Abstract
The Berkeley Pit, an acid mine waste lake, is a source of extremophilic microorganisms that produce interesting bioactive compounds. We have previously reported the isolation of berkeleydione 1, berkeleytrione 2, the berkeleyacetals and the berkeleyamides from the Pit Lake fungus Penicillium rubrum. In this paper we report the isolation and characterization of berkeleyones A-C (4, 5 and 7) as well as previously described preaustinoid A (3) and A1(6) from this same fungus. These compounds were evaluated as inhibitors of the signaling enzyme caspase-1 and as potential inhibitors of interleukin 1-β production by inflammasomes in induced THP-1 cell line assays.
Penicillium rubrum Stoll, an extremophilic fungus isolated from an acid mine waste lake in Montana has yielded several novel compounds including the two berkeleyones, berkeleydione (1) and berkeleytrione (2),1 the berkeleyacetals2 and the berkeleyamides.3 In our continuing studies of this extremophilic fungus 1-3 we have used signal transduction enzyme inhibition to guide the isolation of new compounds. In this case we combined bioassay-guided fractionation (inhibition of caspase-1) with NMR-guided fractionation to direct the isolation of three berkeleyone analogs (4, 5 and 7) as well as the known compounds preaustinoid A (3)4 and A1 (6),5 from this fungus.
Caspase-1, also known as interleukin-1 converting enzyme, is responsible for the activation of IL-1β and IL-18 from precursor molecules.6 Caspase-1 is activated upon binding to the inflammasome, a multiprotein complex that plays a key role in innate immunity by activating the proinflammatory pleiotropic cytokines interleukin 1-β and IL-18.6 There is a strong correlation between dysregulated inflammasome activity and both inherited and acquired inflammatory diseases.6
Recent studies have also shown that activation of the inflammasome might interfere with anticancer vaccines and be responsible for the disappointing performance of anticancer vaccines to date.7 One of the major protein components of most inflammasomes studied to date is NLRP3, which, upon activation (caspase-1 mediated release of interleukin 1-β), induces production of myeloid-derived suppressor cells in tumors (MDSC). MDSCs accumulate in the blood, lymph nodes and tumor sites of cancer patients and interfere with adaptive and innate immunity. Studies have found that NLRP3 was critical for accumulation of MDSCs in tumors and for inhibition of antitumor T-cell immunity after dendritic cell vaccination.7
For several years we have used caspase-1 inhibition assays to select for microbial metabolites with activity against leukemia cell lines. Growing awareness of the key roles the inflammasome and caspase-1 play in autoimmune disorders as well as their potential to interfere with anticancer vaccination protocols led us to evaluate caspase-1 inhibitors as potential mitigators of inflammation-related pathologies or of inflammasome-mediated events.
Penicillium rubrum was grown and extracted as described.1 Flash silica gel column chromatography followed by HPLC yielded berkeleydione (1) and berkeleytrione (2). After compounds 1 and 2 were isolated and characterized, the proton NMR spectra from both bioactive and inactive column fractions were examined for evidence of related analogs. Promising candidates were purified and elucidated. The previously reported preaustinoid A (3)4 and preaustinoid A1 (6)5 as well as three new berkeleyone analogs 4, 5 and 7, were isolated by this methodology.
Comparison of the 1H NMR and 13C NMR spectra of compounds 3-7 with those of 1 and 2 indicated that the C and D rings of all of the compounds were identical. In depth analysis of mass spectra, 1H-1H COSY, HSQC, HMBC, NOESY and NOE difference spectra provided adequate information to determine the structures and the relative configurations of 3-7.
