Abstract
Alanine dehydrogenase (AldA) is the principal enzyme with which pea bacteroids synthesize alanine de novo. In free-living culture, AldA activity is induced by carboxylic acids (succinate, malate, and pyruvate), although the best inducer is alanine. Measurement of the intracellular concentration of alanine showed that AldA contributes to net alanine synthesis in laboratory cultures. Divergently transcribed from aldA is an AsnC type regulator, aldR. Mutation of aldR prevents induction of AldA activity. Plasmid-borne gusA fusions showed that aldR is required for transcription of both aldA and aldR; hence, AldR is autoregulatory. However, plasmid fusions containing the aldA-aldR intergenic region could apparently titrate out AldR, sometimes resulting in a complete loss of AldA enzyme activity. Therefore, integrated aldR::gusA and aldA::gusA fusions, as well as Northern blotting, were used to confirm the induction of aldA activity. Both aldA and aldR were expressed in the II/III interzone and zone III of pea nodules. Overexpression of aldA in bacteroids did not alter the ability of pea plants to fix nitrogen, as measured by acetylene reduction, but caused a large reduction in the size and dry weight of plants. This suggests that overexpression of aldA impairs the ability of bacteroids to donate fixed nitrogen that the plant can productively assimilate. We propose that the role of AldA may be to balance the alanine level for optimal functioning of bacteroid metabolism rather than to synthesize alanine as the sole product of N2 reduction.
Nitrogen fixation by the Rhizobium-legume symbiosis provides a significant proportion of the available nitrogen in the biosphere, making the symbiosis agronomically and ecologically important. N2 is reduced to ammonium by specialized bacterial cells, known as bacteroids, contained in legume nodules. Ammonium is the primary stable product of nitrogen fixation (2), and it has been generally accepted that bacteroids secrete ammonium directly to the plant (6, 17), where it is assimilated by glutamine synthetase-glutamine oxoglutarate aminotransferase (8, 35). However, the recent demonstration that only alanine is secreted by soybean bacteroids suggests a radically different mechanism of nitrogen transport from the bacteroid to the plant (36). This is controversial and has been disputed by others who could not demonstrate alanine secretion by soybean bacteroids (18). Using the R. leguminosarum-pea symbiosis as a tractable genetic and biochemical system, Allaway et al. demonstrated that both alanine and ammonia are secreted by isolated bacteroids (1). The de novo synthesis of alanine by pea bacteroids was shown by mutagenesis and 15N2 labeling studies to be due to alanine dehydrogenase (AldA) (1). AldA activity was not essential for symbiotic nitrogen fixation, as would be expected if alanine were to be the sole nitrogen secretion product, but plants nodulated by aldA mutants showed 20% lower dry weight. Under all conditions tested, ammonium remained the principal secretion product, but the proportion of alanine secreted varied. The rate of alanine synthesis depended on a number of factors, but the key factor was the concentration of ammonium that had built up in the reaction vessel. High bacteroid densities favored ammonium accumulation and activation of alanine synthesis by AldA, which has a Km for ammonium of 5.1 mM (1). The ammonium concentration in soybean bacteroids has been estimated by extrapolation from leakage rates to be 12 mM (34). More recently, nuclear magnetic resonance analysis of intact pea nodules has shown that bacteroids accumulate high concentrations of ammonium (31). Conditions in bacteroids are therefore appropriate for significant alanine synthesis from pyruvate and ammonium. In fact, while the plant phenotype of the R. leguminosarum aldA mutant shows that ammonium is capable of being the sole secretion product of reduced N2 from pea bacteroids, it does not absolutely preclude the possibility that alanine normally has this role in planta.
