Abstract
2-Arachidonoylglycerol is oxygenated by cyclooxygenase-2 to form prostaglandin glyceryl esters. Previous work in this laboratory has suggested that PGE2-G activates a novel G protein-coupled receptor in a murine macrophage-like cell line, RAW 264.7. To probe the structural determinants for the putative receptor in RAW 264.7 cells, a panel of 10 analogs was tested for their ability to increase intracellular calcium. These analogs included PGE2- and PGF2α– ethanolamide, 4 PGE2 glyceryl ester analogs, and 4 PGF2α glyceryl ester analogs. The glyceryl ester analogs differed in the positioning of the hydroxyl groups in the glycerol moiety and the type of linker (ester, amide, or thioester) of the prostaglandin to the glycerol moiety. Compounds were also evaluated in a human non-small cell lung cancer cell line (H1819). The glycerol moiety was required for the calcium response. All glyceryl ester analogs but not ethanolamides caused a concentration-dependent increase in calcium levels in both RAW 264.7 and H1819 cells. An amide or ester linkage was preferable to a thioester linkage. The EC50 values did not significantly change when the positioning of the hydroxyls was varied. This calcium response induced by the glyceryl ester analogs appears to be independent of the putative hydrolysis products, PGE2 and PGF2α, and appears to represent a novel signaling pathway.
Keywords: Prostaglandin glyceryl esters, Prostaglandin receptors, Calcium mobilization, RAW 264.7 cells, H1819 cells
Introduction
Prostaglandin endoperoxide H synthase, or cyclooxygenase (COX), metabolizes arachidonic acid to prostaglandin (PG) H2. PGH2 is converted to PGE2, PGD2, PGF2α, PGI2 and thromboxane (Tx) A2 by a series of isomerases and a reductase. There are two isoforms of COX, COX-1 and COX-2. The endocannabinoids, 2-arachidonoylglycerol (2-AG) and arachidonoylethanolamide (AEA, anandamide) are selectively metabolized by COX-2 to form prostaglandin-glyceryl ester (PGH2-G) and -ethanolamide (PGH2-EA) derivatives, respectively 1–3. Subsequent metabolism of PGH2-G and PGH2-EA produces the glyceryl ester and ethanolamide derivatives of PGE2, PGD2, PGF2α, and PGI2.
Relatively little is known about the biological functions of the glyceryl ester and ethanolamide derivatives of prostaglandins although currently available information suggests potentially important roles for some of the products 4. Products of metabolism of 2-AG by COX-2 and prostacyclin synthase were shown to be important for activation of PPARδ pathway and attenuation of prothrombotic tissue factor gene expression. The available data suggested that the glyceryl ester of PGI2 could be mediating these effects 5. An analog of PGF2α-EA (prostamide F2α) has been shown to cause contraction of cat iris and also reduce intraocular pressure in humans, dogs, and primates 6. PGE2-EA repressed expression of interleukin-12 subunit p40 in RAW 264.7 cells and microglial cells 7. PGE2-G has been found endogenously in rat hind paws treated with carrageenan to induce inflammation 8. At low concentrations, PGE2-G treatment increased NFκB activity but decreased activity at higher concentrations of PGE2-G. PGE2-G induces a concentration-dependent increase in the frequency of miniature inhibitory postsynaptic currents (mIPSCs) 9, potentiates excitatory glutamatergic synaptic transmission and produces neurotoxicity via a non-cannabinoid receptor 10, and enhances long-term potentiation 11. These events are mediated independently of endocannabinoid and prostanoid receptors. PGE2-G and PGE2-serinol amide (PGE2-SA) stimulate calcium mobilization in RAW 264.7 cells in a concentration-dependent manner, increase inositol 1,4,5-triphosphate (IP3) levels approximately twofold, activate protein kinase C (PKC) ~twentyfold, and stimulate phosphorylation of extracellular signal regulated kinases (ERK) 1 and 2 12.
The characteristics of the effects mediated by prostaglandin glyceryl esters or ethanolamides suggest that they activate G protein-coupled receptor(s) (GPCR). The only known receptor for prostaglandin glyceryl esters or ethanolamides is the receptor for the PGF2α ethanolamide analog bimatoprost 13. Bimatoprost has been shown to activate a heterodimeric G protein-coupled receptor consisting of wild type prostaglandin F2α receptor and a splice variant that lacks the intracellular carboxyl terminus (alt-FP). Other prostaglandin glyceryl esters or ethanolamides have little or no affinity for known prostanoid receptors and are most likely signaling through orphan G protein-coupled receptor(s) or unknown heterodimeric receptor complexes. The dependence of the signaling responses on the structures of the glyceryl esters or ethanolamide agonists is not known. We report here the response of RAW264.7 cells and a human non-small cell lung cancer cell line (H1819) to a series of five PGE2 and five PGF2α glyceryl ester or ethanolamide analogs. The results reinforce the concept that PGE2-G and PGF2α-G stimulate Ca2+ mobilization through non-prostaglandin receptors.
Materials and Methods
Materials
PTD15, PTD18, PTD33, PDA44, and PTD53 were synthesized and checked for purity by HPLC and LC/MS/MS analysis and found to be >92% pure. PGE2-G, PGE2 serinol amide, PGE2 ethanolamide, PGF2α-G, and PGF2α ethanolamide were from Cayman Chemical, Ann Arbor, MI, USA.
Cell culture
H1819 cells were maintained as an adherent culture in RPMI (Gibco) with 5% FBS (Atlas). RAW 264.7 cells were maintained in DMEM (Gibco) with 10% heat-inactivated FBS. Cells were cultured at 37°C and 5% CO2. Both cell lines were typically used until passage 15 or 16 and then discarded.