HRESIMS yielded an [M+H]+ ion of m/z 447.2753, which established the molecular formula of 3 as C26H36O6 with nine degrees of unsaturation. This formula indicated an additional degree of saturation and one less oxygen than berkeleytrione 2. Comparison of the 1H NMR and 13C NMR spectra to those of the known compound preaustinoid A, which was also isolated from a Penicillium sp., and 3 indicated that the two compounds were identical.4
Berkeleyone A (4) had a molecular formula of C26H38O6 which was established by HREIMS and which indicated one more degree of saturation than 3. Although the 1H and 13C NMR chemical shifts of the B, C and D rings were virtually identical to those of 3, the 13C NMR spectrum (Table 1) indicated the presence of an additional oxygen-bearing methine at δC 78.2 (C-1) and the loss of a ketone carbon. These data suggested that the A ring ketone was reduced to a secondary alcohol in compound 4. The oxygen-bearing methine proton appeared as a doublet of doublets at δH 3.07 (J = 11.3, 4.2 Hz) and was coupled to a complex two proton multiplet at δH 1.50. The oxygen-bearing methine showed strong 3-bond coupling in the HMBC experiment to the gem-dimethyls at C-16 (δH 0.92, 0.71), confirming the position of the alcohol at C-1. The relative stereoconfiguration of 4 was established by a two-dimensional NOESY experiment followed by one-dimensional difference NOE studies. Specifically, 4 showed mutual NOE enhancements of the H3-25, H3-19 and H3-18 axial methyl protons, as well as mutual enhancement of the 1,3-diaxial methine protons H-1 and H-15.
Table 1.
1H and 13C NMR Data for Berkeleyones A-C, 4, 5, 7(CDCl3)a
| berkeleyone A, 4 | berkeleyone B, 5 | berkeleyone C, 7 | ||||
|---|---|---|---|---|---|---|
| no. | δC multd | δH (J in Hz)c | δC, mult.d | δH (J in Hz)c | δC, mult.d | δH (J in Hz)c |
| 1 | 78.2, CH | 3.07, dd (11.3, 4.2) | b178.2, C | 177.4, C | ||
| 2 | 26.8, CH2 | 1.50, m | 29.7, CH2 | α 1.80, m β 1.90, m |
29.1, CH2 | α 2.00, m β 1.80, m |
| 3 | 38.4, CH2 | α 1.57, m β 0.61, m |
33.7, CH2 | α 2.25, m β 1.40, m |
33.3, CH2 | 1.6, m |
| 4 | 37.7, C | 43.0, C | 40.8, C | |||
| 5 | 52.7, CH | 0.49, m | 43.8, CH | 0.85, dd (12.5, 2.0) | 41.0, CH | 1.00, dd (13.5, 3.4) |
| 6 | 39.0, CH2 | α 1.60, m β 1.90, dd (13.2, 3.0) |
41.4, CH2 | α 1.70, t (13.2) β 2.00, m |
41.2, CH2 | α 1.70, m β 2.00, m |
| 7 | 51.0, C | 51.2, C | 51.2, C | |||
| 8 | 207.9, C | 207.4, C | 207.5, C | |||
| 9 | 79.9, C | 80.0, C | 80.3, C | |||
| 10 | 204.1, C | 204.0, C | 204.0, C | |||
| 11 | 72.5, C | 71.8, C | 71.8, C | |||
| 12 | 47.9, C | 45.9, C | 46.2, C | |||
| 13 | 32.9, CH2 | α 2.18, dt (13.7, 3.5) β 2.00, td (13.7, 3.7) |
31.2, CH2 | α 2.62, bd (17.6, 2.1) β 2.44, dd (17.6, 7.1) |
31.1, CH2 | α 2.71, bd (18.2) β 2.45, dd (18.2, 6.7) |
| 14 | 18.2, CH2 | α 1.40, m β 1.60, m |
123.9, CH | 5.70, dd (7.1, 2.1) | 125.5, CH | 5.59, dd (6.7, 2.3) |
| 15 | 54.7, CH | 0.49, m | 144.5, C | 142.4, C | ||
| 16 | 38.7, C | 75.9, C | 145.4, C | |||
| 17 | 27.8, CH3 | 0.92, s | 35.5, CH3 | 1.40, s | 26.4, CH3 | 1.81, s |
| 18 | 15.5, CH3 | 0.71, s | 33.8, CH3 | 1.36, s | 114.9, CH2 | 4.86, bs 4.60, bs |
| 19 | 17.2, CH3 | 1.19, s | 15.5, CH3 | 1.18, s | 16.3, CH3 | 1.22, s |
| 20 | 168.6, C | 168.6, C | 168.5, C | |||
| 21 | 15.2, CH3 | 1.36, s | 15.0, CH3 | 1.31, s | 15.0, CH3 | 1.33, s |
| 22 | 145.6, C | 145.4, C | 145.7, C | |||
| 23 | 112.4, CH2 | 5.34, bs 4.83, bs |
112.4, CH2 | 5.39, bs 4.87, bs |
112.4, CH2 | 5.39, bs 4.88, bs |
| 24 | 22.1, CH3 | 1.44, s | 22.4, CH3 | 1.49, s | 22.5, CH3 | 1.48, s |
| 25 | 15.8, CH3 | 0.76, s | 21.4, CH3 | 1.18, s | 22.4, CH3 | 0.93, s |
| OCH3 | 52.5, CH3 | 3.70, s | 52.6, CH3 | 3.71, s | 52.6, CH3 | 3.72, s |
All assignments are based on COSY, NOE, HSQC and HMBC experiments.