However, nutrient exchange between the plant cytosol and the bacteroid may be more complex than previously thought. A model in which amino acid cycling is essential to drive nitrogen fixation in pea nodules has been proposed recently (19). The importance of amino acid cycling was first revealed because mutation of the two broad-specificity amino acid uptake systems (Aap and Bra) of Rhizobium leguminosarum causes plants to become severely nitrogen starved, even though bacteroids retain the ability to reduce 15N2 to ammonium (19). In this model, a dicarboxylate and an amino acid such as glutamate need to be taken up by the bacteroid to drive both dicarboxylate oxidation and the secretion of amino acids such as aspartate and alanine. The role of glutamate is to act as the amino group donor for the transamination of oxaloacetate to aspartate and possibly for that of pyruvate to alanine. Disruption of aspartate aminotransferase in alfalfa or pea bacteroids prevents N2 reduction, which is consistent with a central role for transamination (19, 37). Bacteroids with amino acid uptake mutations also become carbon saturated, probably because they become inefficient at using dicarboxylic acids, which instead accumulate as polyhydroxybutyrate (19). Given the proposed importance of amino acid cycling, it is crucial to appreciate the difference between de novo amino acid synthesis by AldA, which results in ammonium assimilation, and amino acid synthesis by transamination. However, it is apparent that de novo amino acid synthesis by AldA could have a significant impact on any transamination cycle, because it would alter the steady-state levels of keto and amino acids.
AldA catalyzes the reversible NADH-dependent synthesis of alanine from NH4+ and pyruvate. Rhizobial AldAs have lower Kms for ammonia (5 to 9 mM) than is common for most bacterial enzymes (20 to 300 mM), consistent with a greater-than-usual ability to assimilate ammonia (1, 32). The aldA gene was identified in R. leguminosarum because, when present in multiple copies, it suppressed a dadR mutant, which does not grow on alanine as the sole source of carbon (1). Thus, while AldA enables alanine catabolism when overexpressed, this does not appear to be its normal role. In agreement with this notion, the dad operon, which consists of three genes (dadR, dadX, and dadA), is present in a wide range of bacteria, where it is the primary pathway for alanine degradation (22, 39). In addition, an aldA mutant grew as well as the wild type on alanine as the sole carbon source, confirming that AldA is not the principal catabolic enzyme in R. leguminosarum (1). In spite of the controversy over the possible importance of ammonium assimilation via alanine synthesis by AldA in bacteroids involved in Rhizobium-legume symbioses, we know very little about what regulates aldA expression. Furthermore, given that AldA may alter the availability of keto and amino acids for amino acid cycling by transamination, we investigated the physiological basis of the regulation of aldA and how it relates to the function of aldA in nodules.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are detailed in Table 1. R. leguminosarum strains were grown at 28°C on either tryptone yeast extract (TY) (3) or acid minimal salts medium (AMS) (25), with carbon and nitrogen sources added to 10 mM. Antibiotics were used at the following concentrations (in micrograms per milliliter): ampicillin, 50; gentamicin, 20; kanamycin, 40; neomycin, 80; spectinomycin, 100; streptomycin, 500; tetracycline, 2 in AMS and 5 in TY.
TABLE 1.
Bacterial strains and plasmids used in this study.
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| 3841 | Rhizobium leguminosarum bv. viciae; Smr derivative of strain 300 | 15 |
| RU1327 | 3841 aldA::TnB20; Smr Kmr | 1 |
| RU1422 | 3841 aldR::Ω; Smr Spr | This study |
| Cosmid, pRU3135 | Cosmid containing aldAR Tcr | 1 |
| Plasmids | ||
| pRU640 | 1.45-kb PCR product (with primers P199 and P200) of aldA in pCR2.1-TOPO; Apr/Kmr | This study |
| pRU679 | 700-bp PCR product (with primers P219 and P220) of aldA-aldR intergenic region in pCR 2.1-TOPO; Apr Kmr | This study |
| pRU693 | 3-kb aldA-aldR SalI fragment from pRU3135 in pSK(−); Apr | This study |
| pRU701 | 0.7-kb aldA-aldR intergenic region in pSK(−); Apr | This study |
| pRU708 | 1.45-kb SacI/KpnI fragment from pRU640 in pTR101; Tetr Apr | This study |
| pRU730 | aldR::gusA fusion in pJP2; Tcr | This study |
| pRU731 | aldA::gusA fusion in pJP2; Tcr | This study |
| pRU734 | Ω cloned in pRU693 (aldR); Apr Spr | This study |
| pRU735 | aldR::Ω cloned in pJQ200SK; Gmr Spr | This study |
| pRU876 | 1-kb PCR product (with primers P287 and P289) of aldA-aldR intergenic region in pCR2.1-TOPO; Apr Kmr | This study |
| pRU877 | gusA in pK19mob; Kmr | This study |
| pRU882 | AldR::gusA fusion in pRU877; Kmr | This study |
| pRU883 | AldA::gusA fusion in pRU877; Kmr | This study |
| pRU889 | 1.9-kb PCR product (primers P280 and P281) of aldA-aldR in pTR101; Tcr | This study |
| pHP45Ω | pBR322 derivative carrying Ω; pHP45 replicon; Apr Spr | 27 |
| pJP2 | Wide-host-range stable gusA transcriptional promoter probe vector; Tcr | 26 |
| pJQ200SK | P15A origin from pACYC184; GmrlacZ sacB traJ | 28 |
| pK19mob | pUC19-derived mobilizable integration vector; Nmr | 30 |
| pCR2.1-TOPO | TA PCR cloning vector, f1 origin, ColE1 replicon; Apr KmrlacZ | Invitrogen |
| pTR101 | Wide-host-range stable mobilizable P-group cloning vector, RK2 derivative; Tcr | 38 |
| pBluescript II SK(−) | Phagemid, pUC19 derivative, f1 origin of replication, ColE1 replicon; Apr | Stratagene |
DNA manipulations.
