Abstract
Purpose: To describe the clinical and genetic characteristics of a Japanese family in which one member exhibited Bietti's crystalline corneoretinal dystrophy (BCD). Methods: Using direct sequencing, mutation screening was performed in the CYP4V2 gene of both the patient with BCD and her daughter. Ophthalmic examinations were performed to determine the clinical features of both subjects. Results: The 64-year-old female patient had a bilateral visual acuity of 0.4. Slit lamp examination revealed bilateral crystalline-like deposits at the superior limbus of the cornea. Fundus examination revealed there was chorioretinal atrophy along with numerous glistening yellowish-white crystalline deposits that were scattered throughout the posterior pole and the mid-peripheral retina. Standard flash electroretinography showed an extinguished electroretinogram and Goldmann kinetic perimetry detected a relative scotoma. Genetic analysis revealed that the patient had a heterozygous mutation in the CYP4V2 gene (IVS6-8delTCATACAGGTCATCGCG/GC), which is the most commonly found mutation in Japanese patients with BCD. Furthermore, the patient was also shown to have a novel heterozygous point mutation in exon 9 of the CYP4V2 gene (c.1168C>T). In contrast, her daughter exhibited no clinical findings for BCD even though she carried the same heterozygous mutation in the CYP4V2 gene (c.1168C>T). Conclusion: A novel compound heterozygous mutation was found in the CYP4V2 gene of a patient with BCD. This previously unreported c.1168C>T mutation causes a missense mutation (p.R390C) in the CYP4V2 protein.
Key Words: Bietti's crystalline corneoretinal dystrophy, CYP4V2 gene, Retinal dystrophy, Retinitis pigmentosa
Introduction
Bietti's crystalline corneoretinal dystrophy (BCD, MIM 210370) is characterized by an autosomal recessive form of progressive chorioretinal degeneration that is associated with numerous crystal deposits in the posterior portion of the retina and peripheral area of the corneal stroma [1]. The disease is known to be caused by mutations in the CYP4V2 gene (HGNC: 23198) [2]. While the protein product of the CYP4V2 gene is a member of the cytochrome p-450 family, CYP4 enzymes, it is generally accepted that these products play a role in lipid metabolism [2, 3]. It has been recently reported that CYP4V2 is a selective omega-hydroxylase of saturated medium-chain fatty acids, in addition to having a relatively high catalytic efficiency toward myristic acid [4]. Nevertheless, the exact mechanism by which the chorioretinal degeneration is able to deposit crystal materials in the retina, cornea, conjunctival fibroblasts and lymphocytes remains unknown [5, 6].
While homozygous mutations of IVS6-8delTCATACAGGTCATC/insGC (c.802-8 810del17insGC (Exon7del)) in the CYP4V2 gene are commonly found in Japanese patients with BCD, they are also found in both Chinese and Indonesian patients with BCD. To date, there have been approximately 31 mutations of the CYP4V2 gene reported [2, 7, 8, 9, 10, 11].
We recently discovered a case with a compound heterozygote for the CYP4V2 gene. This patient had mutations that were situated at exons 7 and 9. One of the mutations that have been found for exon 7 includes the above-mentioned IVS6-8delTCATACAGGTCATC/insGC. The other mutation of exon 9 is p.R390C, which to the best of our knowledge, is a novel mutation that has yet to be reported.
Methods
For molecular genetic analysis, this study was conducted according to the tenets of the declaration of Helsinki and was approved by the Committee on Ethics of Hirosaki University Graduate School of Medicine. Mutation screening of the CYP4V2 gene was performed on both a patient with BCD and her daughter. After obtaining informed consent, genomic DNA was extracted from the peripheral blood leukocytes. The coding region (including the intron-exon boundary) of the CYP4V2 gene was amplified by polymerase chain reaction (PCR). The PCR products were analyzed using direct sequencing. To determine the clinical features that were present, both subjects underwent visual acuity, slit lamp, fundus, fluorescein angiography, Goldmann perimetry (GP) and electroretinography (ERG) examinations.