Required longer D1.
300 MHz for 1H NMR, HMBC
75 MHz for 13C NMR.
The molecular formula of berkeleyone B (5) was established as C26H34O7 by HREIMS which yielded a [M]+ ion at m/z 458.2313 and ten degrees of unsaturation. The typical berkeleyone methylene protons H2-2 and gem-dimethyl protons H3-17 and H3-18 showed long range correlations in the HMBC spectrum to C-1 (δC 178.2) suggesting that the A ring was a lactone. H3-17 and H3-18 were further coupled to the oxygen-bearing quaternary carbon C-16. The H2-2 methylene protons (δH 1.90, 1.80) were clearly J-coupled to diastereotopic methylene protons H-3, (δH 2.25, 1.40) and showed 3-bond correlations (HMBC) to quaternary carbon C-4 (δC 43.0). These correlations established the A-B ring system of 5 as a seven-six ring system, rather than the six-seven ring system of berkeleydione 1. The austalides, isolated from Aspergillus ustus, have an A-B ring system similar to that of 5.8
The molecular formula of compound 6 indicated the presence of two more protons than compound 5. Consideration of the spectral data indicated that 6 was a dihydro-derivative of 5 and identical to the known preaustinoid A1, previously isolated from a Penicillium sp.5
A HREIMS [M]+ peak at m/z 458.2318 indicated that the molecular formula of berkeleyone C (7) was C26H34O7 with ten degrees of unsaturation. Although this compound was isomeric with berkeleyone B, the NMR spectra showed key differences (Table 1). The B, C and D rings were intact, including the C-20 methyl ester, which accounted for eight degrees of unsaturation. The IR spectrum indicated the presence of a carboxylic acid (3528, 1710 cm−1) which was verified by the formation of a dimethyl ester (8) when 7 was treated with diazomethane. Berkeleyone C also contained a second 1,1-disubstituted double bond as seen in the NMR spectra (δC 145.4, C and 114.9, CH2). These two functionalities provided the remaining degrees of unsaturation. All of these data could be accounted for by an opened A ring. All of the HMBC correlations were consistent with this structure. When the chemical shifts of the opened A ring of 7 were compared to those reported for the lanostanoid, elfvingic acid H isolated from the fungus, Elfvingia applanata, these compared very closely (S9, Supporting Information).9 The NOE difference spectra indicated the same relative configuration as that found in the other berkeleyones.
Establishing the absolute configuration of these compounds has been interesting. Although the structure of berkeleydione 1 was confirmed by x-ray crystallography, the data was not sufficiently refined to allow determination of absolute stereoconfiguration.1 Recently the helicity rule of circular dichroism for cisoid homoannular dienes was applied to determine the absolute configuration of 22-epoxyberkeleydione.10 The same approach was used for 1. The negative Cotton effect observed at 267 nm indicates that the diene assumes a left-handed twist.11 In the ORTEP structure of compound 1 a left-handed twist (43°) in the B ring homoannular diene is consistent with the structure as shown and provides the same absolute configuration as 22-epoxyberkeleydione.11 This is also consistent with recently reported dhilirolides A-D.12 Their absolute configurations were determined by single crystal x-ray analysis of dhilirolide A. Careful comparison of berkeleydione 1 and dhilirolide D showed the same stereoconfigurations at C-5, 7, 11 and 12.12 These data supported the absolute configuration of compound 1 as shown.