Standard protocols were used for DNA manipulations (29). Sequencing was carried out by MWG-Biotech AG. Similarity searches were done using the basic local alignment search tool on the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/BLAST/).
To complement aldA mutant RU1327, aldA was PCR amplified by using primers P199 (5′ ATACAAAGAAGGCGGCATCC 3′) and P200 (5′ AGCTCGGCGTTGGTGATGC 3′) and was cloned into pCR2.1-TOPO (pRU640). aldA was transferred as a 1.45-kb SacI/KpnI fragment into the stable plasmid pTR101 (38), producing pRU708.
To mutate aldR, a 3.1-kb SalI fragment carrying aldA and aldR was cloned from cosmid pRU3135 into pBluescript SK(−) (pRU693). aldR was disrupted with a spectinomycin resistance cassette cloned from pHP45Ω into the EcoRI site of aldR (27), producing pRU734. The disrupted gene was transferred into pJQ200SK (28) as a SalI fragment (pRU735), and pRU735 was recombined into strain 3841, creating RU1422. RU1422 was characterized by Southern blotting and hybridization by using a 2.5-kb EcoRV fragment carrying the entire sequence of aldR from pRU693 as a probe.
Primers P280 (TTTTTTGGTACCATACAAAGAAGGCGGCATCCCCTC) and P281 (TTTTTTGAGCTCCGTCCTCTGCGCGTCTGAAAAGAC) were used to amplify aldA and aldR, which was cloned as an SstI/KpnI fragment into pTR101, creating pRU889.
Construction of transcriptional fusions to gusA.
To construct plasmid-based fusions, the aldA-aldR intergenic region was amplified using primers P219 (GAGCGCCTTGTGTGAAAGCC) and P220 (TCCGGCGCCAGATGCAGATAG) and was cloned into pCR2.1-TOPO (pRU679). To construct a gusA fusion to aldR, the region was subcloned into pJP2 (26) as a HindIII/XbaI fragment (pRU730). To construct a gusA fusion to aldA, the region was transferred to pSK(−) as an EcoRI fragment (pRU701) in order to switch the orientation of the aldR-aldA promoter region. The region was subcloned into pJP2 as a HindIII/XbaI fragment (pRU731). To construct chromosomal fusions, a gusA integration vector was made by cloning gusA from pJP2 into pK19mob (30) as a KpnI/PstI fragment, producing pRU877. The ald-aldR intergenic region was amplified using primers P287 (AGCGTCTTGGCGAACTGGC) and P289 (CTGGATGCTGGAATAACGGG) and cloned into pCR2.1-TOPO (pRU876). The intergenic region was cloned in both orientations into the EcoRI site of pRU877, producing pRU882 (aldR-gusA) and pRU883 (aldA-gusA). Plasmids were conjugated into strain 3841, and recombinants were isolated by selecting for neomycin resistance.
RNA extraction and Northern blot analysis.
Total RNA was isolated from strain 3841, which had been grown in 50 ml of AMS to an optical density at 600 nm (OD600) of 0.5, by using the RNeasy Midi kit (Qaigen) and following the protocol for bacteria. The lysis step was modified by incubating cells in 10 mM Tris (pH 8) with 20% sucrose and 1 mg of lysozyme/ml at room temperature for 15 min, followed by a further 20-min incubation with EDTA at 1 mM.