Screening for calcium mobilization using the Flexstation II
RAW 264.7 cells or H1819 cells were seeded in 96 well plates (Corning) 24–48 hours prior to the calcium mobilization assay. Cell confluency was typically 60–70% on day of assay. The FLIPR Calcium 3 Assay Kit (Molecular Devices) was used to measure calcium mobilization. Calcium 3 reagent was dissolved in equal parts Hanks buffered salt solution (HBSS) (Gibco) and modified Tyrode's solution (10 mM HEPES, pH 7.4, 2 mM CaCl2, 10 mM glucose, 150 mM NaCl, 6 mM KCl, and 1 mM MgCl2) with 2.5 mM probenicid (Sigma) and 20 mM HEPES, pH 7.4. Growth medium was removed from the cells in the 96 well plate and replaced with Calcium 3 reagent solution. Cells were allowed to load for 60–70 min. before starting the assay on the FlexStation II (Molecular Devices). To prepare the compound plate, 10 µl of each stock compound in DMSO was added to 190 µl HBSS per well in a non-binding surface 96 well plate (Corning). The Flexstation II transferred 50 μl of compound to the 200 μl of Calcium 3 reagent solution in the cell plate, and the final compound concentration ranged from 0.1 pM to 1 µM. Quantitation of calcium release over time was determined by the SoftMax Pro software that powers the FlexStation. Fold response was calculated by dividing the maximum - minimum fluorescence value for each ligand concentration by the maximum - minimum fluorescence for DMSO vehicle. EC50 values were calculated in GraphPad Prism by plotting the fold responses for increasing ligand concentrations and using the sigmoidal dose-response analysis.
Results
The mouse macrophage-like cell line, RAW 264.7, mobilizes Ca2+ in response to PGE2-G. We recently discovered that the human non-small cell lung cancer cell line, H1819, also mobilizes Ca2+ when treated with PGE2-G. H1819 cells were treated with PGE2-G and PGE2-SA, and calcium release was measured on the FlexStation II instrument as described in "Materials and Methods." H1819 cells displayed a concentration-dependent calcium mobilization upon treatment with PGE2-G (Fig. 1A and 1B), in an analogous fashion to RAW 264.7 cells. PGE2-SA caused a similar calcium effect in H1819 cells (Fig. 2A and 2B). PGE2-SA differs from PGE2-G by having an amide versus an ester linkage from the prostaglandin to the glycerol moiety. The maximum calcium response for PGE2-G and PGE2-SA in H1819 cells typically occurred at 100 pM - 1 nM. Above this concentration, the amplitude of the Ca2+ response decreased. These results suggest that PGE2-G and PGE2-SA are activating a receptor in H1819 cells that is likely the human homolog of the putative PGE2-G receptor in RAW 264.7 cells. The structural determinants of this putative receptor in RAW 264.7 cells and H1819 cells were investigated by testing a panel of ten analogs of PGE2 or PGF2α glyceryl esters or ethanolamides for calcium mobilization.
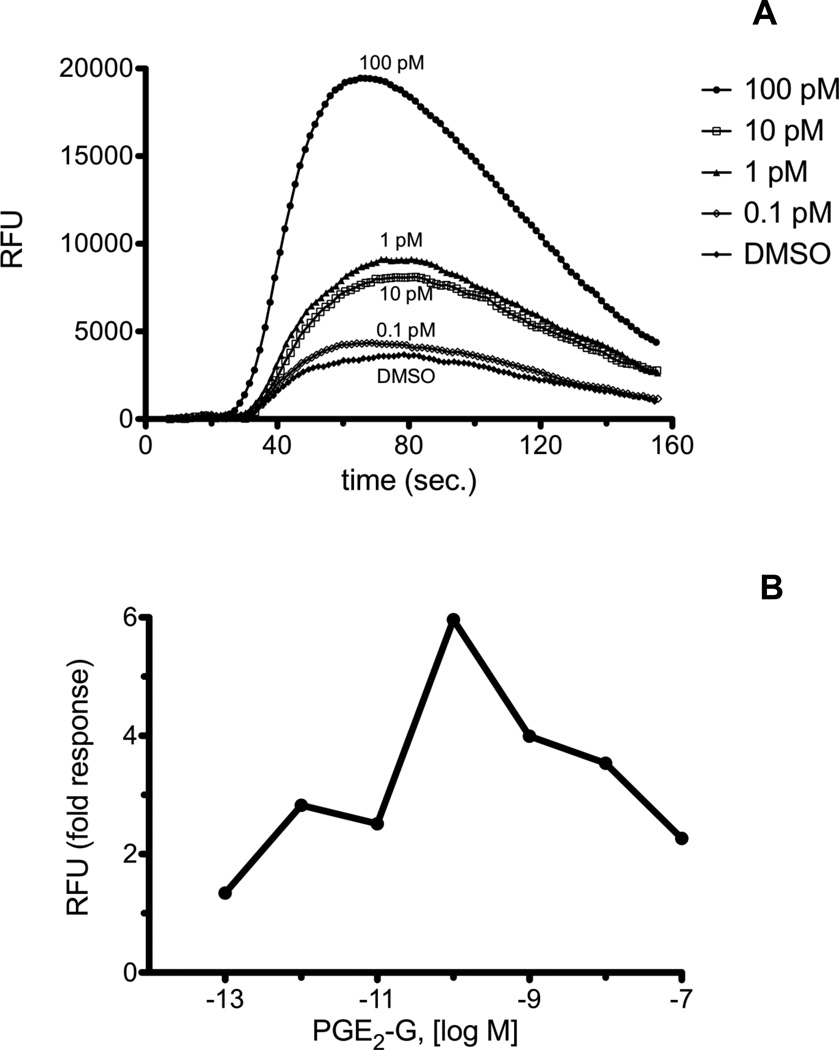
Fig. 1.
A, Calcium mobilization in H1819 cells treated with PGE2-G. H1819 cells were loaded with Calcium 3 reagent in the presence of 2.5 mM probenicid. Samples were excited at 488 nm, and emission spectra were recorded at 525 nm. H1819 cells were treated with PGE2-SA in the concentration range from 0.1 pM to 100 nM. The range from 0.1 pM to 100 pM is shown. Agonist was added at 20 sec., and fluorescence was measured at 1.52 sec. intervals. B, Dose response of PGE2-G from 0.1 pM to 100 nM. Fold response was calculated as described in “Materials and Methods.” Each experiment was performed a minimum of three times with several replicates for each experiment. Shown above are representative plots.
