Abstract
Chronic neuroinflammation is associated with many neurodegenerative and neurocognitive disorders, yet few animal models exist to study the behavioral effects of prolonged neuroinflammation. Therefore, we recently developed a transgenic mouse model harboring an interleukin-1β excisional activation transgene (IL-1βXAT). These mice display localized IL-1β overexpression and resultant neuroinflammation for up to 1 year following transgene induction. Initial behavioral studies demonstrated long-term memory deficits after 2 weeks of hippocampal IL-1β overexpression. In the present studies, we extend these behavioral studies both in scope and timing. We find long-term contextual but not auditory fear memory impairments following 3 months of IL-1β overexpression. On a battery of other behavioral tests, IL-1β overexpression in IL-1βXAT mice increased locomotor activity, especially in female mice, and had slight anxiolytic effects. No differences were found in operant conditioning or in basal or stress-induced CORT levels, despite profound hippocampal glial activation. Interestingly, the volume of discrete hippocampal cell layers was reduced after 6 but not 3 months of IL-1β overexpression. Therefore, this animal model provides a novel tool for examining the effects of chronic inflammation on discrete brain regions.
Introduction
Chronic neuroinflammation is a common component of many neurodegenerative and neurocognitive disorders. Alzheimer’s disease, Parkinson’s disease, HIV-associated dementia, traumatic brain injury, stroke, and even depression have all been associated with increased neuroinflammation, including elevated levels of interleukin-1β (IL-1β) as well as other cytokines and glial cell activation (Griffin et al., 1989; Griffin et al., 1994; Sairanen et al., 1998; Kostulas et al., 1999; Hutchinson et al., 2007; Piletz et al., 2009; Reale et al., 2009; Dowlati et al., 2010). These diseases often have significant and diverse behavioral manifestations, including memory impairments, motor dysfunctions, and mood alterations (Tham et al., 2002; Sachdev et al., 2004; Draper and Ponsford, 2008). Studying the mechanistic role of inflammation in these disease traits has proven difficult due to a lack of animal models with localized, chronic neuroinflammation.
To address the effects of chronic neuroinflammation, we utilized a recently developed mouse model harboring an IL-1β excisional activation transgene (IL-1βXAT). Following intracranial injection of virus expressing Cre, these mice overexpress IL-1β for many months, resulting in prolonged and significant neuroinflammation. Studies to date have characterized the molecular and cellular changes in these mice, demonstrating localized and chronic inflammation lasting up to a year after transgene induction (Shaftel et al., 2007b; Shaftel et al., 2007a). These changes include increases in cytokines, chemokines, and prostaglandins, peripheral immune cell infiltration, and blood brain barrier disruption (Shaftel et al., 2007b; Shaftel et al., 2007a; Matousek et al., 2010). No overt neurodegeneration was detectable at 2 weeks or 2 months after transgene induction (Shaftel et al., 2007b).
While acute elevations in hippocampal IL-1β and neuroinflammation have been shown to impair memory, we wanted to study the effects of a more chronic inflammatory state (Barrientos et al., 2002; Hein et al., 2007). Initial behavioral studies in the IL-1βXAT mice demonstrated memory deficits in contextual fear conditioning and the Morris water maze following 2 weeks of IL-1β overexpression (Moore et al., 2009; Hein et al., 2010). These deficits were specific to long-term and hippocampal-dependent memory, whereas short-term and auditory memory were not affected.
In the studies presented here, we sought to extend these findings by testing later timepoints (up to 6 months following transgene induction), indicative of a truly chronic inflammatory state, and additional indices of behavioral function, including locomotor activity, operant conditioning, and anxiety. We also examined alterations in the hypothalamic-pituitary-adrenal (HPA) axis in these animals, as well as neuroinflammation and hippocampal volume at these late timepoints.
Materials
hIL-1βXAT construct
Creation and genotyping of IL-1βXAT mice on a C57BL/6 background has been described previously (Shaftel et al., 2007a). Briefly, a construct with a murine glial fibrillary acidic protein (GFAP) promoter, loxP flanked transcriptional stop, and downstream signal sequence for the human IL-1RA was fused to the cDNA of human mature IL-1β to allow extracellular release of IL-1β.
Feline immunodeficiency virus (FIV)
The construction and packaging of FIV-Cre has been described previously (Lai et al., 2006). The FIV-Cre virus encodes a modified Cre recombinase protein with a nuclear localization sequence, and V5 epitope tag under the control of a cytomegalovirus promoter. FIV-Cre mediated excision of the transcriptional stop activates the IL-1βXAT transgene. FIV-Cre and FIV-green fluorescent protein (GFP) (System Biosciences, Mountain View, CA) used in these studies had final titers of ~1×107 infectious viral particles per milliliter.
Animals
Experiment 1: 3 months
Male and female IL-1βXAT B/b transgenic (NFIV-GFP = 12 and NFIV-Cre = 11) and wild-type (WT, N = 12) littermates were housed in temperature (23 ± 3 °C) and light (12:12 light:dark) controlled rooms with free access to chow and water. All animal procedures were reviewed and approved by the University Committee on Animal Resources of the University of Rochester Medical Center for compliance with federal regulations prior to the initiation of the study. FIV-Cre injected WT and FIV-GFP injected IL-1βXAT mice served as controls for both genotype and viral injection.
Experiment 2: 6 months
Male and female IL-1βXAT B/b transgenic (Nfemale = 8 and Nmale = 10) and WT (Nfemale = 12 and Nmale = 14) littermates were used and maintained as described above. All mice received FIV-Cre injections and were repeatedly tested before and after these injections.