Results
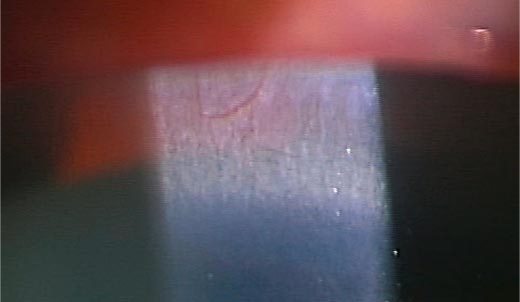
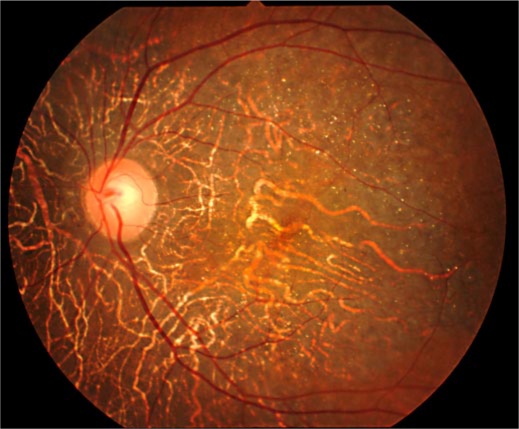
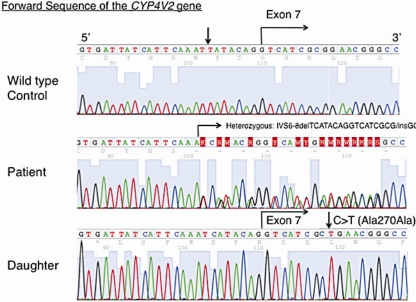
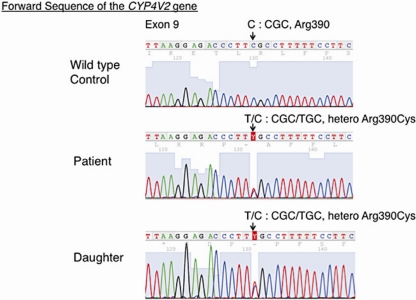
The 64-year-old female patient had a bilateral visual acuity of 0.4. The slit lamp examination revealed bilateral crystalline-like deposits at the superior limbus of the cornea (fig. 1). The fundus examination revealed chorioretinal atrophy and numerous glistening yellowish-white crystalline deposits that were scattered throughout the posterior pole and the mid-peripheral retina (fig. 2). There were no deposits seen in the lens capsule. Full-field standard ERG showed there was an extinguished electroretinogram, and GP indicated the presence of relative scotoma. All these clinical findings indicated that this case had typical features of BCD. Genetic analysis revealed that the patient had a heterozygous mutation in the CYP4V2 gene (IVS6-8delTCATACAGGTCATCGCG/GC), which is the most commonly found mutation in Japanese patients with BCD (fig. 3). Furthermore, this patient had a novel heterozygous mutation in the CYP4V2 gene (c.1168C>T, fig. 4). While her daughter, who has no clinical findings of BCD, exhibited the same heterozygous mutation in the CYP4V2 gene (c.1168C>T) in the exon 9, she lacked the IVS6-8delTCATACAGGTCATCGCG/GC mutation in the gene.
Fig. 1.
Slit lamp biomicroscopic findings showing crystalline-like corneal deposits in the superior limbal area.
Fig. 2.
Fundus photograph of the left eye showing numerous crystalline-like deposits and chorioretinal degeneration in the posterior pole.
Fig. 3.
Nucleotide sequence of the CYP4V2 gene at the exon-intron boundary area of the exon 7, showing a heterozygous IVS6-8delTCATACAGGTCATCGCG/GC mutation in the patient with BCD. Her daughter exhibited a normal sequence similar to the wild-type control. Arrows show positions of silent mutations at IVS6-7(C>T) in a control subject and c.810G>T (p.A270A) in the phenotypically normal daughter.