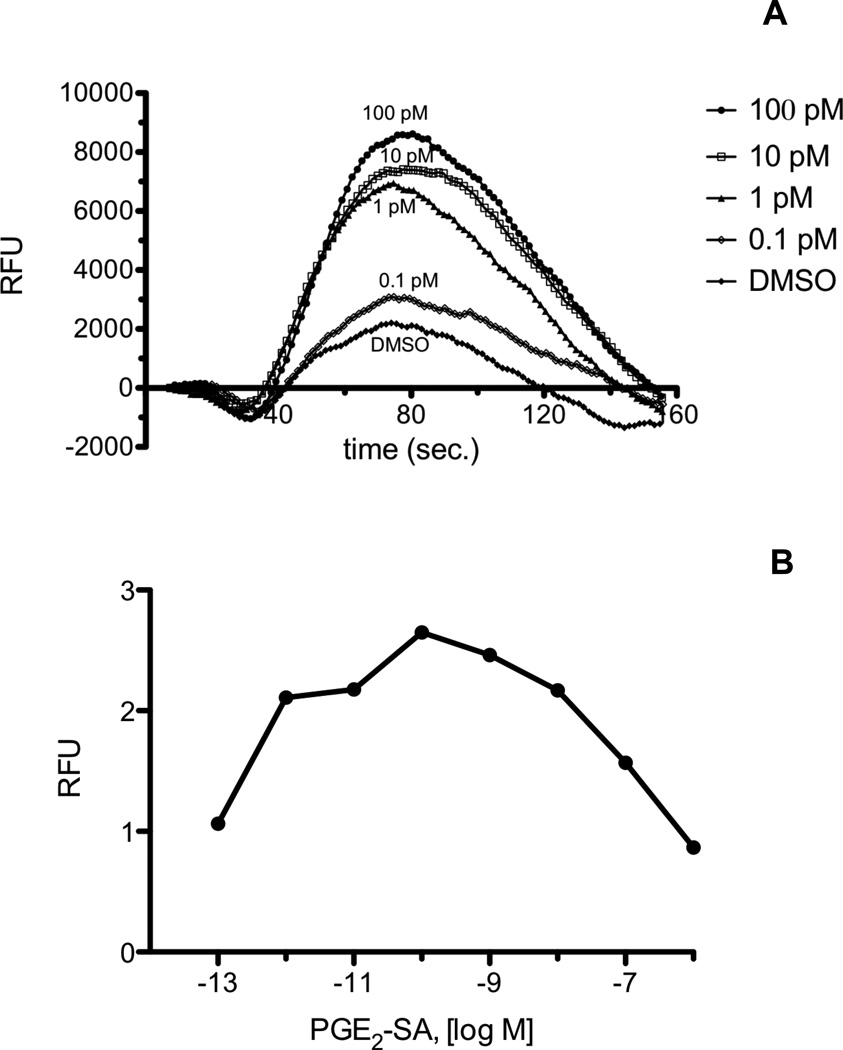
Fig. 2.
A, Calcium mobilization in H1819 cells treated with PGE2-SA. Cells were loaded with Calcium 3 reagent in the presence of 2.5 mM probenicid. Samples were excited at 488 nm, and emission spectra were recorded at 525 nm. H1819 cells were treated with PGE2-SA in the concentration range from 0.1 pM to 1 µM. The range from 0.1 pM to 100 pM is shown. Agonist was added at 20 sec., and fluorescence was measured at 1.52 sec. intervals. B, Dose response of PGE2-SA from 0.1 pM to 1 μM. Fold response was calculated as described in “Materials and Methods.” Each agonist was used in a minimum of three experiments with several replicates for each experiment. Shown above are representative plots.
Calcium mobilization by the PGE2 analogs in H1819 cells and RAW 264.7 cells
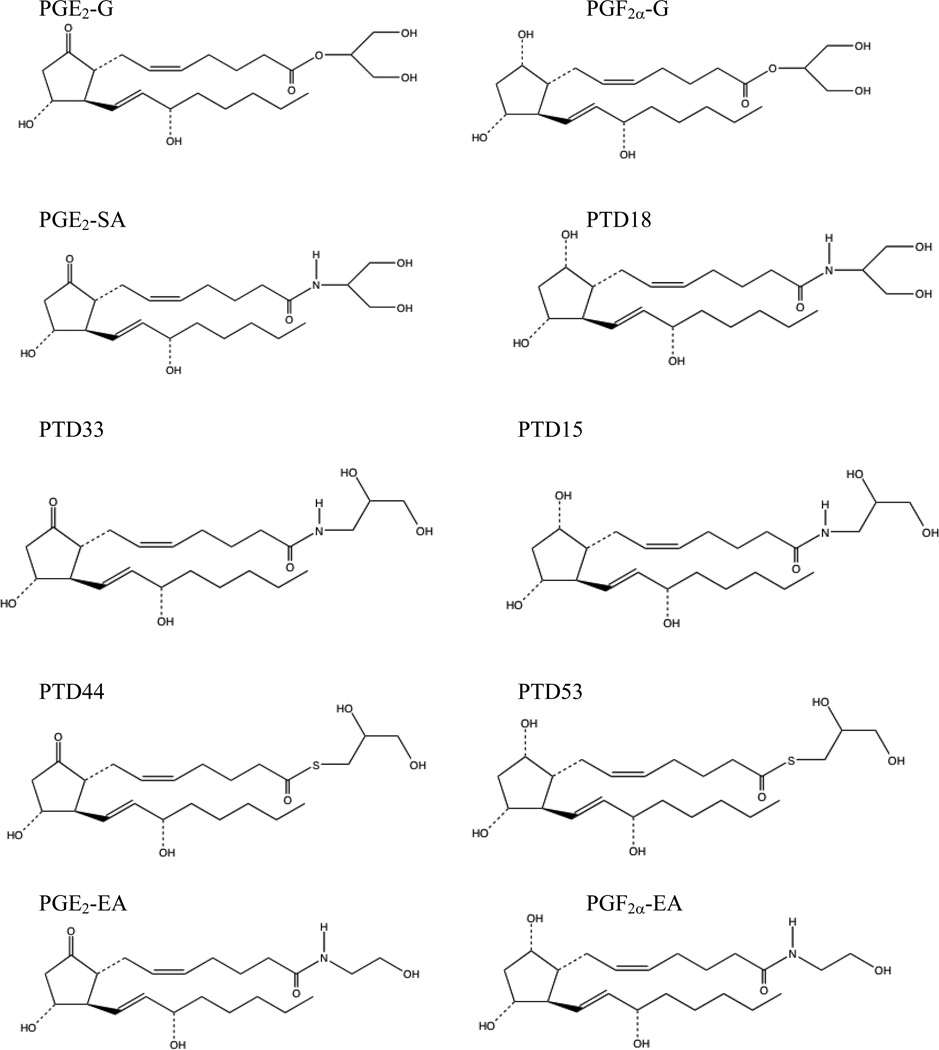
The five PGE2 analogs are PGE2-G, PGE2-SA, PTD33, PTD44, and PGE2 ethanolamide (PGE2-EA) (Fig. 3). Of the glyceryl ester analogs, PTD33 and PTD44 have hydroxyl groups at positions 2 and 3 of the glycerol moiety, and PGE2-G and PGE2-SA have hydroxyls at positions 1 and 3. The type of linker between the prostaglandin and glycerol moiety also differs for these analogs. PGE2-G, PGE2-SA, PTD33, and PTD44 contain an ester, amide, amide, and thioester linker, respectively.
