances the CXC chemokine response to LPS in experimental animals. Pretreatment with G-CSF attenuated the adverse effects of acute ethanol administration on neutrophils (Nelson et al., 1991; Zhang et al., 1998). A recombinant human G-CSF (filgrastim) has been used in a clinical trial of 756 patients with community-acquired pneumonia. Filgrastim administration was found to be safe and well tolerated in these patients. The treatment improved blood neutrophil count accelerated radiologic improvement and reduced serious complications. Despite these beneficial effects, filgrastim failed to alter the mortality or morbidity outcomes (Nelson et al., 1998). Extensive clinical trials are needed to establish and validate the role of these therapies in pneumococcal infections in people with acute or chronic alcohol exposure.
The Advisory Committee on Immunization Practices (ACIP) recommends the use of the 23-valent pneumococcal polysaccharide vaccine in people with a history of alcohol abuse, and also in cirrhosis, for the prevention of invasive pneumococcal disease (2010b). A meta analysis of prospective trials found that although the vaccine is efficacious in low risk adults, it is not as effective in high risk adults (Fine et al., 1994). There are very few studies that have evaluated the effectiveness of pneumococcal vaccine in alcohol abusing individuals and the results of these studies are contradictory. Studies using 14-valent pneumococcal vaccine in patients with alcoholic liver disease and cirrhosis indicate that these patients respond well to the vaccine (Pirovino et al., 1984; Simberkoff et al., 1983; Smith et al., 1980). These results cannot be readily generalized to the currently available 23-valent vaccine. A study comparing the efficacy of the 23-valent vaccine in native Alaskan with a history of chronic alcohol abuse with non alcoholics found that both groups responded adequately to the vaccine although the magnitude of response was better in non alcoholics and this was particularly evident for some serotypes (McMahon et al., 1993). Another study with patients with end stage liver disease (a common outcome of chronic alcohol abuse) indicates that although these patients responded to the vaccine, their mean IgA and IgM levels declined very rapidly. Antibody levels in the patients were at or below the pre-vaccination baseline, three months after transplantation. This study concluded that vaccination with 23-valent pneumococcal vaccine may not be effective for liver transplant patients (McCashland et al., 2000). Increased serum antibody titers in response to the polysaccharide vaccine do not necessarily correlate with protection. This has been validated in another study where increased serum concentration of functional, type-specific anticapsular antibodies in vaccinated cirrhotic rats did not provide protection against type 3 pneumococcal challenge (Preheim et al., 1992). In a study that compared the vaccine response to type 3 pneumococcal polysaccharide and pneumococcal polysaccharide vaccine conjugated to a protein carrier (CRM197) in chronic ethanol rat model, it was found that although the protein conjugated vaccine led to an anamnestic response, this was not accompanied by increased survival. The unconjugated vaccine led to a non significant increase in survival in the ethanol fed group of rats (Henriksen et al., 1997). There are case reports of fatal pneumococcal infections in patients with history of alcohol abuse despite vaccination with polysaccharide vaccine. Pneumococcal isolates from all patients in these two case reports were from serotypes included in the vaccine and these isolates were sensitive to the antibiotics used for management of infection (Hanna et al., 2000; McMahon et al., 1999). All the studies raise doubts about the effectiveness of the current vaccine for this patient population. Given the defects caused by alcohol in immune responses against pneumococcus, this is not entirely surprising. More extensive and well designed studies are needed in this area to help devise specific guidelines for pneumococcal vaccination in people with a history of alcohol abuse. It is possible that multi component protein vaccines may be sufficiently more potent than current capsular vaccines that this could partially alleviate the problems associated with a decreased anamnstetic response and the deranged response of an alcohol exposed host to certain serotypes. Since immune responses to T cell dependent antigens are known to be adversely affected by alcohol abuse (Jerrells, 1991), the efficacy of these protein based vaccines for people with a history of alcohol abuse population also remains questionable. However, this will have to wait until such a vaccine is licensed and its response analyzed for this group of people.
Future directions
As described earlier, both mice and rat models are available to study the effects of alcohol on different organs but all studies evaluating the effect of alcohol in pneumococcal infection have used rat as the animal model. Although both rat and mouse models are available for pneumococcal investigation, mouse is the most commonly used and well characterized model for pneumococcal pneumonia and sepsis (Chiavolini et al., 2008). The immune system of mice and rats is different and the susceptibility of different serotypes of pneumococci also varies for different strains of mice and rats. There is a need for a standardized model, ideally a mouse model, to study the effects of alcohol on pneumococcal immune responses. The availability of a wide range of mouse reagents; inbred and outbred strains; transgenics and knockout strains, and the availability of mouse genomes makes it an attractive research model that provides more flexibility and tractability. Also, there are many studies which have used mouse as a model for evaluating the effect of alcohol on the immune response of the host. A mouse model for chronic and acute ethanol exposure for pneumococcal research will allow the interpretation of the results obtained in light of the current knowledge available in field of pneumococcal immune response.
Studies evaluating the effects of alcohol on the outcome of pneumococcal infections have mostly used serotype 3 pneumococcal isolates for challenge through different routes while some studies have also used LPS for stimulation of immune cells (Lister et al., 1993; Nelson et al., 1989). There are greater than 90 pneumococcal serotypes and it is generally accepted that these serotypes have different invasive abilities. A recent meta-analysis indicates that serotypes 3, 6A, 6B, 9N and 19F are associated with pneumococcal bacteremia (Weinberger et al., 2010). Serotypes causing invasive disease in patients with comorbid conditions like alcoholism are different from those causing disease in normal healthy populations (Harboe et al., 2009; Sjostrom et al., 2006). It has been suggested that serotypes recognized as having high invasive potential (like serotype 1 and 7F) behave as primary pathogens while clones or serotypes with lower invasive potential behave as opportunistic pathogens. These opportunistic pathogens are more prevalent in patients with comorbidities. Infections caused by these `opportunistic' serotypes are much more severe than the invasive serotypes (Sjostrom et al., 2006). Alcohol is one of the most important underlying conditions for pneumococcal disease and it is likely that the serotypes causing infection in alcohol exposed individuals are different from those in the normal populations. The adaptive and innate immune responses evoked by different serotypes of pneumococci are different (Burgess et al., 2008; Soininen et al., 2001). The rarely invasive serotypes initiate a more robust immune response than the commonly invasive serotypes (Burgess et al., 2008). Serotype-dependent bactericidal response to pneumococcal infection in an alcohol exposed host has also been described by one study (Jareo et al., 1995). Therefore, it is important to evaluate the prevalence of serotypes responsible for invasive pneumococcal infections in alcohol abusing populations in order to design appropriate intervention strategies for these group of people.
In summary, the independent interactions of alcohol and pneumococci with host immune system are known. However, despite the documented evidence for increased incidence of pneumococcal infections in alcohol abusing individuals, integrated knowledge to address the intersection of these two important areas of research is limited. Understanding the synergistic effects of alcohol and pneumococci in appropriate animal models with relevant serotypes is critical for translational research in the context of the identification of appropriate intervention points as well as treatment strategies.