The structure and relative stereoconfiguration of preaustinoid A 3 was originally determined in 2002 by spectroscopic methods.4 In a 2009 publication that reported the x-ray structure of preaustinoid A the authors stated that the absolute configuration of the compound was established by the optical rotation reported in the original paper and not by x-ray analysis.13 Optical rotation is generally not sufficient to determine the absolute configuration of a molecule unless it is being compared to a molecule with a clearly defined absolute configuration.14 This was not the case for compound 3.4,13
Of the meroterpenoids that have been reported to date15 compounds 1-7 appear most closely related biosynthetically to the andrastins and citreohybridones.16-18 The absolute configuration of andrastin A was determined by x-ray analysis of the p-bromobenzoyl derivative16 and that of the citreohybridones was determined by the modified Mosher method.17 It is interesting to note that structural variations within the citreohybridones result in wide variations in optical rotation from [α]D −80.5 for citreohybridone D18 to [α]D +85.5 for citreohybridone J.17 Unfortunately, our attempts to use the modified Mosher method on compound 2 were not successful. However, comparison of compounds 1-7 with the andrastins, citreohybridones and berkeleydione has led us to adopt the configurations shown for compounds 1-7.
Compound 1 and 3-7 were evaluated for their ability to inhibit caspase-1 in vitro. Caspase-1 inhibition was determined in a fluorometric assay and percent enzyme inhibition for each compound was determined at 100 μg/mL. Each compound was then evaluated for its ability to mitigate the production of interleukin 1-β in THP-1 cells (pro-monocytic leukemia cell line). Exposure of THP-1 cells to titanium nanowires and bacterial lipopolysaccharide (LPS) resulted in the formation of large numbers of inflammasomes, which in turn produced high levels of IL-1β. Induced THP-1 cells were exposed to compounds 1 and 3-7 and the concentrations of IL-1β post-exposure were determined to establish an IC50 value for each compound (Table 2). It is interesting to note that the inhibitor included in the caspase-1 assay kit (Ac-YVAD-CHO) is several orders of magnitude more potent than the berkeleyones in the enzyme assay, but is comparable to the more potent compounds in the inflammasome assay.
Table 2.
Determination of Inhibition of Caspase-1 and Mitigation of Interleukin-1β Production in Induced THP-1 Cells
| Compound | IL-1β (IC50, μM |
caspase-1a (100 μg/mL) |
|---|---|---|
| Berkeleydione 1 | 4.4 | 89 |
| Preaustinoid A 3 | 15.5 | 97 |
| Berkeleyone A 4 | 2.7 | 68 |
| Berkeleyone B 5 | 3.7 | 100 |
| Preaustinoid A1 6 | 34.3 | 77 |
| Berkeleyone C7 | 37.8 | 0 |
| Ac-YVAD-CHO | 2.0 | 100b |
% inhibition of caspase-1 activity
Ac-YVAD-CHO was tested at 0.0005 μg/mL
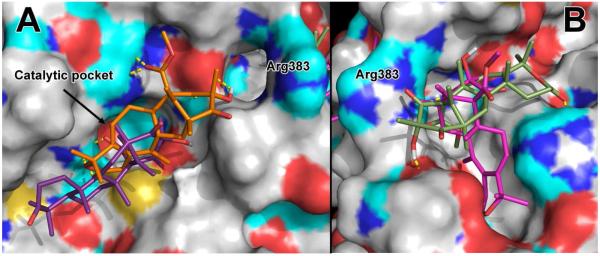
Docking studies were carried out to examine the possible interactions between caspase-1, an important active site in the intact inflammasome, and compounds 1-7. A variety of wild-type holo crystal structures were used in an ensemble fashion in order to avoid ligand tuning. These were selected from the PDB19 based on structural diversity of the co-crystallized ligand: 1RWX,20 1ICE,21 2HBQ,22 1RWK,23 1RWV, 1BMQ,24 and 1ICE.25 The fluorinated substrate assay control molecule Ac-YVAD-AMC was docked against the same ensemble. The top-scoring pose showed the same interactions and motif as the co-crystallized YVAD (1ICE), with the exception of the fluorescent tag, which is too large to fit into the catalytic pocket of caspase-1. Analysis of the top scoring clusters of poses across the family suggests a common site of potential binding. Binding motifs were examined for compounds 1-7. Figure 1A demonstrates such binding motifs of berkeleydione 1 and preaustinoid A 3. Both of these poses represent the collection of top scoring poses, which form interactions along the groove and at the opening to the catalytic site (S18,SI).
Fig 1.
