Abstract
Echinococcosis in humans is a zoonotic infection caused by larval stages (metacestodes) of cestode species of the genus Echinococcus. Cystic echinococcosis (CE) is caused by Echinococcus granulosus, alveolar echinococcosis (AE) is caused by E. multilocularis, and polycystic forms are caused by either E. vogeli or E. oligarthrus. In untreated cases, AE has a high mortality rate. Although control is essentially feasible, CE remains a considerable health problem in many regions of the northern and southern hemispheres. AE is restricted to the northern hemisphere regions of North America and Eurasia. Recent studies have shown that E. multilocularis, the causative agent of AE, is more widely distributed than previously thought. There are also some hints of an increasing significance of polycystic forms of the disease, which are restricted to Central and South America. Various aspects of human echinococcosis are discussed in this review, including data on the infectivity of genetic variants of E. granulosus to humans, the increasing invasion of cities in Europe and Japan by red foxes, the main definitive hosts of E. multilocularis, and the first demonstration of urban cycles of the parasite. Examples of emergence or reemergence of CE are presented, and the question of potential spreading of E. multilocularis is critically assessed. Furthermore, information is presented on new and improved tools for diagnosing the infection in final hosts (dogs, foxes, and cats) by coproantigen or DNA detection and the application of molecular techniques to epidemiological studies. In the clinical field, the available methods for diagnosing human CE and AE are described and the treatment options are summarized. The development of new chemotherapeutic options for all forms of human echinococcosis remains an urgent requirement. A new option for the control of E. granulosus in the intermediate host population (mainly sheep and cattle) is vaccination. Attempts are made to reduce the prevalence of E. multilocualaris in fox populations by regular baiting with an anthelmintic (praziquantel). Recent data have shown that this control option may be used in restricted areas, for example in cities, with the aim of reducing the infection risk for humans.
INTRODUCTION
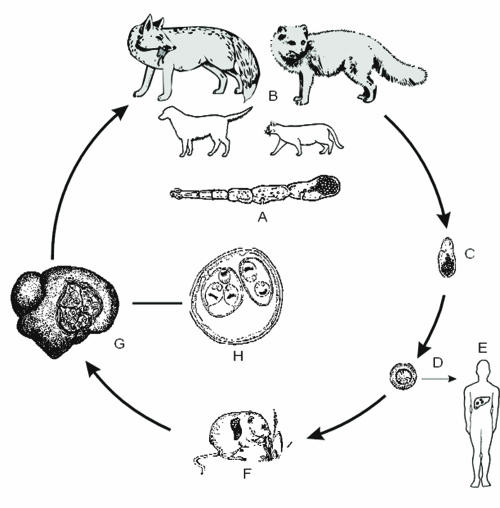
Echinococcosis is a zoonotic infection caused by cestode species of the genus Echinococcus. The life cycles of these parasites involve two mammalian hosts (see Fig. 1). The adult cestode inhabits the small intestine of a carnivore (definitive host) and produces eggs containing infective oncospheres. Either cestode segments (proglottids) containing eggs or free eggs are released from the intestinal tract of the carnivore into the environment. After oral uptake of eggs by an intermediate host animal, a larval stage, the metacestode, develops in internal organs. Typically, the mature metacestode produces numerous protoscoleces, each having the potential to develop into an adult cestode after being ingested by a suitable definitive host. Accidentally, eggs are also ingested by humans and other “aberrant” hosts that do not play a role in the natural cycle. On rare occasions, the spectrum of aberrant hosts may even include definitive hosts (i.e., dogs). Whereas the infection of carnivores with immature or mature intestinal stages of E. granulosus does not cause morbidity, the invasion of various organs (mainly liver and lungs) of intermediate or aberrant hosts by metacestodes can cause severe and even fatal disease (echinococcosis).
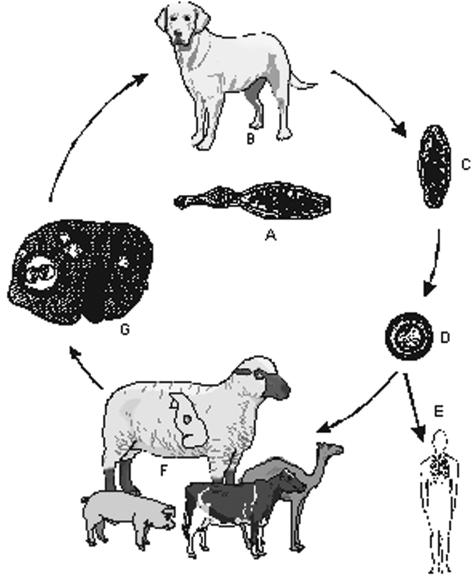
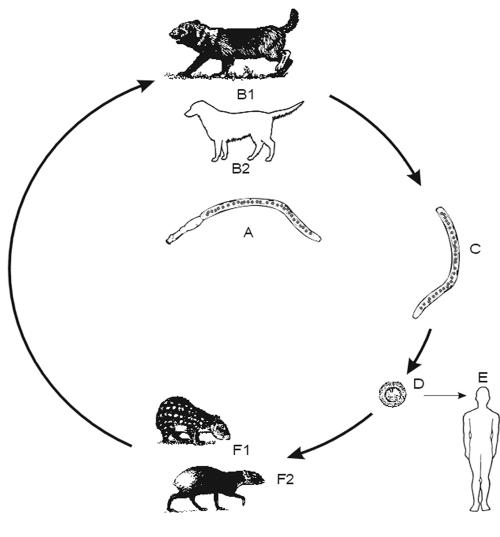
FIG. 1.
Life cycle of E. granulosus (common sheep strain). (A) Adult parasite. (B) Domestic dog as principal definitive host; wild canids (dingo, hyena etc.) can be involved in the cycle. (C) Proglottid with eggs. (D) Egg with oncosphere. (E) Infection of humans. (F) Sheep as principal intermediate hosts; other ungulates are of lower significance. (G) sheep liver with cysts.
Of three forms of echinococcosis occurring in humans (Table 1) cystic echinococcosis (CE) and alveolar echinococcosis (AE) are of special importance due to their wide geographic distribution and their medical and economic impact. Polycystic echinococcosis is less frequent and is restricted to Central and South America.
TABLE 1.
Forms of echinococcosis in humansa
| Name of disease (according to WHO/OIE; [223]) | Cystic echinococcosis | Alveolar echinococcosis | Polycystic echinococcosis | |
|---|---|---|---|---|
| Causative agent | E. granulosus | E. multilocularis | E. vogeli | E. oligarthrus |
| Other names of the disease | Hydatid disease, hydatidosis | Alveolar hydatid disease | E. vogeli echinococcosis, neotropical echinococcosis | E. oligarthrus echinococcosis, neotropical echinococcosis |
| Adult parasite | ||||
| Length (mm) | 2.0-7.0 | 1.2-4.5 | 3.9-5.6 | 2.2-2.9 |
| No. of proglottids | 3 (4-6) | 5 (2-6) | 3 | 3 |
| Definitive hosts | Domestic dog, wild canids (coyote, dingo, red fox, etc.) | Red fox, arctic fox, raccoon dog, coyote, domestic dog, cat | Bush dog, domestic dog | Wild felidae: pampas cat, Geoffroy's cat, ocelot, jaguar, cougar, jaguarundi, puma, bobcat |
| Intermediate hosts | Primarily ungulates, also marsupials | Rodents, other small mammals | Rodents: paca and agouti | Rodents: agouti, spiny rat, paca |
| Geographic distribution of the parasite | Worldwide | North America, northern and central Eurasia | Central and South America | Central and South America |
| Larval parasite in humans | ||||
| Organ localization | Visceral, predominantly liver and lungs | Visceral, primarily liver, metastases in lungs, brain, bones, etc. | Visceral, mainly liver, abdomen, lungs | Orbita, heart |
| Morphology | Fluid-filled mostly solitary (and less frequently multiple) cysts, unilocular or multichambered, diam 1->15 cm (Fig. 2); often with protoscoleces | Masses of numerous small cysts (diam microscopic up to 3 cm), often interconnected, surrounded by dense connective tissue, no cyst fluid, appearance of cheeselike mass, sometimes with central necrosis (Fig. 7); rarely a few protoscoleces | Polycystic; fluid-filled cysts, diam up to 4-6 cm, solitary, but often aggregated, interconnected and multichambered; thick laminated layer; protoscoleces frequently present | Fluid-filled cysts with tendency for multicystic development, less subdivision than in E. vogeli, and laminated layer thinner; protoscoleces formed. |
| Type of growth in humans | Concentric expansion | Exogenous proliferation, tumorlike, infiltrative | Exogenous and endogenous proliferation | Expansive, no indication of exogenous proliferation |
Data from references 156 to 158 and 194.
This review is focused on selected biological, clinical, and edpidemiological aspects, including the emergence or reemergence of infections in regions where they were previously absent or found at lower levels.
E. GRANULOSUS AND CYSTIC ECHINOCOCCOSIS
The Parasite and Its Life Cycle
E. granulosus is a small tapeworm (approximately 2 to 7 mm in length) with typically three segments and other morphological characteristics which allow a species diagnosis (193) (Table 1). In the natural cycle, dogs and other canids are typical definitive hosts and ungulates (sheep, goats, pigs, horses, etc.) intermediate hosts (Fig. 1). The latter harbor the metacestode stage. This stage can also develop in a broad range of other mammals, such as marsupials, hares, rabbits, rodents, carnivores, nonhuman primates, and humans. These and other hosts play a role in the transmission cycle (intermediate hosts) or are dead ends of the development (aberrant hosts).
During the past four decades, considerable phenotypic and genetic variability has been observed within the species E. granulosus and several strains have been identified (Table 2) (150, 194, 195, 210). A common feature of all strains (except the lion strain) is the utilization of dogs and other canids as definitive hosts, but the strains exhibit several differences in intermediate host spectrum, geographic distribution, adult and metacestode morphology, maturation time in definitive hosts, organ localization of metacestodes, and protoscolex production (59). It has to be emphasized that at least seven of nine E. granulosus genotypes are infective to humans. Globally, most human cases of CE are caused by the sheep strain (G1) of E. granulosus. Information on the infectivity of the lion strain and the buffalo strain is not available. Currently, there is no evidence that the horse strain is infective to humans (59,195). This strain is widespread and common in Ireland, but to date autochthonous cases of human CE have not been observed. However, definitive conclusions regarding the infectivity of the horse strain should not be drawn unless strain typing has been performed for a larger number of human CE cases. An example in this respect is the camel strain of E. granulosus. Earlier, it was assumed that humans may not be susceptible to the autochthonous camel strain in Kenya since all 42 E. granulosus isolates of human origin were typed by restriction fragment length polymorphism-PCR as the sheep strain (216). However, the camel strain has recently been identified in human CE cases in Argentina, Nepal, and Iran (64, 175, 195, 224). According to Thompson and McManus (195) and Le et al. (128), special features revealed by genetic comparisons and phylogenetic analyses would justify recognition of the horse and the cattle strain of E. granulosus as separate species, namely, E. equinus and E. ortleppi, respectively.
TABLE 2.
Strains of E. granulosusa
| Strain or isolateb | Definitive and intermediate hostsb | Infectivity for humans | Probable geographic distribution |
|---|---|---|---|
| G1: common sheep strain | D: dog, fox, dingo, jackal, hyena | Yes | Europe, Middle East, Africa, Iran, India, Nepal, China, Russia, Australian mainland, Tasmania, New Zealand, United States, South America |
| I: sheep, cattle, pig, camel, goat, macropods | |||
| G2: Tasmanian sheep strain | D: dog, fox | Yes | Tasmania, Argentina |
| I: sheep, cattle? | |||
| G3: (buffalo strain)? | D: dog, fox? | ? | Asia |
| I: buffalo, cattle? | |||
| G4: horse strainc | D: dog | No/? | Europe, Middle East, South Africa (New Zealand?, United States?) |
| H: horse, other equines | |||
| G5: cattle straind | D: dog | Yes | Europe, South Africa, India, Nepal, Sri Lanka, Russia, South America? |
| I: cattle, buffalo, sheep, goat | |||
| G6: camel strain | D: dog | Yes | Middle East, Iran, Africa, China, Nepal, Argentina |
| I: camel, goat, cattle | |||
| G7: pig strain | D: dog | Yes | Poland, Slovakia, Ukraine, Russia, Argentina |
| I: pig | |||
| G8: cervid strain (G8) | D: wolf, dog | Yes | North America, Eurasia |
| I: cervids | |||
| G9: ? | ? | Yes | Poland |
| Lion strain | D: lion | ? | Africa |
| I: zebra, wildebeest, warthog, bushpig, buffalo, various antelope species, giraffe?, hippopotamus? |
Cystic Echinococcosis in Humans
Course of infection.
CE is caused by the metacestode stage of various strains of E. granulosus, which is a cystic structure typically filled with a clear fluid (hydatid fluid). About 5 days after ingestion of eggs, the metacestode is a small vesicle (60 to 70 μm in diameter) consisting of an internal cellular layer (germinal layer) and an outer acellular, laminated layer. This cyst (endocyst) gradually expands and induces a granulomatous host reaction, followed by a fibrous tissue reaction and the formation of a connective tissue layer (pericyst). The size of cysts in the human body is highly variable and usually ranges between 1 and 15 cm, but much larger cysts (>20 cm in diameter) may also occur (3, 148, 184) (Fig. 2). The exact time required for the development of protoscoleces within cysts in the human host is not known, but it thought to be more than 10 months postinfection. Protoscoleces can already be formed in cysts of 5 to 20 mm in diameter (149); on the other hand, a proportion of cysts do not produce protoscoleces and remain “sterile.” Most of the cysts are univesicular (i.e., unilocular), but in some of them, smaller daughter cysts are formed within larger mother cysts. Mixed infections with metacestodes of E. granulosus and E. multilocularis are rare, although the two species occur simultaneously in large areas of endemic infection (149).
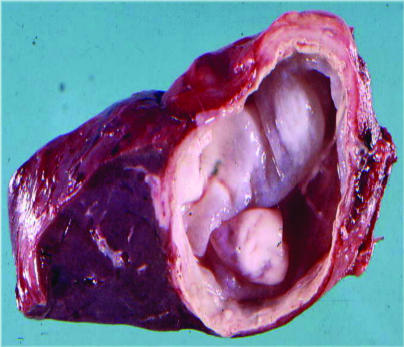
FIG. 2.
Hepatic CE in a patient (endocyst removed; lesion size approximately 3 by 3.5 cm).
In the human host, cysts may develop in many anatomic sites following oral ingestion of E. granulosus eggs. This form of echinococcosis is known as primary CE. Secondary CE, predominantly in the abdominal cavity, results from spontaneous or trauma-induced cyst rupture and the release of protoscoleces and/or small cysts, which can grow to larger cysts. Approximately 40 to 80% of patients with primary CE have single-organ involvement and harbor a solitary cyst (3, 149). Examples of the organ sites of cysts in hospital patients are presented in Table 3.
TABLE 3.
Organ sites of E. granulosus cysts in humansa
| Organ | Study Ab: single-organ involvement in 459 patients
|
Study Bc: single- and multiple-organ involvement in 15,289 Chinese surgical cases
|
||
|---|---|---|---|---|
| No. of cases | % of cases | No. of casesd | % of cases | |
| Liver | 316 | 68.8 | 11,499 | 75.2 |
| Lungs | 79 | 17.2 | 3,432 | 22.4 |
| Kidneys | 17 | 3.7 | 68 | 0.4 |
| Spleen | 15 | 3.3 | 160 | 1.0 |
| Muscles and skin | 10 | 2.2 | 29 | 0.2 |
| Abdominal and pelvic cavity | 9 | 2.0 | 794 | 5.2 |
| Mediastinum, heart | 5 | 1.1 | 4 | 0.03 |
| Brain | 4 | 0.9 | 61 | 0.4 |
| Bones | 3 | 0.6 | 30 | 0.2 |
| Ovarium | 1 | 0.2 | 9 | 0.06 |
| Other organs: skin, eye, spinal cord, pancreas, urinary bladder, testis, etc. | Each <0.1 | |||
Adapted from reference 149.
Data from reference 45. Single-organ involvement in 459 patients, originating predominantly from the Mediterranean region.
Data from reference 140.
The number of cases in this column exceeds the total of 15,289 since many patients had multiple-organ involvement; the same applies to the percentages.
A literature review of 9,970 patients (originating from regions in South America, Africa, Europe, and Australasia where the sheep strain is common and infection is endemic) has revealed that the average liver-to-lung infection ratio was 2.5:1 (126). A different situation exists in infected but asymptomatic individuals. Ultrasonographic and chest X-ray surveys of approximately 10,000 apparently healthy individuals living in areas of Argentina and Uruguay with endemic infection revealed liver-to-lung ratios of 6:1 and 12:1, respectively (126). An explantation for the shift from the higher liver-to-lung ratios in asymptomatic individuals to lower values (2.5:1) in hospitalized patients is that lung cysts cause more frequently morbidity than hepatic cysts (126).
The initial phase of the primary infection is always asymptomatic. Small, well encapsulated, nonprogressive or calcified cysts typically do not induce major pathology, and patients may remain asymptomatic for years or permanently (3, 149). The induction of morbidity depends on the number, size, and developmental status of the cyst(s) (active or inactive), the involved organ, the localization of the cyst(s) within the organ, the pressure of cysts on surrounding tissues and structures, and the defense mechanisms of the infected individual. Ultrasonographic studies in South America have shown that the average diameter of cysts in asymptomatic carriers was significantly smaller (approximately 4 cm) than that in symptomatic patients (approximately 10 cm) (126). According to Perdomo et al. (151), approximately 88% of cysts detectable in asymptomatic carriers were <7.5 cm in diameter. An ultrasonographic survey in Italy revealed that 60% of 424 individuals with CE were asymptomatic (21).
Cyst growth is generally slow. In 14 asymptomatic cyst carriers in Argentina, the diameter of liver cysts increased by <3 to 4 cm in 6 patients and showed no modification in 8 individuals during a 10- to 12-year observation period (72, 126). Low growth rates were also reported for hepatic cysts in another Argentinian study of asymptomatic patients (126). There is evidence that liver cysts grow at a lower rate than lung cysts (126). However, the growth rates of cysts may vary between cysts in the same organ or in the same individual and between individuals in various regions. For example, in the Turkana district of Kenya, a region of high endemicity where CE caused high morbidity, higher growth rates of cysts were recorded. In an ultrasonography study of 66 patients, about 30% of the abdominal cysts grew slowly (1 to 5 mm per year), 43% showed moderate expansion (6 to 15 mm per year), 11% exhibited a more rapid increase (average of 31 mm and maximum of 160 mm per year), and 16% of the cysts did not expand or had collapsed (169,172). The latter finding shows that spontaneous involution of cysts is possible, which leads to changes in the ultrasonographic appearance of the cysts (see below).
Clinical signs may occur after a highly variable incubation period of several months or years. Frider et al. (72) observed that 21 (75%) of 28 carriers of liver cysts in Argentina remained asymptomatic during follow-up periods of 10 to 12 years after the initial diagnosis, while 7 (25%) developed symptoms related to their liver infection. Hepatic cysts can cause pain in the upper abdominal region, hepatomegaly, cholestasis, biliary cirrhosis, portal hypertension, ascites, and a variety of other manifestations (3, 149). Cysts may rupture into the peritoneal cavity, causing anaphylaxis or secondary CE, or into the biliary tree, leading to cholangitis and cholestasis. Abscess formation is possible after bacterial infection of cysts. Chronic cough, expectoration, dyspnea, hemoptysis, pleuritis, and lung abscess are selected symptoms caused by pulmonary cysts, and neurological disorders can be induced by cysts in the brain (3, 149). The modulation of T-lymphocyte responses plays an important role in the outcome of the infection. Th1 and Th2 responses have been associated with resistance and with susceptibility or severe forms of CE, respectively (215). The majority of patients produces various classes of serum antibodies which are not associated with protection but are valuable diagnostic indicators (28, 84).
CE occurs in age groups from younger than 1 to over 75 years. In some areas of endemic infection, most hospital cases are recorded in the age groups between 21 and 40 years, but the highest morbidity may also occur in younger individuals aged between 6 and 20 years (3, 149). An analysis of 8,596 individuals in areas of endemic infection in Uruguay has revealed a significant age-dependent increase of hepatic cysts detectable by ultrasonography from 0.33% in the age group from 0 to 9 years to 3.80% in the age group from 70 to 79 years (151). Similar observations were made in other areas of endemic infection (168). In most of the larger series of patients, there were no significant differences in the gender ratios of individuals with CE (25, 149, 151).
Diagnosis.
The diagnosis of CE in individual patients is based on identification of cyst structures by imaging techniques, predominantly ultrasonography, computed tomography, X-ray examinations, and confirmation by detection of specific serum antibodies by immunodiagnostic tests (28, 84, 90, 114, 149, 192). For clinical practice it should be noted that the enzyme-linked immunosorbent assay (ELISA) using crude hydatid cyst fluid has a high sensitivity (over 95%) but its specificity is often unsatisfactory. If purified antigens (e.g., antigen B) or other techniques (immunoblot analysis, detection of immunoglobulin G4 (IgG4) antibodies, immunoelectropheresis, etc.) are used, specificity is improved but average sensitivity is much lower (Table 4). Furthermore, it should be remembered that approximately 10 to 20% of patients with hepatic cysts and about 40% with pulmonary cysts do not produce detectable specific serum antibodies (IgG) and therefore give false-negative results (3, 149). Cysts in the brain, bone, or eye and calcified cysts often induce no or low antibody responses (3). In routine laboratory practice, usually at least two different tests are used to get the most reliable results (for the differential diagnosis of CE and AE, see the discussion of diagnosis of AE, below). More details are given elsewhere (28, 84, 104, 168, 223).
TABLE 4.