Acknowledgements
We would like to thank Dr David E Briles, University of Alabama at Birmingham for providing Fig 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- http://www.cdc.gov/vaccines/vpd-vac/pneumo.
- Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children - Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb. Mortal. Wkly. Rep. 2010a;59:258–261. [PubMed] [Google Scholar]
- Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23) MMWR Morb. Mortal. Wkly. Rep. 2010b;59:1102–1106. [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Albiger B, Dahlberg S, Henriques-Normark B, Normark S. Role of the innate immune system in host defence against bacterial infections: focus on the Toll-like receptors. J. Intern. Med. 2007;261:511–528. doi: 10.1111/j.1365-2796.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- Alcantara RB, Preheim LC, Gentry MJ. Role of Pneumolysin's complement-activating activity during pneumococcal bacteremia in cirrhotic rats. Infect. Immun. 1999;67:2862–2866. doi: 10.1128/iai.67.6.2862-2866.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony VB, Godbey SW, Hott JW, Queener SF. Alcohol-induced inhibition of alveolar macrophage oxidant release in vivo and in vitro. Alcohol. Clin. Exp. Res. 1993;17:389–393. doi: 10.1111/j.1530-0277.1993.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Arulanandam BP, Lynch JM, Briles DE, Hollingshead S, Metzger DW. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 2001;69:6718–6724. doi: 10.1128/IAI.69.11.6718-6724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O, Howeedy A, Kajdacsy-Balla A. Macrophage function in chronic experimental alcoholism. I. Modulation of surface receptors and phagocytosis. Immunology. 1988;65:405–409. [PMC free article] [PubMed] [Google Scholar]
- Bagby GJ, Zhang P, Stoltz DA, Nelson S. Suppression of the granulocyte colony-stimulating factor response to Escherichia coli challenge by alcohol intoxication. Alcohol. Clin. Exp. Res. 1998;22:1740–1745. [PubMed] [Google Scholar]
- Baghai M, Osmon DR, Wolk DM, Wold LE, Haidukewych GJ, Matteson EL. Fatal sepsis in a patient with rheumatoid arthritis treated with etanercept. Mayo Clin. Proc. 2001;76:653–656. doi: 10.4065/76.6.653. [DOI] [PubMed] [Google Scholar]
- Barocchi MA, Ries J, Zogaj X, Hemsley C, Albiger B, Kanth A, Dahlberg S, Fernebro J, Moschioni M, Masignani V, et al. A pneumococcal pilus influences virulence and host inflammatory responses. Proc. Natl. Acad. Sci. USA. 2006;103:2857–2862. doi: 10.1073/pnas.0511017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton BE. IL-6: insights into novel biological activities. Clin Immunol Immunopathol. 1997;85:16–20. doi: 10.1006/clin.1997.4420. [DOI] [PubMed] [Google Scholar]
- Bernatoniene J, Zhang Q, Dogan S, Mitchell TJ, Paton JC, Finn A. Induction of CC and CXC chemokines in human antigen-presenting dendritic cells by the pneumococcal proteins pneumolysin and CbpA, and the role played by toll-like receptor 4, NF-kappaB, and mitogen-activated protein kinases. J. Infect. Dis. 2008;198:1823–1833. doi: 10.1086/593177. [DOI] [PubMed] [Google Scholar]
- Biffl WL, Moore EE, Moore FA, Barnett CC., Jr. Interleukin-6 delays neutrophil apoptosis via a mechanism involving platelet-activating factor. J. Trauma. 1996;40:575–578. doi: 10.1097/00005373-199604000-00009. discussion 578–579. [DOI] [PubMed] [Google Scholar]
- Boe DM, Nelson S, Zhang P, Bagby GJ. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. J. Infect. Dis. 2001;184:1134–1142. doi: 10.1086/323661. [DOI] [PubMed] [Google Scholar]
- Boe DM, Nelson S, Zhang P, Quinton L, Bagby GJ. Alcohol-induced suppression of lung chemokine production and the host defense response to Streptococcus pneumoniae. Alcohol. Clin. Exp. Res. 2003;27:1838–1845. doi: 10.1097/01.ALC.0000095634.82310.53. [DOI] [PubMed] [Google Scholar]
- Bonten MJ, Froon AH, Gaillard CA, Greve JW, de Leeuw PW, Drent M, Stobberingh EE, Buurman WA. The systemic inflammatory response in the development of ventilator-associated pneumonia. Am. J. Respir. Crit. Care. Med. 1997;156:1105–1113. doi: 10.1164/ajrccm.156.4.9610002. [DOI] [PubMed] [Google Scholar]
- Brecher AS, Riley C, Basista MH. Acetaldehyde-modified lysozyme function: its potential implication in the promotion of infection in alcoholics. Alcohol. 1995;12:169–172. doi: 10.1016/0741-8329(94)00067-0. [DOI] [PubMed] [Google Scholar]
- Brown JS, Hussell T, Gilliland SM, Holden DW, Paton JC, Ehrenstein MR, Walport MJ, Botto M. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc. Natl. Acad. Sci. USA. 2002;99:16969–16974. doi: 10.1073/pnas.012669199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LA, Harris FL, Ping XD, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol. 2004;33:191–197. doi: 10.1016/j.alcohol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Brown SD, Gauthier TW, Brown LA. Impaired terminal differentiation of pulmonary macrophages in a Guinea pig model of chronic ethanol ingestion. Alcohol. Clin. Exp. Res. 2009;33:1782–1793. doi: 10.1111/j.1530-0277.2009.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess TS, Hirschfeld AF, Tyrrell GJ, Bettinger JA, Turvey SE. Commonly invasive serotypes of Streptococcus pneumoniae trigger a reduced innate immune response compared with serotypes rarely responsible for invasive infection. FEMS Immunol. Med. Microbiol. 2008;53:136–139. doi: 10.1111/j.1574-695X.2008.00382.x. [DOI] [PubMed] [Google Scholar]
- Burman LA, Norrby R, Trollfors B. Invasive pneumococcal infections: incidence, predisposing factors, and prognosis. Rev. Infect. Dis. 1985;7:133–142. doi: 10.1093/clinids/7.2.133. [DOI] [PubMed] [Google Scholar]