Microinjections
Intrahippocampal microinjections were described previously (Shaftel et al., 2007a). At 12–16 weeks of age in experiment 1 and at 16 weeks in experiment 2, mice received bilateral, hippocampal FIV-Cre or FIV-GFP injections. Under 1.75% isoflurane in 30% oxygen and 70% nitrogen gas, mice were placed into a Kopf stereotaxic apparatus in a biosafety level 2 approved facility. A 0.5 mm burr hole was drilled at AP: −1.8 mm and ML: ±1.8 mm relative to bregma and a 33 gauge needle attached to a 10 μL syringe was lowered 1.8 mm over 2 min. A Micro-1 microsyringe pump controller (World Precision Instruments, Sarasota, FL, USA) injected 1.5 μl of virus at a constant rate over 10 min and 5 min was allowed for diffusion, resulting in delivery of approximately 1.5 × 104 infectious viral particles to the mouse hippocampus. The needle was raised over 2 min and burr hole sealed with bone wax. The procedure was then repeated on the opposite side to deliver virus bilaterally. Following both injections, the scalp incision was closed with tissue adhesive (Vetbond, St. Paul, MN, USA).
Behavioral procedures
Contextual fear conditioning
In experiment 1, mice underwent contextual and auditory fear conditioning to assess hippocampal-dependent and -independent memory processes 3 months after FIV injections. For 3 days before fear conditioning, mice were transported from the colony room to the testing room, handled for 2 min each, and returned to the colony room to acclimate to experimenter manipulation. On conditioning day, mice were allowed to explore the conditioning context, which consisted of a Plexiglas chamber and metal floor grid (model H10-11M; Coulbourn Instruments, Whitehall, PA, USA). After 3 min, 15 s of white noise (80 dB) was presented coterminating with a 2 s, 0.75 mA foot shock. This noise-shock pairing was repeated twice for a total of 3 shocks with an interval of 30 s between shocks. Twenty four hours later, mice were re-exposed to the conditioning chamber for 5 min each to test long-term hippocampal-dependent context memory. Four hours after the contextual test, mice were placed in a novel context consisting of a 15 cm open-topped plastic cylinder with bedding on the floor for 3 min followed by re-exposure to the white noise for 3 min to test hippocampal-independent auditory memory. All data were video recorded using FreezeFrame Video-Based Conditioned Fear System and analyzed by Actimetrics Software (Coulbourn Instruments). In a pilot study, automated scoring in this program showed greater than 95% concordance with human scoring.
Force-plate actometer
In experiment 2, force-plate actometers were used to measure weight and spontaneous locomotor behavior in WT and IL-1βXAT mice. Mice were tested in 25-min sessions once a week for 3 weeks before FIV-Cre injections and then at 2, 3, 4, 5, 21, and 26 weeks after injections. Testing occurred under red light. Force-plate actometer data were collected by a computer in real time.
Operant conditioning
In experiment 2, custom-made mouse operant chambers were used to test behavioral function in mice 2–3 months after FIV injections. Mouse chambers (130 × 95 × 130mm) were equipped with a photobeam over the access hole (25mm) to the dipper to detect consumatory-related responses. Photobeams behind two “response” holes (15 mm) in the opposite wall recorded pokes by a mouse’s snout, which served as the defined operant responses. Each response hole had a light-emitting diode (LED) recessed into back of the hole. The LEDs were turned on during the session and turned off when the photobeam was broken (i.e. feedback stimulus). The chamber was equipped with a 28 volt incandescent “house” light that was used to signal the start and end of a session. All outputs and inputs were recorded in real time by a computer.
Prior to training, mice were exposed to evaporated milk in their homecages and their food was removed from the homecage 24 hours prior to testing. Mice were fed 1 gram after the first session to maintain their bodyweights and returned to free feeding after the second test session. Operant training consisted of 2 sessions (90 min each) across 2 consecutive days in which rewards were presented on a variable 60 second schedule concomitantly with a fixed ratio 1 schedule for snout entries into the hole on the right side of the chamber.
Elevated zero maze
In experiment 2, an elevated zero maze was used to measure anxiety. The maze consisted of 4 quadrants: 2 open and 2 closed and all testing occurred under red light. Mice were tested in 5-min sessions 4 weeks before FIV-Cre injection to establish a baseline and then at 1 and 6 weeks after injections. Activity in the elevated zero maze was quantified using an automated behavioral analysis video tracking system (TopScan from CleverSys, Inc.; Reston, VA).
Acoustic Stress
In experiment 2, baseline corticosterone (CORT) levels were obtained from a cheek pouch blood sample at 9 weeks after viral injection. Two weeks later (11 weeks after FIV-Cre injections), additional samples were gathered 15 min following an acoustic stressor consisting of a 15 min exposure to 100 dB of white noise.
Tissue Processing
At the end of behavioral testing (3 months for experiment 1 and 6 months for experiment 2), mice were anesthetized with ketamine and xylazine (i.p., 60–90 and 4–8 mg/kg, respectively) and perfused with 0.15 M phosphate buffer (PB) containing 2 IU/ml heparin and 0.5% w/v sodium nitrite. In experiment 1, brains were removed and bisected along the midline. In one hemisphere, the hippocampus was microdissected, frozen in cold isopentane, and stored at −80°C. The other hemisphere was immerse fixed in 4% paraformaldehyde in 0.15M PB (pH 7.2) for 24 h, sunk in 30% sucrose overnight, frozen in cold isopentane, and stored at −80°C. In experiment 2, the PB perfusion was followed by perfusion with 4% paraformaldehyde in 0.15 M PB (pH 7.2). Brains were removed, postfixed for 2 h, sunk in 30% sucrose overnight, frozen in cold isopentane, and stored at −80°C.