Standard protocols were used for RNA blotting and hybridization (29). The DNA probe (a 1.46-kb EcoRI fragment from plasmid pRU640 which carries a full-length copy of aldA) was labeled with [32P]dCTP by using Ready To Go DNA labeling beads and was purified using ProbeQuant G-50 Micro Columns, according to the manufacturer's protocols (Amersham Pharmacia Biotech). After hybridization, the membrane was exposed to a Phosphor Screen for approximately 48 h. The screen was scanned by using a phosphorimager (Molecular Dynamics PhosphorImager TMSI) and was analyzed by ImageQuant (version 5.1) software.
Assay of enzyme activity.
Laboratory cultures were grown to an OD600 of 0.5 in 400 ml of AMS, with the appropriate carbon and nitrogen sources, and were harvested by centrifugation. For assays with bacteroids, nodules were picked from the pea roots and crushed in isolation buffer (100 mM phosphate [pH 7.4], 300 mM sucrose, 2 mM MgCl2). The liberated bacteroids were purified on a prepared Percoll gradient (centrifuged for 45 min at a relative centrifugal force [RCF] of 36,900 with 55% Percoll in isolation buffer) and subjected to centrifugation (15 min at an RCF of 36,900). The bacteroid fraction was carefully isolated, then centrifuged (10 min at an RCF of 3,345), and resuspended twice to remove Percoll.
To prepare enzyme extracts, either free-living cells or bacteroids were centrifuged (10 min at an RCF of 3,345) at 4°C, and pellets were washed twice by repeated centrifugation in cold 10 mM HEPES (pH 7.4) and were then resuspended in 10 ml of ice-cold 40 mM HEPES (pH 7.4) with 2 mM dithiothreitol and 20% glycerol. Cells were lysed by using a French pressure cell (SLM Instruments Inc.) at 69 MPa. Cell debris was removed by centrifugation at an RCF of 28,000 and 4°C for 30 min.
l-Alanine dehydrogenase activity was assayed at 28°C by measuring the change in absorbance at 340 nm due to oxidation of NADH. Substrate concentrations were 0.2 mM NADH, 5 mM pyruvate, and 100 mM NH4Cl in 50 mM Tris-HCl buffer (pH 8.5). Activities were calculated from the initial linear rates (NADH extinction coefficient = 6.22 × 103 liters mol−1 cm−1). KCN (10 mM) was also added to the reaction mixtures to inhibit a high background of NADH oxidase activity.
β-Glucuronidase (GusA) activity was measured as previously described for β-galactosidase reactions (24), except that 5-bromo-4-chloro-3-indoyl-β-d-glucuronide was substituted as the chromogenic substrate.
Protein concentrations of enzyme extracts were determined by the Bradford method with bovine serum albumin as a standard (5).
Intracellular alanine determination.
Cultures (500 ml) of various strains of R. leguminosarum were grown to mid-log phrase (approximately 5 × 108 CFU ml−1) on AMS with either glucose or succinate as the carbon source and NH4Cl as the nitrogen source. Bacteria were harvested by centrifugation at room temperature for 10 min at an RCF of 6,819. The supernatant was discarded, and the pellet was frozen in liquid nitrogen. Frozen pellets were then resuspended in 5 ml of HEPES (250 mM; pH 8.7) and placed in a boiling water bath for 5 min. Disrupted cells were removed by centrifugation at an RCF of 26,200 for 30 min, and the supernatant was removed for alanine determination by the coupled alanine dehydrogenase assay as previously described (1). To ensure that the boiling step did not degrade alanine, internal standards of alanine were added to appropriate samples. French press extracts did not increase the release of alanine. The intracellular concentration of alanine was determined from the known intracellular volume of R. leguminosarum strain 3841 (1.45 ml g−1 [dry weight]) (10).
Plant growth.
Rhizobium leguminosarum bv. viciae strains were used to inoculate surface-sterilized pea seeds (Pisum sativum cv. Avola) at the time of sowing. Plants were grown in a greenhouse in pots (10 liters) filled with a sterile sand-gravel-vermiculite mixture, watered with a nitrogen-free nutrient solution (24), and harvested at 4 weeks for enzyme assay and acetylene reduction and at 6 weeks for dry weight analysis. Acetylene reduction was performed as previously described (1).