Fig. 3.
PGE2 and PGF2α analogs tested for calcium mobilization in RAW 264.7 and H1819 cells.
The analogs were tested for calcium mobilization in both H1819 and RAW 264.7 cells. A concentration-dependent calcium response was seen in both cell lines for all PGE2 analogs with the exception of PGE2-EA. When the fold stimulation of fluorescence was plotted for each ligand concentration, the typical response for the glyceryl ester analogs was a bell-shaped curve (Fig. 1B and 2B). Each glyceryl ester analog displayed increasing fluorescence with increasing ligand concentrations until a maximum level of fluorescence was reached, usually at 2-3-fold over vehicle. Ligand concentrations greater than this were inhibitory towards the calcium response. The ligand concentrations producing the maximum calcium response differed for each analog. In H1819 cells, it was 100 pM - 1 nM for PGE2-G, 100 pM for PGE2-SA, 1–10 nM for PTD33, and 1–10 nM for PTD44. In RAW264.7 cells, it was 10 nM for PGE2-G, 1–10 nM for PGE2-SA, 100 pM for PTD33, and 100 pM - 1 nM for PTD44.
The EC50 value was determined for each ligand. In RAW 264.7 and H1819 cells, the EC50 values for the PGE2 glyceryl ester analogs were all less than 10 pM, suggesting that the glyceryl ester analogs have a high affinity for the putative PGE2-G receptor (Table 1). Since PGE2-EA did not mobilize calcium, the glycerol moiety appeared to be critical for the calcium response. The positioning of the hydroxyls in the glycerol moiety was not a determinant for activation of the putative receptor. PTD33 has hydroxyls at positions 2 and 3; PGE2-SA has hydroxyls at positions 1 and 3. Both contain an amide linker to the glycerol moiety. There was essentially no difference in EC50 values for calcium mobilization between these two analogs. The most dramatic difference was seen with PTD44. The EC50 value for PTD44 was approximately 16-fold higher than the other analogs. PTD44 has hydroxyl groups at positions 2 and 3 and contains a thioester linkage between the prostaglandin and glycerol moiety. Since the thioester linkage is the primary difference between PTD44 and the other analogs, this appeared to be a negative determinant for activation of the putative PGE2-G receptor.
Table 1.
EC50 values and fold responses for calcium mobilization in RAW 264.7 and H1819 cells by PGE2 and PGF2α analogs. EC50 values and fold responses were calculated as described in “Materials and Methods.” Fold responses for analogs are listed in parenthesis after each EC50 value. NR is no response.
| RAW264.7, PM (fold response) | H1819, pM (fold response) | |
|---|---|---|
| PGE2-G | 0.47 ± 0.15 (1.5–3.5) | 0.58 ± 0.12 (2.5–6) |
| PGE2-SA | 0.50 ± 0.14 (1.5–4) | 0.45 ± 0.12 (1.5–3.5) |
| PTD33 | 0.40 ± 0.12 (1.5–3.5) | 0.42 ± 0.12 (1.5–3.5) |
| PTD44 | 7.9 ± 3.9 (1.8–2.5) | 7.2 ± 7.8 (1.5–3.5) |
| PGE2-EA | NR | NR |
| PGF2α-G | 0.32 ± 0.05 (1.5–2.5) | 0.49 ± 0.36 (1.5–2.5) |
| PTD18 | 3.7 ± 1.1 (1.8–2.5) | 1.2 ± 0.85 (1.5–2.5) |
| PTD15 | 0.42 ± 0.12 (1.5–2.3) | 1.0± 0.31 (1.5–2.5) |
| PTD53 | 1.5 ± 1.1 (1.5–2.3) | 0.90 ± 0.23 (1.5–2.5) |
| PGF2α-EA | NR | NR |
There were a few differences in the PGE2 glyceryl ester analogs response between RAW 264.7 cells and H1819 cells. For PGE2-G, both cell lines displayed a concentration-dependent calcium mobilization; however, the response typically peaked at 10 nM in RAW 264.7 cells but peaked at 100 pM - 1 nM for H1819 cells. In addition, the magnitude of the PGE2-G response was greater in H1819 cells. The fold response seen by PGE2-G varied from 2.5-6-fold over vehicle in H1819 cells (Table 1) compared to 1.5-3.5-fold over vehicle in RAW 264.7 cells (Table 1). The maximum response for PTD44 was larger in H1819 cells (3.5-fold) compared to RAW 264.7 cells (2.5-fold).