Immunohistochemistry
Fixed brains were sectioned at 30 μm on a sliding microtome and free-floating sections stored in cryoprotectant until assayed. Sections were washed in 0.15 M PB, reacted in 3% H2O2, blocked with 10% normal serum (Vector), and incubated in rat anti-mouse MHCII (major histocompatibility complex II; Pharmingen, 1:6000) or rabbit anti-bovine GFAP (Dako 1:6000) for 48 h. A biotinylated rabbit anti-rat mouse absorbed or goat anti-rabbit IgG (Vector, 1:2000) secondary antibody was used followed by incubation in ABC Elite (Vector) and Ni-DAB reaction. Sections were mounted, cleared, and coverslipped. Light microscopic images were acquired on an Axioplan IIi (Zeiss) microscope equipped with a Spot RT camera and software (version 4.5.9.8; Diagnostic Instruments).
Real-time RT-PCR
RNA extraction and cDNA synthesis
Total RNA was extracted in hippocampi from experiment 1 using the TRIzol (Invitrogen, Carlsbad, CA, USA) method. Hippocampi were homogenized in 1 mL TRIzol using an Omni 2000 tissue homogenizer. After incubation at room temperature for 5 min, 200 μl chloroform was added, tubes were vortexed, and samples were incubated for 3 min. Samples were then centrifuged (11,900 × g) for 15 min at 4°C to achieve phase separation of nucleic acid. Isopropyl alcohol (500 μl) were added to the aqueous phase to precipitate nucleic acid. Samples were vortexed briefly and incubated for 10 min followed by centrifugation (11,900 × g) for 15 min at 4°C. Nucleic acid precipitate was washed in 75% ethanol (1 ml) and centrifuged (7500 × g) for 5 min at 4°C. Ethanol was decanted and samples air dried. Nucleic acid pellet was resuspended in 20 μl RNase-free water. UV spectrophotometric analysis of nucleic acid was performed at 260 nm to determine concentration. Samples were DNase-treated (DNA-free kit, Ambion) to remove contaminating DNA. cDNA synthesis was completed using the SuperScript III First Strand Synthesis System (Invitrogen).
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR was performed using custom-designed primers (Invitrogen, see table 1) and FAM 490 probes (Biosearch Technologies) on an iCycler (Bio-Rad). For custom-designed sequences, optimal concentrations were determined empirically. Standard curves were generated from serial dilutions of expected products over 5 orders of magnitude. PCR reactions were carried out in a final volume of 20 μl using iQ Supermix (Bio-Rad) and 5 nM FITC dye. PCR reaction conditions were generally as follows: initial denaturation at 95°C for 3 min, followed by 50 cycles of amplification by denaturing at 95°C for 30 s and annealing/extension at 60°C for 30 s. Reaction efficiency (E) was determined from standard curves. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was assessed as a house-keeping gene and used to normalize data. Threshold cycle (Ct) values were transformed using (1 + E)Ct to determine the relative differences in mRNA expression.
Table 1.
RT-PCR primer and probe sequences used for amplification of cDNA.
| Gene | Primer Sequence Upper 5′→3′ | Primer Sequence Lower 5′→3′ | Probe Sequence 5′ → 3′ |
|---|---|---|---|
| COX-1 | gtgccagaaccagggtgtct | gtagcccgtgcgagtacaatc | cgctttggcctcgacactaccagtg |
| COX-2 | tgacccccaaggctcaaata | cccaggtcctcgcttatgatc | ctttgcccgcacttcacccatcagtt |
| GAPDH | cccaatgtgtccgtcgtg | cctgcttcaccaccttcttg | tgtcatcatacttggcaggtttctccagg |
| GFAP | ctggaggtggagagggacaa | ggttggtttcatcttggagctt | tttgcacaggacctcggcaccc |
| mIL-1β | tcgctcagggtcacaagaaa | atcagaggcaaggaggaaacac | catggcacattctgttcaaagagagcctg |
| MHCII | agtcagtcgcagacggtgttt | gataagacagcttgtggaaggaatagt | tgagaccagcttcttcgtcaaccgtg |
Volumetric measurements
Every 6th section through the hippocampus was mounted, cresyl violet stained, and coverslipped. Light microscopic images of the hippocampus were acquired on an Axioplan IIi (Zeiss) microscope equipped with a Spot RT camera and software (version 4.5.9.8; Diagnostic Instruments). Dentate gyrus granule, CA1 pyramidal, and CA3 pyramidal cell layers were outlined separately in each hippocampal section and area was calculated using Image J (NIH). The Cavalieri method was used to calculate a total volume for each animal: V = T × K × ΣA, where V = volume; T = thickness (30 μm); K = every kth section (6); A = area (Gundersen and Jensen, 1987). Because whole brains were sectioned in mice 6 months after viral injection (instead of the half brains that were used at the 3 month timepoint), measurements were taken from one, randomly chosen, hemisphere only. Two of the 7 IL-1βXAT brains used for quantification in experiment 2 showed some disorganization near one injection site. For these 2 mice, volumetric measurements were acquired from the other hemisphere.
CORT analysis
To measure CORT levels, we used double antibody radioimmunoassays (125I, MP Biomedical; Solon, OH) according to the manufacturer’s specifications.
Statistical analyses
Most data were analyzed in Prism (GraphPad Software) using 1-way, 2-way, and 2-way repeated measures analyses of variance (ANOVA). Operant conditioning and force-plate actometer data were analyzed in Systat 10 using repeated measures ANOVA. Bonferroni post-hoc tests were used to further analyze significant ANOVAs. Analyses were considered significant when p < 0.05.