Nodule sections and staining.
Nodules were picked from roots and sectioned in 50 mM sodium phosphate buffer by using a vibratom (Leica VT 1000S). Sections were stained in 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (0.2 mg/ml in 50 mM sodium phosphate buffer, 2 mM ferricyanide, 2 mM ferrocyanide, and 0.1% Triton X-100) as described by Boesten et al. (4). Sections were fixed in 2% glutaraldehyde (50 mM sodium phosphate buffer-0.1% Triton X-100) for 30 min and visualized by microscopy.
Nucleotide sequence accession number.
The sequence of R. leguminosarum aldR has been deposited in EMBL under accession number aj238118.
RESULTS
Induction of l-alanine dehydrogenase activity.
Strain 3841 was grown on a variety of nitrogen and carbon sources, and alanine dehydrogenase activity was measured (Table 2). While there was no detectable activity in strain 3841 grown on glucose-ammonium, activity was induced to 0.074 to 0.084 μmol min−1 mg of protein−1 on monocarboxylic and dicarboxylic acids. This was not simply caused by the lack of a sugar in the medium, because cultures grown on glucose and succinate still induced the enzyme (Table 2). However, the greatest induction was observed in cultures grown on alanine as the sole carbon and nitrogen source (0.77 μmol min−1 mg of protein−1). This is interesting, because such induction suggests that alanine dehydrogenase assists in the degradation of alanine, even though aldA mutants still grow on alanine due to the dad operon (1). It is relevant that when overexpressed, aldA can substitute for the dad operon. The induction levels seen in bacteria grown on organic acids are very similar to those in bacteroids and are consistent with a role in alanine synthesis (Table 2).
TABLE 2.
l-Alanine dehydrogenase activities of various strains grown on different carbon sources
| Carbon source(s) |
l-Alanine dehydrogenase activity (μmol min−1 mg of protein−1)a in:
|
||||
|---|---|---|---|---|---|
| 3841 (WT) | RU1327 (ΔaldA) | RU1422 (ΔaldR) | 3841/pTR101 | 3841/pRU889 | |
| Glc-NH3 | ND | ND | − | − | 0.034 ± 0.013 |
| Succ-NH3 | 0.074 ± 0.014 | ND | − | − | − |
| Glc-Ala | 0.494 ± 0.015 | ND | ND | 0.286 ± 0.054 | 3.96 ± 0.450 |
| Ala | 0.770 ± 0.022 | − | − | 0.645 ± 0.101 | 5.8 ± 0.268 |
| Mal-NH3 | 0.084 ± 0.003 | − | − | 0.136 ± 0.02 | 7.1 ± 0.454 |
| Pyr-NH3 | 0.079 ± 0.004 | − | − | − | − |
| Glc-Succ-NH3 | 0.066 ± 0.006 | − | − | − | − |
| Bacteroids | 0.09b | − | − | 0.069 | 0.463 |
All carbon and nitrogen sources were at 10 mM except when alanine (20 mM) was used as the sole carbon source. Activities from at least three independent cultures ± SEMs are shown. Bacteroid activities are means from two independent harvests. Abbreviations: Glc, glucose; Succ, succinate; Ala, alanine; Mal, malate; Pyr, pyruvate; ND, no detectable activity; −, not tested; WT, wild type.
The activity of AldA in strain 3841 is from previous work (1).
Identification of a regulator of aldA.
In an attempt to look for regulators, we sequenced immediately around the aldA gene contained on cosmid pRU3135 and identified a putative transcriptional regulator transcribed divergently from aldA, which we designated aldR (EMBL accession number aj238118). There is an intergenic region of 142 bp between aldA and aldR. AldR has 58% amino acid identity over its entire length to BkdR, a well-characterized positive transcriptional regulator of the bkd operon in Pseudomonas putida (20, 21). Both AldR and BkdR belong to the AsnC family of bacterial transcriptional regulators, which also includes Lrp of Escherichia coli (40). The predicted AldR protein has 153 amino acids, with a relative molecular weight of 17,186 and a pI of 6.57. As expected for an AsnC regulator, there is a predicted helix-turn-helix motif in the N terminus of AldR at positions 22 to 48.