Tests for antibody detection in human CE and AEa
| Echinococcosis form and test | Antigenb | Sensitivity (%) | Relative specificityc (%) | Cross-reactions |
|---|---|---|---|---|
| Cystic | ||||
| IgG ELISA | Crude EgCF | 80->99 | 61.7 | Cestodes (89%), trematodes (30%), nematodes (39%) |
| Antigen B (native or synthetic peptide) | 63-92 | 85-93 | AE | |
| IgG4 ELISA | Crude EgCF | 61-67 | >99 | AE only (see AE) |
| EITBd | Crude EgCF | 71 | >98e | T. solium cysticercosis only |
| Antigen B fraction | 92 | 100 | None | |
| Antigen B subunits | 34-36 | >90 | ||
| Alveolar | ||||
| IgG ELISA | Crude EgCF | 97.1 | 61.7 | See above |
| Em2PLUS | 97.1 | 98.9 | CE (25%) | |
| Em2/Em2G11 | 89.3 | 100 | CE (5.6%) | |
| Em II/3-10 | 86.4 | 98.4 | CE (6.5%) | |
| IgG4 ELISA | Crude EgCF | 48-67 | >99 | CE (see CE) |
| EITB | Em18 | 97 | 100 | None |
| Glycoproteins | 70-90 | >95 |
Data from references 84, 87, 90, 168, 186, and other sources.
EgCF, E. granulosus cyst fluid; Em, E. multilocularis.
Tested with panels of 80 to 184 sera from patients with different parasitic infections (excluding Echinococcus-infected patients).
EITB, enzyme-linked immunoelectrotransfer blot.
Including additional sera from patients with other diseases.
Ultrasonography-guided fine-needle puncture has been used in recent years as a diagnostic procedure in doubtful cases of CE, i.e., in the absence of detectable anti-Echinococcus antibodies, in patients with small lesions resembling hepatic cysts, and in patients with lesions which cannot be distinguished from liver abscess, neoplasms, or other conditions (149). Aspirated cyst fluid can be examined for protoscoleces, rostellar hooks, and Echinococcus antigens or DNA (186). To prevent secondary echinococcosis if a hydatid cyst is punctured, chemotherapy with albendazole is recommended for 4 days before the procedure. Chemotherapy should be continued for at least 1 month after puncturing a lesion that was diagnosed as an E. granulosus cyst (149), even after its immediate surgical removal.
Classification of cyst types is an important basis for decisions about treatment options. A highly informative review of imaging techniques for diagnosing human echinococcosis has been published by von Sinner and Lewall (213). Recently, the World Health Organization (WHO) Informal Group on Echinococcosis has published an international consensus classification of ultrasonograms of hepatic cysts (222, 223).
Ultrasonography with portable equipment is used for surveys in the field. This technique is well accepted by the population, explores abdominal sites, identifies cyst types, and can be performed at relatively low cost (25, 149, 151, 185). Differential diagnosis of other space-occupying lesions (tumors, liver abscesses, etc.) can be difficult or impossible and may require the use of additional diagnostic techniques. A disadvantage of ultrasonography is that cysts in other sites (lung, brain, etc.) cannot be readily detected. Several comparative field surveys have shown that ultrasonography is much more precise in detecting abdominal cysts than are immunodiagnostic tests since the latter exhibit relatively high rates of false-negative and false-positive results (22, 28, 35, 168, 179, 185).
Treatment.
There are several major options for treatment of CE, including surgery, puncture aspiration injection reaspiration (PAIR), and chemotherapy. For asymptomatic individuals, a “wait-and-observe” approach may be considered with supervision of the patient (101, 102, 115, 149).
(i) Surgery.
Surgery, using various technical approaches (3, 142, 149), has the potential to remove the cysts and lead to complete cure. It can be successfully performed in a high proportion of patients with simple forms of CE (cyst number and organ involvement limited, cysts not in risky locations, disease not too far advanced). However, surgery may be impractical in other cases, predominantly in patients with multiple cysts in several organs, in patients with a high surgical risk, and if facilities for advanced surgery are inadequate. In such situations, PAIR or chemotherapy can be considered as alternative options of treatment.
The effect of pre- or postoperative chemotherapy for preventing secondary CE after spillage of cyst fluid during surgery is still unclear. For the PAIR technique (see below), experts recommend chemotherapy with albendazole 24 to 4 h before intervention and 15 to 30 days afterwards (222). The number of E. granulosus cysts developing from intraperitoneally inoculated protoscoleces in rodents could be reduced by 80 to 90% if albendazole treatment (10 mg/kg of body weight/day) for 1 week was initiated immediately after inoculation, but treatment starting 15 days after inoculation was ineffective (143). Data on praziquantel treatment are contradictory. In a previous study (165), the drug reduced protoscolex viablity by 65 to 82% when applied in vitro at a concentration of 0.1% for 10 min. According to a recent publication, a 0.1% praziquantel solution had no marked protoscolicidal effect in vitro after 1 h but was strongly effective at 1% after 30 min (100). In mice, treatment with high doses of praziquantel (600 mg/kg of body weight/day on 5 days per week) for 4 months did not significantly influence established E. granulosus cysts. When the same treatment was initiated 43 h after intraperitoneal application of protoscoleces, a high prophylactic effect was achieved, as indicated by a 99% reduction in cyst numbers (208). However, the drug doses used in this experiment are much higher than those used in humans (e.g., for a Schistosoma mansoni infection, a single dose of 40 mg/kg of body weight was recommended).
(ii) Puncture-aspiration-injection-reaspiration.
PAIR was introduced in the mid-1980s (14, 67, 69, 73, 117). It is a minimally invasive technique and includes the following steps: (i) percutaneous puncture of the cyst under ultrasonographic guidance, (ii) aspiration of a substantial portion (for example, 10 to 15 ml) of the cyst fluid, (iii) injection of a parasitocidal solution (95% ethanol; approximately an equivalent of one-third of the amount aspirated), and (iv) reaspiration of the fluid content after 5 min (222). Hypertonic NaCl solution (at least 15% [final concentration] in the cyst fluid) can also be used as a parasitocidal solution, but its action is slower, so that reaspiration is performed only after 15 to 20 min (116, 222).
A guideline for the performance of PAIR has recently been published (222). PAIR should always be performed by skilled and experienced physicians well prepared to deal with complications. According to expert recommendations, PAIR should be accompanied by chemotherapeutic coverage to minimize the potential risk of secondary echinococcosis (149, 221, 222). In this indication, albendazole is applied in daily oral doses of 10 mg/kg of body weight 24 to 4 h before and 15 to 30 days after the intervention (222). More studies are needed to evaluate the efficacy and optimize this treatment schedule.
PAIR is indicated for univesicular hepatic cysts of ≥5 cm in diameter (types CEL and CE1 according to the international classification [222]), for cysts with daughter cysts (type CE2) (Fig. 3), for cysts with detached membranes (type CE3), and also for multiple cysts if accessible to puncture (222). The main contraindications for PAIR are cysts communicating with the biliary tree, cysts in a risky or inaccessible location in the liver, cysts free in the abdominal cavity, and cysts in the lungs, heart, brain, or spine (149, 222).
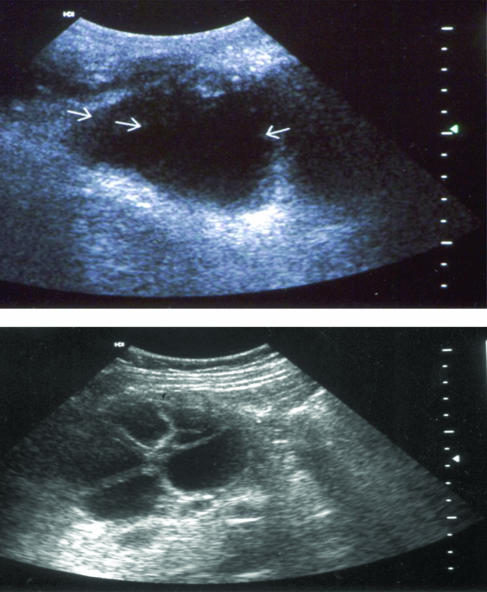
FIG. 3.
Ultrasonograms of hepatic cysts of E. granulosus (Top) Type CL of WHO-IGWE classification (see text): lesion (arrows) with uniform anechoic content, not clearly delimited by an hyperechoic rim (cvst wall not visible). (Bottom) Type CE2: multivesicular cysts. Reprinted with permission from P. Kern and Dr. W. Kratzer, University Hospital and Medical Center, University of Ulm, Ulm, Germany.
Great care must be taken to detect cysts with biliary communications in order to prevent an influx of parasitocidal solution into the biliary tree and the risk of chemical cholangitis. Therefore, it is a basic requirement to examine aspirates from liver cysts for traces of bilirubin. Furthermore, endoscopic retrograde cholangiopancreatography is used to rule out cyst-biliary communications (222). If bilirubin is present in the cyst fluid, the PAIR procedure must be discontinued.
PAIR interventions in more than 2000 patients had a high rate of efficacy and generally a low rate of complications (67, 116, 137, 209, 222). There are indications that PAIR has several detrimental effects to the parasite, including (partial) detachment of endocyst membranes from the pericyst and/or damage of the germinal layer and protoscoleces by the parasitocidal solution. A recent international survey of PAIR treatments performed in various hospitals showed the following results (68). Treatment of 765 abdominal cysts, mostly hepatic, almost always resulted in various degrees of size reduction (at least 50%) and involution of the cysts, except for two failures (0.26%). A >5-year follow-up of approximately 10% of the cysts and a <5-year follow-up of 90% revealed 12 recurrences (1.6%). Major complications occurred in four patients (0.52%), with one death (0.13%) and spillage of hydatid fluid in four patients (0.52%); 13.7% of the patients had minor complications. Ustunsoz et al. (209) have reported a 97% cure rate of PAIR in 70 Turkish patients with a mean follow-up of 37 months and only two (3%) recurrences.
(iii) Percutaneous thermal ablation.
A new approach of treatment involves percutaneous thermal ablation (PTA) of the germinal layer in the cyst by using a radiofrequency ablation device. Brunetti and Filice (18) have used PTA for treating two patients with hepatic cysts; more experience with this technique is needed. PTA would have the advantage that injection of parasitocidal substances into the cyst is unnecessary.
(iv) Chemotherapy.
Chemotherapy with benzimidazoles (albendazole or mebendazole) is indicated for patients with inoperable CE and for those with multiple cysts in two or more organs. Cysts located in bones are less susceptible to chemotherapy. According to WHO recommendations, albendazole is given in daily doses of 10 to 15 mg/kg of body weight in two divided doses postprandially for 3 to 6 months. The usual dose of mebendazole is 40 to 50 mg/kg of body weight per day for at least 3 to 6 months (115, 221, 222). Results for over 2,000 well-controlled cases treated with benzimidazoles and evaluated for up to 12 months have shown that cysts disappeared in 10 to 30% of the patients (cure), there was objective evidence of response in 50 to 70% (degeneration or size reduction of cysts), and 20 to 30% did not exhibit morphological changes of the cysts (101, 102, 149, 197, 198). Relapses after chemotherapy have been observed in 14 to 25% of patients, but are usually sensitive to retreatment (149). In a comparative study with 448 patients, Franchi et al. (70) assessed the efficacy of mebendazole and albendazole treatment (3 to 6 months) and found degenerative changes in 82% of the cysts in the albendazole group and in 56% in the mebendazole group (P < 0.001). Relapses were observed in 25% of the cysts. Side effects of chemotherapy are generally mild and rarely treatment limiting (3, 101, 221, 223). Although the efficacy of chemotherapy is not satisfactory and the costs are high, it is an option of treatment, predominantly for inoperable cases.
E. granulosus Infection in Animals
Defintive hosts.
The domestic dog is the principal defintitive host of E. granulosus, but in certain regions wild canids may be involved in the life cycle of the parasite (Table 1). Although E. granulosus penetrates deep between the villi of the small intestine of a definitive host, there are no pathogenic effects even in animals with a heavy infection (54). Therefore, infected definitive hosts are typically asymptomatic carriers of the parasite.
The diagnosis of intestinal E. granulosus infection in living dogs is difficult because the small proglottids spontaneously discharged with feces are usually overlooked and eggs detected by routine coproscopic techniques cannot be differentiated by light microscopy from the eggs of other Echinococcus species or of Taenia species. ELISAs for detecting parasite antigens in fecal samples (coproantigens) have been used in specialized laboratories for the last few years (29, 40). Recently, a PCR for specific detection of DNA from E. granulosus eggs has been developed (A. Mathis and P. Deplazes, unpublished data) (Table 5). The coproantigen ELISA has a reasonable sensitivity and a high specificity and can be used as a screening test for individual dogs or for dog populations (Table 5). One of the advantages of this test is that about 200 samples can be examined by one person per day (41). The more sophisticated PCR can be used as a highly sensitive and specific secondary test for confirming or excluding an E. granulosus infection (Table 5). Postmortem examination of definitive hosts for Echiococcus species requires special techniques (Table 5), which are described elsewhere in detail (54). Praziquantel is the drug of choice for treating infected dogs (reviewed in reference 54) (see “Control options and prevention” below).
TABLE 5.
Options for the diagnosis of E. granulosus in animals
| Animal group | Material required | Test, sensitivity and specificitya | Reference(s) |
|---|---|---|---|
| Live animals | |||
| Individual dogs | Feces in buffer | Screening: coproantigen ELISA. S, 65-77%; SP, >90%. | 28, 40 |
| Secondaryb: copro-PCR. S, under evaluation; SP, close to 100%. | Mathis and Deplazes, unpublished | ||
| Dog populations | Fecal material discharged by dogs after arecoline treatment | Standard option: macroscopic examination of discharged material. S, 65% after single-dose arecoline, 78% after second dose; SP, close to 100%. | 179 |
| Feces in buffer | New option. Screening: Coproantigen-ELISA. | 28, 40 | |
| S and SP, as for individual dogs. | |||
| Secondary: copro-PCR. S and SP as for individual dogs. | Mathis and Deplazes, unpublished | ||
| Intermediate hosts: sheep, goat, cattle, horse etc. | No reliable in vivo method for detecting the infection in individual animals, except rare cases in which cysts can be identified by ultrasonography in conjunction with antibody detection, for example in individual horses. | 54 | |
| A new ELISA might be useful for the detection of E. granulosus in sheep flocks (sensitivity, 50-60%). | 120 | ||
| Dead animals | |||
| Dogs and other carnivores | Small intestine | Standard option: Parasite detection at necropsy by direct examination of the intestine or by sedimentation technique (S and SP, close to 100%). | 54 |
| Feces from rectum or content from intestine in buffer | New option: coproantigen ELISA in conjunction with copro-PCR (details as for small intestine). | ||
| Intermediate hosts | Viscera | Cyst detection at meat inspection or necropsy; in doubtful cases histology and/or PCR. | 54 |
S, sensitivity, SP, specificity.
Secondary test for confirmation or exclusion.
Intermediate hosts.
Infections with E. granulosus cysts in intermediate hosts (sheep, goat, cattle, horses, etc.) are typically asymptomatic, except a few cases of long-standing and heavy infections, for example in horses (Fig. 4). There are no reliable methods for the routine diagnosis of the infection in living animals, but in rare cases cysts have been identified by ultrasonography alone or in conjunction with serum antibody detection (54). A new ELISA with a high specificity and a sensitivity of 50 to 60% might be useful for detecting E. granulosus cysts in sheep on a flock basis but cannot be used for reliable diagnosis of infected individual animals (120) (Table 5). The most reliable diagnostic method is cyst detection during meat inspection or at postmortem examination (Table 5). CE in farm animals causes considerable economic problems due to loss of the edible liver. Significant loss of meat and milk production and value of the fleece from infected sheep may also occur. These losses are of especial significance in countries of low economic output where sheep production is of particular importance (201).
FIG. 4.
Horse liver with multiple cysts of E. granulosus (cyst diameters approximately 1 to 10 cm). The horse exhibited clinical signs of the disease.
Epidemiology
Life cycle patterns.
The life cycles of E. granulosus strains (Table 2) can be classified as domestic, involving the domestic dog as the principal definitive host and various species of domestic ungulates as intermediate hosts, or as sylvatic, involving wild carnivores and ungulates as hosts (the wildlife cycle). Within the cycles, the specific role of various host species may differ considerably between regions of endemic infection. In many areas of endemic infection, domestic and sylvatic life cycles coexist or overlap (155). For example, the dog-sheep strain is globally the most widespread and important strain of E. granulosus and exists in its domestic form (dog-sheep/goat) in many regions. However, in Australia this strain is transmitted between domestic animals (dog/sheep) and wild-animal hosts (definitive hosts are dingoes [Canis lupus dingo], dingo-domestic dog hybrids, less frequently red foxes [Vulpes vulpes]; intermediate hosts are mainly macropod marsupials, also feral pigs [Sus scrofa] and wombats [Vombatus ursinus]) (105). On the other hand, the cervid strain of E. granulosus in the Arctic is transmitted almost exclusively between wolves and wild Cervidae (elk [Alces alces], reindeer [Rangifer tarandus], and red deer [Cervus elaphus]) but domestic dogs and domesticated reindeer can replace the wild hosts (155). Complex situations of coexisting or overlapping domestic and sylvatic cycles also exist in other regions (for example, in Africa and Eurasia) and represent special problems in echinococcosis control (105, 132, 133, 155).
Transmission dynamics.
During the past four decades, considerable advances have been made in understanding the epidemiological key factors and the transmission dynamics of E. granulosus and other members of the family Taeniidae (notably Taenia hydatigena and T. ovis). A mathematical model was developed which allows us to quantify various factors contributing to the regulation and stability of the parasite populations and to draw conclusions for control (74, 78, 85, 166, 167, 199, 200). For Taenia species, the following key factors have been identified (78): (i) biotic potential of the parasite, (ii) immunity acquired by the intermediate host as a density-dependent constraint, and (iii) environmental factors as density-independent constraints in the free-living egg-phase.
T. hydatigena and T. ovis have high biotic potentials with the production of large numbers of eggs and large numbers of metacestode cysts developing in sheep. In contrast, the biotic potential of E. granulosus is relatively low, representing less than 5% of the potentials of T. hydatigena and T. ovis (75). Results of previous studies have suggested that the degree of immunity acquired by definitive hosts during natural infections with E. granulosus is negligible and does not play a role in regulating the intestinal parasite population (75). Experiments to stimulate strong immunity against intestinal stages of E. granulosus in dogs have failed so far (92). Recent observations from Tunisia and Kazakhstan, where young dogs are more heavily infected than older dogs, should stimulate new basic studies of the immune responses of dogs to intestinal cestode infections (199).
On the other hand, acquired immunity in intermediate hosts has clearly been identified as a density-dependent constraint reducing the parasite (metacestode) population of Taenia species. A considerable degree of immunity against T. hydatigena and T. ovis is acquired by sheep within about 2 weeks after ingestion of small numbers of eggs (as few as 10 eggs per animal); it persists life-long in the presence of eggs in the environment but is lost between 6 and 12 months in the absence of eggs, and it does not depend on the presence of metacestodes from a previous infection (74, 75). Strong immunity can also be experimentally induced against E. granulosus, but it requires much larger numbers of eggs (approximately 50,000 eggs per animal) (199). Consequently, sheep populations do not develop strong immunity against E. granulosus under natural infection pressure, as indicated by the fact that both the prevalence and intensity of the infection with cysts of E. granulosus increase with age of sheep (123, 124, 206).
Environmental temperature and humidity influence egg survival and infectivity but do not regulate the parasite population. E. granulosus eggs can survive under humid conditions for several weeks or months in areas of warm and cold climates, but they are sensitive to desiccation (55, 75). Several factors play a role in egg dispersal (75, 78) (see also “Eggs in the environment” below).
Infection risk for humans.
Humans acquire primary CE by oral uptake of E. granulosus eggs excreted by infected carnivores. The infection may be acquired by handling infected definitive hosts, egg-containing feces, or egg-contaminated plants or soil followed by direct hand-to-mouth transfer. It has been shown that Echinococcus eggs adhere to the coat of dogs, particularly to the hairs around the anus and on the thighs, muzzles, and paws. The same applies to dogs infected with Taenia species and to foxes infected with E. multilocularis (55). Eggs can also be ingested with vegetables, salads, uncooked fruits, and other plants which have become contaminated. Foodstuffs or surfaces may possibly be secondarily contaminated with Echinococcus eggs via wind, birds, beetles, and flies (55). Also, drinking water contaminated with Echinococcus eggs by the feces of infected carnivores is a potential source of infection (see below). Prenatal transfer of E. granulosus does not play a role (26).
Very little is known about the relevant modes of E. granulosus egg transmission to humans. Campos-Bueno et al. (20) in Spain have evaluated several risk factors in a case-control study involving 127 patients with proven CE cases and 127 controls matched by sex, age, and residence. The risk of infection was highest in small places with up to 500 inhabitants and increased with the number of dogs in the family and the number of years of coexistence with them. Further important risk factors were dogs having access to raw viscera of slaughter animals and dogs kept loose and able to enter dwellings. Surprisingly, the ingestion of products from family vegetable gardens over prolonged periods was not associated with an increased risk for CE. In a case-control study in Argentina, one of the risk factors for CE was spending the first years of life surrounded by a large number of dogs (125). In Tibetan aeras of China (Sichuan), increased risks for CE were associated with nomadic life, age, playing with dogs, not protecting food from flies, and raising yaks or sheep (217). In studies in Jordan (43) and Kyrgystan (204), multivariate analysis revealed the use of potentially contaminated water as the only statistically significant risk factor for humans, but confounding factors could not be excluded. Water wells were also suspected as sources of infection with E. granulosus in arid areas of Africa where humans and carnivores frequently use the same water points (132). In a cross-sectional survey in an area of endemicity in mid-Wales (United Kingdom), no significant association could be found between treatment of humans for CE and many of the well-established risk factors, such as dog or farm ownership (44). The identification of risk factors is generally difficult for various reasons, such as small sample sizes, long time interval between infection and diagnosis of CE, and high mobility of patients and migration of definitive hosts. Molecular approaches allowing the species-specific identification of Echinococcus eggs in the environment will open up new opportunities for the study of transmission routes (see “E. multilocularis infection in animals” below).