Results
Behavioral Results
Experiment 1: 3 months
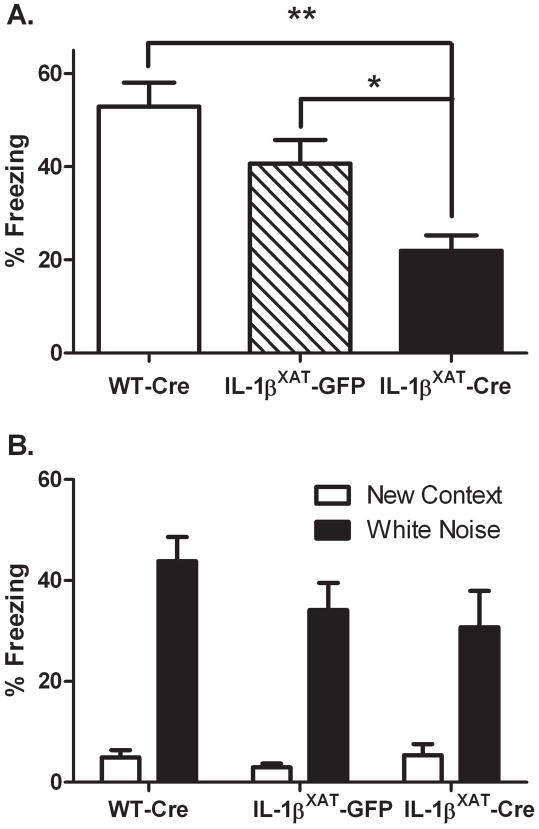
In experiment 1, we examined the effects of chronic hippocampal inflammation on hippocampal-dependent and –independent memory function. Adult WT and IL-1βXAT mice received bilateral intra-hippocampal injections of FIV-Cre or FIV-GFP at 12–16 weeks of age resulting in three experimental groups: WT-Cre, IL-1βXAT-GFP, and IL-1βXAT-Cre. Three months following viral injection, mice underwent fear conditioning. IL-1βXAT-Cre injected mice displayed significantly less contextual freezing than either WT-Cre or IL-1βXAT-GFP injected mice, indicating a hippocampal-dependent memory deficit (F (2,31) = 10.27, p < 0.0005; figure 1). When placed in a novel context, all mice displayed low levels of freezing, which were increased by the presentation of the conditioned auditory stimulus (F (2,31) = 55.01, p < 0.0001). IL-1βXAT-Cre injected mice, however, did not differ in their freezing to the auditory stimulus, indicating normal hippocampal-independent memory function.
Figure 1.
Three months of hippocampal IL-1β overexpression impairs contextual but not auditory fear memory. IL-1βXAT-Cre injected mice show reduced freezing to a conditioned context compared to WT-Cre and IL-1βXAT-GFP injected mice (A). All mice display low freezing to a novel context and increased freezing with a conditioned white noise (B). Error bars show SEM. * p < .01, ** p < .0001.
Experiment 2: 6 months
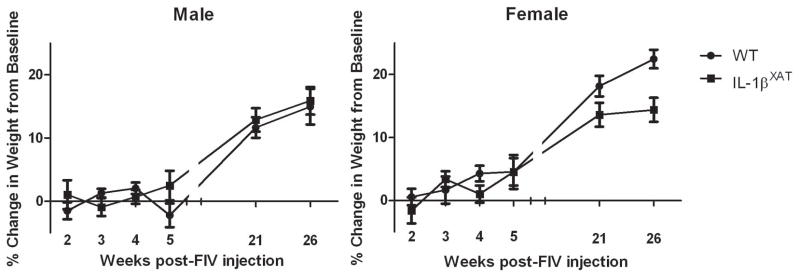
In experiment 2, adult WT and IL-1βXAT mice underwent extensive testing to examine potential effects of chronic hippocampal inflammation on various behaviors. Both male and female IL-1βXAT transgenic mice weighed significantly less than WT littermates at baseline, before viral injection (Fmales (1,52) = 43.89, p < 0.0001, Ffemales (1,79) = 45.97, p < 0.0001, data not shown). Following FIV-Cre injection, male WT and IL-1βXAT mice did not differ in the percent change in body weight (figure 2). However, female IL-1βXAT mice showed a trend for less weight gain compared to WT-Cre injected mice, especially at later ages (F (1,80) = 2.02, p < .07).
Figure 2.
Hippocampal IL-1β overexpression does not significantly affect body weight over 6 months. However, female IL-1βXAT-Cre injected mice showed a trend for reduced weight gain. Error bars show SEM.
Animals underwent repeated testing on the force-plate actometer, including 3 baseline sessions before viral injection and 6 sessions at various timepoints from 2 to 26 weeks after FIV-Cre injection. Distance traveled and time spent in the center 25% portion of the chamber were dependent measures and activity plots were generated, showing total movement during each session. No differences were found between genders or genotypes during the baseline sessions (figure 3A). However, following FIV-Cre injection, male and female IL-1βXAT mice ambulated further total distances during their sessions (Fmales (1,172) = 14.51, p < .0003; Ffemales (1,153) = 48.55, p < .0001). These differences reached significance in post-hoc analyses at 21 weeks in males and were significant at 3 weeks following FIV-Cre injection and at all timepoints thereafter in females (figure 3A).
Figure 3.
Hippocampal IL-1β overexpression increases locomotor activity in the force-plate actometer. Both male and female IL-1βXAT-Cre injected mice travelled a greater total distance than WT-Cre injected mice (A). Female, but not male, IL-1βXAT-Cre injected mice spent a greater percentage of time in the center 25% of the force plate actometer (B). No differences between genotypes were found during baseline testing before FIV-Cre injection. Error bars show SEM. * p < 0.05. Mapping of total activity demonstrates the increased activity seen in male and female IL-1βXAT-Cre injected mice (C).