The AldR sequence from R. leguminosarum enabled the identification by BLAST analysis of other rhizobial aldR genes divergent from aldA. Sinorhizobium meliloti, Mesorhizobium loti, and Bradyrhizobium japonicum have proteins with 81% (SMc01168_AA at http://sequence.toulouse.inra.fr/meliloti.html), 72% (mlr0363 at http://www.kazusa.or.jp/rhizobase/), and 48% (bll3178 at http://www.kazusa.or.jp/rhizobase/) amino acid identity to R. leguminosarum AldR, respectively, over their entire lengths (12, 16).
S. meliloti, like R. leguminosarum, has one chromosomal copy of aldA and a divergent aldR. However, M. loti has two putative copies of aldA, one divergent from aldR on the chromosome (mll0362 at http://www.kazusa.or.jp/rhizobase/) and a second (pmll9089 at http://www.kazusa.or.jp/rhizobase/) on plasmid pMLa, not associated with a regulator. Likewise, B. japonicum has a single isolated copy of aldA (blr1738 at http://www.kazusa.or.jp/rhizobase/) on the symbiotic island (14) and a second copy of aldA (blr3179 at http://www.kazusa.or.jp/rhizobase/), divergent from a putative aldR. R. leguminosarum AldA has 85% amino acid identity to its S. meliloti homologue, 84% amino acid identity to both proteins in M. loti, and 69 and 72% identities to the symbiotic island and aldR-associated copies of aldA in B. japonicum, respectively, over their entire lengths (12, 16). The presence of two copies of aldA in M. loti and B. japonicum, both of which form determinate nodules, contrasts dramatically with the situation in R. leguminosarum and S. meliloti, which form indeterminate nodules. The absolute AldA enzyme activity in R. leguminosarum is approximately an order of magnitude lower in both free-living cultures and bacteroids than that in B. japonicum, consistent with the presence of multiple copies of aldA in B. japonicum (1, 33). The specific importance of AldA in rhizobia is highlighted by the absence of aldA in both Agrobacterium tumefaciens and Brucella melitensis, even though these organisms are closely related to the rhizobia (9, 13).
Mutation of aldR.
To investigate the role of AldR in the regulation of aldA, an aldR mutant (RU1422) was isolated by cloning an Ω interposon into the EcoRI site of aldR and recombining this into the chromosome. Mutant RU1422 had no detectable AldA activity (Table 2). In agreement with a previous study of the aldA mutant (RU1327) (1), growth of the aldR mutant RU1422 on glucose, succinate, pyruvate, or alanine as the sole carbon source was unaffected.
Transcriptional regulation of aldA and aldR was investigated by cloning a 641-bp PCR product in both orientations in the stable broad-host-range gusA transcriptional vector pJP2. The vector alone had no detectable GusA activity in strain 3841, but with either an aldA (pRU731) or an aldR (pRU730) fusion, there was considerable activity on a variety of carbon sources (Table 3). Both aldA::gusA and aldR::gusA fusions were induced approximately twofold on succinate- compared to glucose-grown cells. Growth of strain 3841 on alanine resulted in a sevenfold induction of gusA transcription, consistent with the induction of AldA enzyme activity (Table 2). When either the aldA::gusA or aldR::gusA fusions were measured in the aldR mutant, RU1422, there was no detectable activity on a wide variety of carbon sources (Table 3). Thus, aldR regulates both itself (auotoregulatory) and aldA. The autoregulation of aldR, in conjunction with variations in plasmid copy number, may explain the large errors in GusA activity for both aldA and aldR fusions (Table 3). Of 15 independent cultures of each strain assayed, it was common to obtain either high induction or no induction at all for both aldA::gusA and aldR::gusA. This effect is expected if the plasmid containing the ald fusions titrates out the positive transcriptional regulator AldR. Minor changes in the plasmid copy number will have a large effect on the concentration of available AldR. It should be noted that the plasmid fusions do not contain a full-length copy of aldR. Cultures containing either aldA::gusA or aldR::gusA that failed to induce GusA activity after growth on succinate-ammonium also failed to show detectable AldA enzyme activity.
TABLE 3.