Global distribution of E. granulosus and CE in humans.
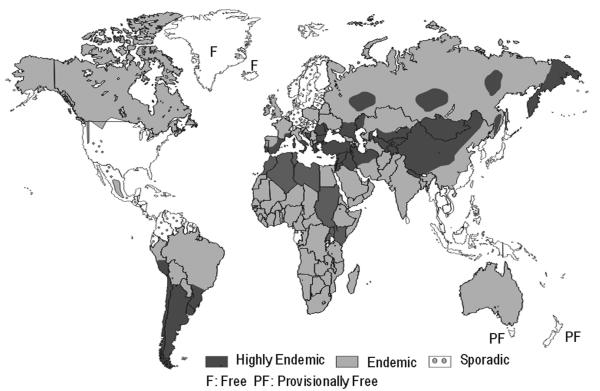
Due to the lack of well-documented data from many countries, the global picture of the current situation is incomplete. However, a recent review (58) has shown that E. granulosus is known to occur on all continents and in at least 100 countries (Fig. 5). High parasite prevalences are found in parts of Eurasia (for example, the Mediterranean region, the Russian Federation and adjacent independent states, and China), Africa (northern and eastern regions), Australia, and South America. In some European countries or regions, the annual incidences (AI) of hospital cases of human CE vary between <1 and >8 per 100,000 population. In China, CE is regarded as one of the major public health problems (220). In Xinjiang, the average AI was 8.7 per 100,000 in 1990 but the AI was up to 42 per 100,000 in one of the counties (140). In Sichuan province, human CE had a prevalence of 2.1% (85/3,998) in 1997 to 1998 (217). High incidence rates or prevelances have also been recorded from countries in northern and eastern Africa (prevalences of >3%) and South America (for example; an AI of 9.2 per 100,000 population in Uruguay in 1995). A few islands are free of E. granulosus (Iceland and Greenland), and in some islands only very sporadic, cases have been detected in domestic animals in recent years (“provisionally free”) (New Zealand, Tasmania, southern Cyprus). The occurrence of E. granulosus is sporadic or has not been reported from other regions, including countries or regions in northern and central Europe, in North and Central America, in the Pacific Region, and in the Caribbean.
FIG. 5.
Approximate global distribution of E. granulosus (as of 2002). The exact identification of areas of normal and high endemicity is difficult because of incomplete or lacking data. Modified from WHO/OIE 2001 (223) with permission.
Factors associated with persistence, emergence, or reemergence.
Key factors associated with persistence, emergence, or reemergence of CE have recently been described in Bulgaria (196) and the Mediterranean region (12). They include (i) the presence of large numbers of dogs (especially stray dogs) infected with E. granulosus, (ii) easy access of dogs to organs of livestock infected with E. granulosus cysts, (iii) insufficient facilities for slaughter and destruction of infected viscera, (iv) illegal or uninspected home slaughter, (v) a close association of dogs and other animals on small rural lots of land, (vi) uncontrolled animal trade and movements within and between countries, (vii) poor living conditions (especially lack of tap water), (viii) lack of adequate health education, and (ix) economic instability and financial restrictions in control and prevention. In central Asian countries (Kazakhstan and Kyrgystan), the reemergence of CE is clearly associated with the transition from a planned to a free-market economy since their independence from the former Soviet Union and its several consequences, such as a decline of the economy and living standards, deterioration of veterinary and medical services owing to the lack of adequate funding, and reforms in agriculture with increase of smaller livestock enterprises, uncontrolled slaughter, and offal disposal (183, 203, 204, 205).
Examples of emergence or reemergence.
Reports from several countries provide documented evidence for the emergence or reemergence of E. granulosus and CE in recent years. For example, in Bulgaria the annual incidence of CE in children has increased from 0.7 per 100,000 in 1971 to 1982 to 5.4 in 1995 (196). Other reports indicate an alarmingly high prevalence of E. granulosus in humans and animals in some countries of the Mediterranean region (12). In Kazakhstan the annual surgical incidence of CE over the whole country was below 1.4 per 100,000 inhabitants from 1988 until 1995 but has increased to approximately 2.5 in 1997 and to 5.9 in 2000; 29% of the cases were in children younger than 14 years, indicating recent transmission (182, 183, 203). In the South Kazakhstan Oblast, the prevalence of E. granulosus cysts was 13.6% in 5,968 sheep prior to independence and 37.0% in 917 sheep in the same area in 1999 to 2000 (203). A similar trend has been identified in Kyrgystan (203, 204), where the annual incidence of CE per 100,000 inhabitants has increased over the whole country approximately threefold from 5.4 cases in 1991 to 18 in 2000. Hospital admissions due to CE in the capital, Biskek, have increased 5.9-fold from 21 cases in 1990 to approximately 124 in 1999; the increase of pediatric cases was 41 times higher in 2000 (82 cases) than in 1990 (2 cases) (204). These data are supported by ultrasonography cross-sectional surveys of 8,777 persons between 1989 and 1994 and 1,486 subjects between 1991 and 2000; in this period, the prevalence had increased significantly, from 0.42 to 1.35% (204).
The data from Bulgaria, Kazakhstan, Kyrgystan, and some other regions provide strong evidence for a real increase or reemergence of the incidences and prevalences in recent years, which is not attributable to improved diagnosis or reporting.
Control Options and Prevention
Several options for the control of E. granulosus have been thoroughly evaluated and are described in detail elsewhere (13, 61, 62, 74-77, 153). One option (type I) emphasizes long-term measures of public health education with primary health care (147) and veterinary public health activities, such as the improvement of slaughter hygiene and meat inspection, dog registration and sanitation measures (77). Experience from several countries has shown that this option alone may not be sufficient and may be too slow for effective E. granulosus control (77). Another option (type II) is based on legislation and includes specific measures targeted to interruption of parasite transmission. Prior to the “attack phase” of the program, base-line data are collected to serve as references for measuring control progress. Important base-line data are the prevalence of E. granulosus in dog populations, the age-dependent prevalences of cysts in sheep and other domestic ungulates, and human cases of CE. Modern techniques can now be used for surveys; for example, the coproantigen ELISA can be used to detect E. granulosus in dog populations (instead of arecoline testing) and ultrasonography alone or in combination with serology can be used for mass diagnosis of CE in humans (24, 28, 71, 168, 179, 223). Specific control measures include stray-dog control, registration of all owned dogs, spaying of bitches, and treatment of all (or most) dogs with praziquantel at predetermined intervals, for example every 6 or 8 weeks. These measures are complemented by upgrading of meat inspection, slaughter hygiene, slaughter offal disposal, public health education, and other measures. Control programs in various countries have shown that the attack phase can be successfully concluded in less than 15 years if the necessary measures can be performed without major constraints and financial restrictions (76-78). Under suboptimal conditions, the attack phase can last much longer. For example, in the Rio Negro Province of Argentina, a control program including dog treatment with praziquantel (5 mg/kg of bodyweight) at 2-month intervals has, within about 20 years (1979/1980 to 1999), reduced the prevalence of cysts in sheep from 61 to 18% and of intestinal stages in rural dogs from an estimated 40 to 2-3% (124). After the parasite has been driven close to extinction during the attack phase, further measures are necessary in the following consolidation and maintenance phases (76, 77). Major problems of control programs against E. granulosus are long-term funding and perturbations due to political and administrative reasons. Control options of type II were predominantly successful in island situations (New Zealand, Tasmania, Falkland Islands, and Cyprus), and partially effective in continental regions (Argentina, Chile, Uruguay, Bulgaria, Spain, parts of China, and some other areas) (24, 61, 62, 76, 77, 124, 219).
Great efforts have been made in Australia and New Zealand to develop vaccines which can protect sheep or cattle against infections with metacestode stages of taeniid cestodes. A recombinant vaccine against Taenia ovis in sheep has been successfully developed by using antigens derived from oncopheres (108, 129). Similar vaccines were developed against T. saginata in cattle, T. solium in pigs, and E. granulosus in sheep and cattle (93, 129). Large controlled studies with sheep have shown that vaccination with a recombinant oncospheral E. granulosus antigen (EG95) induces high degrees of protection, reducing the cyst numbers in vaccinated animals by approximately 90 to 100% (93, 95, 107, 129, 130). A high degree of immunity (about 80%) persists for 6 months (in the absence of reinfection), and pregnant ewes vaccinated before lambing transfer high levels of antibody to their lambs (94).
A recent mathematical simulation suggests that a combination of regular dosing of dogs with praziquantel and vaccination of intermediate hosts would destabilize the system more rapidly than other control options (200). Regrettably, the vaccine is not yet commercially available.
Problems of safety precautions and disinfection have been neglected in research. A review of the current state of art has been published in the WHO/OIE Manual on Echinococcosis (55). Essential precautions are summarized as follows. (i) Personnel involved in handling animals infected with mature intestinal stages of Echinococcus spp. or egg-contaminated materials should weare protective clothing (cap, face mask, safety glasses, single-use overall, plastic apron, rubber gloves, and boots). (ii) For work with infected definitive hosts (for example, treatment of dogs), their intestines, fecal matter, or other materials possibly containing infective Echinococcus eggs, special rooms or sterile bench systems should be used. In some countries, a BL-3 biohazard safety level is required. (iii) Whole carcasses of carnivores, intestines, or fecal sample possibly containing infective Echinococcus eggs can be decontaminated by deep-freezing at −70 to −80°C. Care should be taken that the effective temperature reaches all parts of the material and is maintained for at least 96 or 48 h, respectively. The temperatures of household deep-freezers are too high to inactivate eggs. Echinococcus eggs are killed within 5 min at +60 to 80°C and instantly at + 100°C. (iv) Persons who have had a single exposure to infected final hosts or egg-contaminated materials or who have ingested apparently contaminated food should receive serological screening for specific antibodies at the following intervals after the suspected contact: 4 weeks and 6, 12, and 24 months. Individuals with repeated infection risk (for example, laboratory personnel, field workers in echinococcosis control) should be serologically examined twice a year.
In areas which are free of E. granulosus or have only sporadic occurrence of the parasite, special measures should be taken in order to prevent the introduction of the parasite by living definitive or intermediate host animals.
E. MULTILOCULARIS AND ALVEOLAR ECHINOCOCCOSIS
The Parasite and Its Life Cycle
The adult stage of E. multilocularis is characterized by its small size (length of up to 4.5 mm), a mean number of five segments, a sack-like uterus, and other morphological features, allowing its differentiation from E. granulosus and other Echinococcus species (193, 223) (Table 1). Transmission of E. multilocularis occurs in a sylvatic cycle, which is sometimes linked via infected small mammals to domestic dogs and cats (Fig. 6). In the sylvatic cycle, foxes (mainly the Arctic fox [Alopex lagopus] and the red fox [Vulpes vulpes]) play a key role as definitive hosts and small mammals, mainly rodents, are the intermediate hosts. In some areas, other wild canids, such as coyotes (Canis latrans), raccoon dogs (Nyctereutes procyonoides), and wolves (Canis lupus f. familiaris), or wild felidae (wild cats) can also serve as definitive hosts. Among the many species (>40 species) of small mammals that are susceptible to E. multilocularis under natural conditions, members of the family Arvicolidae (voles and lemmings) and Cricetidae (hamsters, gerbils, and related rodents) are most important (79, 155, 165). Aberrant host animals and humans can also become infected with the metacestode stage, which has the potential to cause AE, one of the most lethal helminthic infection in humans. Although some variation between E. multiolocularis isolates from North America and Eurasia has been described, there is no evidence for distinct genetic strain differences (91). This is in accordance with the fact that E. multilocularis in various regions, including large areas of the northern hemisphere (Asia, Europe, and North America), is infective to humans.
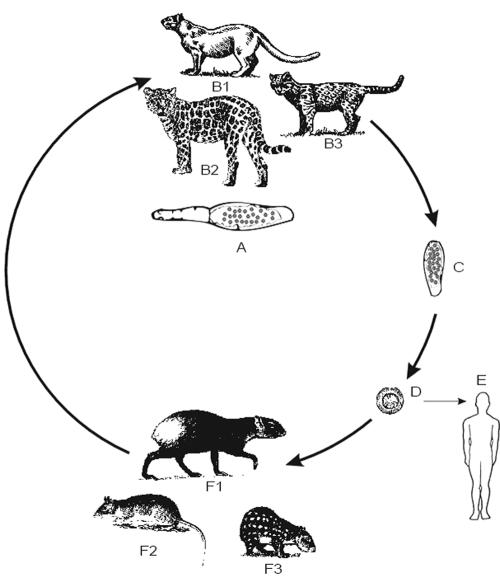
FIG. 6.
Life cycle of E. multilocularis. (A) Adult parasite. (B) Foxes (left, red fox; right, Arctic fox) as principal definitive hosts; dogs, other canids, and cats can be involved in the cycle. (C) Proglottid with eggs. (D) Egg with oncosphere. (E) Infection of humans. (F) Rodent infected with metacestodes. (G) Rodent liver with metacestodes. (H) single metacestode cyst with protoscoleces.
Alveolar Echinococcosis in Humans
Course of infection.
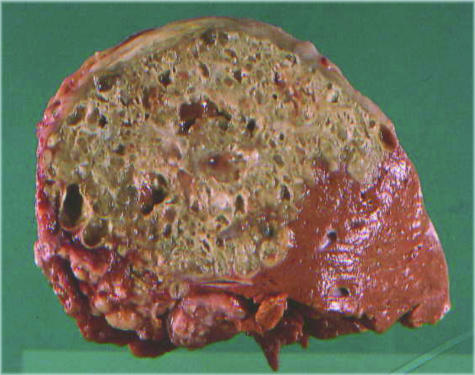
The metacestode stage of E. multilocularis in natural intermediate hosts or aberrant hosts is characterized by an alveolar structure, composed of numerous small vesicles (<1 mm to 3 cm in diameter). A characteristic feature of this stage is its exogenous tumour-like proliferation, which leads to infiltration of the affected organs and, in progressive cases, to severe disease and even death (Fig. 7). The single vesicle has a wall structure similar to that of the metacestode of E. granulosus (germinal and laminated layer) (48). In intermediate or aberrant hosts, the metacestodes can proliferate from initially minor lesions (microscopic up to a few millimetres) to diameters of 15 to 20 cm (in the human host) (3, 48). Data from patients with single-organ involvement indicate that metacestodes initially establish themselves almost exclusively in the liver (approximately 99% of the cases) and are rarely found in extra-hepatic sites (48). Later in the infection, metastases may spread from the liver to adjacent locations (abdomen, retroperitoneum, etc.) or to distant organs (lungs, brain, bones, etc.). In a Swiss series of 70 patients with advanced AE, the liver alone was involved in 67% of patients, the liver and adjacent organs were involved in 20%, and the liver and distant organs were involved in 13% (48). Recently, a new clinical classification system has been proposed (related to a similar system used for malignant tumour diseases) that is based on imaging and clinical parameters and takes into account the parasite mass (and hepatic localization of the parasite), the extrahepatic involvement of neighbouring organs, and the absence or presence of distant metastases (122, 223). This system provides a defined basis for decisions about therapeutic options, for follow-up studies after treatment, and for prognostic considerations.
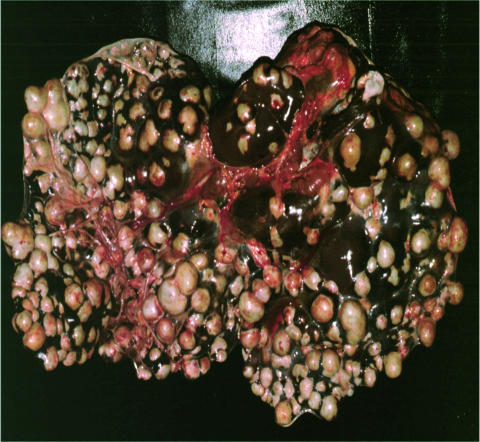
FIG. 7.
Hepatic alveolar echinococcosis in a 62-year-old Swiss patient (maximum diameter of single cysts approximately 1.5 cm).
In humans, several main phases of the infection (initial, progressive, advanced, stability, abortive course) and several pathological entities can be distinguished. The initial phase is always asymptomatic and may be cured spontaneously or turn to a progressive course. Estimates of the incubation period vary between less than 5 and up to 15 years (3, 178); the shortest period between oral uptake of E. multilocularis eggs and seroconversion is unknown. In the progressive phase, symptoms occur when the metacestode has infiltrated larger parts of the liver or influences important functions. Initial symptoms include abdominal pain, jaundice, hepatomegaly, sometimes fever and anaemia, weight loss, and pleural pain (3, 48, 178). The advanced stage is characterized by severe hepatic dysfunction, often associated with portal hypertension. The duration of the disease is variable between weeks and years. Mortality rates in untreated or inadequately treated AE patients can be very high; in three groups of such patients (a total of 107 individuals), the average survival rate 10 years after diagnosis was 29% (range, 0 to 23%) and the survival rate after 15 years was 0% (reference 3 and references therein). The reported ages of patients at diagnosis ranged from 5 to 89 years, with means of 45 ± 15 years in a Japanese series (178) and 52 ± 17 years in a Swiss series (56). The most frequent ages at diagnosis are usually between 35 and 65 years, and the gender ratio is often close to 1:1 (48). A stable phase of the infection can occur in patients undergoing long-term chemotherapy, and this phase inhibits further parasite proliferation (see below). Abortive cases have been observed in asymptomatic patients, when the parasite dies out and is mineralized (9, 89, 149, 162).
During the infection with E. multilocularis metacestodes, parasite-specific humoral and cell-mediated immune responses are both progressively established. The latter may play a crucial role in host defense (86). Immunological studies of mice experimentally infected with E. multilocularis have revealed an initial Th1 dominant cytokine secretion, including interleukin-2 (IL-2) and gamma interferon. This is associated with slow parasite proliferation. Rapid growth of the parasite was associated with secretion of Th1- and Th2-type cytokines including IL-5 and IL-10. Finally, in animals with advanced AE, suppression of immunity was observed associated with almost complete inhibition of cytokine secretion by lymphocytes on specific or unspecific in vitro stimulation, (63, 214, 215). A varying, spontaneous cytokine secretion was also observed in patients in different phases of AE. In patients with progressive disease, IL-10 secretion by peripheral mononuclear cells was more pronounced and IL-10 levels in serum were higher than in individuals with abortive forms (82, 83, 218).
Other studies suggest that the formation of the laminated layer by the metacestode inhibits or prevents immunological control of parasite proliferation. In recent studies of a laminated layer-associated antigen (Em2G11), a high-molecular-weight mucin-type glycosylated protein could be identified (103). This protein stimulates antibody production in the absence of major histocompatibility complex class II- restricted T-cell help. In addition, it does not stimulate cell proliferation in vitro. Therefore, it is claimed to be a relevant factor contributing to the lack of protection against the metacestode in vivo (31). The mouse model has provided much useful information; however, due to the rapid parasite development in mice, it might not be an appropriate model for the study of all mechanisms involved in human AE.
Diagnosis.
Essentially the same procedures are used for diagnosing AE in human patients as for diagnosing CE (3, 112, 149). Among the imaging techniques, ultrasonography is the method of choice for screening; it is usually complemented by computed tomography, which detects the largest number of lesions and characteristic calcifications. Magnetic resonance imaging may facilitate the diagnosis in unclear cases (164). Immunodiagnostic tests (for primary diagnosis or confirmation of imaging results) are more reliable in the diagnosis of AE than of CE since more specific antigens are available (Table 4). For example, the Em2plus-ELISA, using a mixture of affinity-purified E. multilocularis metacestode antigens (Em2-antigen) and a recombinant antigen (EmII/3-10), exhibited a diagnostic sensitivity of 97% in 140 patients with confirmed AE and an overall specificity of 99% for infections due to other helminths (87). However, the use of this test in a broader spectrum of patients has revealed lower sensitivity (approximately 85%) and specificity (>95%) (168). Immunoblot tests using the EM18 antigen are reported to have sensitivities between 50 and 90% and specificities of >95% (168) (for further details, see references 84, and 104).
The diagnosis can be confirmed by parasite identification in surgical or biopsy material. In most of the cases, histological examination is sufficient, but recognition of characteristic structures in fine-needle biopsy specimens or calcified samples may be difficult or impossible. In such cases, the specific Em2G11 antigen can either be detected immunhistologically or in an ELISA using a monoclonal antibody (39). This antigen persists in calcified lesions within fragments of the laminated layer even after the death of the parasite. Furthermore, specific PCRs targeting the U1 small nuclear RNA gene or the mitochondrial 12S rRNA gene (16, 42) can be used. The viability of the parasite can be assessed by transplanting metacestode tissue samples to laboratory rodents (50). Another option is the use of reverse transcription-PCR (111). The sensitivities of both techniques depend on the amount and quality of the sample material. Puncture of the liver of a human with AE includes a risk of dissemination of parasite material and may require postinterventional chemotherapy.
Immunodiagnostic tests (alone or in combination with ultrasonography) have been used for screening human populations for AE in various countries, for example in Alaska, Austria, China, France, Germany, Japan, and Switzerland (10, 17, 30, 81, 88, 174, 189). In view of the low prevalence of the infection in most areas of endemic infection, it is essential to use highly sensitive and specific test systems. The largest mass-screening program was carried out in Japan (55, 178, 189). In Hokkaido (population of 5.8 million), 715,841 individuals received serological primary screening during 1984 to 1993. Overall, 5,159 individuals had a positive ELISA reaction, 1,272 persons underwent secondary ultrasonographic screening, and, finally, 60 persons (0.008% of the total) were shown to have asymptomatic AE (189). Eearly detection of AE by mass screening can be cost-effective and reduces morbidity, suffering, and mortality (55). In Hokkaido, the rate of complete surgical excision of liver lesions in the group of screened individuals was 100%, in contrast to a resectability rate of only 20% in nonscreened patients in whom AE was detected at a later stage (55).
Treatment.