Calcium mobilization by the PGF2α analogs in H1819 cells and RAW 264.7 cells
The five PGF2α analogs are PGF2α-G, PTD18 (PGF2α 2-serinol amide), PTD15, PTD53, and PGF2α ethanolamide (PGF2α-EA). PTD15 and 53 have hydroxyl groups at positions 2 and 3 of the glycerol moiety as opposed to positions 1 and 3 for PGF2α-G and PTD18. PGF2α-G, PTD18, PTD15, and PTD53 contain an ester, amide, amide, and thioester linkage, respectively, between the prostaglandin and glycerol moiety (Fig. 3).
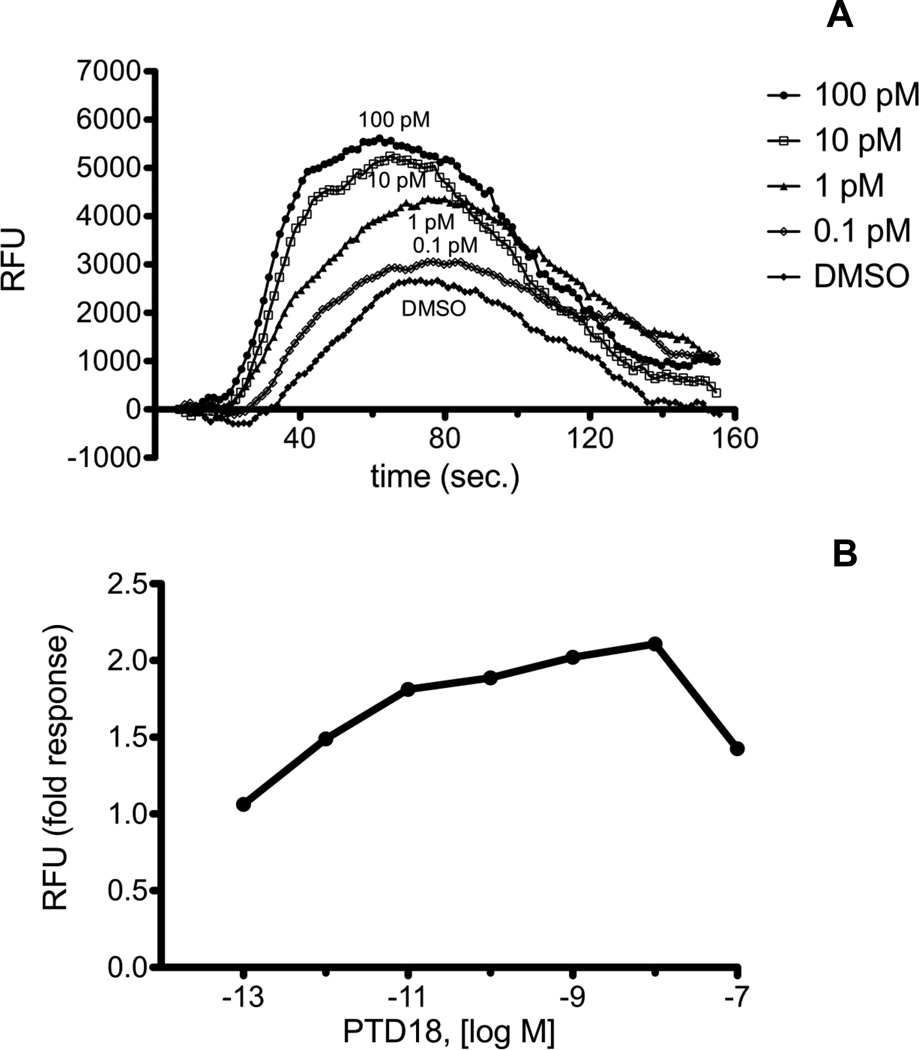
The PGF2α analogs all elicited a calcium response in both RAW 264.7 and H1819 cells with the exception of PGF2α-EA. The response for PTD18 in RAW 264.7 and H1819 cells is shown in Fig. 4 and Fig. 5. The maximum fold response for PGF2α glyceryl ester analogs was less than that seen for the PGE2 glyceryl ester analogs. The calcium response peaked at 2.3–2.5 fold over vehicle versus 3.5–6 fold over vehicle for the PGE2 analogs (Table 1).
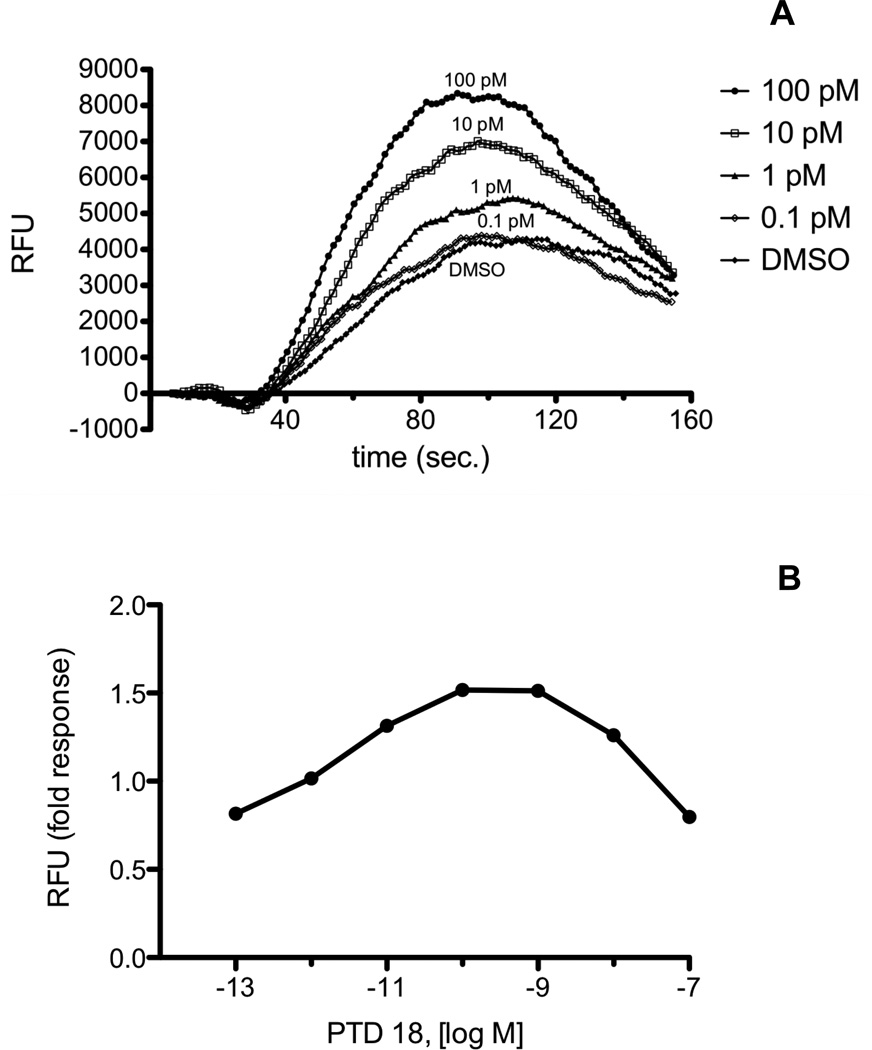
Fig. 4.