- Burnham EL, Gaydos J, Hess E, House R, Cooper J. Alcohol Use Disorders Affect Antimicrobial Proteins and Anti-pneumococcal Activity in Epithelial Lining Fluid Obtained via Bronchoalveolar Lavage. Alcohol Alcohol. 2010;45:414–421. doi: 10.1093/alcalc/agq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara M, Boulnois GJ, Andrew PW, Mitchell TJ. A neuraminidase from Streptococcus pneumoniae has the features of a surface protein. Infect. Immun. 1994;62:3688–3695. doi: 10.1128/iai.62.9.3688-3695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Chen T, Gong Y, Ying B, Li D, Xu W, Zhang X, Wang L, Yin Y. Molecular mechanisms of the secretion of cytokines and chemokines from human monocytes activated by pneumococcal surface protein A (PspA): Roles of mitogen-activated protein kinases and NF-kappaB. Microb. Pathog. 2010;48:220–229. doi: 10.1016/j.micpath.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson EJ, Pruett SB. Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol. Clin. Exp. Res. 1996;20:132–138. doi: 10.1111/j.1530-0277.1996.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Chapman SJ, Khor CC, Vannberg FO, Frodsham A, Walley A, Maskell NA, Davies CW, Segal S, Moore CE, Gillespie SH, et al. IkappaB genetic polymorphisms and invasive pneumococcal disease. Am. J. Respir. Crit. Care Med. 2007;176:181–187. doi: 10.1164/rccm.200702-169OC. [DOI] [PubMed] [Google Scholar]
- Chiavolini D, Pozzi G, Ricci S. Animal models of Streptococcus pneumoniae disease. Clin. Microbiol. Rev. 2008;21:666–685. doi: 10.1128/CMR.00012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Young BM, Turner LE, Cook RT. A practical method of chronic ethanol administration in mice. Methods Mol. Biol. 2008;447:49–59. doi: 10.1007/978-1-59745-242-7_4. [DOI] [PubMed] [Google Scholar]
- Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen TH, Waldschmidt TJ. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion--absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol. Clin. Exp. Res. 2007;31:1746–1758. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol. Clin. Exp. Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Cruse G, Fernandes VE, de Salort J, Pankhania D, Marinas MS, Brewin H, Andrew PW, Bradding P, Kadioglu A. Human lung mast cells mediate pneumococcal cell death in response to activation by pneumolysin. J. Immunol. 2010;184:7108–7115. doi: 10.4049/jimmunol.0900802. [DOI] [PubMed] [Google Scholar]
- Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- D'Souza El-Guindy NB, Kovacs EJ, De Witte P, Spies C, Littleton JM, de Villiers WJ, Lott AJ, Plackett TP, Lanzke N, Meadows GG. Laboratory models available to study alcohol-induced organ damage and immune variations: choosing the appropriate model. Alcohol. Clin. Exp. Res. 2010;34:1489–1511. doi: 10.1111/j.1530-0277.2010.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza NB, Nelson S, Summer WR, Deaciuc IV. Alcohol modulates alveolar macrophage tumor necrosis factor-alpha, superoxide anion, and nitric oxide secretion in the rat. Alcohol. Clin. Exp. Res. 1996;20:156–163. doi: 10.1111/j.1530-0277.1996.tb01059.x. [DOI] [PubMed] [Google Scholar]
- Dai Q, Pruett SB. Different effects of acute and chronic ethanol on LPS-induced cytokine production and TLR4 receptor behavior in mouse peritoneal macrophages. J. Immunotoxicol. 2006a;3:217–225. doi: 10.1080/15476910601080156. [DOI] [PubMed] [Google Scholar]
- Dai Q, Pruett SB. Ethanol suppresses LPS-induced Toll-like receptor 4 clustering, reorganization of the actin cytoskeleton, and associated TNF-alpha production. Alcohol. Clin. Exp. Res. 2006b;30:1436–1444. doi: 10.1111/j.1530-0277.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- Dave S, Brooks-Walter A, Pangburn MK, McDaniel LS. PspC, a pneumococcal surface protein, binds human factor H. Infect. Immun. 2001;69:3435–3437. doi: 10.1128/IAI.69.5.3435-3437.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli LM, Lieber CS. Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J. Nutr. 1967;91:331–336. doi: 10.1093/jn/91.3_Suppl.331. [DOI] [PubMed] [Google Scholar]
- Dessing MC, Hirst RA, de Vos AF, van der Poll T. Role of Toll-like receptors 2 and 4 in pulmonary inflammation and injury induced by pneumolysin in mice. PLoS One. 2009;4:e7993. doi: 10.1371/journal.pone.0007993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G, Beckloff N, Weinberg A, Kisich KO. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 2009;15:2377–2392. doi: 10.2174/138161209788682325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droemann D, Goldmann T, Branscheid D, Clark R, Dalhoff K, Zabel P, Vollmer E. Toll-like receptor 2 is expressed by alveolar epithelial cells type II and macrophages in the human lung. Histochem. Cell Biol. 2003;119:103–108. doi: 10.1007/s00418-003-0497-4. [DOI] [PubMed] [Google Scholar]
- Eisen DP, Dean MM, Boermeester MA, Fidler KJ, Gordon AC, Kronborg G, Kun JF, Lau YL, Payeras A, Valdimarsson H, et al. Low serum mannose-binding lectin level increases the risk of death due to pneumococcal infection. Clin. Infect. Dis. 2008;47:510–516. doi: 10.1086/590006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feger F, Varadaradjalou S, Gao Z, Abraham SN, Arock M. The role of mast cells in host defense and their subversion by bacterial pathogens. Trends Immunol. 2002;23:151–158. doi: 10.1016/s1471-4906(01)02156-1. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sola J, Junque A, Estruch R, Monforte R, Torres A, Urbano-Marquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch. Intern. Med. 1995;155:1649–1654. doi: 10.1001/archinte.1995.00430150137014. [DOI] [PubMed] [Google Scholar]
- Fine MJ, Smith MA, Carson CA, Meffe F, Sankey SS, Weissfeld LA, Detsky AS, Kapoor WN. Efficacy of pneumococcal vaccination in adults. A meta-analysis of randomized controlled trials. Arch. Intern. Med. 1994;154:2666–2677. doi: 10.1001/archinte.1994.00420230051007. [DOI] [PubMed] [Google Scholar]
- Fitzgerald TJ, Repesh LA. The hyaluronidase associated with Treponema pallidum facilitates treponemal dissemination. Infect. Immun. 1987;55:1023–1028. doi: 10.1128/iai.55.5.1023-1028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher PM, Lowe G, Fitzgerald T, Bella A, Greene CM, McElvaney NG, O'Neill SJ. Association of IL-10 polymorphism with severity of illness in community acquired pneumonia. Thorax. 2003;58:154–156. doi: 10.1136/thorax.58.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- Gentry MJ, Snitily MU, Preheim LC. Phagocytosis of Streptococcus pneumoniae measured in vitro and in vivo in a rat model of carbon tetrachloride-induced liver cirrhosis. J. Infect. Dis. 1995;171:350–355. doi: 10.1093/infdis/171.2.350. [DOI] [PubMed] [Google Scholar]
- Goral J, Karavitis J, Kovacs EJ. Exposure-dependent effects of ethanol on the innate immune system. Alcohol. 2008;42:237–247. doi: 10.1016/j.alcohol.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goral J, Kovacs EJ. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J. Immunol. 2005;174:456–463. doi: 10.4049/jimmunol.174.1.456. [DOI] [PubMed] [Google Scholar]