There are several options for treatment, including surgery, chemotherapy, and interventional procedures (2, 3, 113, 149, 221). Liver transplantation is rarely indicated and has a relatively high risk of postoperative metastasis formation (149). This is due to minor parasite remnants, which cannot be resected and proliferate under immunosuppression. Recommendations for treatment have been published by the WHO Working Group on Echinococcosis (221, 223).
(i) Surgery.
The first-choice treatment in all operable cases is radical resection of the entire parasite lesion(s) from the liver and other affected organs by using procedures of radical tumour surgery (3, 113, 149, 221). Radical surgery is possible in virtually all cases diagnosed in an early stage but in only 20 to 40% of the advanced cases (3, 113, 178, 221). It may lead to complete cure, but resection is often incomplete because of diffuse and undetected parasite infiltration into host tissues. Therefore, according to WHO recommendations, postsurgical chemotherapy should be carried out for at least 2 years, with monitoring of the patients for a minimum of 10 years for possible recurrence (221, 223). In one case, despite postoperative medication with mebendazole for 2.5 years, a recurrence was observed in a patient 14 years after radical surgery (3).
(ii) Chemotherapy.
Chemotherapy is recommended for a limited period (at least 2 years) after radical surgery (see above) and for long-term periods (from many years to life-long) after incomplete resection of lesions in inoperable cases and after liver transplantation (see the above remarks on liver transplantation) (221, 223). Chemotherapy of infected laboratory rodents with mebendazole and albendazole, applied at high oral doses over at least 2 months, causes destruction of protoscoleces, damage of the germinal layer, inhibition of metacestode proliferation, and metastasis formation but does not usually kill the parasite (46).
For chemotherapy of AE in humans, mebendazole is given at daily doses of 40 to 50 mg/kg of body weight in three divided doses. Albendazole is given at daily doses of 10 to 15 mg/kg of body weight in two divided doses (149, 221). Albendazole is licensed for cyclic treatment, i.e., 28 days of treatment followed by 14 days without chemotherapy, but continuous treatment is also practised (see below). Both drugs should be taken with a fat-containing meal (221, 223). The evaluation of treatment success in human AE is difficult because there are no methods allowing a direct assessment of the parasite's activity status (alive or dead). The most reliable indirect criteria are (i) long-term survival rates of patients, (ii) lack of disease progression and improvement of the clinical status, (iii) decrease or arrest of parasite proliferation, and (iv) lack of recurrence. The size and structure of the parasite lesions are evaluated at regular intervals (approximately 6 to 12 months) by ultrasonography and computed tomography. Serology using specific tests has only some value in cases after radical operation. In a Swiss study, 110 patients with nonresectable AE had received long-term chemotherapy (mainly with mebendazole) for 9.4 ± 5.8 years and were under follow-up observation for 12.8 ± 6.9 years (4). This therapy resulted in an increased 10-year survival rate of approximately 80% (versus 29% in untreated historical controls) and a 16- to 20-year survival rate of approximately 70% (versus 0% in historical controls) (4). The optimal duration of treatment has not yet been determined. In a group of 19 patients with nonresectable AE, long-term mebendazole therapy (average duration, 4.3 years) was ceased because there was no evidence of parasite activity (6). Recurrence occurred in seven patients (37%) within an average of 1.6 years after discontinuation of chemotherapy. On the other hand, there is evidence that in some cases long-term chemotherapy can be parasiticidal rather than merely parasitostatic (5).
Recently, Reuter et al. (163) have compared the efficacies of mebendazole and albendazole in 35 AE patients, 12 of whom had extrahepatic lesions. The average time of treatment was 24 months for mebendazole, 25 months for cyclic albendazole (see above), and 28 months for continuous albendazole. The duration of treatment and the average follow-up period (3.2 years) were relatively short compared with long-term treatment (see above) but were sufficient for the purpose of the study. The criteria for success of treatment were lack of disease progression (i.e., stability or regression of AE lesions) for >1 year and lack of effects necessitating a change of treatment (progression of disease or intolerable side effects). There was no significant difference between initial treatment with mebendazole, which was successful in 71% of cases (12 of 17), and albendazole therapy, which was successful in 78% (14 of 18). Four of five progressive cases could be stabilized by changing the treatment regimen (change from mebendazole to albendazole or vice versa or from cyclic to continuous albendazole). The overall success was 97% (34 of 35). Continuous albendazole treatment of seven patients for >12 months was successful and well tolerated. In this study, the use of albendazole has reduced costs considerably (approximate costs in Germany per patient per year are $4,300 to $6,350 for albendazole and $8,200 to $16,300 for mebendazole) (163). Furthermore, the authors (163) have summarized data from 19 published studies involving approximately 500 AE patients treated with mebendazole or albendazole. Most of the patients were treated for >20 months. The success rates were over 74% (up to 100%) in 18 trials and lower (55%) in only one study.
E. multilocularis Infection in Animals
Defintive hosts.
Like E. granulosus, infection with E. multilocularis is typically asymptomatic in definitive hosts (54). The repertoire for the diagnosis of the infection in living dogs, cats, and foxes (Table 6) has been considerably improved in recent years and includes the coproantigen ELISA and PCR techniques for the specific detection of DNA in E. multilocularis eggs and/or in parasite tissue fragments released into the intestinal contents (see references 36, 49, 53, and 134 and references therein). These techniques can also be used for the examination of fecal samples collected in the environment (154, 188, 207). Praziquantel is highly effective against intestinal stages of E. multilocularis in definitive hosts, but chemotherapy of dogs and cats infected with the parasite requires a careful risk assessment and special safety precautions (55) (see “Control options and intervention” above).
TABLE 6.
Options for the diagnosis of E. multilocularis in animals
| Animal group | Material required | Test, sensitivity and specificitya |
|---|---|---|
| Live animals | ||
| Dogs and cats: individual animals and populations | Feces in buffer | Screening: coproantigen ELISA. S, 84-95%; SP, 96-99% |
| Secondaryb: copro-PCR. S, 89-94%; SP, ∼100%. | ||
| Population of foxes | Feces collected in the environment | Screening: coprogantigen ELISA. Secondary: copro-PCR. S and SP similar to above. |
| Aberrant hosts: dogs, monkeys, pigs | Serum, fine needle biopsy | Abdominal ultrasonography, other imaging techniques, serology possible but not validated for all species detection of specific antigens and DNA in liver biopsy samples |
| Dead animals | ||
| Dogs, cats, foxes, etc. | Small intestine | Standard option: parasite detection at necropsy by intestinal smear technique (S, 78%; SP, ∼100%) or by sedimentation technique and counting technique (S, ∼100%; SP, ∼100%). |
| Feces from rectum or contents from intestine in buffer | New option: coprogantigen ELISA and/or copro-PCR can also be used for routine screening. | |
| Aberrant and intermediate hosts | Internal organs | Normal necropsy procedures, histology, in conjunction with PCR in doubtful cases. |
Average values of various studies. Data from references 36, 37, 41, 49, and 134. S, sensitivity; SP, specificity.
Secondary test for confirmation or exclusion.
Intermediate and aberrant hosts.
A relatively high proportion of the natural intermediate hosts (small mammals), infected with the metacestode of E. multilocularis, will die of the infection if not previously captured by a carnivore. According to earlier reports, metacestodes of E. multilocularis can be found under natural conditions in various mammalian hosts that do not play a role in the transmission cycle, such as horses, domestic and wild pigs, nutria (Myocastor coypus), several species of monkeys in captivity, and some others (references 54 and 118 and references therein). In Japan, E. multilocularis lesions were diagnosed in 0.82% of 1,100 horses and 0.14% of 1 million slaughtered pigs during 1993 to 1994 (145). In our Institute in Zurich, 15 cases of AE were diagnosed in monkeys and 10 cases were diagnosed in dogs during the period from 1987 to 2000 (37). A finding of peculiar interest was that the domestic dog, a natural definitive host of E. multilocularis, can also harbor the metacestode stage, which causes severe lesions in the liver or other organs and produce symptoms very similar to human AE (Fig. 8). In two of these cases it was observed for the first time that dogs can be simultaneously infected with adult stages in the intestine and metacestodes in the liver. Furthermore, lesions caused by E. multilocularis metacestodes were detected in the liver of 10% of 90 slaughter pigs which had been kept outdoors, and severe pathology and disease were observed in several genera of monkeys in capitivity (Hylobates, Macaca, Miopithecus, and Lemur) (37). These infections in aberrant host animals are important indicators for the existing infection risk for humans.
FIG. 8.
Hepatic alveolar echinococcosis in a dog.
Epidemiology
Transmission dynamics of E. multilocularis are based on the predator-prey relationship between carnivores and small mammals and depend on a large number of interacting factors. Some of the major factors are presented in Table 7. Since only a few of these factors have been studied in detail, our understanding of the whole epidemiological system is still limited.
TABLE 7.
Variable factors in the epidemiology of E. multilocularis
| Key factors | Selected contributing factors (defined or suspected) |
|---|---|
| Definitive hosts and the adult parasite | Animal species: geographic and spatial distribution, population size and dynamics, interactions with prey animals, feeding habits, behavior, landscape characters of the habitat, relation to human habitats |
| E. multilocularis: prevalence, intensity, and duration of the infection; intensity of egg production and release; host immunity | |
| Parasite eggs in the environment | Dispersal and survival of eggs, conditions of climate and weather |
| Intermediate hosts and the metacestode stage | Animal species: biology and longevity, geographic and spatial distribution, population size and dynamics, feeding habits, role as prey animals of carnivores, interactions with other small mammals, landscape characters of the habitat |
| E. multilocularis: prevalence, intensity, and duration of the infection; intensity of protoscolex production; host immunity | |
| Humans and other aberrant hosts | Exposure to eggs, behavior, relationship to carnivores, susceptibility, resistance, and immunity |
Parasite-host assemblages.
In various regions of endemic infection, different definitive and intermediate host assemblages are involved in the transmission cycles. This is exemplified by an outline of two different epidemiological situations in the Arctic tundra region and in sub-Arctic regions.
(i) Arctic region.
On St. Lawrence Island, Alaska, the epidemiological situation is characterized by rather uniform biotope conditions. The Arctic fox is the sole definitive host of E. multilocularis in a wildlife cycle (155, 160, 180). Arctic foxes are highly susceptible to E. multilocularis, as indicated by high parasite pervalences (overall prevalence, approximately 77%) and heavy worm burdens (burdens in excess of 100,000 in an individual fox were not uncommon) (160). Voles, predominantly the northern vole (Microtus oeconomus), are intermediate hosts and represent the major prey of foxes. Prevalences of E. multilocularis metacestodes in these voles increase with age and can reach approximately 40% (160). In villages, the Arctic fox is replaced by the domestic dog as definitive host. In previous years, large numbers of dogs were kept in the villages and had access to voles living in these synanthropic habitats. Up to 83% of the voles and 12% of the dogs were found to be infected with E. multilocularis. This synanthropic cycle represented the major risk factor for humans (160).
(ii) Sub-Arctic regions.
In sub-Arctic regions of North America and Eurasia, the situation is more complex than in the Arctic tundra. Landscape characters, biotopes, and climatic conditions are more diverse, populations of small mammals often consist of several species susceptible to E. multilocularis, and sometimes more than one carnivore species is suited as the definitive host of the parasite. For example, in central Europe, red foxes play the key role as definitive hosts. They have a wide geographic range in European countries but fox densities per square kilometer are highly variable. In larger regions of the area of endemicity, the average prevalences of E. multilocularis in foxes vary from approximately 1 to >60% (51, 52, 79, 131, 170, 172). Information on parasite prevalences in foxes is available from several central European countries, but exact data on infection intensities are scarce. Some data show that the distribution of parasite numbers in a given fox population is rather heterogeneous, with a small number of foxes carrying most of the parasite biomass. For example, in a Swiss study the average worm number in 133 adult and subadult foxes was approximately 3,000, ranging between 1 and approximately 57,000; 69% of the foxes had worm burdens below 1,000 per animal and 31% had burdens above this number. Only 7.5% of the foxes (10 of 133) harbored more than 10,000 worms, corresponding to 72% of the total biomass (99). Previous data on differences of E. multilocularis prevalences and infection intensities in young and older foxes were contradictory. It has now been documented in several studies that juvenile foxes are more frequently and/or intensively infected than are adult foxes (99, 190). These findings suggest that older foxes acquire partial immunity under high-endemicity conditions but that under low infection pressure immunity may not be expressed.
Domestic dogs and cats have been found infected with E. multilocularis in several European countries (19, 47, 51, 152). In a previous study in France, 5.6% of 36 dogs were identified as carriers of E. multilocularis, and in a further five studies in Germany and France, 0.5 to 3.7% of cats (58 of 498) were identified (by necropsy) as carriers (51). In an area of endemic infection in eastern Switzerland with an average prevalence of E. multilocularis in foxes of approximately 33%, only 0.30% of 660 dogs and 0.38% of 265 cats from “normal” populations were parasite carriers (36). In a model calculation, these data were referred to the population sizes of these hosts in a given area (Canton of Zurich), and it was estimated that 1,551 foxes, 145 dogs, and 552 cats could have hypothetically been carriers of the parasite (52). Besides prevalence and population sizes, further factors have to be considered in assessing the relative risks posed by the various definitive hosts. These include the duration of the E. multilocularis infection, worm burdens, capacity of egg production, habitat and behavior of the hosts, and their relationships with humans.
Dogs are highly susceptible to E. multilocularis, but cats appear to have a lower and a more variable degree of susceptibility, as observed both in several older and in more recent experimental studies (60, 106). However, naturally infected cats can harbor small numbers of E. multilocularis worms containing fully developed eggs and are therefore potential sources of infection (48).
In areas of endemic infection in central Europe, the average prevalences of E. multilocularis metacestodes in rodents are generally low (<1%) (48), but they may be higher locally. For example, prevalences up to 21 and 39% have been recorded in two studies of water voles (Arvicola terrestris) in high-endemicity foci in Switzerland (89, 188) and prevalances of 12 to 14% have been recorded in Microtus arvalis in a focus in France (79). These data are indicative for a heterogeneous (”patchy“) spatial distribution of infected rodents. Different rodent species differ in their susceptibility to the meacestode stage of E. multilocularis (215) and capacity for protoscolex production (see below). Typically, the prevalences of E. multilocularis metacestodes in rodents increase with age (79, 160, 188).
Influences of landscape characters and rodent populations.
In view of the wide distribution of foxes and the ubiquity of susceptible small mammals in most European habitats, one could anticipate that virtually all regions might be suitable for E. multilocularis transmission. However, in some European regions, the parasite has so far not been recorded.
(i) Factors related to larger regions.
Based on observations in Alaska, China, Japan, and France, a group of authors has put forward the hypothesis that on a regional scale intensive transmission of E. multilocularis occurs if one or two species of rodents are dominant, their populations reach high densities for several months or years, and foxes use these rodents as main prey animals (79, 212).
Studies in France have shown that prevalences of E. multilocularis in foxes are higher in areas of medium altitude (600 to 900 m) where permanent grassland is prominent (79). In such areas, the field vole (M. arvalis) and the water vole (Arvicola terrestris), the two most important intermediate hosts in central Europe, undergo multiannual cyclic population outbreaks which lead to high population densities (79). During the high-density phase of M. arvalis and A. terrestris, several kilograms per hectare of biomass is available as prey for foxes. In this phase, foxes prey mainly on these species. For example, in the Swiss Jura and in Franche-Comté, A. terrestris represented 87% of the mammalian prey items, and 54% of all dietary items of foxes (79). During the cyclical decline of the dominant rodent species, the diet of foxes is more diversified. In spite of annual and cyclic fluctuations of rodent populations, a high level of transmission is maintained in certain areas which provide favorable conditions for dominant rodent species. Such conditions also exist on permanent grassland of suburban recreation areas, vegetable gardens, orchards, and similar habitats.
(ii) Factors related to macro- and microfoci.
Evidence has accumulated in recent years that a heterogeneous spatial distribution of definitive and intermediate hosts is a particular epidemiological feature of E. multilocularis. This heterogeneity can lead to highly variable parasite prevalences and the formation of larger foci (macrofoci) or small foci (microfoci) of intensive transmission (”hot spots“). For example, within an area of endemicity of approximately 4,450 km2 in northern Germany, two high-endemicity macrofoci have been identified, with an E. multilocularis prevalence in foxes of 23.8%, which was significantly higher than the prevalence of 4.9% in the surrounding area (190).
Microfoci of some 10 m2 have been identified in France in which E. multilocularis prevalences in M. arvalis exceed 12 to 14% (79). Such foci were found in places where the density of deposits of fox feces was high and the parasite eggs could be covered with soil by ploughing (79). In Zurich, Switzerland, high contamination with fox feces was found in the recreational area, and it was observed that foxes deposit their feces directly on ground systems of A. terrestris (38). In Hokkaido, Japan, foxes breed and defecate predominantly in areas where Clethrionomys rufocanus is abundant (79). These data indicate that several factors contribute to the formation of macro- and microfoci.
Eggs in the environment.
Exact knowledge of the intensity and dynamics of E. multilocularis egg excretion by definitive hosts is lacking. The method by which the eggs are dispersed in the environment has not been adequately investigated, mainly because morphological differentiation of eggs from E. granulosus and other taeniids is not possible. Recent developments of new techniques for detecting E. multilocularis antigens or DNA in fecal samples of foxes and other definitive hosts have opened new options for transmission studies. In one study in Switzerland a coproantigen ELISA was used as a screening method and a PCR (134) was used as a confirmation assay. It was found that 10 to 60% of 647 fecal samples from foxes in an urban area were coproantigen positive and that the spatial distribution of these samples was in accordance with E. multilocularis prevalences found in necropsied foxes (188).
Eggs of E. multilocularis remain infective for approximately 1 year in a suitable, moist environment at lower temperatures, but they are sensitive to desiccation and high temperatures (211). Their high resistance to low temperatures is a precondition for their survival in Arctic regions. These eggs can survive at −50°C for 24 h but are killed at −70°C within 96 h and at −80 to 83°C within 48 h. Deep-freezing at −70°C for at least 4 days or at −80°C for at least 2 days is recommended for inactivating E. multilocularis eggs in carcasses or intestines of final hosts or in fecal material before examination in the laboratory (55).
Infection risk for humans.
It is generally assumed that humans can become exposed to the eggs of E. multilocularis by handling infected definitive hosts or by ingesting food or water contaminated with eggs (55, 180). However, studies of the epidemiological significance of the various potential methods of transmission are lacking.
Biostatistical studies of suspected risk factors are scarce. Stehr-Green et al. (187) have assessed risk factors on St. Lawrence Island, Alaska, and found that people who have owned dogs, kept dogs around dwellings, and have houses built on the tundra were at higher risk from AE. In an area of endemicity in Austria, Kreidl et al. (121) found cat ownership and hunting to be independent risk factors for AE and rejected the hypothesis that eating of mushrooms or certain wild berries is a main risk factor. Such conclusions are difficult to prove, mainly because of the long incubation period of AE and possible data corruption by undetected confounding factors.
Exposure of humans to eggs may be influenced by occupational and behavioral factors. Hunters, trappers, and people working with fur may frequently be exposed to the eggs of E. multilocularis, but there is no evidence that these groups are at increased risk (98, 160). Older data from Austria, Germany, France, and Switzerland have indicated that persons working in agriculture were at increased risk of infection (57, 110). Living in the countryside in close proximity to infected foxes (dogs or cats) and/or frequent contacts with egg-contaminated soil or plants may be reasons for a higher infection risk. Egg-contaminated plants were obviously the infection source for monkeys in a zoo in Switzerland. The monkeys suffering from AE had been regularly fed with grass harvested from a meadow in an area of high endemicity accessible to foxes (37). There are some hints that higher incidences of AE in humans might be associated with high E. multilocularis prevalence in foxes (57, 66, 79, 144). A high proportion of grassland can result in high rodent populations over prolonged periods, favoring disease transmission (79). According to studies in France, high densities of A. terrestris yielded a 10-fold-higher risk for AE in humans (212).
Both the wide distribution and high prevalences of E. multilocularis in wild carnivores and the low incidence of human AE suggest that the infection risk for humans is limited by unknown factors. There is evidence that humans have a relatively high degree of innate resistance to infection with eggs of E. multilocularis, as indicated by the slow development of the metacestode stage in internal organs, the reduced capacity of protoscolex formation, and the type of histopathological reaction. Further factors may include low exposure rates of humans and immunogenetic factors of the human population (215).
Global distribution in humans.
E. multilocularis in carnivores is widely distributed in the northern hemisphere, including regions of central Europe, most of northern and central Eurasia (extending eastward to Japan), and parts of North America (Fig. 9). Currently, the parasite is found in at least 30 countries, including large territories, for example in Russia, Kazakhstan, China, parts of Europe, and North America (51, 58, 180).
FIG. 9.
Approximate global distribution of E. multilocularis (as of 2002). Exact identification of areas of normal and high endemicity is difficult becaue of incomplete or lacking data. Modified from WHO/OIE 2001 (223) with permission.
The annual incidence of human AE is not documented in most of the countries where infection is endemic (58). Reliable data from central Europe (Austria, France, Germany, and Switzerland) and Japan (from 1970 to 1992) have indicated low annual incidences of new AE cases of between 0.02 and 1.4 per 100,000 population for entire countries or larger regions of endemicity (52). In Switzerland, the countrywide annual incidences showed only minor variation between 0.10 and 0.18/100,000 for the 37 years between 1956 and 1992 (52). Much higher incidences have been reported from St. Lawrence Island, Alaska (180), but these data refer only to villages where AE cases have been diagnosed and are local group incidences. Ultrasonographic and immunodiagnostic surveys (1991 to 1997) have revealed a very high prevalence within a focus in Gansu, China, where 135 (4%) of 3,331 persons examined had documented AE (30). Although generally rare, AE deserves public health attention due to the high fatality rate of untreated patients and the high costs of treatment.
Emergence and spread of E. multilocularis?