Docking of berkeleydione 1 (orange, up conformation in A, pink, down conformation in B), preaustinoid A 3 (purple, A), berkeleyone B 2 (green, B) into 1RWV, showing the top scoring pose across the discussed ensemble of caspase-1 structures. Atoms in the protein are colored according to atom type (oxygen, red; nitrogen, blue; sulfur, yellow), with polar hydrogens colored cyan. Arg383, which bisects the active site groove, is indicated. The catalytic pocket can be seen behind the ligands in A.
The use of signal transduction enzymes to guide compound isolation has led to the discovery of promising bioactive metabolites. The rising importance of the inflammasome as a key component in the development of inflammation associated pathologies has provided the next logical step in the investigation of these compounds. The docking studies suggest that the berkeleyone analogs could inhibit caspase-1 by binding into the active site cleft. While they do not fit into the catalytic pocket, the number of favorable contacts made by the conformationally constrained ligands could displace the far more flexible and larger tetrapeptide YVAD or natural substrate. It is also interesting to note that the hydrogen-bond rich substituents of the berkeleyones are too short to reach into the catalytic pocket when the bulky fused ring system binds to the cleft. This suggests possible synthetic routes for increasing efficacy of this family, which is currently being explored.
Experimental Section
General Experimental Procedures
Collection, Extraction, and Isolation Procedures
The collection of water samples from the Berkeley Pit, the isolation of the various organisms, the pilot growths and biological testing of the extracts, and the fermentation and extraction of Penicillium rubrum have been previously described.1 The chloroform extract (1.13 g) was chromatographed in a gradient mode on a flash Si gel column with hexanes to which increasing amounts of IPA were added. The column was washed with 100% IPA and finally with MeOH. The fraction that eluted with 5% IPA/hexanes was further purified with Si gel HPLC using IPA/hexanes mixtures to give the known preaustinoid A 3 (2.5 mg), preaustinoid A1 6 (3.3 mg) and the three new berkeleyones 4 (3.1 mg), 5 (2.3 mg) and 7 (5.5 mg).
Berkeleydione (1): CD (2.55 × 10−5 M, MeOH), λmax (Δε) 318 (0.95), 267 (−3.56), 237 (14.27) nm.
Preaustinoid A (3): [α]25D −3.5 (c 0.17, CHCl3), [lit. [α]25D −4.97 (c 0.11, CH2Cl2)]4
Berkeleyone A (4): colorless oil; [α]25D −3.7 (c 0.27, MeOH); IR (CHCl3) νmax 3528, 2940, 2852, 1736, 1710, 1384, 1120 cm−1; 1H NMR and 13C NMR see Table 1; ESIMS m/z 447 (100) [M+1]+ , 429 (40), 411 (10); HREIMS m/z 447.2753 [M + H]+, (calcd for C26H39O6, 447.2747).
Berkeleyone B (5): colorless oil; [α]20D −14.3 (c 0.14, MeOH); IR (CHCl3) νmax 3532, 2928, 2856, 1734, 1710, 1130, 903 cm−1; 1H NMR and 13C NMR see Table 1; CIMS (NH3) m/z 459 (80), 441 (60), 329 (71), 233 (100); HREIMS m/z 458.2313 [M]+, (calcd for C26H34O7, 458.2304).
Preaustinoid A1 (6): [α]25D −41.7 (c 0.06, CHCl3), [lit. [α]25D −25.5 (c 1.7, CH2Cl2]5
Berkeleyone C (7): colorless oil; [α]20D −25.4 (c 0.44, MeOH); IR (CHCl3) νmax 3528, 3029, 2953, 1734, 1710, 1456, 1383, 1126, 908 cm−1; 1H NMR and 13C NMR see Table 1; HREIMS m/z 458.2318 [M]+, (calcd for C26H34O7, 458.2304).