- Goss CH, Rubenfeld GD, Park DR, Sherbin VL, Goodman MS, Root RK. Cost and incidence of social comorbidities in low-risk patients with community-acquired pneumonia admitted to a public hospital. Chest. 2003;124:2148–2155. doi: 10.1378/chest.124.6.2148. [DOI] [PubMed] [Google Scholar]
- Goto Y, Hogg JC, Whalen B, Shih CH, Ishii H, Van Eeden SF. Monocyte recruitment into the lungs in pneumococcal pneumonia. Am. J. Respir. Cell Mol. Biol. 2004;30:620–626. doi: 10.1165/rcmb.2003-0312OC. [DOI] [PubMed] [Google Scholar]
- Grossman CJ, Mendenhall CL, Roselle GA. Alcohol and immune regulation. I. In vivo effects of ethanol on concanavalin A sensitive thymic lymphocyte function. Int. J. Immunopharmacol. 1988;10:187–195. doi: 10.1016/0192-0561(88)90094-x. [DOI] [PubMed] [Google Scholar]
- Gruenberg DA, Anover-Sombke S, Gern JE, Holland SM, Rosenzweig SD, Torgerson TR, Seroogy CM. Atypical presentation of IL-12 receptor beta1 deficiency with pneumococcal sepsis and disseminated nontuberculous mycobacterial infection in a 19-month-old girl born to nonconsanguineous US residents. J. Allergy Clin. Immunol. 2010;125:264–265. doi: 10.1016/j.jaci.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt S, Bethe G, Remane PH, Chhatwal GS. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect. Immun. 1999;67:1683–1687. doi: 10.1128/iai.67.4.1683-1687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt S, Talay SR, Brandtzaeg P, Chhatwal GS. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol. Microbiol. 1997;25:1113–1124. doi: 10.1046/j.1365-2958.1997.5391899.x. [DOI] [PubMed] [Google Scholar]
- Hanna JN, Wenck DJ, Murphy DN. Three fatal pneumococcal polysaccharide vaccine failures. Med. J. Aust. 2000;173:305–307. doi: 10.5694/j.1326-5377.2000.tb125662.x. [DOI] [PubMed] [Google Scholar]
- Happel KI, Rudner X, Quinton LJ, Movassaghi JL, Clark C, Odden AR, Zhang P, Bagby GJ, Nelson S, Shellito JE. Acute alcohol intoxication suppresses the pulmonary ELR-negative CXC chemokine response to lipopolysaccharide. Alcohol. 2007;41:325–333. doi: 10.1016/j.alcohol.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe ZB, Thomsen RW, Riis A, Valentiner-Branth P, Christensen JJ, Lambertsen L, Krogfelt KA, Konradsen HB, Benfield TL. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 2009;6:e1000081. doi: 10.1371/journal.pmed.1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heermans EH. Booze and blood: the effects of acute and chronic alcohol abuse on the hematopoietic system. Clin. Lab Sci. 1998;11:229–232. [PubMed] [Google Scholar]
- Heidemann E, Nerke O, Waller HD. Alcohol induced changes in hemopoiesis (author's transl) Klin. Wochenschr. 1981;59:1303–1312. doi: 10.1007/BF01711180. [DOI] [PubMed] [Google Scholar]
- Henriksen JL, Preheim LC, Gentry MJ. Vaccination with protein-conjugated and native type 3 capsular polysaccharide in an ethanol-fed rat model of pneumococcal pneumonia. Alcohol. Clin. Exp. Res. 1997;21:1630–1637. [PubMed] [Google Scholar]
- Hill DB, Barve S, Joshi-Barve S, McClain C. Increased monocyte nuclear factor-kappaB activation and tumor necrosis factor production in alcoholic hepatitis. J. Lab. Clin. Med. 2000;135:387–395. doi: 10.1067/mlc.2000.106451. [DOI] [PubMed] [Google Scholar]
- Homann C, Varming K, Hogasen K, Mollnes TE, Graudal N, Thomsen AC, Garred P. Acquired C3 deficiency in patients with alcoholic cirrhosis predisposes to infection and increased mortality. Gut. 1997;40:544–549. doi: 10.1136/gut.40.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J, Volanakis JE, Briles DE. Blood clearance of Streptococcus pneumoniae by C-reactive protein. J. Immunol. 1987;138:2598–2603. [PubMed] [Google Scholar]
- Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357:763–767. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J. Immunol. 2006;176:3578–3584. doi: 10.4049/jimmunol.176.6.3578. [DOI] [PubMed] [Google Scholar]
- Jareo PW, Preheim LC, Gentry MJ. Ethanol ingestion impairs neutrophil bactericidal mechanisms against Streptococcus pneumoniae. Alcohol. Clin. Exp. Res. 1996;20:1646–1652. doi: 10.1111/j.1530-0277.1996.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Jareo PW, Preheim LC, Lister PD, Gentry MJ. The effect of ethanol ingestion on killing of Streptococcus pneumoniae, Staphylococcus aureus and Staphylococcus epidermidis by rat neutrophils. Alcohol Alcohol. 1995;30:311–318. [PubMed] [Google Scholar]
- Jerrells TR. Immunodeficiency associated with ethanol abuse. Adv. Exp. Med. Biol. 1991;288:229–236. doi: 10.1007/978-1-4684-5925-8_26. [DOI] [PubMed] [Google Scholar]
- Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J. Immunol. 2005;175:7530–7535. doi: 10.4049/jimmunol.175.11.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S, Musher DM, Chapman A, Goree A, Lawrence EC. Phagocytosis and killing of common bacterial pathogens of the lung by human alveolar macrophages. J. Infect. Dis. 1985;152:4–13. doi: 10.1093/infdis/152.1.4. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Applewhite L, Ritzenthaler JD, Roman J, Fernandez AL, Eaton DC, Brown LA, Guidot DM. Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. J Immunol. 2005;175:6837–6845. doi: 10.4049/jimmunol.175.10.6837. [DOI] [PubMed] [Google Scholar]
- Karavitis J, Murdoch EL, Gomez CR, Ramirez L, Kovacs EJ. Acute ethanol exposure attenuates pattern recognition receptor activated macrophage functions. J. Interferon Cytokine Res. 2008;28:413–422. doi: 10.1089/jir.2007.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch. Intern. Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr AR, Irvine JJ, Search JJ, Gingles NA, Kadioglu A, Andrew PW, McPheat WL, Booth CG, Mitchell TJ. Role of inflammatory mediators in resistance and susceptibility to pneumococcal infection. Infect. Immun. 2002;70:1547–1557. doi: 10.1128/IAI.70.3.1547-1557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AQ, Shen Y, Wu ZQ, Wynn TA, Snapper CM. Endogenous pro- and anti-inflammatory cytokines differentially regulate an in vivo humoral response to Streptococcus pneumoniae. Infect. Immun. 2002;70:749–761. doi: 10.1128/iai.70.2.749-761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby AC, Coles MC, Kaye PM. Alveolar macrophages transport pathogens to lung draining lymph nodes. J. Immunol. 2009;183:1983–1989. doi: 10.4049/jimmunol.0901089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby AC, Raynes JG, Kaye PM. The role played by tumor necrosis factor during localized and systemic infection with Streptococcus pneumoniae. J. Infect. Dis. 2005;191:1538–1547. doi: 10.1086/429296. [DOI] [PubMed] [Google Scholar]
- Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, van Rooijen N, van der Poll T. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 2003;167:171–179. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- Knapp S, Wieland CW, van 't Veer C, Takeuchi O, Akira S, Florquin S, van der Poll T. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J. Immunol. 2004;172:3132–3138. doi: 10.4049/jimmunol.172.5.3132. [DOI] [PubMed] [Google Scholar]
- Kolling UK, Hansen F, Braun J, Rink L, Katus HA, Dalhoff K. Leucocyte response and anti-inflammatory cytokines in community acquired pneumonia. Thorax. 2001;56:121–125. doi: 10.1136/thorax.56.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls JK, Xie J, Lei D, Greenberg S, Summer WR, Nelson S. Differential effects of in vivo ethanol on LPS-induced TNF and nitric oxide production in the lung. Am. J. Physiol. 1995;268:L991–998. doi: 10.1152/ajplung.1995.268.6.L991. [DOI] [PubMed] [Google Scholar]
- Kuranaga N, Kinoshita M, Kawabata T, Habu Y, Shinomiya N, Seki S. Interleukin-18 protects splenectomized mice from lethal Streptococcus pneumoniae sepsis independent of interferon-gamma by inducing IgM production. J. Infect. Dis. 2006;194:993–1002. doi: 10.1086/507428. [DOI] [PubMed] [Google Scholar]
- Laso FJ, Vaquero JM, Almeida J, Marcos M, Orfao A. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcohol. Clin. Exp. Res. 2007;31:846–854. doi: 10.1111/j.1530-0277.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- Latifi SQ, O'Riordan MA, Levine AD. Interleukin-10 controls the onset of irreversible septic shock. Infect. Immun. 2002;70:4441–4446. doi: 10.1128/IAI.70.8.4441-4446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AH, Abe M, Thomson AW. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. J. Leukoc. Biol. 2006;79:941–953. doi: 10.1189/jlb.0905517. [DOI] [PubMed] [Google Scholar]
- Lauw FN, Branger J, Florquin S, Speelman P, van Deventer SJ, Akira S, van der Poll T. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J. Immunol. 2002;168:372–378. doi: 10.4049/jimmunol.168.1.372. [DOI] [PubMed] [Google Scholar]
- Lee KS, Scanga CA, Bachelder EM, Chen Q, Snapper CM. TLR2 synergizes with both TLR4 and TLR9 for induction of the MyD88-dependent splenic cytokine and chemokine response to Streptococcus pneumoniae. Cell Immunol. 2007;245:103–110. doi: 10.1016/j.cellimm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Lister PD, Gentry MJ, Preheim LC. Ethanol impairs neutrophil chemotaxis in vitro but not adherence or recruitment to lungs of rats with experimental pneumococcal pneumonia. J. Infect. Dis. 1993a;167:1131–1137. doi: 10.1093/infdis/167.5.1131. [DOI] [PubMed] [Google Scholar]
- Lister PD, Gentry MJ, Preheim LC. Granulocyte colony-stimulating factor protects control rats but not ethanol-fed rats from fatal pneumococcal pneumonia. J. Infect. Dis. 1993b;168:922–926. doi: 10.1093/infdis/168.4.922. [DOI] [PubMed] [Google Scholar]
- Littmann M, Albiger B, Frentzen A, Normark S, Henriques-Normark B, Plant L. Streptococcus pneumoniae evades human dendritic cell surveillance by pneumolysin expression. EMBO Mol. Med. 2009;1:211–222. doi: 10.1002/emmm.200900025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JM, Briles DE, Metzger DW. Increased protection against pneumococcal disease by mucosal administration of conjugate vaccine plus interleukin-12. Infect. Immun. 2003;71:4780–4788. doi: 10.1128/IAI.71.8.4780-4788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor RR, Safford M, Shalit M. Effect of ethanol on functions required for the delivery of neutrophils to sites of inflammation. J. Infect. Dis. 1988;157:682–689. doi: 10.1093/infdis/157.4.682. [DOI] [PubMed] [Google Scholar]
- Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA. 2003;100:1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malley R, Srivastava A, Lipsitch M, Thompson CM, Watkins C, Tzianabos A, Anderson PW. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect. Immun. 2006;74:2187–2195. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. USA. 2005;102:4848–4853. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr., Musher DM, Niederman MS, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J. Immunol. 2004;173:3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Girouard L, Szabo G. Human monocyte IL-10 production is increased by acute ethanol treatment. Cytokine. 1996;8:567–577. doi: 10.1006/cyto.1996.0076. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Szabo G. Alcohol-induced regulation of nuclear regulatory factor-kappa beta in human monocytes. Alcohol. Clin. Exp. Res. 1997;21:988–994. [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Szabo G. Inhibition of lipopolysaccharide-mediated NFkappaB activation by ethanol in human monocytes. Int. Immunol. 1999;11:1781–1790. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, White B, Szabo G. Moderate alcohol intake in humans attenuates monocyte inflammatory responses: inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcohol. Clin. Exp. Res. 2006;30:135–139. doi: 10.1111/j.1530-0277.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Martner A, Dahlgren C, Paton JC, Wold AE. Pneumolysin released during Streptococcus pneumoniae autolysis is a potent activator of intracellular oxygen radical production in neutrophils. Infect. Immun. 2008;76:4079–4087. doi: 10.1128/IAI.01747-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martner A, Skovbjerg S, Paton JC, Wold AE. Streptococcus pneumoniae autolysis prevents phagocytosis and production of phagocyte-activating cytokines. Infect. Immun. 2009;77:3826–3837. doi: 10.1128/IAI.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason CM, Dobard E, Kolls JK, Nelson S. Ethanol and murine interleukin (IL)-12 production. Alcohol. Clin. Exp. Res. 2000;24:553–559. [PubMed] [Google Scholar]
- McCashland TM, Preheim LC, Gentry MJ. Pneumococcal vaccine response in cirrhosis and liver transplantation. J. Infect. Dis. 2000;181:757–760. doi: 10.1086/315245. [DOI] [PubMed] [Google Scholar]