A, Calcium mobilization over time in RAW 264.7 cells treated with PTD18 (PGF2α serinol amide). Cells were loaded with Calcium 3 reagent in the presence of 2.5 mM probenicid. Samples were excited at 488 nm, and emission spectra were recorded at 525 nm. RAW 264.7 cells were treated with PTD18 in the concentration range from 0.1 pM to 100 nM. The range from 0.1 pM to 100 pM is shown. Agonist was added at 20 sec., and fluorescence was measured at 1.52 sec. intervals. B, Dose response of PTD18 from 0.1 pM to 100 nM. Fold response was calculated as described in “Materials and Methods.” Each agonist was used in a minimum of three experiments with several replicates for each experiment. Shown above are representative plots.
Fig. 5.
A, Calcium mobilization over time in H1819 cells treated with PTD18 (PGF2α serinol amide). Cells were loaded with Calcium 3 reagent in the presence of 2.5 mM probenicid. Samples were excited at 488 nm, and emission spectra were recorded at 525 nm. H1819 cells were treated with PTD18 in the concentration range from 0.1 pM to 100 nM. The range from 0.1 pM to 100 pM is shown. Agonist was added at 20 sec., and fluorescence was measured at 1.52 sec. intervals. B, Dose response of PTD18 from 0.1 pM to 100 nM. Fold response was calculated as described in “Materials and Methods.” Each agonist was used in a minimum of three experiments with several replicates for each experiment. Shown above are representative plots.
In H1819 cells, the EC50 values for all of the PGF2α analogs (PGF2α-G, PTD18, PTD15, and PTD53) were very similar. Since all displayed calcium mobilization except PGF2α-EA, this suggested that the defining structural characteristic was a glycerol moiety. There was no significant difference when the position of the hydroxyl groups on the glycerol moiety was varied or with varying linkers between the prostaglandin and glycerol moiety (ester, amide, or thioester) (Table 1).
In RAW 264.7 cells, the PGF2α glyceryl ester analogs with hydroxyls at positions 2 and 3 of the glycerol moiety (PTD15 and PTD53) had 8-9-fold lower EC50 values than PTD18, which has the hydroxyl groups at positions 1 and 3. Varying the type of linker between the prostaglandin and glycerol moiety (ester, amide, or thioester) did not significantly change the EC50 for calcium mobilization. The defining structural characteristics of the PGF2α analogs for the putative PGE2-G receptor in RAW 264.7 cells was a glycerol moiety with hydroxyls at positions 2 and 3. There is a slight difference in PGF2α analog specificity between RAW 264.7 and H1819 cells.
Discussion
PGE2-G has been previously shown to mobilize calcium in a concentration-dependent manner in RAW 264.7 cells 12. PGE2-G increased IP3 levels, PKC activity, ERK phosphorylation, and Ca2+ mobilization was blocked by an IP3 receptor antagonist. These events suggest that PGE2-G is activating a G protein-coupled receptor. Heterotrimeric G proteins Gi and Gq are known to activate phospholipase Cβ (PLCβ) through their βγ and α subunits, respectively, causing a rise in IP3 levels. IP3 is known to mobilize calcium from intracellular stores and lead to events causing PKC activation and ERK phosphorylation. A non-small cell lung carcinoma cell line, H1819, was also found to mobilize calcium in response to PGE2-G in a similar manner as the PGE2-G response in RAW 264.7 cells. These results suggest that a putative PGE2-G receptor(s) is present in both H1819 and RAW 264.7 cells.
The amount of calcium mobilization seen with the activated PGE2-G receptor varies depending on the ligand used and also the cell type. Previous investigations in RAW 264.7 cells found a 2–3 fold difference in calcium levels in cells treated with a concentration range of PGE2-G from 0.1 pM to 10 μM compared to vehicle-treated cells. In H1819 cells, the calcium response is slightly more robust with a rise in calcium levels of up to 6-fold over vehicle treatment. Increasing calcium mobilization was seen from 0.1 pM to 100 pM PGE2-G with a decrease in calcium when concentrations greater than 100 pM were used. Although the magnitude of the calcium response of the putative PGE2-G receptor does not vary more than 6-fold over a concentration range of 0.1 pM to 100 pM ligand, there is also precedent in the literature of calcium responses of this magnitude over a similar scale of ligand concentration. For example, leukotriene B4 activates two different receptors, BLT1 and BLT2, but the calcium response induced varies depending on which receptor is present. With BLT2, a 4–5 fold stimulation in calcium levels is seen with a concentration range of LTB4 of 4 orders of magnitude 14,15; however, with BLT1, the calcium response is at least twice as great as BLT2 over the same concentration range of LTB4. Differences in calcium signaling can occur from several different sources including cell type, different receptors for the same ligand, compartmentalization of signaling proteins involved in calcium mobilization, coupling to G proteins, interaction with regulators of G protein signaling (RGS) proteins, and modification of calcium release and uptake mechanisms 16. It is not known whether the putative PGE2-G receptor(s) is the same in RAW 264.7 and H1819 cells, but the magnitude of response does differ between these two cell lines.