Fig. 4.
Nucleotide sequence of the CYP4V2 gene around nucleotide 1168 in the exon 9, showing the heterozygous missense mutations (c.1168C>T) that were found in both the patient and her daughter. These mutations resulted in p.R390C.
Discussion
BCD is a progressive chorioretinal degeneration that has been demonstrated to be caused by mutations in the CYP4V2 gene [2]. Although BCD phenotypes vary among patients, even when the same mutations are seen in a similar age group [8, 12], basic clinical findings including chorioretinal degeneration associated with crystalline deposits are common features [13].
The mutation of exon 7 (IVS6-8delTCATACAGGTCATCGCG/GC) has been commonly found not only in Japanese but also in Chinese and Indonesian patients with BCD [2, 7, 8, 9, 10, 11, 12, 13]. A molecular genetic study disclosed that this mutation breaks the splicing acceptor site of the exon 7, resulting in the skipping of exon 7 (Exon7del) [2]. As a result of this mutation, the translated protein is truncated and lacks peptides that are normally derived from the entire part of exon 7 [2]. It has been postulated that this truncated peptide causes a decreased fatty acid binding and removal of the cell attachment site [2]. We further speculated that there might be severe deterioration of the enzyme activity of the putative CYP4V2 protein, which is known as omega-hydroxylase. However, the molecular mechanisms for the defective form of the CYP4V2 protein that produce the characteristic BCD phenotypes are, as of yet, largely unknown.
On the other hand, the c.1168C>T mutation in the CYP4V2 gene is located at exon 9. This mutation causes a missense mutation (p.R390C) in the CYP4V2 protein. Thus it is feasible that this could lead to changes in the cubic structure of the CYP4V2 protein, because cysteine is classified into the thiol group leading to disulfide bond. In addition, a putative substitution of cysteine for arginine could cause changes in the polarity of the CYP4V2 protein at the amino acid 390 position, resulting in structural and/or functional disorganization of the protein. In the literature, 6 other mutations have been reported in the exon 9 including c.1091-2A>G (Exon9del), c.1157A>C (p.K386T), c.1169G>A (p.R390H), c.1187C>T (p.P396L), c.1198C>T (p.R400C), c.1199G>A (p.R400H) [13]. Our cases add an additional point mutation to the exon 9. Previous studies regarding the relationship between genotypes and phenotypes have revealed that there is no apparent rule in phenotypes of BCD in relation to kinds and locations of mutations in the CYP4V2 gene [13]. Our present case also demonstrated typical features of BCD, supporting previous findings.
Since BCD is thought to be an autosomal recessive disease, heterozygous carriers show no clinical symptoms. As the current patient had double heterozygous mutations, and her daughter only had a heterozygous missense mutation of exon 9, this suggests that these mutations are located at different alleles.
Several compound heterozygous mutations have been previously reported for the CYP4V2 gene. The mutation of exon 7 (IVS6-8delTCATACAGGTCATCGCG/GC) is often detected as one of these. Because the 1168C>T mutation has not previously been reported, the present study added a novel 1168C>T (R390C) mutation in the CYP4V2 gene as a possible causative mutation of BCD.
Disclosure Statement
The authors report no conflicts of interest.