- McMahon BJ, Parkinson AJ, Bulkow L, Davidson M, Wainwright K, Wolfe P, Schiffman GS. Immunogenicity of the 23-valent pneumococcal polysaccharide vaccine in Alaska Native chronic alcoholics compared with nonalcoholic Native and non-Native controls. Am. J. Med. 1993;95:589–594. doi: 10.1016/0002-9343(93)90354-r. [DOI] [PubMed] [Google Scholar]
- McMahon BJ, Parkinson AJ, Rudolph K, Davidson M, Grabman J. Sepsis due to Streptococcus pneumoniae in a patient with alcoholism who received pneumococcal vaccine. Clin. Infect. Dis. 1999;28:1162–1163. doi: 10.1086/517764. [DOI] [PubMed] [Google Scholar]
- Mellencamp MA, Preheim LC. Pneumococcal pneumonia in a rat model of cirrhosis: effects of cirrhosis on pulmonary defense mechanisms against Streptococcus pneumoniae. J. Infect. Dis. 1991;163:102–108. doi: 10.1093/infdis/163.1.102. [DOI] [PubMed] [Google Scholar]
- Mitchell AM, Mitchell TJ. Streptococcus pneumoniae: virulence factors and variation. Clin. Microbiol. Infect. 2010;16:411–418. doi: 10.1111/j.1469-0691.2010.03183.x. [DOI] [PubMed] [Google Scholar]
- Mitchell TJ, Andrew PW, Saunders FK, Smith AN, Boulnois GJ. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute-phase protein. Mol. Microbiol. 1991;5:1883–1888. doi: 10.1111/j.1365-2958.1991.tb00812.x. [DOI] [PubMed] [Google Scholar]
- Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin. Immunol. 2002;14:123–132. doi: 10.1006/smim.2001.0349. [DOI] [PubMed] [Google Scholar]
- Mogensen TH, Paludan SR, Kilian M, Ostergaard L. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J. Leukoc. Biol. 2006;80:267–277. doi: 10.1189/jlb.1105626. [DOI] [PubMed] [Google Scholar]
- Moscoso M, Claverys JP. Release of DNA into the medium by competent Streptococcus pneumoniae: kinetics, mechanism and stability of the liberated DNA. Mol. Microbiol. 2004;54:783–794. doi: 10.1111/j.1365-2958.2004.04305.x. [DOI] [PubMed] [Google Scholar]
- Nagy LE. Acute and chronic ethanol exposure. Preface. Methods Mol. Biol. 2008;447:v–vi. doi: 10.1007/978-1-59745-242-7. [DOI] [PubMed] [Google Scholar]
- Nelson AL, Roche AM, Gould JM, Chim K, Ratner AJ, Weiser JN. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect. Immun. 2007;75:83–90. doi: 10.1128/IAI.01475-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S, Bagby G, Summer WR. Alcohol suppresses lipopolysaccharide-induced tumor necrosis factor activity in serum and lung. Life Sci. 1989;44:673–676. doi: 10.1016/0024-3205(89)90472-4. [DOI] [PubMed] [Google Scholar]
- Nelson S, Belknap SM, Carlson RW, Dale D, DeBoisblanc B, Farkas S, Fotheringham N, Ho H, Marrie T, Movahhed H, et al. A randomized controlled trial of filgrastim as an adjunct to antibiotics for treatment of hospitalized patients with community-acquired pneumonia. CAP Study Group. J. Infect. Dis. 1998;178:1075–1080. doi: 10.1086/515694. [DOI] [PubMed] [Google Scholar]
- Nelson S, Summer W, Bagby G, Nakamura C, Stewart L, Lipscomb G, Andresen J. Granulocyte colony-stimulating factor enhances pulmonary host defenses in normal and ethanol-treated rats. J. Infect. Dis. 1991;164:901–906. doi: 10.1093/infdis/164.5.901. [DOI] [PubMed] [Google Scholar]
- Noske N, Kammerer U, Rohde M, Hammerschmidt S. Pneumococcal interaction with human dendritic cells: phagocytosis, survival, and induced adaptive immune response are manipulated by PavA. J. Immunol. 2009;183:1952–1963. doi: 10.4049/jimmunol.0804383. [DOI] [PubMed] [Google Scholar]
- Nurmi K, Methuen T, Maki T, Lindstedt KA, Kovanen PT, Sandler C, Eklund KK. Ethanol induces apoptosis in human mast cells. Life Sci. 2009;85:678–684. doi: 10.1016/j.lfs.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J. Immunol. 2006;176:7628–7635. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- Olsen KM, Gentry-Nielsen M, Yue M, Snitily MU, Preheim LC. Effect of ethanol on fluoroquinolone efficacy in a rat model of pneumococcal pneumonia. Antimicrob. Agents Chemother. 2006;50:210–219. doi: 10.1128/AAC.50.1.210-219.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidvari K, Casey R, Nelson S, Olariu R, Shellito JE. Alveolar macrophage release of tumor necrosis factor-alpha in chronic alcoholics without liver disease. Alcohol. Clin. Exp. Res. 1998;22:567–572. doi: 10.1111/j.1530-0277.1998.tb04294.x. [DOI] [PubMed] [Google Scholar]
- Ortqvist A, Hedlund J, Wretlind B, Carlstrom A, Kalin M. Diagnostic and prognostic value of interleukin-6 and C-reactive protein in community-acquired pneumonia. Scand. J. Infect. Dis. 1995;27:457–462. doi: 10.3109/00365549509047046. [DOI] [PubMed] [Google Scholar]
- Pelaez A, Bechara RI, Joshi PC, Brown LA, Guidot DM. Granulocyte/macrophage colony-stimulating factor treatment improves alveolar epithelial barrier function in alcoholic rat lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;289:L106–111. doi: 10.1152/ajplung.00148.2003. [DOI] [PubMed] [Google Scholar]
- Perez-Castellano M, Penaranda M, Payeras A, Mila J, Riera M, Vidal J, Pujalte F, Pareja A, Villalonga C, Matamoros N. Mannose-binding lectin does not act as an acute-phase reactant in adults with community-acquired pneumococcal pneumonia. Clin. Exp. Immunol. 2006;145:228–234. doi: 10.1111/j.1365-2249.2006.03140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, Soudais C, Dupuis S, Feinberg J, Fieschi C, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- Pirovino M, Lydick E, Grob PJ, Arrenbrecht S, Altorfer J, Schmid M. Pneumococcal vaccination: the response of patients with alcoholic liver cirrhosis. Hepatology. 1984;4:946–949. doi: 10.1002/hep.1840040527. [DOI] [PubMed] [Google Scholar]
- Plackett TP, Kovacs EJ. Acute models of ethanol exposure to mice. Methods Mol. Biol. 2008;447:3–9. doi: 10.1007/978-1-59745-242-7_1. [DOI] [PubMed] [Google Scholar]
- Preheim LC, Gentry MJ, Snitily MU. Pulmonary recruitment, adherence, and chemotaxis of neutrophils in a rat model of cirrhosis and pneumococcal pneumonia. J. Infect. Dis. 1991;164:1203–1206. doi: 10.1093/infdis/164.6.1203. [DOI] [PubMed] [Google Scholar]
- Preheim LC, Mellencamp MA, Snitily MU, Gentry MJ. Effect of cirrhosis on the production and efficacy of pneumococcal capsular antibody in a rat model. Am. Rev. Respir. Dis. 1992;146:1054–1058. doi: 10.1164/ajrccm/146.4.1054. [DOI] [PubMed] [Google Scholar]