Little is known about the structural determinants required for activation of the putative PGE2-G receptor. This report investigates the structural determinants required for the calcium response in RAW 264.7 and H1819 cells by testing a panel of PGE2 and PGF2α glyceryl ester and ethanolamide derivatives. All of the PGE2 and PGF2α glyceryl ester analogs elicited a calcium response in both RAW 264.7 and H1819 cells, but the two ethanolamide derivatives (PGE2-EA and PGF2α-EA) did not. As described previously, an analog of PGF2α-EA (prostamide F2α) caused contraction of cat iris muscle and reduced intraocular pressure in humans, dog, and primates 6. This compound activated a heterodimeric G protein-coupled receptor complex composed of wild type FP and a truncated splice variant of FP, termed alt-FP 13. Alt-FP lacks the carboxyl terminal intracellular tail present in wild type FP and requires dimerization to an intact GPCR for activation of downstream G proteins.
Receptor dimerization has been established as another regulatory mechanism for ligand recognition, second messenger signaling, and receptor trafficking 17–19. Heterodimerization of at least 40 GPCR pairs has been described, including prostanoid receptors 17. A heterodimeric complex of EP1 receptor and the β2-adrenergic receptor exists 20, and IP and TPα receptors have also been shown to dimerize 21. PGE2-G could be activating a single uncharacterized orphan GPCR or a dimeric GPCR complex as is the case for bimatoprost. The bimatoprost receptor and the putative PGE2-G receptor are potentially two members of a group of heterodimeric GPCRs activated by glyceryl ester or ethanolamide derivatives of prostaglandins. The PGE2-G receptor appears to be distinct from the bimatoprost receptor. An antagonist of the feline iris contraction elicited by bimatoprost was unable to disrupt contraction caused by treatment with PGE2-G 22. Prostaglandin glycerol ester and ethanolamide receptors could potentially represent a new group of heterodimeric GPCR complexes activated by a new class of signaling compounds. These lipid signaling compounds arise from oxygenation of endocannabinoids by COX-2. Since COX-2 is upregulated in inflammation and cancer, the prostaglandin glyceryl esters and ethanolamides could be important mediators of novel signal transduction pathways in these sites.
The putative receptor PGE2-G appears to have a very high affinity for its ligand. The EC50 values for all the glyceryl ester analogs were less than 10 pM in both H1819 and RAW 264.7 cells with the lowest being approximately 0.5 pM. Murine resident peritoneal macrophages induced to express COX-2 produced low levels of PG glyceryl esters compared to levels of PGs produced 23. Thus, for PG glyceryl esters to activate a receptor and produce a physiological effect, the EC50 value for the receptor would have to be very low, which appears to be the case. The concentrations of PGE2-G generated by RAW cells following lipopolysaccharide and zymosan treatment exceed the values reported here to activate Ca2+ mobilization. Prostaglandins act in an autocrine or paracrine fashion and bind to and activate the prostanoid receptors of cells close by the site of prostaglandin synthesis. The prostaglandin glycerol esters are also likely to act in an autocrine or paracrine manner. Prostaglandin glycerol esters have been shown to have a short half life in some media. In rat plasma or skin, PGE2-G is rapidly hydrolyzed (t1/2 = 14 sec) 8. For PGE2-G to have a physiological effect in such a setting, it would need to activate receptors close to the site of its synthesis before it is hydrolyzed. However, the half-life of PGE2-G is much greater in other media (for example, its t1/2 is greater than 10 min in human plasma, and it is stable indefinitely in cerebrospinal fluid 2), but since PGE2-G has been shown to be produced in low levels, it is unlikely that it would be synthesized in quantities sufficient to effectively function as an endocrine agent. Given the high affinity of the putative PGE2-G receptor for its ligand, PGE2-G would be most effective when synthesized locally in sufficient amounts to activate its putative receptor by a paracrine or autocrine mechanism.
The structural determinants required for the calcium response by the PGE2 and PGF2α glyceryl ester analogs were similar between H1819 and RAW 264.7 cells. The defining structural feature was the glycerol moiety, and the different positioning of the hydroxyls (positions 1 and 3 versus positions 2 and 3) did not change the EC50 values. A thioester linkage between the prostaglandin and glycerol moiety was a negative structural determinant for the PGE2 but not the PGF2α glyceryl ester analogs. There was no difference between analogs with amide or ester linkages. One exception to these rules occurred with PTD18. In RAW 264.7 cells, PTD18 had a higher EC50 value than the other two PGF2α glyceryl ester analogs.
Since the PGE2 glyceryl ester analogs induced a slightly greater calcium response than the PGF2α glyceryl ester analogs, the cyclopentane ring of PGE2 must also be a structural determinant for the putative receptor. Full agonists of receptors produce a more robust physiological response than partial agonists and are better able to stabilize the active conformation of the receptor. The PGE2 glyceryl ester analogs appear to be full agonists, and the PGF2α glyceryl ester analogs are partial agonists. The glycerol moiety is the main structural determinant responsible for activation of the receptor, and it is sufficient for partial activation as is the case with the PGF2α glyceryl ester analogs. For full activation of the receptor, the PGE2 cyclopentane ring and an amide or ester linker to the glycerol moiety are required.
Acknowledgements
This work was supported by a research grant from the National Institutes of Health (GM15431) to L.J.M and a training grant (E5007028) to R.R.J. We are grateful to P.J. Conn for use of the FlexStation.