When examining locomotor patterns, male WT and IL-1βXAT mice did not differ in the proportion of time spent in the center 25% of the activity box (figure 3B). Female IL-1βXAT mice, on the other hand, spent slightly more time in the center 25% compared to their WT littermates (F (1,153) = 5.44, p < .002). However, post-hoc analyses did not find significance at any specific timepoint. Activity plots for the total locomotion in the final session (26 weeks) for representative mice demonstrate the increased activity of the IL-1βXAT mice 6 months following transgene induction (figure 3C).
WT and IL-1βXAT mice performed similarly in an operant conditioning paradigm. Both groups showed increasing numbers of correct responses and rewards over the 2-day testing schedule. No differences between WT and IL-1βXAT mice were found in the rate of learning or in the total number of correct responses or rewards (data not shown).
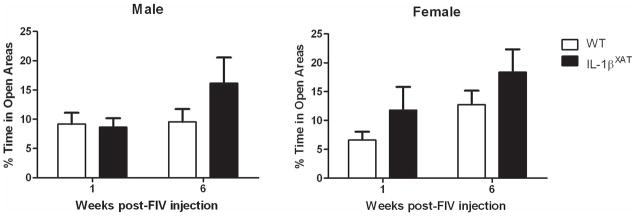
WT and IL-1βXAT mice were tested for percent time spent in the open areas of an elevated zero maze to examine the effect of hippocampal neuroinflammation on anxiety-like behavior. During the baseline session before FIV-Cre injections, no differences between genders or genotypes were observed (data not shown). When animals were tested again at 1 and 6 weeks after FIV-Cre injections, no significant differences were found between genotypes or genders. However when the data were collapsed across genders, IL-1βXAT mice spent significantly more time in the open areas (F (1,39) = 5.64, p < .05, figure 4C).
Figure 4.
Hippocampal IL-1β overexpression did not significantly affect time spent in the open areas of a zero maze. No difference was found between genotypes during baseline trials. Error bars show SEM.
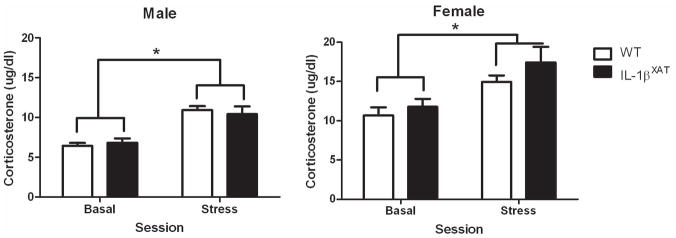
No basal differences in CORT were found between WT and IL-1βXAT mice 2 months after FIV-Cre injection (figure 5). When acoustic stress was given, a significant increase in CORT occurred 15 min post-stress in both male and female mice. However, no differences were observed between WT and IL-1βXAT mice in this response to stress. CORT levels were significantly elevated in females compared to males at both time points (F (1,42) = 31.71, p < .0001).
Figure 5.
Hippocampal IL-1β overexpression did not affect basal or stress-induced CORT levels. Females showed elevated CORT levels compared to males, regardless of genotype. Error bars show SEM.
Hippocampal Changes
Three months of IL-1β overexpression in IL-1βXAT-Cre injected mice induced substantial neuroinflammation. We measured increased hippocampal mRNAs for the murine cytokine IL-1β (40 fold increase over controls, F (2,27) = 13.88, p < 0.0001), astrocytic and microglial activation markers GFAP (3 fold increase over controls, F (2,29) = 12.52, p < 0.0001) and MHCII (80 fold increase over controls, F (2,29) = 19.69, p < 0.0001), and the prostaglandin synthesizing enzyme COX-1 (4 fold increase over controls, F (2,28) = 16.78, p < 0.0001, N = 10–12). COX-2 was the only mRNA examined that did not increase in the hippocampus with transgene induction, similar to previous findings observed 2 and 4 weeks after transgene induction (data not shown) (Hein et al., 2010; Matousek et al., 2010). WT-Cre and IL-1βXAT-GFP injected mice did not differ significantly for any gene examined.
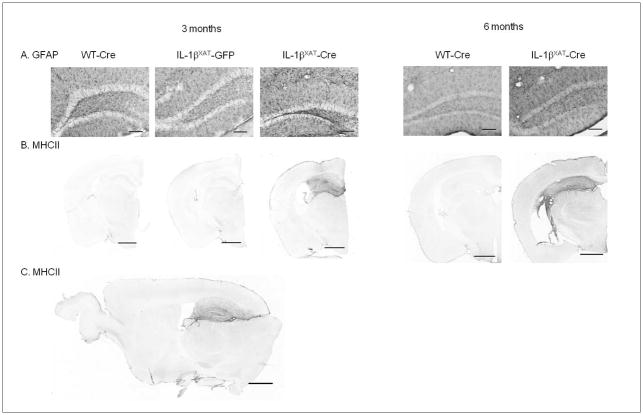
Neuroinflammation as measured by glial activation was also evident 3 and 6 months after transgene induction. IL-1βXAT-Cre injected mice showed elevated levels of MHCII and GFAP protein, which were restricted to the hippocampus (figure 6).
Figure 6.
Three and 6 months after viral injection, IL-1βXAT-Cre injected mice showed astrocytic and microglial activation within the hippocampus. GFAP-IR (A, coronal, 20× magnification of dentate gyrus) and MHCII-IR (B, coronal, 2.5× magnification) is increased with 3 and 6 months of hippocampal IL-1β overexpression. Elevated MHCII-IR is restricted to the hippocampus 3 months after viral injection (C, sagittal). Scale bars are 100 μm (A) and 1 mm (B and C).