- Preheim LC, Olsen KM, Yue M, Snitily MU, Gentry-Nielsen MJ. Effect of cirrhosis on antibiotic efficacy in a rat model of pneumococcal pneumonia. Diagn. Microbiol. Infect. Dis. 2005;51:103–111. doi: 10.1016/j.diagmicrobio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Preheim LC, Snitily MU, Gentry MJ. Effects of granulocyte colony-stimulating factor in cirrhotic rats with pneumococcal pneumonia. J. Infect. Dis. 1996;174:225–228. doi: 10.1093/infdis/174.1.225. [DOI] [PubMed] [Google Scholar]
- Propst-Graham KL, Preheim LC, Vander Top EA, Snitily MU, Gentry-Nielsen MJ. Cirrhosis-induced defects in innate pulmonary defenses against Streptococcus pneumoniae. BMC Microbiol. 2007;7:94. doi: 10.1186/1471-2180-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett BS, Pruett SB. An explanation for the paradoxical induction and suppression of an acute phase response by ethanol. Alcohol. 2006;39:105–110. doi: 10.1016/j.alcohol.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q, Schwab C. Differences in IL-10 and IL-12 production patterns and differences in the effects of acute ethanol treatment on macrophages in vivo and in vitro. Alcohol. 2005;37:1–8. doi: 10.1016/j.alcohol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Schwab C, Zheng Q, Fan R. Suppression of innate immunity by acute ethanol administration: a global perspective and a new mechanism beginning with inhibition of signaling through TLR3. J. Immunol. 2004a;173:2715–2724. doi: 10.4049/jimmunol.173.4.2715. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol. 2004b;33:147–155. doi: 10.1016/j.alcohol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Raasch CE, Zhang P, Siggins RW, 2nd, Lamotte LR, Nelson S, Bagby GJ. Acute Alcohol Intoxication Impairs the Hematopoietic Precursor Cell Response to Pneumococcal Pneumonia. Alcohol. Clin. Exp. Res. 2010 doi: 10.1111/j.1530-0277.2010.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijneveld AW, Lauw FN, Schultz MJ, Florquin S, Te Velde AA, Speelman P, Van Deventer SJ, Van Der Poll T. The role of interferon-gamma in murine pneumococcal pneumonia. J. Infect. Dis. 2002;185:91–97. doi: 10.1086/338122. [DOI] [PubMed] [Google Scholar]
- Roselle GA, Mendenhall CL, Grossman CJ, Weesner RE. Lymphocyte subset alterations in patients with alcoholic hepatitis. J. Clin. Lab. Immunol. 1988;26:169–173. [PubMed] [Google Scholar]
- Roy S, Hill AV, Knox K, Griffiths D, Crook D. Research pointers: Association of common genetic variant with susceptibility to invasive pneumococcal disease. BMJ. 2002a;324:1369. doi: 10.1136/bmj.324.7350.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Knox K, Segal S, Griffiths D, Moore CE, Welsh KI, Smarason A, Day NP, McPheat WL, Crook DW, Hill AV. MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet. 2002b;359:1569–1573. doi: 10.1016/S0140-6736(02)08516-1. [DOI] [PubMed] [Google Scholar]
- Saitz R, Ghali WA, Moskowitz MA. The impact of alcohol-related diagnoses on pneumonia outcomes. Arch. Intern. Med. 1997;157:1446–1452. [PubMed] [Google Scholar]
- Samokhvalov AV, Irving HM, Rehm J. Alcohol consumption as a risk factor for pneumonia: a systematic review and meta-analysis. Epidemiol. Infect. 2010;138:1789–1795. doi: 10.1017/S0950268810000774. [DOI] [PubMed] [Google Scholar]
- Sander M, Irwin M, Sinha P, Naumann E, Kox WJ, Spies CD. Suppression of interleukin-6 to interleukin-10 ratio in chronic alcoholics: association with postoperative infections. Intensive Care Med. 2002;28:285–292. doi: 10.1007/s00134-001-1199-9. [DOI] [PubMed] [Google Scholar]
- Schaaf BM, Boehmke F, Esnaashari H, Seitzer U, Kothe H, Maass M, Zabel P, Dalhoff K. Pneumococcal septic shock is associated with the interleukin-10–1082 gene promoter polymorphism. Am. J. Respir. Crit. Care. Med. 2003;168:476–480. doi: 10.1164/rccm.200210-1164OC. [DOI] [PubMed] [Google Scholar]
- Segura E, Villadangos JA. Antigen presentation by dendritic cells in vivo. Curr. Opin. Immunol. 2009;21:105–110. doi: 10.1016/j.coi.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- Seppa K, Sillanaukee P, Saarni M. Blood count and hematologic morphology in nonanemic macrocytosis: differences between alcohol abuse and pernicious anemia. Alcohol. 1993;10:343–347. doi: 10.1016/0741-8329(93)90018-j. [DOI] [PubMed] [Google Scholar]
- Shellito JE, quan Zheng M, Ye P, Ruan S, Shean MK, Kolls J. Effect of alcohol consumption on host release of interleukin-17 during pulmonary infection with Klebsiella pneumoniae. Alcohol. Clin. Exp. Res. 2001;25:872–881. [PubMed] [Google Scholar]
- Simberkoff MS, Schiffman GS, Spicehandler JR, Moldover NH, Rahal JJ., Jr. Radioimmunoassay and opsonic antibody responses to pneumococcal capsular polysaccharide vaccine in serum and ascitic fluid of cirrhotic patients. J. Clin. Microbiol. 1983;18:154–159. doi: 10.1128/jcm.18.1.154-159.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JH. Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. Am. J. Physiol. 1995;268:L596–600. doi: 10.1152/ajplung.1995.268.4.L596. [DOI] [PubMed] [Google Scholar]
- Sisson JH, May K, Wyatt TA. Nitric oxide-dependent ethanol stimulation of ciliary motility is linked to cAMP-dependent protein kinase (PKA) activation in bovine bronchial epithelium. Alcohol. Clin. Exp. Res. 1999;23:1528–1533. [PubMed] [Google Scholar]
- Sjostrom K, Spindler C, Ortqvist A, Kalin M, Sandgren A, Kuhlmann-Berenzon S, Henriques-Normark B. Clonal and capsular types decide whether pneumococci will act as a primary or opportunistic pathogen. Clin. Infect. Dis. 2006;42:451–459. doi: 10.1086/499242. [DOI] [PubMed] [Google Scholar]
- Smith WI, Jr., Van Thiel DH, Whiteside T, Janoson B, Magovern J, Puet T, Rabin BS. Altered immunity in male patients with alcoholic liver disease: evidence for defective immune regulation. Alcohol. Clin. Exp. Res. 1980;4:199–206. doi: 10.1111/j.1530-0277.1980.tb05635.x. [DOI] [PubMed] [Google Scholar]
- Soininen A, Pursiainen H, Kilpi T, Kayhty H. Natural development of antibodies to pneumococcal capsular polysaccharides depends on the serotype: association with pneumococcal carriage and acute otitis media in young children. J. Infect. Dis. 2001;184:569–576. doi: 10.1086/322794. [DOI] [PubMed] [Google Scholar]
- Standiford TJ, Danforth JM. Ethanol feeding inhibits proinflammatory cytokine expression from murine alveolar macrophages ex vivo. Alcohol. Clin. Exp. Res. 1997;21:1212–1217. [PubMed] [Google Scholar]