The question of the emergence and spread of E. multilocularis is of actual public interest and has been addressed in recent reviews (51, 52, 79, 80, 131, 170). In this context, some key questions are discussed here.
(i) Risk areas and spreading.
According to Rausch (155), E. multilocularis has been indigenous since post-glacial times in the Arctic tundra zone of North America and has spread southward. The genetic homogeneity of E. multilocularis isolates originating from North America, Europe, and Japan (91) has been interpreted as an indication that an invasion of the parasite from the Arctic to sub-Arctic and temperate regions may have occurred relatively recently (170). Indeed, there are indications from various regions for the spreading of E. multilocularis.
In Japan, E. multilocularis is thought to have been introduced into the northern part of the country in 1924 to 1926 in red foxes translocated from one of the Kurile Islands to Rebun Island (northwest of Hokkaido) for the purpose of controlling the vole population. Another outbreak occurred in about 1960 in eastern Hokkaido (180), followed by the spread of the parasite between 1981 and 1991 from approximately 8 to 90% of the area (189). In North America, E. multilocularis occurs in the Northern Tundra Zone (NTZ) of Alaska and Canada and further south, in the North Central Region (NCR). It is assumed that prior to the 1960s, E. multilocularis spread from the NTZ and became established in central North America, including parts of three Canadian provinces (Alberta, Saskatchewan, and Manitoba) and 13 contiguous U.S. states (Fig. 9). Previous surveys of endoparasites of canids and rodents in the NCR had failed to reveal E. multilocularis (180).
Recent surveys in central Europe have revealed that E. multilocularis has a much wider geographical distribution than previously thought. By the end of the 1980s, areas of endemic infection with E. multilocularis were known to exist in only four countries (Austria, France, Germany, and Switzerland), but by the end of 2001 the parasite was known to occur in red foxes in at least nine further central European countries (Belgium, Czech Republic, Denmark, Italy, Liechtenstein, Luxembourg, Poland, Slovak Republic, and The Netherlands) (references 51, 58, and 173 and references therein). Findings of E. multilocularis metacestodes in rodents have been reported from several countries (Slovenia, Bulgaria, Romania, etc.) and more recently from Spitsbergen (Norwegian Svalbard Island group in the Barents Sea). Since surveys were not conducted in previous years, it cannot be determined if the new records reflect a recent extension of the parasite's range or only the first identification of hitherto unnoticed areas of endemic infection.
(ii) AE in humans as a risk indicator.
The real infection risk for humans in areas of endemic infection is reflected by AE cases in the human population. Such cases have been diagnosed in many Eurasian areas and in Alaska, but only two cases are known from the NTZ in North America (58, 180). Apparently, the infection risk can differ considerably between and within various regions. For example, since the middle of the 19th century, most AE cases in central Europe have been recorded from regions in western Austria, southwestern Germany, eastern France, and northern Switzerland, where recent surveys have revealed high prevalences of E. multilocularis in foxes (58, 66, 110). Since 1993, human cases of AE have been recorded from at least 8 (61%) of 13 countries with endemic infection, namely, from Austria, Belgium, France, Germany, Slovak Republic, Switzerland, Liechtenstein, and Poland (51, 119) but most of the human cases have been reported so far from Switzerland, France, Germany, and Austria (58, 110). Between 1982 and 2000, 559 human cases of AE were reported on a voluntary basis from nine European countries to the European Echinococcosis Registry (110) and 202 new cases were diagnosed in Turkey between 1980 and 1998 (1). These data probably do not include all cases, but they do indicate a considerable and persistent infection risk for humans in certain Europen regions. In France, cases of human AE are found predominantly in areas of intermediate altitudes (600 to 900 m) where grassland is dominant and the E. multilocularis prevalence in foxes is high (40 to 60% in winter) (79). In Japan, with the spread of E. multilocularis in Hokkaido, the number of human cases of AE had increased in that area from only 6 new cases between 1937 and 1964 to a total of 110 cases between 1985 and 1994 (118).
(iii) Increasing fox populations and parasite prevalences.
Evidence is accumulating that in parts of Europe the fox population densities have increased in recent years (23), possibly due to reduced fox mortality after the introduction of anti-rabies vaccination. For example, in Baden-Württemberg, southwestern Germany, the number of foxes sampled by hunting has increased approximately 2.7-fold between 1975/1976 and 1997 (data extracted from a graph) (170). In several other Federal States of Germany, increasing fox populations have been observed in recent years, associated with increasing prevalences of E. multilocularis (15, 109, 136, 170, 173).
(iv) Invasion of urban areas by foxes.
Invasion of urban areas by foxes has been observed in the United Kingdom since the middle of the 1940s; in recent years it has also been observed in continental Europe (reference 38 and references therein) and Japan (207). Such foxes have been identified as carriers of E. multilocularis in several European cities, for example in Copenhagen, Geneva, and Zurich. The number of foxes shot or found dead in Zurich increased 20-fold between 1985 and 1997. Ecological studies in this area revealed high fox population densities, small home ranges, formation of family groups, and a low immigration rate. The overall prevalence of E. multilocularis in urban foxes was found to be 47% (99). Furthermore, the highest contamination with fox feces positive for E. multilocularis coproantigens was found in adjacent recreational areas. In such places, A. terrestris and Clethrionomys glareolus, common voles of the urban periphery, were infected with E. multilocularis metacestodes. The average prevalences of infection in these two species were 9.1 and 2.4%, respectively (188). Metacestode prevalences in A. terrestris did not show significant seasonal variation. An average of 3.5% of voles were infected with metacestodes containing between 14 and 244,400 protoscoleces per animal. These data have provided the first evidence of an urban cycle of E. multilocularis. Hence, the contamination of a densely populated urban environment, including recreational areas, with E. multilocularis eggs by foxes represents a potential new risk factor for humans. Moreover, dogs preying on metacestode-infected rodents in the urban area can acquire the E. multilocularis infection and may pose a further infection risk, especially due to their close contacts with humans.
(v) Potential modes of spreading.
There is little knowledge about the modes and dynamics of E. multilocularis spreading, but epidemiological evidence suggests that migration of infected foxes can play a role. In the Arctic region of North America, E. multilocularis may be transported and dispersed by foxes migrating on the sea ice or on land (155). In Japan, the parasite evidently became established on Hokkaido by migrating foxes from the Kurile Islands (reference 180 and references therein). In Europe, the spreading of E. multilocularis, if it happened at all, could be associated with spatial migration of red foxes out of areas of endemic infection. Most foxes migrate less than 5 to 10 km, and only a few migrate more than 50 to 70 km (190). There are good reasons to assume that the establishment of urban cycles of E. multilocularis (see above) was initially caused by the invasion of infected foxes. If rodents populate new habitats, they normally migrate short distances. For example, A. terrestris migrates 30 to 50 m (97). However, a wider extension of rodent habitats, induced by human activities or other factors, may well contribute to a long-term dissemination of the parasite. Furthermore, dislocation of hosts infected with E. multilocularis may result in parasite spreading. Evidently, E. multilocularis has been imported to northern Japan by dislocation of foxes from one of the Kurile Islands to Rebun Island. In the United States, foxes and coyotes have been moved from areas of endemic infection to other areas without infection for hunting purposes, posing the risk of disease spreading (180). Parasite dispersal by dislocation of infected domestic dogs and cats has also to be considered, but its significance is unknown. On the Arctic Norwegian island of Svalbad (Spitsbergen), E. multilocularis apparently became endemic after the accidental introduction of a suitable intermediate host, Microtus rossiaemeridionalis (170). Transportation of E. multilocularis eggs sticking to people's shoes and mechanical dispersal by flies and birds could be other modes of dispersal which are proven for E. granulosus and Taenia species (127, 202).
Control Options and Prevention
Control in definitive hosts.
Currently, there is no reliable and cost-effective method for sustainable control or eradication of E. multilocularis in the sylvatic cycle. Large field trials have been conducted since 1995 in Germany for mass treatment of red foxes with baits containing 50 mg of praziquantel. The baits are repeatedly distributed by light aircraft (171). According to first reports, it is possible to reduce the prevalence of E. multilocularis in the fox population significantly (181). These and other trials (171, 191) suggest that long-term control of E. multilocularis, in the sylvatic cycle in large areas, is extremely difficult and costly. Since the urban cycle of E. multilocularis seems to depend on locally restricted factors, we assume that a reduction of the infection pressure in distinct risk areas should be feasible. This hypothesis was tested in Zurich by distributing praziquantel-containing baits every month for 2 years in six areas of 1 km2 inhabited by foxes. Preliminary results indicate a significant decrease of the environmental contamination with E. multilocularis eggs and of parasite prevalences in voles in baited areas compared to those in control areas (96).
In areas of endemic infection with low prevalences of E. multilocularis infection in dog and cat populations, mass treatment of these animals may currently be neither indicated nor feasible. The effect of such a strategy for preventing human cases of AE has not been evaluated, and cost-benefit considerations were not made. In these circumstances it may be appropriate to give regular praziquantel treatment every 4 weeks only to dogs and cats having access to infected rodents. New options should be considered: for example, mass screening of dog and cat populations by using the coproantigen ELISA with the aim of obtaining better baseline data for control strategies, or the development of a new scheme for regular drug treatments directed against E. multilocularis and other endoparasites of domestic carnivores.
Precautions for individuals.
For persons at especial risk (for example, laboratory personnel working with E. multilocularis eggs or handling infected definitive hosts, children after exposure to feces of infected foxes, etc.), repeated serological screening for anti-E. multilocularis antibodies by using highly sensitive and specific tests (if necessary in conjunction with ultrasonography) is recommended, with the aim of detecting an infection in an early stage or excluding it with a reasonable degree of probability (52, 55) (see “Control options and prevention” above). In our experience, such precautions can help to reduce the anxiety of exposed persons in fear of having contracted a severe disease. Special safety precautions are recommended for laboratory and field workers possibly exposed to infective E. multilocularis eggs (55).
Measures to reduce morbidity and mortality.
Mass screening of populations with the aim of detecting human AE cases at an early stage have been discussed above. The stepwise introduction of regular screening programs could be considered for areas of endemic infection with relatively high incidence rates of human AE or high prevalence rates of the parasite in definitive hosts (52, 55).
Surveillance.
In view of the current lack of effective measures for control and prevention and the seriousness of AE in humans, long-term epidemiologic surveillance is extremely important as a basis for continuous risk analysis and the development of countermeasures. Furthermore, spreading and emergence of AE can be detected or excluded only on the basis of adequate surveillance. Until recently, the prevalence of E. multilocularis in foxes, dogs, and cats was determined only by parasite identification at necropsy. The use of the recently developed coproantigen ELISA allows mass screening of populations of living foxes, dogs, and cats (36, 52, 141, 188). Long-term monitoring of the parasite prevalence by ELISA should be carried out with selected and representative populations of foxes and, if necessary, of other definitive hosts. PCR for detecting parasite DNA in fecal samples may be used as a confirmation test in selected cases. Surveillance should also include mandatory reporting and centralized analysis of all cases of AE in humans and of E. multilocularis infections diagnosed in animals. A first step in this direction was recently made in Europe within the framework of a research program, initially sponsored by the European Union, by the establishment of European Echinococcosis Registry centers for AE patients and E. multilocularis-infected definitive hosts (110).
E. VOGELI, E. OLIGARTHRUS, AND POLYCYSTIC ECHINOCOCCOSIS
Human polycystic echinococcosis, caused by E. vogeli or E. oligarthrus, is confined to Central and South America and is therefore also denoted as neotropical echinococcosis.
Life Cycles of the Parasites and Epidemiology
E. vogeli is perpetuated in a life cycle with the bush dog (Spoethos venaticus) as the natural definitive host and pacas (Cuniculus papca) and agoutis (Dasyprocta spp.) as intermediate hosts (Fig. 10). Adult cestodes have also been found in one naturally infected domestic dog, and dogs could be experimentally infected (158). Intestinal stages of E. vogeli apparently do not develop in cats or in other felids. In a survey in Columbia, 30% of 325 pacas were infected with the metacestode stage of E. vogeli (34). Since the bush dog is a shy and rarely observed wild animal, its role as source of human infections remains obscure. It is assumed that domestic dogs may play a role in the transmission of parasite eggs to humans. Dogs are probably unable to capture pacas, but they may become infected with metacestodes when fed viscera of pacas at the hunting site or at home (11, 158). As shown in a recent study in which the Mongolian gerbil was used as an experimental host for E. vogeli, this parasite can be detected by the coproantigen ELISA developed for the detection of E. multilocularis (135). This would open the possibility for large-scale examinations of domestic dogs.
FIG. 10.
Life cycle of E. vogeli. (A): Adult parasite. (B) Bush dog (Speothos venaticus) as principal definitive host (B1); domestic dogs are rarely infected (B2). (C) Proglottid with eggs. (D) Egg with oncosphere. (E) Infection of humans. (F) Intermediate hosts: paca (Cuniculus paca) (F1) and agouti (Dasyprocta spp.) (F2). Data from reference 180.
Definitive hosts of E. oligarthrus are wild felids (Fig. 11). Of 10 species indigenous to Central and South America, 6 have been identified as definitive hosts of this species: pampas cat (Felis colocolo), Geoffroy's cat (F. geoffroyi), ocelot (F. pardalis), jaguarundi (F. yagouaroundi), jaguar (Panthera onca), and puma (Puma concolor). In addition, E. oligarthrus was found in one bobcat (Lynx rufus) in northern Mexico. The parasite reached maturity in experimentally infected cats (158). Agoutis (Dasyprocta spp.), spiny rats (Proechimys spp.), and pacas (Cuniculus paca) are the documented intermediate hosts. The metacestode prevalences found in several studies and countries were 18% (7 of 39) in agoutis (Dasyprocta punctata) (Panama), 0.17% (2 of 1168) in spiny rats (Colombia), and 0.92% (3 of 325) in pacas (Colombia) (158). The mode of transmission to humans is unknown.
FIG. 11.
Life cycle of E. oligarthrus. (A) adult parasite. (B) Wild felids as definitive hosts: cougar (Felis concolor) (B1), jaguar (Panthera onca) (B2), and ocelot (Felis pardalis) (B3) as examples. (C) Proglottid with eggs. (D) Egg with oncosphere. (E) Infection of humans. (F) Intermediate hosts: agouti (Dasyprocta spp.) (F1) spiny rat (Proechimys spp.) (F2), and paca (Cuniculus paca) (F3). Data from reference 180.
Geographic Distribution and Prevalence
According to information published in 2002 (158), 99 human cases of PE have been diagnosed in 11 countries including a region from Nicaragua in Central America to Chile, Argentina, and Uruguay in South America (32, 158). Of 72 cases diagnosed by August 1996, the majority were from Brazil (24 cases), Colombia (16 cases), Ecuador (11 cases), and Argentina (11 cases); 1 to 3 sporadic cases were identified in seven other countries).
PE occurs predominantly in rural, sylvatic areas where E. granulosus is not endemic (32). E. oligarthrus has been found in natural intermediate or definitive hosts in a wide range as far north as Costa Rica and far south as La Pampas Province in Argentina (180). Furthermore, this parasite was found in a bobcat in northeastern Mexico, approximately 100 km south of the border with Texas (177). As far as is currently known, E. vogeli occurs in the range of the bush dog, i.e., from Panama in the north to Paraguay and southern Brazil in the south. The distribution of these two species overlaps in some areas where suitable hosts are present (32).
Polycystic Echinococcosis in Humans
In 39 of the 99 diagnosed human cases, E. vogeli was identified as the causative agent, and in 3 further cases E. oligarthrus was identified; no species diagnosis was made in the remaining 57 cases (158). Although human infections with these parasites are summarized under the term “polycystic echinococcosis,” it should be considered that we have to deal with two disease entities characterized by distinctive features of adult and larval parasite morphology, epidemiology, and manifestation in the human host (see also Table 1).
Course of the infection.
(i) E. vogeli echinococcosis.
In the natural intermediate host, the paca, the metacestode stage of E. vogeli usually develops in the liver and consists of subspherical, fluid-filled cysts, up to 3 cm in diameter, which are often interconnected and multichambered. The cysts may occur singly or in aggregations. The thin internal germinal layer is surrounded by a thick laminated layer (159, 161). In contrast to cysts of E. granulosus, the metacestode stage of E. vogeli has the capacity for exogenous proliferation, which does not occur in the natural intermediate host but is pronounced in accidental hosts such as primates. Endogenous proliferation leads to multichambered cysts and endogenous daughter cyst formation (157, 159).
In a group of 59 patients with PE, the liver was involved in 78%, either alone (41%), with other abdominal organs (29%), or with chest organs (8%). The second most frequently affected organ was the lungs alone (14%) or with the liver and other organs (32). After growth and proliferation in the liver, cysts may penetrate the liver capsule and continue exogenous proliferation in the peritoneal cavity, eventually affecting various abdominal and chest organs such as the omentum, spleen, pancreas, diaphragm, intercostal muscles, pericardium, and lungs (32, 157). Penetration of the diaphragm, with subsequent cyst development in the pleural cavity and in the lungs, has been observed in experimentally infected gerbils (157). The exogenous proliferation of the E. vogli metacestode accounts for its considerable pathogenicity in other hosts than natural intermediate hosts, including humans and nonhuman primates (157). Single cysts usually vary in diameter between 0.5 and 6 cm, but larger cysts (>15 cm) may also occur (146). Cyst aggregates can attain much larger dimensions: in one case, a total of 2 kg of cysts was surgically excised (32). Caseous degeneration, partial necrosis, and calcifications of cyst masses have been observed in several patients (32).
Clinical manifestations of E. vogeli infection in humans are similar to those of AE. Typically, E. vogeli infection is a chronic disease lasting for long periods. In a group of 59 patients, the duration of illness reported at the first consultation ranged between 1 month and 22 years; the median age of 51 patients was 44 years (range, 6 to 78 years) without gender specificity (32). The main clinical manifestations include abdominal pain, palpable abdominal masses, loss of weight, jaundice, hepatomegaly, anaemia, fever, hemoptysis (in cases of lung involvement), and, in about 25% of patients, signs of portal hypertension and biliary obstruction. In some cases laboratory tests revealed high serum levels of blirubin, gamma-globulin, and liver enzymes, eosinophilia, and low levels of albumin (32, 65, 138, 139, 146). In advanced cases, mortality is high. According to one report, 22% of 46 patients with PE died during liver lobectomy or from postsurgical complications (32).
(ii) E. oligarthrus infection.
In experimentally infected natural intermediate hosts (agoutis), the metacestode of E. oligarthrus consisted of subspherical, fluid-filled cysts located in muscles and subcutaneous tissues, in the heart, and in the lungs, with a tendency to become multichambered. However, E. oligarthrus has less subdivision into secondary chambers, the germinal layer is thicker, and the laminated layer is much thinner than in cysts of E. vogeli. Exogenous proliferation has not been observed (157).
To date, only three human cases have been diagnosed, two orbital (in Suriname and Venezuela) and one cardiac (in Brazil) (32). In the patient in Suriname (a 6-year-old boy), a large fluid-filled cyst (2.7 by 3.2 cm, filled with 10 ml of fluid) surrounded by a thick fibrotic capsule was located at the apex of the orbit and had caused exophthalmus, extensive chemosis, mydriasis, other symptoms, and blindness of the affected eye (11). In two patients with submandibular cysts reported from India (158, 176), E. oligarthrus was described as causative agent, but there are doubts about the correctness of the diagnosis.
Diagnosis.
Typically, polycystic structures can be detected by ultrasonography or computed tomography of the liver and abdomen or by X-ray examination of the chest. Antibodies against Echinococcus antigen could be detected in 12 of 16 patients by the indirect hemagglutination test (32, 138), but this test does not discriminate between Echinococcus species. In one study, discrimination between PE and CE was achieved by using a purified antigen of E. vogeli in the ELISA (85). The current standard technique for diagnosis of the involved Echinococcus species is the isolation of protoscoleces at surgical intervention or postmortem and the identification of rostellar hooks by the shape and dimensions (159). A new option for diagnosing the Echinococcus species is DNA analysis by PCR using protoscoleces. Four genetic sequences from E. oligarthrus (three mitochondrial and one nuclear) are published in data banks. Although the corresponding sequences are known for E. vogeli, differing, e.g., by 13.8% in the identified 471 bp of the mitochondrial ND1 gene, we are not aware of published reports exploiting the genetic difference for diagnostic species identification.
Treatment.
Because of the high risk of surgical intervention in advanced cases of PE (see above), it has been recommended to consider chemotherapy with albendazole as the first treatment option. Surgery should be performed when the lesions are small, when biliary drainage is required, or when chemotherapy has failed (32). Experience with albendazole treatment of PE is limited but has shown some promising results. In one study, six patients were treated with 10 mg/kg of body weight per day, either continuously for 3 to 8 months or in several cycles. Reduction in cyst size or disappearance and clinical improvement were observed in four patients and clinical improvement alone was observed in two patients after a follow-up period of 10 to 30 months. Side effects were generally minor (32, 139). Studies of inoperable cases of AE have shown that long-term treatment over several years or life-long with mebendazole (or albendazole) is needed to achieve improvement or stabilization of the disease in the majority of cases (4).
Emergence or Spreading of Polycystic Echinococcosis?
By the end of the 1970s, human PE was recognized as a distinct disease entity. In 1979, D'Alessandro et al. (33) reported 13 human cases from four countries. By 2002, a total of 99 cases of human PE had been diagnosed in 11 countries (158), but some experts think that the published cases probably represent only a small proportion of the actual number (146). Although the recent increase in case numbers may at least partially reflect improved awareness of the disease and the results of better diagnostic methods, it is indicative of an infection risk existing in a larger area than was previously acknowledged.