References
- 1.Bietti G. Ueber familiaeres Vorkommen von 'Retinitis punctata albescens' (verbunden mit 'Dystrophia marginalis cristallinea corneae'), Glitzern des Glaskoerpers und anderen degenerativen Augenveraenderungen. Klin Mbl Augenheilk. 1937;99:737–753. [Google Scholar]
- 2.Li A, Jiao X, Munier FL, Schorder et DF, Yao W, Iwata F, Hayakawa M, Kanai A, Chen MS, Lewis RA, Heckenlively J, Weleber RG, Traboulsi EI, Zhang Q, Xiao X, Kaise-Kupfer M, Sergeev YV, Hejtmancik JF. Bietti crystalline corneoretinal dystrophy is caused by mutations in the novel gene CYP4V2. Am J Hum Genet. 2004;74:817–826. doi: 10.1086/383228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J, Jiao X, Hejtmancik JF, Kaiser-Kupfer M, Gahl WA, Markello TC, Guo J, Chader GJ. The metabolism of fatty acids in human Bietti crystalline dystrophy. Invest Ophthalmol Vis Sci. 2001;42:1707–1714. [PubMed] [Google Scholar]
- 4.Nakano M, Kelly EJ, Rettie AE. Expression and characterization of CYP4V2 as a fatty acid omega-hydroxylase. Drug Metab Dispos. 2009;37:2119–2122. doi: 10.1124/dmd.109.028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaiser-Kupfer MI, Chan CC, Markello TC, Crawford MA, Caroso RC, Csaky KG, Guo J, Gahl WA. Clinical biochemical and pathologic correlations in Bietti's crystalline dystrophy. Am J Ophthalmol. 1994;118:569–582. doi: 10.1016/s0002-9394(14)76572-9. [DOI] [PubMed] [Google Scholar]
- 6.Wilson DJ, Weleber RG, Klein ML, Welch RB, Green WR. Bietti's crystalline dystrophy: a clinicopathologic correlative study. Arch Ophthalmol. 1989;107:213–221. doi: 10.1001/archopht.1989.01070010219026. [DOI] [PubMed] [Google Scholar]
- 7.Wada Y, Itabashi T, Sato H, Kawamura M, Tada A, Tamai M. Screening for mutations in CYP4V2 gene in Japanese patients with Bietti's crystalline corneoretinal dystrophy. Am J Ophthalmol. 2005;139:894–899. doi: 10.1016/j.ajo.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 8.Lin J, Nishiguchi KM, Nakamura N, Dryja TP, Berson EL, Miyake Y. Recessive mutations in the CYP4V2 gene in East Asian and Middle Eastern patients with Bietti crystalline corneoretinal dystrophy. J Med Genet. 2005;42:e38. doi: 10.1136/jmg.2004.029066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gekka T, Hayashi T, Takeuchi T, Goto-Omoto S, Kitahara K. CYP4V2 mutations in two Japanese patients with Bietti's crystalline dystrophy. Ophthalmic Res. 2005;37:262–269. doi: 10.1159/000087214. [DOI] [PubMed] [Google Scholar]
- 10.Lee KY, Koh AH, Aung T, Yong VHK, Yeung K, Ang CL, Vitharna EN. Characterization of Bietti crystalline dystrophy patients with CYP4V2 mutations. Invest Ophthalmol Vis Sci. 2005;46:3812–3816. doi: 10.1167/iovs.05-0378. [DOI] [PubMed] [Google Scholar]
- 11.Jin ZB, Ito S, Saito Y, Inoue Y, Yanagi Y, Nao-i N. Clinical and molecular findings in three Japanese patients with crystalline retinopathy. Jpn J Ophthalmol. 2006;50:426–431. doi: 10.1007/s10384-006-0350-0. [DOI] [PubMed] [Google Scholar]
- 12.Lai TYY, Ng TK, Tam POS, Yam GH, Ngai JW, Chan WM, Liu DT, Lam DS, Pang CP. Genotype-phenotype analysis of Bietti's crystalline dystrophy in patients with CYP4V2 mutations. Invest Ophthalmol Vis Sci. 2007;48:5212–5220. doi: 10.1167/iovs.07-0660. [DOI] [PubMed] [Google Scholar]
- 13.Xiao X, Mai G, Li S, Guo X, Zhang Q. Identification of CYP4V2 mutation in 21 families and overview of mutation spectrum in Bietti crystalline corneoretinal dystrophy. Biochem Biophys Res Com. 2011;409:181–188. doi: 10.1016/j.bbrc.2011.04.112. [DOI] [PubMed] [Google Scholar]