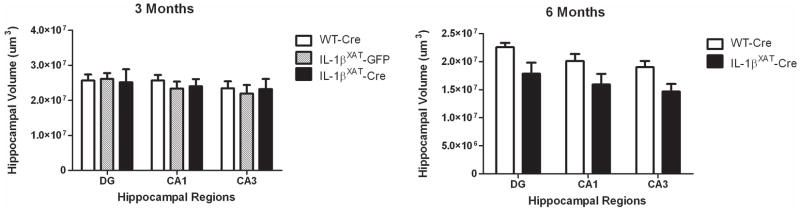
Because neuroinflammation can be detrimental to neurons, we measured the volume of discrete cell layers in the hippocampus 3 and 6 months after viral injection (N = 6–8). No differences were found in the volumes of the dentate gyrus granule, CA1 pyramidal, or CA3 pyramidal cell layers between WT-Cre, IL-1βXAT-GFP, or IL-1βXAT-Cre injected mice 3 months after viral injection. Conversely, a main effect of genotype was found 6 months following viral injection, demonstrating a significant reduction in hippocampal volume after 6 months of IL-1β overexpression (F (1,26) = 6.34, p < 0.03, figure 7). However, with a Bonferroni correction, no post-hoc tests were significant for individual cell layers. The reduction occurred throughout all layers.
Figure 7.
Six, but not 3 months of hippocampal IL-1β overexpression significantly reduced the volume of dentate gyrus granule, CA1 pyramidal, and CA3 pyramidal cell layers of the hippocampus. Error bars show SEM.
Discussion
The experiments presented here extend the scope and timing of previous studies in the recently developed IL-1βXAT mouse model of neuroinflammation. We induced IL-1β overexpression locally within the hippocampus of adult mice and measured a variety of behavioral endpoints up to 6 months following induction. After 3 months of hippocampal IL-1β overexpression, we found long-term contextual fear memory deficits and from 2 weeks to 6 months after transgene induction, locomotor activity was increased. However, body weight, operant conditioning, anxiety, and CORT indices were modestly or not at all affected by chronic hippocampal inflammation. Hippocampal glial activation was confirmed following 3 and 6 months of IL-1β overexpression, and reduced volume of hippocampal cell layers was detected 6 months after transgene induction. These results demonstrate a complex picture, where chronic hippocampal inflammation alters some key behaviors while leaving others unaffected.
Acute elevations in brain IL-1β are well-known to impair hippocampal-dependent memory (Oitzl et al., 1993; Gibertini et al., 1995; Barrientos et al., 2002; Goshen et al., 2007; Hein et al., 2007). Fewer studies have examined the cognitive effects of chronic neuroinflammation. Intracerebroventricular infusion of lipopolysaccharide for 28 or 37 days has been used as one such model of chronic neuroinflammation. With this model, multiple groups have found robust deficits in spatial memory in water and T-mazes (Hauss-Wegrzyniak et al., 1998; Hauss-Wegrzyniak et al., 2000b;Hauss-Wegrzyniak et al., 2000a; Min et al., 2009). In addition, we previously reported that 2-weeks of sustained IL-1β overexpression in the IL-1βXAT mouse model significantly impaired hippocampal-dependent memory in contextual fear conditioning and Morris water maze paradigms (Moore et al., 2009; Hein et al., 2010). Similar to our previous data, we find significant deficits in long-term, hippocampal-dependent but not –independent fear memory with chronic neuroinflammation. Intact auditory fear memory in these mice indicates that reduced contextual freezing observed in IL-1βXAT mice is due specifically to impaired hippocampal-dependent memory and not a general increase in activity or decrease in anxiety. These data indicate that following 3 months of inflammation, the hippocampus is still impaired in its ability to encode contextual information into long-term memories.
Despite chronic hippocampal inflammation, typical sickness behavior, such as reduced food intake and locomotor activity, is not apparent in IL-1βXAT-Cre mice. While a trend exists for decreased weight in the female IL-1βXAT-Cre mice at 6 months, overall, weights remain relatively unaffected by hippocampal inflammation. This result is consistent with literature showing that hypothalamic inflammation regulates sickness-related hypophagia (Kent et al., 1994; Bretdibat et al., 1995; Kent et al., 1996). Hippocampal IL-1β overexpression also increased locomotor activity. While all groups performed equivalently prior to FIV-Cre injection, after injection, both IL-1βXAT males and females were more active than WT mice. This increased locomotion was particularly pronounced in the females. Peripheral infection and whole brain inflammation have been shown to reduce animal activity (Swiergiel and Dunn, 2007) with the exception of one report showing intraperitoneal IL-1β injection increasing activity (Song et al., 2006). However, to our knowledge, this data is the first to examine locomotor behavior following localized hippocampal inflammation.
Similar to the locomotor data, our results on anxiety-like behavior differ from findings in other models of inflammation. Neuroinflammation resulting from intracerebroventricular or intraperitoneal IL-1β or lipopolysaccharide injection have been shown to increase anxiety-like behavior and CORT in rodents (Song et al., 2003; Song et al., 2004; De La Garza et al., 2005; Song et al., 2006; Swiergiel and Dunn, 2007). In contrast, we find that localized hippocampal IL-1β overexpression has little effect on anxiety-like behavior in IL-1βXAT mice. We also find no difference in basal or stress-induced CORT with chronic hippocampal inflammation. However, we only tested one specific stressor, an auditory stressor, and one particular timepoint. Hippocampal inflammation may alter the shape of the CORT response or its induction by other stressors. The differences between our anxiety- and HPA-related data and the literature most likely are explained by the regional localization of inflammation in our model. Inflammation may need to be more widespread, including other brain regions such as the amygdala, hypothalamus, or others to incite normal sickness response behaviors and hormonal alterations.