Methylation of Berkeleyone C (7)
Berkeleyone C (7, 0.2 mg) was dissolved in MeOH (200 μL) and a solution of diazomethane in ether added dropwise until the yellow color remained. The reaction was stirred for two more minutes and the solvents removed to give the methyl ester, 8, as an oil. 1H NMR (CDCl3, 500 MHz) δ 5.58 (dd, J= 6.8, 2.3 Hz, H-14), 5.39 (brs, H-21), 4.88 (brs, H-21), 4.86 (brs, H-18), 4.59 (brs, H-18), 3.72 (s, 3H, OCH3), 3.61 (s, 3H, OCH3), 2.68 (brd, H-13), 2.44 (dd, J= 6.6, 17.9 Hz, H-13), 2.02-1.93 (m, 2 H), 1.81 (brs, 3H, H-17), 1.80 - 1.58 (m, 4 H), 1.50 (s, 3H, H-23), 1.33 (s, 3H, H-22), 1.22 (s, 3H, H-19), 1.00 (dd, J= 3.0, 13.1 Hz, H-5), 0.92 (s, 3H, H-24); APCIMS m/z 473 [M+H]+, m/z 471 [M-H]−.
In Vitro Assay
Human monocyte cell line THP-1 was purchased from ATCC (#TIB-202), The cells were suspended at 2-4 × 105 viable cells/ml in RPMI media supplemented with 10% fetal bovine serum, 0.05 mM 2-mercaptoethanol, sodium pyruvate, supplemented with an antimycotic/antibiotic cocktail (Mediatech, VWR). The cells were differentiated into macrophage-like cells by the phorbol ester, PMA (1 μg/mL, Sigma, St. Louis, MO), 24 hour prior to experimentation. The transformed cells were removed from the flask by scraping, centrifuged at 450 × g for 5 min. The resulting cell pellet was suspended at 1.0 × 106 cells/mL and exposed to caspase-1 inhibitors at concentrations described below (0.5%-0.005%), LPS [20 ng/mL] and TiO2 nanowires (100 μg/ml). Experiments were conducted in 96-well plates for 24 h in 37° C water-jacketed CO2 incubators (ThermoForma).
Toxicity Assay
Cell viability was determined by MTS reagent using the CellTiter96 assay (Promega), according to the manufacturer’s protocol. The plate was read at 490 nm.
Cytokine Assays
Human IL-1β DuoSet was obtained from R&D Systems and ELISA assays performed according to the manufacturer’s protocol. The plate was read at 490 nm.
Protein Docking
Proteins were prepared by adding hydrogens and removing waters. Geometries and residues were checked for library values. Docking was carried out using GOLD26 with 150% search efficiency. Ligands were docked with full flexibility (all flexible options enabled). The active site was defined by a 6Å radius around the co-crystallized ligand of 1RWX. Poses were scored with GOLDscore26 using default parameters and assessed as groups of poses.
Supplementary Material
Acknowledgment
We thank Ms. B. Parker (University of Montana) for high resolution mass spectrometry data. We thank NSF grant #CHE-9977213 for acquisition of an NMR spectrometer and the MJ Murdock Charitable Trust Ref # 99009:JVZ:11/18/99 for acquisition of the mass spectrometer. The project described was supported by NIH grants # P20RR16455-04 and P20RR017670 from the National Center for Research Resources, and grants # R01CA139159, 5P30NS055022 and RC2ES018742.
Footnotes
Supporting Information Available: Experimental details including 1H NMR, 13C NMR, COSY, HMBC and HSQC spectra for berkeleyones 4, 5 and 7. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Stierle D, Stierle A, Hobbs JD, Stokken J, Clardy J. Org. Lett. 2004;6:1049–1052. doi: 10.1021/ol049852k. [DOI] [PubMed] [Google Scholar]
- (2).Stierle D, Stierle A, Patacini B. J. Nat. Prod. 2007;70:1820–1823. doi: 10.1021/np070329z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Stierle D, Stierle A, Patacini B. J. Nat. Prod. 2008;71:856–860. doi: 10.1021/np0705054. [DOI] [PubMed] [Google Scholar]
- (4).Santos RMG, Rodrigues-Fo E. Phytochemistry. 2002;61:907–912. doi: 10.1016/s0031-9422(02)00379-5. [DOI] [PubMed] [Google Scholar]