The first report of E. oligarthrus in a bobcat in Mexico (177), where human cases of PE have not been observed so far, may be an indicator of a hitherto unknown area of parasite endemicity. The distribution of the bush dog, the only natural definitive host of E. vogeli, includes tropical sylvatic areas from Panama to Paraguay and southern Brazil (32). Interestingly, human cases of PE, in which the causative Echinococcus species could not be determined, were also found in regions outside the range of the bush dog, for example in Nicaragua, Chile, and Argentina. There are indications that some of these cases may have been caused by E. oligarthrus. However, it cannot be excluded that other, unidentified canids may be involved as definitive hosts in transmission of E. vogeli. According to reports from areas of endemic infection, viscera from hunted pacas are often fed to domestic dogs (hunting dogs) (158). This behavior increases the risk that dogs may become carriers of E. vogeli and infection sources for humans. In view of the gaps in our current epidemiological knowledge, further studies and continuous surveillance is needed.
CONCLUSIONS
During the last three decades, considerable progress has been achieved in various fields of echinococcosis research as described in this review. For example, various strains of E. granulosus have been identified and characterised; imaging and immunological techniques for diagnosing CE and AE in humans could be improved and have achieved high degrees of sensitivity and specificity; mass screening of human populations for abdominal CE or AE is now possible by ultrasonography alone or in conjunction with serum antibody detection; the coproantigen ELISA is a new tool for the identification of E. granulosus or E. multilocularis carriers in populations of carnivores (dogs, foxes, and cats); PCR techniques are now available for detecting DNA of E. granulosus and E. multilocularis in faecal samples of definitive hosts; and new options for treatment of human CE and AE have been developed. These options include improved surgical techniques, chemotherapy with albendazole or mebendazole, and PAIR as a minimally invasive technique for treating various forms of human CE. Although not fully satisfactory, chemotherapy is an option, predominantly in inoperable cases, and can be life saving, especially in patients with advanced AE. Praziquantel, a drug highly effective against intestinal stages of E. granulosus and E. multilocularis in carnivores, is now available in various formulations, improving the modes of drug application. A research highlight was the development of a recombinant vaccine which induces high degrees of protection against the establishment of E. granulosus cysts in domestic ruminants. Furthermore, excellent studies of transmission dynamics of E. granulosus and Taenia species have resulted in the development of a mathematical model of transmission and improved strategies for E. granulosus control. The long-term evaluation of various control strategies against E. granulosus has produced a large amount of data, providing a scientific basis for the improvement of control schemes. Our knowledge of polycystic echinococcosis, which occurs in Central and South America, has significantly increased in recent years, and has shown that this form of echinococcosis is apparently of increasing importance. Progress has also been made in some other fields, including our understanding of host immuninty to the metacestode stage.
Nevertheless, there are many open questions and unresolved problems waiting for further research. For example, there is a need to improve the chemotherapy of human echinococcosis, to better understand immunology, and to evaluate the potentials of immunotherapy or immunoprophylaxis. Since our knowledge of the epidemiological key factors of E. multilocularis, E. vogeli, and E. oligarthrus is insufficient, further research is urgently required. Reliable parameters for measuring the actual infection risk posed by Echinococcus species for humans are needed. Furthermore, surveillance and monitoring systems are required which can detect changes in the risk of infection and which can monitor parasite spreading. The large-scale use of the recombinant vaccine for immunizing domestic ruminants against E. granulosus should be supported. The lack of methods for effective and feasible control of E. multilocularis and prevention of human AE requires special research efforts in a broad spectrum of relevant fields.
Recent publications (see, for example, references 7, 8, 12, 27, 51, and 223) have shown that human CE is persisting with high incidences in many parts of the world and that it is an emerging problem in some areas. AE, although rare in most of the regions of endemicity, represents a considerable public health burden since the infection is lethal in most untreated patients and treatment is very costly (223). Moreover, the risk that the causative agent of AE, E. multilocularis, might spread to further regions of the northern hemisphere cannot be excluded. In Central and South America, PE appears to be an emerging problem. Therefore, WHO and the public health authorities in the affected countries should make every possible effort to develop and establish countermeasures.
Acknowledgments
We thank P. Köhler and P. Torgerson for their advice and F. Grimm for his help in creating some of the figures.
REFERENCES
- 1.Altintas, N. 2003. Past to present: echinococcosis in Turkey. Acta Trop. 85:105-112. [DOI] [PubMed] [Google Scholar]
- 2.Ammann, R. W., and J. Eckert. 1995. Clinical diagnosis and treatment of echinococcosis in humans, p. 411-463. In R. C. A. Thompson and A. J. Lymbery (ed.), Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom.
- 3.Ammann, R. W., and J. Eckert. 1996. Cestodes: Echinococcus. Gastroenterol. Clin. North Am. 25:655-689. [DOI] [PubMed] [Google Scholar]
- 4.Ammann, R. W., A. Fleiner-Hoffmann, J. Eckert, and Schweizerische Echinokokkose-Studiengruppe (SESG). 1999. Schweizerische Studie für Chemotherapie der alveolären Echinokokkose—Rückblick auf ein 20 jähriges klinisches Forschungsprojekt. Schweiz. Med. Wochenschr. 129:323-332. [PubMed] [Google Scholar]
- 5.Ammann, R. W., A. Fleiner-Hoffmann, F. Grimm, and J. Eckert. 1998. Long-term mebendazole therapy may be parasitocidal in alveolar echinococcosis. J. Hepatol. 29:994-998. [DOI] [PubMed] [Google Scholar]
- 6.Ammann, R. W., R. Hirsbrunner, J. Cotting, U. Steiger, P. Jacquier, and J. Eckert. 1990. Recurrence rate after discontinuation of long-term mebendazole therapy in alveolar echinococcosis (preliminary results). Am. J. Trop. Med. Hyg. 43:506-515. [DOI] [PubMed] [Google Scholar]
- 7.Andersen, F. L., J.-J. Chai, and F.-J. Liu. 1993. Compendium on cystic echinococcosis with special reference to the Xinjiang Uygur Autonomous Region, The People's Republic of China. Brigham Young University Print Service, Provo, Utah.
- 8.Andersen, F. L., H. Ouhelli, and M. Kachani. 1997. Compendium on cystic echinococcosis in Africa and in Middle Eastern Countries with Special Reference to Morocco. Brigham Young University Print Services, Provo, Utah.
- 9.Anonymus. 1991. Alveolar hydatid disease. Alveolar hydatid disease in Franche-Comte. Wkly. Epidemiol. Rec. 66:86. [PubMed] [Google Scholar]
- 10.Bartholomot, B., D. Vuitton, S. Harraga, D. Z. Shi, P. Giraudoux, G. Barnish, Y. H. Wang, C. N. L. Macpherson, and P. S. Craig. 2002. Combined ultrasound and serologic screening for hepatic alveolar echinococcosis in central China. Am. J. Trop. Med. Hyg. 66:23-29. [DOI] [PubMed] [Google Scholar]
- 11.Basset, D., C. Girou, I. P. Nozais, F. D'-Hermies, C. Hoang, R. Gordon, and A. D'Alessandro. 1998. Neotropical echinococcosis in Suriname: Echinococcus oligarthrus in the orbit and Echinococcus vogeli in the abdomen. Am. J. Trop. Med. Hyg. 59:787-790. [DOI] [PubMed] [Google Scholar]
- 12.Battelli, G., A. Mantovani, and A. Seimenis. 2002. Cystic echinococcosis and the Mediterranean Region: a long-lasting association. Parassitologia 44:43-57. [PubMed] [Google Scholar]
- 13.Beard, T., A. J. Bramble, and M. J. Middleton. 2001. Eradication in our lifetime. A log book of the Tasmanian Hydatid Control Programs 1962-1996. Department of Primary Industry, Water and Environment, Hobart, Tasmania, Australia.
- 14.Ben Amor, N., M. Gargouri, H. A. Gharbi, Y. J. Golvan, K. Ayachi, and H. Kchouk. 1986. Trial therapy of inoperable hydatid cysts by puncture. Ann. Parasitol. Hum. Comp. 61:689-692. [PubMed] [Google Scholar]
- 15.Berke, O., and M. von Keyserlingk. 2001. Aufwärtstrend bei der Prävalenz des kleinen Fuchsbandwurmes (Echinococcus multilocularis) in Niedersachen. Dtsch. Tierärztl. Wochenschr. 108:193-232. [PubMed] [Google Scholar]
- 16.Bretagne, S., J. P. Guillou, M. Morand, and R. Houin. 1993. Detection of Echinococcus multilocularis DNA in fox faeces using DNA amplification. Parasitology 106:193-199. [DOI] [PubMed] [Google Scholar]
- 17.Bresson-Hadni, S., J.-J. Laplante, D. Lenys, P. Rohmer, B. Gottstein, P. Jacquier, P. Mercet, J.-P. Meyer, J.-P. Miguet, and D.-A. Vuitton. 1994. Seroepidemiologic screening of Echinococcus multilocularis infection in a European area endemic for alveolar echinococcosis. Am. J. Trop. Med. Hyg. 51:837-846. [DOI] [PubMed] [Google Scholar]
- 18.Brunetti, E., and C. Filice. 2001. Radiofrequency thermal ablation of echinococcal liver cysts. Lancet 358:1464. [DOI] [PubMed] [Google Scholar]
- 19.Cada, F., K. Martinek, and L. Kolarova. 1999. Echinococcus multilocularis in a domestic cat (Felis catus f. dom.). Veterinarstvi 49:6-7. [Google Scholar]
- 20.Campos-Bueno, A., G. Lopez-Abente, and A. M. Andres-Cercadillo. 2000. Risk factors for Echinococcus granulosus infection: a case-control study. Am. J. Trop. Med. Hyg. 62:329-334. [DOI] [PubMed] [Google Scholar]
- 21.Caremani, M., R. Maestrini, U. Occhini, S. Sassoli, A. Accorsi, A. Giorgio, and C. Filice. 1993. Echographic epidemiology of cystic hydatid disease in Italy. Eur. J. Epidemiol. 9:401-404. [DOI] [PubMed] [Google Scholar]
- 22.Carmona, C., R. Perdomo, A. Carbò, C. Alvarez, J. Monti, R. Grauert, D. Stern, G. Perera, S. Lloyd, R. Bazini, M. A. Gemmell, and L. Yarzabal. 1998. Risk factors associated with human cystic echinococcosis in Florida, Uruguay: results of a mass screening study using ultrasound and serology. Am. J. Trop. Med. Hyg. 58:599-605. [DOI] [PubMed] [Google Scholar]
- 23.Chautan, M., D. Pontier, and M. Artois. 2000. Role of rabies in recent demographic changes in red fox (Vulpes vulpes) populations in Europe. Mammalia 64:391-410. [Google Scholar]
- 24.Christofi, G., P. Deplazes, N. Christofi, I. Tanner, P. Economides, and J. Eckert. 2002. Screening of dogs for Echinococcus granulosus coproantigen in a low endemic situation in Cyprus. Vet. Parasitol. 104:299-306. [DOI] [PubMed] [Google Scholar]
- 25.Cohen, H., E. Paolillo, R. Bonifacino, B. Botta, L. Parada, P. Cabrera, K. Snowden, R. Gasser, R. Tessier, L. Dibarboure, H. Wen, J. C. Allan, H. S. De Alfaro, M. T. Rogan, and P. S. Craig. 1998. Human cystic echinococcosis in a Uruguayan community: a sonographic, serologic, and epidemiologic study. Am. J. Trop. Med. Hyg. 59:620-627. [DOI] [PubMed] [Google Scholar]
- 26.Conn, D. B. 1994. Cestode infections of mammary glands and female reproductive organs: potential for vertical transmission? J. Helminthol. Soc. Wash. 61:162-168. [Google Scholar]
- 27.Craig, P., and Z. Pawlowski (ed.). 2002. Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 28.Craig, P. S. 1997. Immunodiagnosis of Echinococcus granulosus and a comparison of tech-niques for diagnosis of canine echinococcosis, p. 85-118. In F. L. Andersen, H. Ouhelli, and M. Kachani (ed.), Compendium on cystic echinococcosis in Africa and Middle Eastern Countries with Special Reference to Morocco. Brigham Young University Print Services, Provo, Utah.
- 29.Craig, P. S., R. B. Gasser, L. Parada, P. Cabrera, S. Parietti, C. Borgues, A. Acuttis, J. Agulla, K. Snowden, and E. Paolillo. 1995. Diagnosis of canine echinococcosis: comparison of coproantigen and serum antibody tests with arecoline purgation in Uruguay. Vet. Parasitol. 56:293-301. [DOI] [PubMed] [Google Scholar]
- 30.Craig, P. S., P. Giraudoux, D. Shi, B. Batholomot, G. Barnish, P. Delattre, J. P. Quere, S. Harraga, G. Bao, Y. Wang, F. Lu, A. Ito, and D. A. Vuitton. 2000. An epidemiological and ecological study of human alveolar echinococcosis transmission in south Gansu, China. Acta Trop. 77:167-177. [DOI] [PubMed] [Google Scholar]
- 31.Dai, W. J., A. Hemphill, A. Waldvogel, K. Ingold, P. Deplazes, H. Mossmann, and B. Gottstein. 2001. Major carbohydrate antigen of Echinococcus multilocularis induces an im-munoglobulin G response independent of αβ+ CD4+ T cells. Infect. Immun. 69:6074-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Alessandro, A. 1997. Polycystic echinococcosis in tropical America: Echinococcus vogeli and E. oligarthrus. Acta Trop. 67:43-65. [DOI] [PubMed] [Google Scholar]
- 33.D'Alessandro, A., R. L. Rausch, and C. A. N. Cuello. 1979. Echinococcus vogeli in man, with a review of polycystic hydatid disease in Colombia and neighboring countries. Am. J. Trop. Med. Hyg. 28:303-317. [DOI] [PubMed] [Google Scholar]
- 34.D'Alessandro, A., R. L. Rausch, G. A. Morales, S. Collet, and D. Angel. 1981. Echinococcus infections in Colombian animals. Am. J. Trop. Med. Hyg. 30:1263-1276. [DOI] [PubMed] [Google Scholar]
- 35.Del Carpio, M., S. Moguilansy, M. T. Costa, M. Lezcanco, H. Panomarenko, C. Bianchi, S. Bendersky, M. Lazcano, B. Frider, and E. Larrieu. 2000. Diagnosis of human hydatidosis: predictive values of a rural ultrasonographic survey in an apparently healthy population. Medicina (Buenos Aires) 60:466-468. [PubMed] [Google Scholar]
- 36.Deplazes, P., P. Alther, I. Tanner, R. C. A. Thompson, and J. Eckert. 1999. Echinococcus multilocularis coproantigen detection by enzyme-linked immunosorbent assay in fox, dog, and cat populations. J. Parasitol. 85:115-121. [PubMed] [Google Scholar]
- 37.Deplazes, P., and J. Eckert. 2001. Veterinary aspects of alveolar echinococcosis—a zoonosis of public health significance. Vet. Parasitol. 98:65-87. [DOI] [PubMed] [Google Scholar]
- 38.Deplazes, P., S. Gloor, C. Stieger and D. Hegglin. 2002. Urban transmission of Echinococcus multilocularis, p. 287-297. In P. Craig and Z. Pawlowski. (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 39.Deplazes, P., and Gottstein, B. 1991. A monoclonal antibody against Echinococcus multilocularis Em2 antigen. Parasitology 103:41-49. [DOI] [PubMed] [Google Scholar]
- 40.Deplazes, P., S. Jimenez-Palacios, B. Gottstein, J. Skaggs, and J. Eckert. 1994. Detection of Echinococcus granulosus coproantigens in stray dogs of northern Spain. Appl. Parasitol. 35:297-301. [PubMed] [Google Scholar]
- 41.Deplazes, P., A. Dinkel, and A. Mathis. Molecular tools for studies on the transmission biology of Echinococcus multilocularis. Parasitology, in press. [DOI] [PubMed]
- 42.Dinkel, A., M. von Nickisch-Rosenegk, B. Bilger, M. Merli, R. Lucius, and T. Romig. 1998. Echinococcus multilocularis in the definitive host: coprodiagnosis by PCR is an alternative to necropsy. J. Clin. Microbiol. 36:1871-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dowling, P. M., M. N. Abo-Shehada, and P. R. Torgerson. 2000. Risk factors associated with human cystic echinococcosis in Jordan: results of a case-control study. Ann. Trop. Med. Parasitol. 94:69-75. [DOI] [PubMed] [Google Scholar]
- 44.Dowling, P. M., and P. R. Torgerson. 2000. A cross-sectional survey to analyse the risk factors associated with human cystic echinococcosis in an endemic area of mid-Wales. Ann. Trop. Med. Parasitol. 94:241-245. [DOI] [PubMed] [Google Scholar]
- 45.Drolshammer, I., E. Wiesmann, and J. Eckert. 1973. Echinokokkose beim Menschen in der Schweiz 1956-1969. Schweiz. Med. Wochenschr. 103:1337-1341, 1386-1392. [PubMed] [Google Scholar]
- 46.Eckert, J. 1986. Prospects for treatment of the metacestode stage of Echinococcus, p. 250-284. In R. C. A. Thompson (ed.), The biology of Echinococcus and hydatid disease. Allen and Unwin, London, United Kingdom.
- 47.Eckert, J. 1996. Der “gefährliche Fuchsbandwurm” (Echinococcus multilocularis) und die alveoläre Echinokokkose des Menschen in Mitteleuropa. Berl. Münch. Tierärztl. Wochenschr. 109:202-210. [PubMed] [Google Scholar]
- 48.Eckert, J. 1998. Alveolar echinococcosis (Echinococcus multilocularis) and other forms of echinococcosis (Echinococcus oligarthrus and Echinococcus vogeli), p. 689-716. In S. R. Palmer, E. J. L. Soulsby, and D. I. H. Simpson (ed.), Zoonoses. Oxford University Press, Oxford, United Kingdom.
- 49.Eckert, J. 2003. Predictive values and quality control of techniques for the diagnosis of Echinococcus multilocularis in definitive hosts. Acta Trop. 85:157-163. [DOI] [PubMed] [Google Scholar]
- 50.Eckert, J., G. Barandun, and J. Pohlenz. 1978. Chemotherapie der larvalen Echinokokkose bei Labortieren. Schweiz. Med. Wochenschr. 108:1104-1112. [PubMed] [Google Scholar]
- 51.Eckert, J., F. J. Conraths, and K. Tackmann. 2000. Echinococcosis: an emerging or reemerging zoonosis? Int. J. Parasitol. 30:1283-1294. [DOI] [PubMed] [Google Scholar]
- 52.Eckert, J., and P. Deplazes. 1999. Alveolar echinococcosis in humans: the current situation in Central Europe and the need for countermeasures. Parasitol. Today 15:315-319. [DOI] [PubMed] [Google Scholar]
- 53.Eckert, J., and P. Deplazes. 2001. Immunological and molecular techniques for diagnosing the Echinococcus multilocularis infection in definitive and intermediate hosts. Acta Parasitol. 46:1-7. [Google Scholar]
- 54.Eckert, J., P. Deplazes, P. S. Craig, M. A. Gemmell, B. Gottstein, D. Heath, D. J. Jenkins, M. Kamiya and M. Lightowlers. 2001. Echinococcosis in animals: clinical aspects, diagnosis and treatment, p. 72-99. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHO/OIE Manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 55.Eckert, J., B. Gottstein, D. Heath, and F.-J. Liu. 2001. Prevention of echinococcosis in humans and safety precautions, p. 238-247. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 56.Eckert, J., P. Jacquier, D. Baumann, and P. A. Raeber. 1995. Echinokokkose des Menschen in der Schweiz, 1984-1992. Schweiz. Med. Wochenschr. 125:1989-1998. [PubMed] [Google Scholar]
- 57.Eckert, J., R. L. Rausch, M. A. Gemmell, P. Giraudoux, M. Kamiya, F.-J. Liu, P. M. Schantz, and T. Romig. 2001. Epidemiology of Echinococcus multilocularis, Echinococcus vogeli and Echinococcus oligarthrus, p. 164-182. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 58.Eckert, J., P. M. Schantz, R. B. Gasser, P. R. Torgerson, A. S. Bessonov, S. O. Movses-sian, A. Thakur, F. Grimm, and M. A. Nikogossian. 2001. Geographic distribution and prevalence, p. 100-142. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 59.Eckert, J., and R. C. A. Thompson. 1997. Intraspecific variation of Echinococcus granulosus and related species with emphasis on their infectivity to humans. Acta Trop. 64:19-34. [DOI] [PubMed] [Google Scholar]
- 60.Eckert, J., R. C. A. Thompson, H. Bucklar, B. Bilger, and P. Deplazes. 2001. Prüfung der Wirkung von Episprantel (Cestex(R)) gegen Echinococcus multilocularis bei Hunden und Katzen. Berl. Münch. Tierärztl. Wochenschr. 114:121-126. [PubMed] [Google Scholar]
- 61.Economides, P., and G. Christofi. 2002. Experience gained and evaluation of the echinococcosis/hydatidosis ercadication programmes in Cyprus 1971-1999, p. 367-379. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 62.Economides, P., E. J. Larrieu, and D. Orlando. 2001. Evolution of programmes for control of Echinococcus granulosus (examples), p. 204-209. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 63.Emery, I., M. Liance, E. Deriaud, D. A. Vuitton, R. Houin, and C. Leclerc. 1996. Characterization of T-cell immune responses of Echinococcus multilocularis-infected C57BL/6J mice. Parasite Immunol. 18:463-472. [DOI] [PubMed] [Google Scholar]
- 64.Fasihi Harandi, M., R. P. Hobbs, P. J. Adams, I. Mobedi, U. M. Morgan-Ryan, and R. C. A. Thompson. 2002. Molecular and morphological characterization of Echinococcus granulosus of human and animal origin in Iran. Parasitology 125:367-373. [DOI] [PubMed] [Google Scholar]
- 65.Ferreira, M. S., S. A. deNishioka, A. Rocha, and A. D' Alessandro. 1995. Echinococcus vogeli polycystic hydatid disease: report of two Brazilian cases outside the Amazon region. Trans. R. Soc. Trop. Med. Hyg. 89:286-287. [DOI] [PubMed] [Google Scholar]
- 66.Fesseler, M. 1990. Vergleich der Endemiegebiete von Echinococcus multilocularis und Tollwut in Mitteleuropa. Veterinary doctoral thesis. University of Zürich, Zürich, Switzerland.