Six months of IL-1β overexpression caused chronic inflammation, which was restricted to the hippocampus. Previous studies in unilaterally injected IL-1βXAT mice found robust glial activation for up to a year after transgene induction (Shaftel et al., 2007a). Here, we confirmed this glial activation at 6 months after transgene activation in bilaterally injected animals. Importantly, we found that this inflammatory response remained localized to the hippocampus. No evidence of astrocytic or microglial activation was found outside of the hippocampus, indicating regional specificity of the neuroinflammation. This finding is consistent with our behavioral results and previous data from mice 2 weeks after viral injection (Hein et al., 2010).
Severe neuroinflammation and IL-1β itself have proven detrimental to neurons in various in vivo settings, including ischemia, traumatic brain injury, and neurodegenerative disease (see (Allan et al., 2005) for review). In vitro, IL-1β and tumor necrosis factor alpha, specifically, have been shown to cause neuronal death (Chao et al., 1995). However, previous work in the IL-1βXAT mice failed to find evidence of neuronal apoptosis or alterations in the density of the dentate gyrus granule cell layer following 2 months of unilateral IL-1β overexpression (Shaftel et al., 2007b). Consistent with our previous findings, at 3 months we find no change in volume in any hippocampal cell layer. However, after 6 months of IL-1β overexpression, we did observe a reduction in hippocampal volume. Hippocampal inflammation in IL-1βXAT mice may cause low levels of neurodegeneration, that we are unable to detect immunohistochemically, and may require many months to accumulate (Shaftel et al., 2007b). Alternatively, inflammation can impair neurogenesis, which indeed occurs in this model, and may result in reduced hippocampal volume over time (Ekdahl et al., 2003; Monje et al., 2003; Wu et al., 2010). Finally, chronic neuroinflammation may interact with normal aging processes to accelerate age-related neurodegeneration. Further studies will be needed to examine the mechanism for the observed reduction in hippocampal volume in this model following 6 months of inflammation.
Given the published detrimental effects of IL-1β and neuroinflammation on neurons, it is surprising that we do not see bigger effects on hippocampal volume earlier in our IL-1βXAT mouse model. However, we have artificially elevated IL-1β without any other peripheral or central injury. Other, secondary ‘danger signals’ may be necessary to produce more profound hippocampal neurodegeneration (Trendelenburg, 2008). Regardless, it seems unlikely that a severe loss of neurons themselves is responsible for the memory and other behavioral deficits observed within 3 months of transgene induction.
This IL-1βXAT mouse model provides an invaluable tool to examine the effects of localized and chronic neuroinflammation on behavioral, cellular, and molecular endpoints. Here we have characterized the influence of chronic neuroinflammation on a variety of behavioral indices and found that localized hippocampal IL-1β overexpression impairs contextual fear memory and increases activity while not affecting other behavioral measures. In addition, hippocampal volume was reduced by 6 but not 3 months of IL-1β overexpression. The negative effects on many behavioral indices are likely due to the brain region specificity of the induced inflammation, both a potential strength and weakness of this model. Further studies examining the effect of chronic inflammation in other brain regions, such as the hypothalamus, substantia nigra, striatum, or thalamus may help to confirm this idea. Future studies will also examine the mechanism behind these behavioral and cellular changes, testing the roles of different inflammatory cells and mediators.
Acknowledgments
Funding: NIH RO1 AG030149, P30 ES001247, and T32 NS051152.
We would like to thank S. Kyrkanides and J. Miller for FIV packaging, M. Olschowka and J. Walter for animal colony management, and L. Trojancyzk for help with tissue processing. The present work was supported by NIH RO1 AG030149 and P30 ES001247. A. Hein was supported by an NINDS T32 training grant in neuroinflammation and glial cell biology (NIH T32 NS051152).
Footnotes
Guarantors: Amy Hein and M. Kerry O’Banion
Conflict of interest disclosures: None
References
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Bretdibat J, Bluthe R, Kent S, Kelley K, Dantzer R. Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav Immun. 1995;9:242–246. doi: 10.1006/brbi.1995.1023. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Ehrlich L, Peterson PK. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun. 1995;9:355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Asnis GM, Fabrizio KR, Pedrosa E. Acute diclofenac treatment attenuates lipopolysaccharide-induced alterations to basic reward behavior and HPA axis activation in rats. Psychopharmacology. 2005;179:356–365. doi: 10.1007/s00213-004-2053-x. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Draper K, Ponsford J. Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychology. 2008;22:618–625. doi: 10.1037/0894-4105.22.5.618. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein T. Spatial Learning Impairment in Mice Infected with Legionella pneumophila or Administered Exogenous Interleukin-1-β. Brain Behav Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Griffin DE, Wesselingh SL, McArthur JC. Elevated central nervous system prostaglandins in human immunodeficiency virus-associated dementia. Ann Neurol. 1994;35:592–597. doi: 10.1002/ana.410350513. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Vraniak PD, Wenk GL. LPS-induced neuroinflammatory effects do not recover with time. Neuroreport. 2000a;11:1759–1763. doi: 10.1097/00001756-200006050-00032. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Vannucchi MG, Wenk GL. Behavioral and ultrastructural changes induced by chronic neuroinflammation in young rats. Brain Res. 2000b;859:157–166. doi: 10.1016/s0006-8993(00)01999-5. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- Hein AM, Stutzman DL, Bland ST, Barrientos RM, Watkins LR, Rudy JW, Maier SF. Prostaglandins are necessary and sufficient to induce contextual fear learning impairments after interleukin-1 beta injections into the dorsal hippocampus. Neuroscience. 2007;150:754–763. doi: 10.1016/j.neuroscience.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O’Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson PJ, O’Connell MT, Rothwell NJ, Hopkins SJ, Nortje J, Carpenter KL, Timofeev I, Al-Rawi PG, Menon DK, Pickard JD. Inflammation in human brain injury: intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J Neurotrauma. 2007;24:1545–1557. doi: 10.1089/neu.2007.0295. [DOI] [PubMed] [Google Scholar]