- Sun K, Salmon SL, Lotz SA, Metzger DW. Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect. Immun. 2007;75:1196–1202. doi: 10.1128/IAI.01403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Catalano D, White B, Mandrekar P. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alcohol. Clin. Exp. Res. 2004;28:824–828. doi: 10.1097/01.alc.0000127104.80398.9b. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P. Human monocytes, macrophages, and dendritic cells: alcohol treatment methods. Methods Mol. Biol. 2008;447:113–124. doi: 10.1007/978-1-59745-242-7_9. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Girouard L, Catalano D. Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-alpha, interleukin-1 beta and elevated interleukin-10, and transforming growth factor-beta production. Alcohol. Clin. Exp. Res. 1996;20:900–907. doi: 10.1111/j.1530-0277.1996.tb05269.x. [DOI] [PubMed] [Google Scholar]
- Takashima K, Tateda K, Matsumoto T, Iizawa Y, Nakao M, Yamaguchi K. Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumonia in mice. Infect. Immun. 1997;65:257–260. doi: 10.1128/iai.65.1.257-260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Rudolph D, Du Clos TW, Snapper CM, Mold C. C-reactive protein enhances immunity to Streptococcus pneumoniae by targeting uptake to Fc gamma R on dendritic cells. J. Immunol. 2007;178:7283–7291. doi: 10.4049/jimmunol.178.11.7283. [DOI] [PubMed] [Google Scholar]
- Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- Travis SM, Singh PK, Welsh MJ. Antimicrobial peptides and proteins in the innate defense of the airway surface. Curr. Opin. Immunol. 2001;13:89–95. doi: 10.1016/s0952-7915(00)00187-4. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, French SW. Evolution of intragastric ethanol infusion model. Alcohol. 1993;10:437–441. doi: 10.1016/0741-8329(93)90060-2. [DOI] [PubMed] [Google Scholar]
- van der Poll T, de Waal Malefyt R, Coyle SM, Lowry SF. Antiinflammatory cytokine responses during clinical sepsis and experimental endotoxemia: sequential measurements of plasma soluble interleukin (IL)-1 receptor type II, IL-10, and IL-13. J. Infect. Dis. 1997;175:118–122. doi: 10.1093/infdis/175.1.118. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Marchant A, Keogh CV, Goldman M, Lowry SF. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J. Infect. Dis. 1996;174:994–1000. doi: 10.1093/infdis/174.5.994. [DOI] [PubMed] [Google Scholar]
- Vander Top EA, Wyatt TA, Gentry-Nielsen MJ. Smoke exposure exacerbates an ethanol-induced defect in mucociliary clearance of Streptococcus pneumoniae. Alcohol. Clin. Exp. Res. 2005;29:882–887. doi: 10.1097/01.alc.0000164364.35682.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dossow V, Schilling C, Beller S, Hein OV, von Heymann C, Kox WJ, Spies CD. Altered immune parameters in chronic alcoholic patients at the onset of infection and of septic shock. Crit. Care. 2004;8:R312–321. doi: 10.1186/cc2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterer GW, Quasney MW, Cantor RM, Wunderink RG. Septic shock and respiratory failure in community-acquired pneumonia have different TNF polymorphism associations. Am. J. Respir. Crit. Care Med. 2001;163:1599–1604. doi: 10.1164/ajrccm.163.7.2011088. [DOI] [PubMed] [Google Scholar]
- Weinberger DM, Harboe ZB, Sanders EA, Ndiritu M, Klugman KP, Ruckinger S, Dagan R, Adegbola R, Cutts F, Johnson HL, et al. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin. Infect. Dis. 2010;51:692–699. doi: 10.1086/655828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortham C, Grinberg L, Kaslow DC, Briles DE, McDaniel LS, Lees A, Flora M, Snapper CM, Mond JJ. Enhanced protective antibody responses to PspA after intranasal or subcutaneous injections of PspA genetically fused to granulocyte-macrophage colony-stimulating factor or interleukin-2. Infect. Immun. 1998;66:1513–1520. doi: 10.1128/iai.66.4.1513-1520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderink RG, Waterer GW, Cantor RM, Quasney MW. Tumor necrosis factor gene polymorphisms and the variable presentation and outcome of community-acquired pneumonia. Chest. 2002;121:87S. [PubMed] [Google Scholar]
- Wyatt TA, Forget MA, Sisson JH. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am. J. Pathol. 2003;163:1157–1166. doi: 10.1016/S0002-9440(10)63475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Droemann D, Rupp J, Shen H, Wu X, Goldmann T, Hippenstiel S, Zabel P, Dalhoff K. Modulation of the inflammatory response to Streptococcus pneumoniae in a model of acute lung tissue infection. Am. J. Respir. Cell Mol. Biol. 2008;39:522–529. doi: 10.1165/rcmb.2007-0328OC. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- Yother J, Volanakis JE, Briles DE. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in mice. J. Immunol. 1982;128:2374–2376. [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Xie M, Stoltz DA, Summer WR, Nelson S. Acute ethanol intoxication inhibits neutrophil beta2-integrin expression in rats during endotoxemia. Alcohol. Clin. Exp. Res. 1998;22:135–141. [PubMed] [Google Scholar]
- Zhang P, Nelson S, Summer WR, Spitzer JA. Acute ethanol intoxication suppresses the pulmonary inflammatory response in rats challenged with intrapulmonary endotoxin. Alcohol. Clin. Exp. Res. 1997;21:773–778. [PubMed] [Google Scholar]
- Zhang P, Zhong Q, Bagby GJ, Nelson S. Alcohol intoxication inhibits pulmonary S100A8 and S100A9 expression in rats challenged with intratracheal lipopolysaccharide. Alcohol. Clin. Exp. Res. 2007;31:113–121. doi: 10.1111/j.1530-0277.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Bagby GJ, Stoltz D, Oliver P, Schwarzenberger PO, Kolls JK. Prolonged ethanol treatment enhances lipopolysaccharide/phorbol myristate acetate-induced tumor necrosis factor-alpha production in human monocytic cells. Alcohol. Clin. Exp. Res. 2001;25:444–449. [PubMed] [Google Scholar]
- Zhao XJ, Marrero L, Song K, Oliver P, Chin SY, Simon H, Schurr JR, Zhang Z, Thoppil D, Lee S, et al. Acute alcohol inhibits TNF-alpha processing in human monocytes by inhibiting TNF/TNF-alpha-converting enzyme interactions in the cell membrane. J. Immunol. 2003;170:2923–2931. doi: 10.4049/jimmunol.170.6.2923. [DOI] [PubMed] [Google Scholar]