Transcriptional activities of aldA and aldR fusions in strains grown on various carbon and nitrogen sources
| Strain and fusion | GusA activity (nmol min−1 mg of protein−1)a on:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Glc-NH3 | Mal-NH3 | Succ-NH3 | Pyr-NH3 | Ala-Glc | Ala | Glu-Glc | Glu | |
| 3841/pRU730 (aldR::gusA in pJP2) | 163 ± 20 | − | 325 ± 64 | − | 555 ± 145 | 1,144 ± 244 | − | − |
| 3841/pRU731 (aldA::gusA in pJP2) | 104 ± 9 | − | 227 ± 31 | − | 349 ± 100 | 757 ± 152 | − | − |
| 3841/pRU882 (aldR::gusA genome) | 112 ± 18 | 237 ± 55 | 220 ± 10 | 310 ± 8 | 519 ± 40 | 1,142 ± 46 | 140 ± 1 | 366 ± 22 |
| 3841/pRU883 (aldA::gusA genome) | 124 ± 8 | 194 ± 40 | 184 ± 3 | 219 ± 9 | 598 ± 30 | 1,082 ± 29 | 109 ± 3.11 | 318 ± 3 |
| RU1422/pRU730 (aldR::gusA in pJP2) | ND | − | ND | − | ND | ND | − | − |
| RU1422/pRU731 (aldA::gusA in pJP2) | ND | − | ND | − | ND | ND | − | − |
Values shown are means ± SEMs. Strains carrying pJP2 fusions (pRU730 and pRU731) were assayed in at least 15 independent cultures, while strains carrying integrated fusions (pRU882 and pRU883) were assayed in at least 3 independent cultures. Strain 3841 containing pJP2 without an insert had no detectable GusA activity. Abbreviations: Glc, glucose; Mal, malate; Succ, succinate; Ala, alanine; Pyr, pyruvate; Glu, glutamate; ND, no detectable activity; −, not tested.
While the use of replicating plasmid fusions was highly illuminating with regard to the autoregulation of AldR, we decided to prevent the problems associated with their copy number by making integrated gusA fusions to both aldR and aldA. To enable this, a new gusA transcriptional integration vector, pRU877, was made by cloning the gusA gene from pJP2 into pK19mob. Primers p287 and p289 were then used to amplify a 1,070-bp fragment spanning the aldA-aldR intergenic region, which was cloned in both orientations in pRU877. The integrated aldA::gusA (pRU883) and aldR::gusA (pRU882) fusions were induced approximately twofold in cultures grown on organic acids and as much as ninefold in those grown on alanine as the sole carbon source, relative to growth on glucose (Table 3).
Total RNA was extracted from cultures of strain 3841 grown in minimal medium containing either glucose, succinate, glucose-alanine, or 20 mM alanine as the carbon source(s). The isolated RNA was probed with a 1.46-kb EcoRI fragment from plasmid pRU640, which carries a full-length copy of aldA. The aldA transcript was between 1.1 and 1.2 kb, consistent with the length of the aldA gene (1,133 bp). Quantitation with a phosphorimager showed that there was a 2.1-fold induction of aldA mRNA levels on succinate, a 2.0-fold induction on alanine-glucose, and a 3.8-fold induction on 20 mM alanine, with respect to cells grown on glucose. These results were consistent over three independent RNA extractions, blotting, and hybridizations and are reasonably consistent with both enzyme activity and gusA fusion results. The presence of a good hybridization signal for cultures grown on glucose-ammonium, measured with a phosphorimager (data not shown), confirms the GusA transcriptional data indicating that aldA is transcribed in cultures grown on glucose-ammonium (Table 3). This is true even though AldA enzyme activity was not detected. The absence of detectable enzyme activity is likely to be due to the insensitivity of NADH-linked enzyme assays, particularly in crude extracts where there is high background NADH oxidase activity.
In planta expression.