- Kent S, Rodriguez F, Kelley KW, Dantzer R. Reduction in food and water intake induced by microinjection of interleukin-1 beta in the ventromedial hypothalamus of the rat. Physiol Behav. 1994;56:1031–1036. doi: 10.1016/0031-9384(94)90339-5. [DOI] [PubMed] [Google Scholar]
- Kent S, Bret-Dibat JL, Kelley KW, Dantzer R. Mechanisms of sickness-induced decreases in food-motivated behavior. Neurosci Biobehav Rev. 1996;20:171–175. doi: 10.1016/0149-7634(95)00037-f. [DOI] [PubMed] [Google Scholar]
- Kostulas N, Pelidou SH, Kivisakk P, Kostulas V, Link H. Increased IL-1beta, IL-8, and IL-17 mRNA expression in blood mononuclear cells observed in a prospective ischemic stroke study. Stroke. 1999;30:2174–2179. doi: 10.1161/01.str.30.10.2174. [DOI] [PubMed] [Google Scholar]
- Lai YC, Shaftel SS, Miller JN, Tallents RH, Chang Y, Pinkert CA, Olschowka JA, Dickerson IM, Puzas JE, O’Banion MK, Kyrkanides S. Intraarticular induction of interleukin-1beta expression in the adult mouse, with resultant temporomandibular joint pathologic changes, dysfunction, and pain. Arthritis Rheum. 2006;54:1184–1197. doi: 10.1002/art.21771. [DOI] [PubMed] [Google Scholar]
- Matousek SB, Hein AM, Shaftel SS, Olschowka JA, Kyrkanides S, O’Banion MK. Cyclooxygenase-1 mediates prostaglandin E(2) elevation and contextual memory impairment in a model of sustained hippocampal interleukin-1beta expression. J Neurochem. 2010;114:247–258. doi: 10.1111/j.1471-4159.2010.06759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SS, Quan HY, Ma J, Han JS, Jeon BH, Seol GH. Chronic brain inflammation impairs two forms of long-term potentiation in the rat hippocampal CA1 area. Neurosci Lett. 2009;456:20–24. doi: 10.1016/j.neulet.2009.03.079. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Moore AH, Wu M, Shaftel SS, Graham KA, O’Banion MK. Sustained expression of interleukin-1beta in mouse hippocampus impairs spatial memory. Neuroscience. 2009;164:1484–1495. doi: 10.1016/j.neuroscience.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl M, Van Oers H, Schobitz B, Ron de Kloet E. Interleukin-1 β, but not interleukin-6, impairs spatial navigation learning. Brain Res. 1993;613:160–163. doi: 10.1016/0006-8993(93)90468-3. [DOI] [PubMed] [Google Scholar]
- Piletz JE, Halaris A, Iqbal O, Hoppensteadt D, Fareed J, Zhu H, Sinacore J, Devane CL. Pro-inflammatory biomakers in depression: treatment with venlafaxine. World J Biol Psychiatry. 2009;10:313–323. doi: 10.3109/15622970802573246. [DOI] [PubMed] [Google Scholar]
- Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, Onofrj M. Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun. 2009;23:55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz LM, Koschera A. Progression of cognitive impairment in stroke patients. Neurology. 2004;63:1618–1623. doi: 10.1212/01.wnl.0000142964.83484.de. [DOI] [PubMed] [Google Scholar]
- Sairanen T, Ristimaki A, Karjalainen-Lindsberg ML, Paetau A, Kaste M, Lindsberg PJ. Cyclooxygenase-2 is induced globally in infarcted human brain. Ann Neurol. 1998;43:738–747. doi: 10.1002/ana.410430608. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O’Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007a;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O’Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci. 2007b;27:9301–9309. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Leonard BE, Horrobin DF. Dietary ethyl-eicosapentaenoic acid but not soybean oil reverses central interleukin-1-induced changes in behavior, corticosterone and immune response in rats. Stress. 2004;7:43–54. doi: 10.1080/10253890410001667188. [DOI] [PubMed] [Google Scholar]
- Song C, Horrobin DF, Leonard BE. The comparison of changes in behavior, neurochemistry, endocrine, and immune functions after different routes, doses and durations of administrations of IL-1beta in rats. Pharmacopsychiatry. 2006;39:88–99. doi: 10.1055/s-2006-941557. [DOI] [PubMed] [Google Scholar]
- Song C, Li X, Leonard BE, Horrobin DF. Effects of dietary n-3 or n-6 fatty acids on interleukin-1beta-induced anxiety, stress, and inflammatory responses in rats. J Lipid Res. 2003;44:1984–1991. doi: 10.1194/jlr.M300217-JLR200. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ. Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav. 2007;86:651–659. doi: 10.1016/j.pbb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham W, Auchus AP, Thong M, Goh ML, Chang HM, Wong MC, Chen CP. Progression of cognitive impairment after stroke: one year results from a longitudinal study of Singaporean stroke patients. J Neurol Sci. 2002;203–204:49–52. doi: 10.1016/s0022-510x(02)00260-5. [DOI] [PubMed] [Google Scholar]
- Trendelenburg G. Acute neurodegeneration and the inflammasome: central processor for danger signals and the inflammatory response? J Cereb Blood Flow Metab. 2008;28:867–881. doi: 10.1038/sj.jcbfm.9600609. [DOI] [PubMed] [Google Scholar]
- Wu MD, Hein AM, Moravan M, Olschowka JA, O’Banion MK. Sustained interleukin-1beta expression ablates adult subgranular zone neurogenesis while sparing subventricular zone neurogenesis. Proc Soc Neurosci. 2010:879.21. [Google Scholar]