- 67.Filice, C., and E. Brunetti. 1997. Use of PAIR in human cystic echinococcosis. Acta Trop. 64:95-107. [DOI] [PubMed] [Google Scholar]
- 68.Filice, C., E. Brunetti, R. Bruno, F. G. Crippa, and WHO Informal Working Group on Echinococcosis: PAIR Network. 2000. Percutaneous drainage of echinococcal cysts (PAIR-puncture, aspiration, injection, reaspiration): results of a worldwide survey for assessment of its saftey and efficacy. Gut 47:156-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Filice, C., F. Pirola, E. Brunetti, S. Dughetti, M. Strosselli, and C. S. Foglieni. 1990. A new therapeutic approach for hydatid liver cysts. Aspiration and alcohol injection under sonographic guidance. Gastroenterology 98:1366-1368. [DOI] [PubMed] [Google Scholar]
- 70.Franchi, C., B. Di Vico, and A. Teggi. 1999. Long-term evaluation of patients with hydatidosis treated with benzimidazole carbamates. Clin. Infect. Dis. 29:304-309. [DOI] [PubMed] [Google Scholar]
- 71.Fraser, A., F. Elayoubi, and P. S. Craig. 2002. Detection of cestode infections in definitive hosts: present status and future advances, p. 157-175. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 72.Frider, B., E. Larrieu, and M. Odriozola. 1999. Long-term outcome of asymptomatic liver hydatidosis. J. Hepatol. 30:228-231. [DOI] [PubMed] [Google Scholar]
- 73.Gargouri, N. Ben Amor, F. Ben Chehida, A. Hammou, H. A. Gharbi, M. B. Ben Cheikh, H. Kchouck, K. Ayachi, and Y. J. Golvan. 1990. Percutaneous treatment of hydatid cysts (Echinococcus granulosus). Cardiovasc. Intervent. Radiol. 13:169-173. [DOI] [PubMed] [Google Scholar]
- 74.Gemmell, M. A. 1997. Quantifying transmission dynamics of the family Taeniidae with particular reference to Echinococcus spp.: an update, p. 54-71. In F. L. Andersen, H. Ouhelli, and M. Kachani. (ed.), Compendium on cystic echinococcosis in Africa and Middle Eastern Countries with Special Reference to Morocco. Brigham Young University Print Services, Provo, Utah.
- 75.Gemmell, M. A., and M. G. Roberts. 1998. Cystic echinococcosis (Echinococcus granulosus), p. 665-688. In S. R. Palmer, E. J. L. Soulsby, and D. I. H. Simpson (ed.), Zoonoses. Oxford University Press, Oxford, United Kingdom.
- 76.Gemmell, M. A., M. G. Roberts, T. C. Beard, S. Campano Diaz, J. R. Lawson, and J. M. Nonnemaker. 2001. Formulating effective and cost-effective policies in the planning phase for permanent control of Echinococccus granulosus, p. 209-219. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 77.Gemmell, M. A., M. G. Roberts, T. C. Beard, S. Campano Diaz, J. R. Lawson, and J. M. Nonnemaker. 2001. Control of echinococcosis: control of Echinococcus granulosus, p. 195-204. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 78.Gemmell, M. A., M. G. Roberts, T. C. Beard, and J. R. Lawson. 2001. Quantitative epidemiology and transmission dynamics with special reference to Echinococcus granulosus, p. 143-156. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 79.Giraudoux, P., P. Delattre, K. Takahashi, F. Raoul, J. P. Quéré, P. Craig, and D. Vuitton. 2002. Transmission ecology of Echinococcus multilocularis in wildlife: what can be learned from comparative studies and multiscale approaches? p. 251-266. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 80.Giraudoux, P., F. Raoul, K. Bardonnet, P. Vuillaume, F. Tourneux, F. Cliquet, P. Delattre, and D. A. Vuitton. 2001. Aleolar echinococcosis: characteristics of a possible emergence and new perspectives in epidemiosurveillance. Méd. Mal. Infect. 31(Suppl.):247-256. [Google Scholar]
- 81.Giraudoux, P., D. A. Vuitton, S. Bresson-Hadni, P. Craig, B. Bartholomot, G. Barnish, J.-J. Laplante, S. D. Zhong, and D. Lenys. 1996. Mass screening and epidemiology of alevolar echinococcosis in France, western Europe, and Gansu, central China: from epidemiology towards transmission ecology, p. 197-211. In J. Uchino and N. Sato (ed.), Alveolar echinococcosis. Strategy for eradication of alveolar echinococcosis of the liver. Fuji Shoin, Sapporo, Japan.
- 82.Godot, V., S. Harraga, I. Beurton, C. Pater, M. E. Sarciron, B. Gottstein, and D. A. Vuitton. 2000. Resistance/susceptibility to Echinococcus multilocularis infection and cytokine profile in humans. I. Comparisons of patients with progressive and abortive lesions. Clin. Exp. Immunol. 121:484-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Godot, V., S. Harraga, M. Deschaseaux, S. Bresson-Hadni, B. Gottstein, D. Emilie, and D. A. Vuitton. 1997. Increased basal production of interleukin-10 by peripheral blood mononuclear cells in human alveolar echinococcosis. Eur. Cytokine Netw. 8:401-408. [PubMed] [Google Scholar]
- 84.Gottstein, B. 2000. Immunodiagnosis of infections with cestodes, p. 347-373. In Y. H. Hui, S. A. Sattar, K. D. Murrell, W.-K. Nip, and P. S. Stanfield (ed.), Foodborne disease handbook. Marcel Dekker, Inc., New York, N.Y.
- 85.Gottstein, B., A. D' Alessandro, and R. L. Rausch. 1995. Immunodiagnosis of polycystic hydatid disease/polycystic echinococcosis due to Echinococcus vogeli. Am. J. Trop. Med. Hyg. 53:558-563. [DOI] [PubMed] [Google Scholar]
- 86.Gottstein, B., and A. Hemphill. 1997. Immunopathology of echinococcosis. Chem. Immunol. 66:177-208. [DOI] [PubMed] [Google Scholar]
- 87.Gottstein, B., P. Jacquier, S. Bresson Hadni, and J. Eckert. 1993. Improved primary immunodiagnosis of alveolar echinococcosis in humans by an enzyme-linked immunosorbent assay using the Em2plus antigen. J. Clin. Microbiol. 31:373-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gottstein, B., C. Lengeler, P. Bachmann, P. Hagemann, P. Kocher, M. Brossard, F. Witassek, and J. Eckert. 1987. Sero-epidemiological survey for alveolar echinococcosis (by Em2-ELISA) of blood donors in an endemic area of Switzerland. Trans. R. Soc. Trop. Med. Hyg. 81:960-964. [DOI] [PubMed] [Google Scholar]
- 89.Gottstein, B., F. Saucy, P. Deplazes, J. Reichen, G. Demierre, A. Busato, C. Zürcher, and P. Pugin. 2001. Is high prevalence of Echinococcus multilocularis in wild and domestic animals associated with disease incidence in humans? Emerg. Infect. Dis. 7:408-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grimm, F., F. E. Maly, J. A. Lü, and R. Ilano. 1998. Analysis of specific immunoglobulin G subclass antibodies for serological diagnosis of echinococcosis by a standard enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 5:613-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haag, K. L., A. Zaha, A. M. Araujo, and B. Gottstein. 1997. Reduced genetic variability within coding and non-coding regions of the Echinococcus multilocularis genome. Parasitology 115:521-529. [DOI] [PubMed] [Google Scholar]
- 92.Heath, D. D. 1986. Immunobiology of Echinococcus infections, p. 164-188. In R. C. A Thompson (ed.), The biology of Echinococcus and hydatid disease. Allen & Unwin, London, United Kingdom.
- 93.Heath, D. D., O. Jensen, and M. W. Lightowlers. 2003. Progress in control of hydatidosis using vaccination—a review of formulation and delivery of the vaccine and recommendations for practical use in control programmes. Acta Tropica 85:133-143. [DOI] [PubMed] [Google Scholar]
- 94.Heath, D. D., and M. W. Lightowlers. 1997. Vaccination on hydatidology—state of the art. Arch. Int. Hidatid. 33:14-16. [Google Scholar]
- 95.Heath, D. D., Q. Pusheng, Z. Zhuangzhi, W. Jincheng, F. Jinglan, and M. W. Lightowlers. 1999. Role of immunization of the intermediate host in hydatid disease control. Arch. Int. Hidatid. 33:14-16. [Google Scholar]
- 96.Hegglin, D., P. Ward, and P. Deplazes. 2003. Anthelmintic baiting of foxes against urban contamination with Echinococcus multilocularis. Emerg. Infect. Dis. 9:1266-1272. [DOI] [PMC free article] [PubMed]
- 97.Heusser, J. 1995. Säugetiere der Schweiz, p. 1-501. Birkhäuser Verlag, Basel, Switzerland.
- 98.Hildreth, B., S. Sriram, B. Gottstein, M. Wilson, and P. M. Schantz. 2000. Failure to identify alveolar echinococcosis in trappers from South Dakota in spite of high prevalence of Echinococcus multilocularis in wild canids. J. Parasitol. 86:75-77. [DOI] [PubMed] [Google Scholar]
- 99.Hofer, S., S. Gloor, U. Müller, A. Mathis, D. Hegglin, and P. Deplazes. 2000. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zurich, Switzerland. Parasitology 120:135-142. [DOI] [PubMed] [Google Scholar]
- 100.Hokelek, M., K. Erzurumlu, Y. Uyar, and A. Birinci. 1999. The effect of praziquantel as a scolecidal agent on the protoscoleces of Echinococcus granulosus. Turk Hij. Deneysel Biyol. Derg. 56:129-133. [Google Scholar]
- 101.Horton, R. J. 1997. Albendazole in treatment of human cystic echinococcosis: 12 years of experience. Acta Trop. 64:79-93. [DOI] [PubMed] [Google Scholar]
- 102.Horton, J. 2003. Albendazole for treatment of echinococcosis. Fundam. Clin. Pharmacol. 17:205-212. [DOI] [PubMed] [Google Scholar]
- 103.Hüslmeier, A. J., P. M. Gehrig, R. Geyer, R. Sack, B. Gottstein, P. Deplazes, and P. Köhler. 2002. A major Echinococcus multilocularis antigen is a mucin-type glycoprotein. J. Biol. Chem. 277:5742-5749. [DOI] [PubMed] [Google Scholar]
- 104.Ito, A., Y. Sako, H. Yamasaki, H., W. Mamuti, K. Nakaya, M. Nakao, and Y. Ishikawa. 2003. Delopment of Em18-immoblot and Em18-ELISA fo specific diagnosis of alveolar echinococcosis. Acta Trop. 85:173-182. [DOI] [PubMed] [Google Scholar]
- 105.Jenkins, D. J. 2002. Echinococcus in Australia: the role of wildlife in transmission, with particular reference to South Eastern Australia, p. 327-332. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 106.Jenkins, D. J., and T. Romig. 2000. Efficacy of Droncit Spot-on (praziquantel) 4 % w/v against immature and mature Echinococcus multilocularis in cats. Int. J. Parasitol. 30:959-962. [DOI] [PubMed] [Google Scholar]
- 107.Jensen, O. 1999. Immunizacion ovina: factibilidad técnica en Argentina. Arch. Int. Hidatid. 33:17-19. [Google Scholar]
- 108.Johnson, K. S., G. B. L. Harrison, M. W. Lightowlers, K. L. O'Hoy, W. G. Cougle, R. P. Dempster, S. B. Lawrence, J. G. Vinton, D. D. Heath, and M. D. Rickard. 1989. Vaccination against ovine cysticercosis using a defined recombinant antigen. Nature 338:585-587. [DOI] [PubMed] [Google Scholar]
- 109.Jonas, C., and K. Dräger. 1998. Untersuchung von Füchsen auf Echinococcus multilocularis: Entwicklung seit 1982 und Situation 1996/97 in Rheinland-Pfalz. Tierärztl. Umsch. 53:214-221. [Google Scholar]
- 110.Kern, P., K. Bardonet, E. Renner, H. Auer, Z. Pawlowski, R. W. Ammann, D. A. Vuitton, and European Echinococcosis Registry. 2003. European Echinococcosis Registry: human alveolar echinococcosis, Europe, 1982-2000. Emerg. Infect. Dis. 9:343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kern, P., Frosch, P., Helbig, M., J. G. Wechsler, S. Usadel, K. Beckh, R. Kunz, R. Lucius, and M. Frosch. 1995. Diagnosis of Echinococcus multilocularis infection by reverse-transcription polymerase chain reaction. Gastroenterology 109:596-600 [DOI] [PubMed] [Google Scholar]
- 112.Kern, P., W. Kratzer, and S. Reuter. 2000. Alveoläre Echinokokkose: Diagnostik. Dtsch. Med. Wochenschr. 125:59-62. [DOI] [PubMed] [Google Scholar]
- 113.Kern, P., W. Kratzer, and S. Reuter. 2000. Alveoläre Echinokokkose: Therapie. Dtsch. Med. Wochenschr. 125:87-89. [DOI] [PubMed] [Google Scholar]
- 114.Kern, P., S. Reuter, K. Buttenschoen, and W. Kratzer. 2001. Diagnostik der zystischen Echinokokkose. Dtsch. Med. Wochenschr. 126:20-23. [DOI] [PubMed] [Google Scholar]
- 115.Kern, P., S. Reuter, W. Kratzer, and K. Buttenschoen. 2001. Therapie der zystischen Echinokokkose. Dtsch. Med. Wochenschr. 126:51-54. [DOI] [PubMed] [Google Scholar]
- 116.Khuroo, M. S., N. A. Wani, G. Javid, B. A. Khan, G. N. Yattoo, A. H. Shah, and S. G. Jeelani. 1997. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N. Eng. J. Med. 337:881-887. [DOI] [PubMed] [Google Scholar]
- 117.Khuroo, M. S., S. A. Zargar, and R. Mahajan. 1991. Echinococcus granulosus cysts in the liver: management with percutaneous drainage. Radiology 180:141-145. [DOI] [PubMed] [Google Scholar]
- 118.Kimura, H., K. Furuya, S. Kawase, C. Sato, K. Takahashi, K. Uraguchi, T. Ito, and K. Yagi. 1999. Epidemiology of alveolar echinococcosis in Hokkaido, Japan. Arch. Int. Hidatid. 33:85-89. [PubMed] [Google Scholar]
- 119.Kincekova, J., K. Reiterova, P. Dubinsky, M. Szilagyiova, R. Johanes, and M. Gottas. 2002. A second case of autothonous human alveolar echinococcosis in the Slovak Republic. Helminthologia 39:193-196. [Google Scholar]
- 120.Kittelberger, R., M. P. Reichel, J. Jenner, D. Heath, M. W. Lightowlers, P. Moro, M. M. Ibrahem, S. Craig, and J. S. O'Keefe. 2002. Evaluation of three enzyme-linked immunosorbent assays (ELISAs) for the detection of serum antibodies in sheep infected with Echinococcus granulosus. Vet. Parasitol. 110:57-76. [DOI] [PubMed] [Google Scholar]
- 121.Kreidl, P., F. Allersberger, G. Judmaier, H. Auer, H. Aspöck, and A. J. Hall. 1998. Domestic pets as risk factors of alveolar hydatid disease in Austria. Am. J. Epidemiol. 147:978-981. [DOI] [PubMed] [Google Scholar]
- 122.Kurz, S. 2000. Alveoläre Echinokokkose: Entwicklung einer Klassifikation in Anlehnung an das TNM-System. Dissertation, Universität Ulm. H. Utz Verlag, München, Germany.
- 123.Lahmar, S., M. Kilani, P. R. Torgerson, and M. A. Gemmell. 1999. Echinococcus granulosus larvae in the livers of sheep in Tunisia: the effects of host age. Ann. Trop. Med. Parasitol. 93:75-81. [DOI] [PubMed] [Google Scholar]
- 124.Larrieu, E., M. T. Costa, G. Cantoni, R. Alvarez, L. Cavagion, J. L. Labanchi, R. Bigatti, D. Araya, E. Herrero, E. Alvarez, S. Mancini, and P. Cabrera. 2001. Ovine Echinococcus granulosus transmission dynamics in the province of Rio Negro, Argentina, 1980-1999. Vet. Parasitol. 98:263-272. [DOI] [PubMed] [Google Scholar]
- 125.Larrieu, E. J., M. T. Costa, M. Del Carpio, S. Moguillansky, G. Bianchi, and Z. E. Yadon. 2002. A case-control study of the risk factors for cystic echinococcosis among children of Rio Negro province, Argentina. Ann. Trop. Med. Parasitol. 96:43-52. [DOI] [PubMed] [Google Scholar]
- 126.Larrieu, E. J., and B. Frider. 2001. Human cystic echinococcosis: contributions to the natural history of the disease. Ann. Trop. Med. Parasitol. 95:679-687. [DOI] [PubMed] [Google Scholar]
- 127.Lawson, J. R., and M. A. Gemmell. 1990. Transmission of taeniid tapeworm egg via blowflies to intermediate hosts. Parasitology 100:143-146. [DOI] [PubMed] [Google Scholar]
- 128.Le, T. H., M. S. Pearson, D. Blair, N. Dai, L. H. Zhang, and D. P. McManus. 2002. Complete mitochondrial genomes confirm the distinctiveness of the horse-dog and the sheep-dog strains of Echinococcus granulosus. Parasitology 124:97-112. [DOI] [PubMed] [Google Scholar]
- 129.Lightowlers, M. W. 2002. Vaccines for control of cysticercosis and hydatidosis, p. 381-391. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 130.Lightowlers, M. W., D. D. Heath, O. Jensen, E. Fernandez, and J. A. Iriarte. 1999. Intermediate host vaccination and prospects for a human vaccine. Arch. Int. Hidatidosis 33:13-14. [Google Scholar]
- 131.Lucius, R., and B. Bilger. 1995. Echinococcus multilocularis in Germany: increased awareness or spreading of a parasite? Parasitol. Today 11:430-434. [DOI] [PubMed] [Google Scholar]
- 132.Macpherson, C. N. L. 2001. Epidemiology of Echinococcus granulosus in transhumant situations, p. 156-163. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 133.Macpherson, C. N. L., and T. W. M. Wachira. 1997. Cystic echinococcosis in Africa south of the Sahara, p. 245-277. In F. L. Andersen, H. Ouhelli, and M. Kachani (ed.), Compendium on cystic echinococcosis in Africa and Middle Eastern Countries with special reference to Morocco. Brigham Young University Print Services, Provo, Utah.
- 134.Mathis, A., and P. Deplazes. 2002. Role of PCR-DNA detection of Echinococcus multilocularis, p. 195-204. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 135.Matsuo, K., M. Shimizu, N. Nonaka, Y. Oku, and M. Kamiya. 2000. Development and sexual maturation of Echinococcus vogeli in an alternative definitive host, Mongolian gerbil (Meriones unguiculatus). Acta Trop. 75:323-330. [DOI] [PubMed] [Google Scholar]
- 136.Meine, K., and P. Müller. 1996. Zum Vorkommen des Kleinen Fuchsbandwurmes Echinococcus multilocularis (Leuckart 1863) im Saarland. Z. Jagdwiss. 42:274-283. [Google Scholar]
- 137.Men, S., B. Hekimoglu, C. Yucesoy, I. S. Arda, and I. Baran. 1999. Percutaneous treatment of hepatic hydatid cysts: an alternative to surgery. Am. J. Roentgenol. 172:83-89. [DOI] [PubMed] [Google Scholar]
- 138.Meneghelli, U. G., A. L. C. Martinelli, A. D. Bellucci, M. G. Villanova, M. A. S. Llorach-Velludo, and J. E. Magro. 1992. Polycystic hydatid disease (Echinococcus vogeli). Treatment with albendazole. Ann. Trop. Med. Parasitol. 86:151-156. [DOI] [PubMed] [Google Scholar]
- 139.Meneghelli, U. G., A. L. C. Martinelli, M. A. S. L. Velludo, A. D. Bellucci, J. E. Magro, and M. L. P. Barbo. 1992. Polycystic hydatid disease (Echinococcus vogeli). Clinical, laboratory and morphological findings in nine Brazilian patients. J. Hepatol. 14:203-210. [DOI] [PubMed] [Google Scholar]
- 140.Menghebat, L., L. Jiang, and J. Chai. 1993. A retrospective survey for surgical cases of cystic echinococcosis in the Xinjiang Uygur Autonomous Region, PRC (1951-90), p. 135-145. In F. L. Andersen, J. Chai, and F. Liu. (ed.), Compendium on cystic echinococcosis with special referencet to the Xinjiang Ugur Autonomous Region, the People's Republic of China. Brigham Young University Print Services, Provo, Utah.
- 141.Morishima, Y., H. Tsukada, N. Nonaka, Y. Oku, and M. Kamiya. 1999. Coproantigen survey for Echinococcus multilocularis prevalence of red foxes in Hokkaido, Japan. Parasitol. Int. 48:121-134. [DOI] [PubMed] [Google Scholar]
- 142.Morris, D. L., and K. S. Richards. 1992. Hydatid disease. Current medical and surgical management. Butterworth-Heinemann Ltd., Oxford, United Kingdom.
- 143.Morris, D. L., and D. H. Taylor. 1988. Optimal timing of post-operative albendazole prophylaxis in E. granulosus. Ann. Trop. Med. Parasitol. 82:65-66. [DOI] [PubMed] [Google Scholar]
- 144.Nothdurft, H. D., T. Jelinek, A. Mai, B. Sigl, F. von Sonnenburg, and T. Löscher. 1995. Epidemiology of alveolar echinococcosis in Southern Germany (Bavaria). Infection 23:85-88. [DOI] [PubMed] [Google Scholar]
- 145.Ohbayashi, M. 1996. Host animals of Echinococcus multilocularis in Hokkaido, p. 59-64. In J. Uchino and N. Sato (ed.), Alveolar echinococcosis. Strategy for eradication of alveolar echinococcosis of the liver. Fuji Shoin, Sapporo, Japan.