Abbreviations
- COX
cyclooxygenase
- 2-AG
2- arachidonoylglycerol
- AEA
arachidonoylethanolamide
- PG
prostaglandin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glycerol prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275(43):33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- 2.Kozak KR, Crews BC, Ray JL, Tai HH, Morrow JD, Marnett LJ. Metabolism of prostaglandin glycerol esters and prostaglandin ethanolamide in vitro and in vivo. J Biol Chem. 2001;276(40):36993–36998. doi: 10.1074/jbc.M105854200. [DOI] [PubMed] [Google Scholar]
- 3.Kozak KR, Crews BC, Morrow JD, et al. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into plastaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277(47):44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 4.Woodward DF, Carling RW, Cornell CL, et al. The pharmacology and therapeutic relevance of endocannabinoid derived cyclo-oxygenase (COX)-2 products. Pharmacology & Therapeutics. 2008;120(1):71–80. doi: 10.1016/j.pharmthera.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh M, Wang H, Ai Y, et al. COX-2 suppresses tissue factor expression via endocannabinoid-directed PPARdelta activation. J Exp Med. 2007;204(9):2053–2061. doi: 10.1084/jem.20070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodward DF, Krauss AH, Chen J, et al. The pharmacology of bimatoprost (Lumigan) Survey of Ophthalmology. 2001;45 Suppl 4:S337–S345. doi: 10.1016/s0039-6257(01)00224-7. [erratum appears in Surv Ophthalmol 2002 May–Jun;47(3):295] [DOI] [PubMed] [Google Scholar]
- 7.Correa F, Docagne F, Clemente D, Mestre L, Becker C, Guaza C. Anandamide inhibits IL-12p40 production by acting on the promoter repressor element GA-12: possible involvement of the COX-2 metabolite prostamide E(2) Biochemical Journal. 2008;409(3):761–770. doi: 10.1042/BJ20071329. [DOI] [PubMed] [Google Scholar]
- 8.Hu SS, Bradshaw HB, Chen JS, Tan B, Walker JM. Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates NFkappaB activity. British Journal of Pharmacology. 2008;153(7):1538–1549. doi: 10.1038/bjp.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sang N, Zhang J, Chen C. PGE2 glycerol ester, a COX-2 oxidative metabolite of 2-arachidonoyl glycerol, modulates inhibitory synaptic transmission in mouse hippocampal neurons. Journal of Physiology. 2006;572(Pt 3):735–745. doi: 10.1113/jphysiol.2006.105569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sang N, Zhang J, Chen C. COX-2 oxidative metabolite of endocannabinoid 2-AG enhances excitatory glutamatergic synaptic transmission and induces neurotoxicity. Journal of Neurochemistry. 2007;102(6):1966–1977. doi: 10.1111/j.1471-4159.2007.04668.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Zhang J, Andreasson K, Chen C. COX-2 oxidative metabolism of endocannabinoids augments hippocampal synaptic plasticity. Molecular & Cellular Neurosciences. 2008;37(4):682–695. doi: 10.1016/j.mcn.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nirodi CS, Crews BC, Kozak KR, Morrow JD, Marnett LJ. The glyceryl ester of prostaglandin E2 mobilizes calcium and activates signal transduction in RAW264.7 cells. Proc Natl Acad Sci U S A. 2004;101(7):1840–1845. doi: 10.1073/pnas.0303950101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang Y, Woodward DF, Guzman VM, et al. Identification and pharmacological characterization of the prostaglandin FP receptor and FP receptor variant complexes. British Journal of Pharmacology. 2008;154(5):1079–1093. doi: 10.1038/bjp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tryselius Y, Nilsson NE, Kotarsky K, Olde B, Owman C. Cloning and characterization of cDNA encoding a novel human leukotriene B(4) receptor. Biochemical Biophysical Research Communications. 2000;274(2):377–382. doi: 10.1006/bbrc.2000.3152. [DOI] [PubMed] [Google Scholar]
- 15.Yokomizo T, Kato K, Hagiya H, Izumi T, Shimizu T. Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. Journal of Biological Chemistry. 2001;276(15):12454–12459. doi: 10.1074/jbc.M011361200. [DOI] [PubMed] [Google Scholar]
- 16.Kiselyov K, Shin DM, Muallem S. Signalling specificity in GPCR-dependent Ca2+ signalling. Cell Signaling. 2003;15(3):243–253. doi: 10.1016/s0898-6568(02)00074-8. [DOI] [PubMed] [Google Scholar]
- 17.Prinster SC, Hague C, Hall RA. Heterodimerization of g protein-coupled receptors: specificity and functional significance. Pharmacological Reviews. 2005;57(3):289–298. doi: 10.1124/pr.57.3.1. [DOI] [PubMed] [Google Scholar]
- 18.Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends in Pharmacological Sciences. 2005;26(3):131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Levoye A, Dam J, Ayoub MA, Guillaume JL, Jockers R. Do orphan G-protein-coupled receptors have ligand-independent functions? New insights from receptor heterodimers. EMBO Reports. 2006;7(11):1094–1098. doi: 10.1038/sj.embor.7400838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGraw DW, Mihlbachler KA, Schwarb MR, et al. Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. Journal of Clinical Investigation. 2006;116(5):1400–1409. doi: 10.1172/JCI25840. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson SJ, Roche AM, Kostetskaia E, Smyth EM. Dimerization of the human receptors for prostacyclin and thromboxane facilitates thromboxane receptor-mediated cAMP generation. Journal of Biological Chemistry. 2004;279(51):53036–53047. doi: 10.1074/jbc.M405002200. [DOI] [PubMed] [Google Scholar]
- 22.Woodward DF, Krauss AH, Wang JW, et al. Identification of an antagonist that selectively blocks the activity of prostamides (prostaglandin-ethanolamides) in the feline iris. British Journal of Pharmacology. 2007;150(3):342–352. doi: 10.1038/sj.bjp.0706989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rouzer CA, Marnett LJ. Glycerylprostaglandin synthesis by resident peritoneal macrophages in response to a zymosan stimulus. Journal of Biological Chemistry. 2005;280(29):26690–26700. doi: 10.1074/jbc.M501021200. [DOI] [PubMed] [Google Scholar]