- (5).Santos RMG, Rodrigues-Fo E. Z. Naturforsch. C. 2003;58:663–669. doi: 10.1515/znc-2003-9-1012. [DOI] [PubMed] [Google Scholar]
- (6).Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. Nat. Immunol. 2009;10:241–256. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).van Deventer HW, Burgents JE, Wu QP, Woodford R-MT, Brickey WJ, Allen IC, McElvania-Tekippe E, Serody JS, Ting JP-Y. Cancer Res. 2010;70:10161–10169. doi: 10.1158/0008-5472.CAN-10-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Horak RM, Steyn PS, Vlaggeer R, Rabbie CJ. J. Chem. Soc. Perkin Trans. I. 1985:363–367. [Google Scholar]
- (9).Yoshikawa K, Nishimura N, Bando S, Arihara S, Matsumaura E, Katayama S. J. Nat. Prod. 2002;65:548–552. doi: 10.1021/np0103160. [DOI] [PubMed] [Google Scholar]
- (10).Iida M, Ooi T, Kito K, Yoshida S, Kanoh K, Shizuri Y, Kusumi T. Org. Lett. 2008;10(5):845–848. doi: 10.1021/ol7029867. [DOI] [PubMed] [Google Scholar]
- (11).Moscowitz A, Charney E, Weiss U, Ziffer H. J. Am. Chem. Soc. 1961;83:4661–4663. [Google Scholar]
- (12).de Silva ED, Williams DE, Jayanetti DR, Centko RM, Patrick BO, Wijesundera RLC, Andersen RJ. Org. Lett. 2011;13:1174–1177. doi: 10.1021/ol200031t. [DOI] [PubMed] [Google Scholar]
- (13).Maganhi SH, Fill TP, Rodrigues-Fo E, Caracellib I, Zukerman-Schpector J. Acta Cryst. 2009:E65, o221. doi: 10.1107/S1600536808043481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Stephens PJ, Devlin FJ, Cheeseman JR, Frisch MJ, Rosini C. Org. Lett. 2002;4:4595–4598. doi: 10.1021/ol0201714. [DOI] [PubMed] [Google Scholar]
- (15).Geris R, Simpson TJ. Nat. Prod. Rep. 2009;26:1063–1094. doi: 10.1039/b820413f. [DOI] [PubMed] [Google Scholar]
- (16).Shiomi K, Uchida R, Inokoshi J, Tanaka H, Iwai Y, Omura S. Tetrahedron Lett. 1996;37:1265–1268. [Google Scholar]
- (17).Kosemura S. Tetrahedron Lett. 2002;43:1253–1256. [Google Scholar]
- (18).Kosemura S, Yamamura S. Tetrahedron Lett. 1997;38:6221–6224. [Google Scholar]
- (19).Bernstein FC, Koetzle TF, Williams GF, Meyer EE, Jr., Brice MD, Rodgers JR, Kennard O, Shimanouchi T, Tasumi M. J. Mol. Biol. 1977;112:535. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- (20).Fahr BT, O’Brien T, Pham P, Waal ND, Baskaran S, Raimundo BC, Lam JW, Sopko MM, Purkey HE, Romanowski MJ. Bioorg. Med. Chem. Lett. 2006;16:559–562. doi: 10.1016/j.bmcl.2005.10.048. [DOI] [PubMed] [Google Scholar]
- (21).Wilson KP, Black JA, Thomson JA, Kim EE, Griffith JP, Navia MA, Murcko MA, Chambers SP, Aldape RA, Raybuck SA. Nature. 1994;370:270–275. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]
- (22).Scheer JM, Romanowski MJ, Wells JA. Proc. Nat. Acad. Sci. U. S. A. 2006;103:7595–7600. doi: 10.1073/pnas.0602571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).O’Brien T, Fahr BT, Sopko MM, Lam JW, Waal ND, Raimundo BC, Purkey HE, Pham P, Romanowski MJ. Acta Crystallogr., Sect. F: Struct. Biol. Cryst. Commun. 2005;61:451–458. doi: 10.1107/S1744309105010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Okamoto Y, Anan H, Nakai E, Morihira K, Yonetoku Y, Kurihara H, Sakashita H, Terai Y, Takeuchi M, Shibanuma T, Isomura Y. Chem. Pharm. Bull. 1999;47:11–21. doi: 10.1248/cpb.47.11. [DOI] [PubMed] [Google Scholar]
- (25).Wilson KP, Black JA, Thomson JA, Kim EE, Griffith JP, Navia MA, Murcko MA, Chambers SP, Aldape RA, Raybuck SA. Nature. 1994;370:270–275. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]
- (26).Verdonk ML, Cole JC, Hartshorn MJ, Murray CW, Taylor RD. Proteins. 2003;52:609–623. doi: 10.1002/prot.10465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.