- 146.Oostburg, B. F. J., M. A. Vrede, and A. E. Bergen. 2000. The occurrence of polycystic echinococcosis in Suriname. Ann. Trop. Med. Parasitol. 94:247-252. [DOI] [PubMed] [Google Scholar]
- 147.Parodi, P., A. Mantovani, and A. Seimenis. 2001. Public health education and training in control programmes, p. 219-225. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 148.Pawlowski, Z. S. 1997. Critical points in the clinical management of cystic echinococcosis: a revised review, p. 119-135. In F. L. Andersen, H. Ouhelli, and M. Kachani (ed.), Compendium on cystic echinococcosis in Africa and Middle Eastern Countries with special reference to Morocco. Brigham Young University Print Services, Provo, Utah.
- 149.Pawlowski, Z. S., J. Eckert, D. A. Vuitton, R. W. Ammann, P. Kern, P. S. Craig, F. K. Dar, F. De Rosa, C. Filice, B. Gottstein, F. Grimm, C. N. L. Macpherson, N. Sato, T. Todorov, J. Uchino, W. von Sinner, and H. Wen. 2001. Echinococcosis in humans: clinical aspects, diagnosis and treatment, p. 20-66. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 150.Pearson, M., T. H. Le, L. H. Zhang, D. Blair, T. H. N. Dai, and D. P. McManus. 2002. Molecular taxonomy and strain analysis in Echinococcus, p. 205-219. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 151.Perdomo, R., C. Alvarez, J. Monti, C. Ferreira, A. Chiesa, A. Carbò, R. Alvez, R. Grauert, D. Stern, C. Carmona, and L. Yarzabal. 1997. Principles of the surgical approach in human liver cystic echinococcosis. Acta Trop. 64:109-122. [DOI] [PubMed] [Google Scholar]
- 152.Pétavy, A. F., F. Tenora, S. Deblock, and V. Sergent. 2000. Echinococcus multilocularis in domestic cats in France: a potential risk factor for alveolar hydatid disease contamination in humans. Vet. Parasitol. 87:151-156. [DOI] [PubMed] [Google Scholar]
- 153.Polydorou, K. 1992. Echinococcosis/hydatidosis. The problem and its control. Case study, Cyprus. K. Polydorou, Nicosia, Cyprus.
- 154.Raoul, F., P. Deplazes, N. Nonaka, R. Piarroux, D. A. Vuitton, and P. Giraudoux. 2001. Assessment of the epidemiological status of Echinococcus multilocularis in foxes in France using ELISA coprotests on fox feces collected in the field. Int. J. Parasitol. 31:1579-1588. [DOI] [PubMed] [Google Scholar]
- 155.Rausch, R. L. 1995. Life cycle patterns and geographic distribution of Echinococcus species, p. 88-134. In R. C. A. Thompson and A. J. Lymbery (ed.), Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom.
- 156.Rausch, R. L., and J. J. Bernstein. 1972. Echinococcus vogeli sp. n. (Cestoda: Taeniidae) from the bush dog, Speothos venaticus (Lund). Z. Tropenmed. Parasitol. 23:25-34. [PubMed] [Google Scholar]
- 157.Rausch, R. L., and A. D'Alessandro. 1999. Histogenesis in the metacestode of Echinococcus vogeli and mechanism of pathogenesis in polycystic hydatid disease. J. Parasitol. 85:410-418. [PubMed] [Google Scholar]
- 158.Rausch, R. L., and A. D'Alessandro. 2002. The epidemiology of echinococcosis caused by Echinococcus oligarthrus and E. vogeli in the Neotropics, p. 107-113. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 159.Rausch, R. L., A. D'Alessandro, and V. R. Rausch. 1981. Characteristics of the larval Echinococcus vogeli (Rausch & Bernstein 1972) in the natural intermediate host, the paca, Cuniculus paca L. (Rodentia: Dasyproctidae). Am. J. Trop. Med. Hyg. 30:1043-1052. [DOI] [PubMed] [Google Scholar]
- 160.Rausch, R. L., and F. H. Fay. 2002. Epidemiology of alveolar echinococcosis, with reference to St. Lawrence Island, Bering Sea, p. 309-325. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 161.Rausch, R. L., V. R. Rausch, and A. D'Alessandro. 1978. Discrimination of the larval stages of Echinococcus oligarthrus (Diesing, 1863) and E. vogeli Rausch and Bernstein, 1972 (Cestoda: Taeniidae). Am. J. Trop. Med. Hyg. 27:1195-1202. [DOI] [PubMed] [Google Scholar]
- 162.Rausch, R. L., J. F. Wilson, P. M. Schantz, and B. J. McMahon. 1987. Spontaneous death of Echinococcus multilocularis: cases diagnosed serologically (by Em2 ELISA) and clinical significance. Am. J. Trop. Med. Hyg. 36:576-585. [DOI] [PubMed] [Google Scholar]
- 163.Reuter, S., B. Jensen, K. Buttenschoen, W. Kratzer, and P. Kern. 2000. Benzimidazoles in the treatment of alveolar echinococcosis: a comparative study and review of the literature. J. Antimicrob. Chemother. 46:451-456. [DOI] [PubMed] [Google Scholar]
- 164.Reuter, S., K. Nüssle, O. Kolokythas, U. Haug, A. Rieber, P. Kern, and W. Kratzer. 2001. Alveolar liver echinococcosis: a comparative study of three imaging techniques. Infection 29:119-125. [DOI] [PubMed] [Google Scholar]
- 165.Richards, K. S., E. M. Riley, D. H. Taylor, and D. L. Morris. 1988. Studies on the effect of short term, high dose praziquantel treatment against protoscoleces of ovine and equine Echinococcus granulosus within the cyst and in vitro. Tropenmed. Parasitol. 39:269-272. [PubMed] [Google Scholar]
- 166.Roberts, M. G., J. R. Lawson, and M. A. Gemmell. 1986. Population dynamics in echinococcosis and cysticercosis: mathematical model of the life-cycle of Echinococcus granulosus. Parasitology 92:621-641. [DOI] [PubMed] [Google Scholar]
- 167.Roberts, M. G., J. R. Lawson, and M. A. Gemmell. 1987. Population dynamics in echinococcosis and cysticercosis: mathematical model of the life-cycles of Taenia hydatigena and T. ovis. Parasitology 94:181-197. [DOI] [PubMed] [Google Scholar]
- 168.Rogan, M. T., and P. S. Craig. 2002. Immunological approaches for transmission and epidemiological studies in cestode zoonoses—the role of serology in human infection, p. 135-145. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 169.Romig, T. 1990. Beobachtungen zur zystischen Echinokokkose des Menschen im Turkana-Gebiet, Kenia. Doctoral thesis, Universität Hohenheim, Hohenheim, Germany.
- 170.Romig, T. 2002. Spread of Echinococcus multilocularis in Europe? p. 65-80. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 171.Romig, T., B. Bilger, B., M. Merli, A. Dinkel, R. Lucius, and U. Mackenstedt. 1999. Bekämpfung von Echinococcus multilocularis in einem Hochendemiegebiet Süddeutschlands. In Neuere Methoden und Ergebnisse zur Epidemiologie von Parasitosen. Tagung der Fachgruppe Parasitologie und parasitäre Krankheiten, p. 172-183. Deutsche Veterinärmedizinische Gesellschaft, Giessen, Germany.
- 172.Romig, T., E. Zeyhle, C. N. L. Macpherson, P. H. Rees, and J. B. O. Were. 1986. Cyst growth and spontaneous cure in hydatid disease. Lancet i:861. [DOI] [PubMed] [Google Scholar]
- 173.Romig, T., B. Bilger, A. Dinkel, M. Merli, and U. Mackenstedt. 1999. Echinococcus multilocularis in animal hosts: new data from western Europe. Helminthologia 36:185-191. [Google Scholar]
- 174.Romig, T., W. Kratzer, P. Kimming, M. Frosch, W. Gaus, W. A. Flegel, B. Gottstein, R. Lucius, K. Beckh, and P. Kern. 1999. An epidemiologic survey of human alveolar echinococcosis in southwestern Germany. Am. J. Trop. Med. Hyg. 61:566-573. [DOI] [PubMed] [Google Scholar]
- 175.Rosenzvit, M. C., L. H. Zhang, L. Kamenetzky, S. G. Canova, E. A. Guarnera, and D. P. McManus. 1999. Genetic variation and epidemiology of Echinococcus granulosus in Argentina. Parasitology 118:523-530. [DOI] [PubMed] [Google Scholar]
- 176.Sahni, J. K., M. Jain, Y. Bajaj, V. Kumar, and A. Jain. 2000. Submandibular hydatid cyst caused by Echinococcus oligarthrus. J. Laryngol. Otol. 114:473-476. [DOI] [PubMed] [Google Scholar]
- 177.Salinas-Lopez, N., F. Jimenez-Guzman, and A. Cruz-Reyes. 1996. Presence of Echinococcus oligarthrus (Diesing, 1863) Luhe, 1910 in Lynx rufus texensis Allen, 1895, from San Fernando, Tamaulipas State, in north-east Mexico. Int. J. Parasitol. 26:793-796. [DOI] [PubMed] [Google Scholar]
- 178.Sato, N., S. Aoki, M. Matsushita, and J. Uchino. 1993. Clinical features, p. 63-68. In J. Uchino and N. Sato (ed.), Alveolar echinococcosis of the liver. Hokkaido University School of Medicine, Sapporo, Japan.
- 179.Schantz, P. M. 1997. Sources and uses of surveillance data for cystic echinococcosis, p. 72-84. In F. L. Andersen, H. Ouhelli, and M. Kachani (ed.), Compendium on cystic echinococcosis in Africa and Middle Eastern Countries with special reference to Morocco. Brigham Young University Print Services, Provo, Utah.
- 180.Schantz, P. M., J. Chai, P. S. Craig, J. Eckert, D. J. Jenkins, C. N. L. Macpherson, and A. Thakur. 1995. Epidemiology and control of hydatid disease, p. 233-331. In R. C. A. Thompson and A. J. Lymbery (ed.), Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom.
- 181.Schelling, U., W. Frank, R. Will, T. Roming, and R. Lucius. 1997. Chemotherapy with praziquantel has the potential to reduce the prevalence of Echinococcus multilocularis in wild foxes (Vulpes vulpes). Ann. Trop. Med. Parasitol. 91:179-186. [DOI] [PubMed] [Google Scholar]
- 182.Shaikenov, B. S., and P. R. Torgerson. 2002. Distribution of Echinococcus multilocularis in Kazakhstan, p. 299-307. In P. Craig and Z. Pawlowski. (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 183.Shaikenov, B. S., T. F. Vaganov, and P. R. Torgerson. 1999. Cystic echinococcosis in Kazakhstan: an ermerging disease since independence from the Soviet Union. Parasitol. Today 15:172-174. [DOI] [PubMed] [Google Scholar]
- 184.Shambesh, M. K. A. 1997. Human cystic echinococcosis in North Africa (excluding Morocco), p. 223-244. In F. L. Andersen, H. Ouhelli, and M. Kachani (ed.), Compendium on cystic echinococcosis in Africa and Middle Eastern Countries with special reference to Morocco. Brigham Young University Print Services, Provo, Utah.
- 185.Shambesh, M. A., P. S. Craig, C. N. L. Macpherson, M. T. Rogan, A. M. Gusbi, and E. F. Echtuish. 1999. An extensive ultrasound and serologic study to investigate the prevalence of human cystic echinococcosis in Northern Libya. Am. J. Trop. Med. Hyg. 60:462-468. [DOI] [PubMed] [Google Scholar]
- 186.Siles-Lucas, M. M., and B. Gottstein. 2001. Molecular tools for the diagnosis of cystic and alveolar echinococcosis. Trop. Med. Int. Health 6:463-475. [DOI] [PubMed] [Google Scholar]
- 187.Stehr-Green, J. K., P. A. Stehr-Green, P. M. Schantz, J. F. Wilson, and A. Lanier. 1988. Risk factors for infection with Echinococcus multilocularis in Alaska. Am. J. Trop. Med. Hyg. 38:380-385. [DOI] [PubMed] [Google Scholar]
- 188.Stieger, C., D. Hegglin, G. Schwarzenbach, A. Mathis, and P. Deplazes. 2002. Spatial and temporal aspects of urban transmission of Echinococcus multilocularis. Parasitology 124:631-640. [DOI] [PubMed] [Google Scholar]
- 189.Suzuki, K., J. Uchino, N. Sato, and H. Takahashi. 1996. Development and efficacy of mass screening of alveolar echinococcosis, p. 213-217. In J. Uchino, J. and N. Sato (ed), Alveolar echinococcosis. Strategy for eradication of alveolar echinococcosis of the liver. Fuji Shoin, Sapporo, Japan.
- 190.Tackmann, K., U. Löschner, H. Mix, C. Staubach, H.-H. Thulke, and F. J. Conraths. 1998. Spatial distribution patterns of Echinococcus multilocularis (Leuckart 1863) (Cestoda: Cyclophyllidea: Taeniidae) among red foxes in an endemic focus in Brandenburg, Germany. Epidemiol. Infect. 120:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tackmann, K., U. Löschner, H. Mix, C. Staubach, H. H. Thulke, M. Ziller, and F. J. Conraths. 2001. A field study to control Echinococcus multilocularis infections of the red fox (Vulpes vulpes) in an endemic focus. Epidemiol. Infect. 127:577-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Teggi, A., and B. DiVico. 2002. The natural history of human cystic echinococcosis (CE) by imaging methods, p. 125-134. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 193.Thompson, R. C. A. 1995. Biology and systematics of Echinococcus, p. 1-50. In R. C. A. Thompson and A. J. Lymbery (ed.), The biology of Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom.
- 194.Thompson, R. C. A., and D. P. McManus. 2001. Aetiology: parasites and life-cyles, p. 1-19. In J. Eckert, M. A. Gemmell, F.-X. Meslin, and Z. S. Pawlowski (ed.), WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organisation for Animal Health, Paris, France.
- 195.Thompson, R. C. A., and D. P. McManus. 2002. Towards a taxonomic revision of the genus Echinococcus. Trends Parasitol. 18:452-457. [DOI] [PubMed] [Google Scholar]
- 196.Todorov, T., and V. Boeva. 1999. Human echinococcosis in Bulgaria: a comparative epidemiological analysis. Bull. W. H. O. 77:110-118. [PMC free article] [PubMed] [Google Scholar]
- 197.Todorov, T., P. Georgiev, S. Handjiev, and K. Vutova. 1997. Potentials of benzimidazole compounds in treatment of human echinococcosis. Arch. Int. Hidatidosis 32:164-167. [Google Scholar]
- 198.Todorov, T., G. Mechkov, P. Georgiev, S. Handjiev, K. Vutova, D. Petkov, A. Ivanov, and V. Boeva. 1998. Chemotherapy of human cystic hydatid disease: indications, effectiveness, prognosis. Mediterran. J. Infect. Parasit. Dis. 13:89-94. [Google Scholar]
- 199.Torgerson, P. 2002. Transmission dynamics of taeniid parasites in animal hosts, p. 221-235. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 200.Togerson, P. 2003. The use of mathematical models to simulate control options for echinococcosis. Acta Trop. 85:211-221. [DOI] [PubMed] [Google Scholar]
- 201.Torgerson, P. R., P. M. Dowling, and M. N. Abo-Shehada. 2001. Estimating the economic effects of cystic echinococcosis. 3. Jordan, a developing country with lower-middle income. Ann. Trop. Med. Hyg. 95:595-603. [DOI] [PubMed] [Google Scholar]
- 202.Torgerson, P. R., J. Pilkington, F. M. D. Gulland, and M. A. Gemmell. 1995. Further evidence for the long distance dispersal of taeniid eggs. Int. J. Parasitol. 25:265-267. [DOI] [PubMed] [Google Scholar]
- 203.Torgerson, P., B. Shaikenov, and O. Kuttybaev. 2002. Cystic echinococcosis in Central Asia: new epidemic in Kazakhstan and Kyrgystan, p. 99-105. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, and emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 204.Torgerson, P. R., R. R. Karaeva, N. Corkeri, T. A. Abdyjaparov, and O. T. Kuttubaev. 2003. Human cystic echinococcosis in Kyrgystan: an epidemiological study. Acta Trop. 85:51-61. [DOI] [PubMed] [Google Scholar]
- 205.Torgerson, P. R., B. Shaikenov, K. K. Baitursinov, and A. M. Abdybekova. 2002. The emerging epidemic of echinococcosis in Kazakhstan. Trans. R. Soc. Trop. Med. Hyg. 96:124-128. [DOI] [PubMed] [Google Scholar]
- 206.Torgerson, P. R., D. H. Williams, and M. N. Abo-Shehada. 1998. Modelling the prevalence of Echinococcus and Taenia species in small ruminants of different ages in northern Jordan. Vet. Parasitol. 79:35-51. [DOI] [PubMed] [Google Scholar]
- 207.Tsukada, H., Morishima, Y., Nonaka, N., Oku, Y. and Kamiya, M. 2000. Preliminary study of the role of red foxes in Echinococcus multilocularis transmission in the urban area of Sapporo, Japan. Parasitology 120:423-428. [DOI] [PubMed] [Google Scholar]
- 208.Urrea-Paris, M. A., M. J. Moreno, N. Casado, and F. Rodriguez-Caabeiro. 1999. Echinococcus granulosus: praziquantel treatment against the metacestode stage. Parasitol. Res. 85:999-1006. [DOI] [PubMed] [Google Scholar]
- 209.Ustunsoz, B., O. Akhan, M. A. Kamiloglu, I. Somuncu, M. S. Ugurel, and S. Cetiner. 1999. Percutaneous treatment of hydatid cysts of the liver: long-term results. Am. J. Roentgenol. 172:91-96. [DOI] [PubMed] [Google Scholar]
- 210.van Herwerden, L., R. B. Gasser, and D. Blair. 2000. ITS-1 ribosomal DNA sequence variants are maintained in different species and strains of Echinococcus. Int. J. Parasitol. 30:157-169. [DOI] [PubMed] [Google Scholar]
- 211.Veit, P., B. Bilger, V. Schad, J. Schäfer, W. Frank, and R. Lucius. 1995. Influence of environmental factors on the infectivity of Echinococcus multilocularis eggs. Parasitology 110:79-86. [DOI] [PubMed] [Google Scholar]
- 212.Viel, J.-F., P. Giraudoux, V. Abrial, and S. Bresson-Hadni. 1999. Water vole (Arvicola terrestris scherman) density as risk factor for human alveolar echinococcosis. Am. J. Trop. Med. Hyg. 61:559-565. [DOI] [PubMed] [Google Scholar]
- 213.von Sinner, W. N., and D. Lewall. 2001. Hydatid disease (echinococcosis), p. 205-353. In P. E. S. Palmer and M. M. Reeder (ed.), The imaging of tropical diseases, vol. 1. Springer-Verlag KG, Berlin, Germany.
- 214.Vuitton, D. A. 2003. The ambiguous role of immunity in echinicoccosis: protection of the host or the parasite? Acta Trop. 85:119-132. [DOI] [PubMed] [Google Scholar]
- 215.Vuitton, D. A., V. Godot, S. Zhang, S. Harraga, A. Penfornis, I. Beurton, B. Gottstein, P. Kern, and the Euechinoreg Network. 2002. Host immunogenetics and role in epidemiolgy of larval cestodes, p. 183-194. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 216.Wachira, T. M., J. Bowles, E. Zeyhle, and D. P. McManus. 1993. Molecular examination of the sympatry and distribution of sheep and camel strains of Echinococcus granulosus in Kenya. Am. J. Trop. Med. Hyg. 48:473-479. [DOI] [PubMed] [Google Scholar]
- 217.Wang, Q., J. Qiu, P. Schantz, J. He, A. Ito, and F. Liu. 2001. Investigation of risk factors for development of human hydatidosis among households raising livestock in Tibetan areas of western Sichuan province. Chin. J. Parasitol. Parasit. Dis. 19:93-96. [PubMed] [Google Scholar]
- 218.Wellinghausen, N., P. Gebert, and P. Kern. 1999. Interleukin (IL)-4, IL-10 and IL-12 profile in serum of patients with alveolar echinococcosis. Acta Trop. 73:165-174. [DOI] [PubMed] [Google Scholar]
- 219.Wen, H., J. J. Chai, J. C. Wang, S. H. Wang, X. Y. Wang, X. H. Feng, and H. X. Zhou. 2002. Hydatid control within a continental system in P.R. China, p. 355-366. In P. Craig and Z. Pawlowski (ed.), Cestode zoononoses: echinococcosis and cysticercosis, an emergent and global problem. IOS Press, Amsterdam, The Netherlands.
- 220.Wen, H., and W.-G. Yang. 1997. Public health importance of cystic echinococcosis in China. Acta Trop. 67:133-145. [DOI] [PubMed] [Google Scholar]
- 221.World Health Organization. 1996. Guidelines for treatment of cystic and alveolar echinococcosis. WHO Informal Working Group on Echinococcosis. Bull. W. H. O. 74:231-242. [PMC free article] [PubMed] [Google Scholar]
- 222.World Health Organization Informal Working Group of Echinococcosis. 2001. Puncture, aspiration, injection, re-aspiration. An option for the treatment of cystic echinococcosis, p. 1-40. Document WHO/CDS/CSR/APH/2001.6. World Health Organization, Geneva, Switzerland.
- 223.World Health Organization Office International des Epizooties. 2001. WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organization for Animal Health, Paris, France.
- 224.Zhang, L. H., D. D. Joshi, and D. P. McManus. 2000. Three genotypes of Echinococcus granulosus identified in Nepal using mitochondrial DNA markers. Trans. R. Soc Trop. Med. Hyg. 94:258-260. [DOI] [PubMed] [Google Scholar]