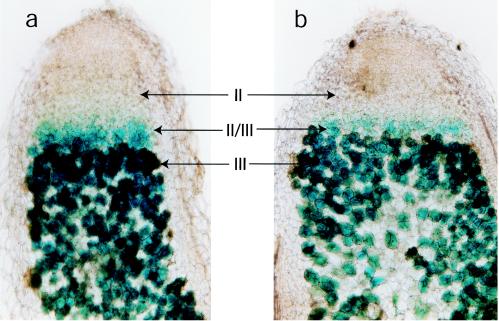
Strain 3841 containing the aldA::gusA or aldR::gusA fusion was inoculated onto plants, and 3-week-old nodules were removed, sectioned, and stained for GusA. The staining shows that GusA is active in the II/III interzone, where bacteroid maturation is occurring, as well as in zone III, which contains the mature nitrogen-fixing bacteroids (Fig. 1).
FIG. 1.
Histochemical staining of longitudinal sections of pea nodules for GusA activity. Pea plants were infected with R. leguminosarum strain 3841 carrying either an aldA::gusA (pRU731) (a) or an aldR::gusA (pRU730) (b) fusion in the stable plasmid pJP2.
Since the level of expression of aldA is low in pea versus soybean bacteroids, we wanted to examine the effect of aldA overexpression on the symbiotic performance of pea plants. If alanine really is the sole secreted product of N2 reduction, as has been reported for soybean bacteroids (36), then overexpression of aldA might increase nitrogen supply to the plant. Given the results of the work presented here, showing that AldR is autoregulatory, we decided to express both aldR and aldA together. This should prevent problems associated with several copies of the aldR-aldA intergenic region causing removal of AldR by titration. Therefore, aldA and aldR were cloned into the stable broad-host-range vector pTR101, creating pRU889, and conjugated into strain 3841. Strain 3841 containing pTR101 was used as a control. Strains 3841/pRU889 and 3841/pTR101 grew with virtually identical mean generation times on glucose-ammonia (3.8 versus 3.8 h), glucose-alanine (4.0 versus 3.9 h), malate-ammonium (4.2 versus 4.3 h), and alanine (5.5 versus 5.4 h), respectively, indicating that overexpression of these genes was not deleterious to growth. The enzyme activity of AldA was elevated 9- to 52-fold in strain 3841/pRU889 relative to that in 3841/pTR101, depending on growth conditions; in addition, there was clearly detectable activity in cultures grown on glucose-ammonium (Table 2). However, a more modest (6.7-fold) elevation in AldA activity was observed in bacteroids of strain 3841/pRU889 versus 3841/pTR101 (Table 2). This suggests that nongrowing bacteroids do not support the same levels of aldA expression as exponentially growing cells in laboratory culture. The lower activity was not due to plasmid curing, since 36 of 36 colonies of 3841/pRU889 reisolated from nodules retained the plasmid, 6 of 6 colonies of 3841/pRU889 reisolated from separate plants all retained high AldA activity in free-living culture, and finally, examination of gusA expression in nodule sections has shown that pJP2, which is derived from pTR101, is not cured in individual bacteroids (26).
Four-week-old pea plants inoculated with strain 3841/pTR101 or 3841/pRU889 reduced acetylene at 4.55 μmol h−1 plant−1 (standard error of the mean [SEM], ±0.53 μmol h−1 plant−1; n = 8) or 5.9 μmol h−1 plant−1 (SEM, ±0.38 μmol h−1 plant−1; n = 8), respectively. These results are not significantly different (P > 0.05), indicating that there is no change in the ability of 3841/pRU889 to reduce N2. However, the dry weights of 6-week-old pea plants inoculated with 3841/pTR101 or 3841/pRU889 were 1.51 g (SEM, ±0.16; n = 11) or 1.08 g (SEM, ±0.09; n = 11), respectively. These differences are highly significant (P = 0.00021). Thus, while the overall capacity of strain 3841/pRU889 to reduce N2 is unimpaired, overexpression of aldA dramatically reduces plant growth. This suggests that increasing the de novo synthesis of alanine from pyruvate and ammonium in bacteroids reduces the ability of the bacteroid to donate fixed nitrogen to the plant.
Intracellular concentration of alanine.
Since aldA is induced by both organic acids and alanine, and the reaction it catalyzes is reversible, it is difficult to predict whether AldA contributes to net alanine synthesis or degradation in vivo. It is also possible that alanine might be the true intracellular inducer of aldA, because cultures grown on it are more highly induced than those grown on organic acids (Tables 2 and 3). One way to investigate these questions is to measure the effect of expression of aldA on the intracellular concentration of alanine.
Surprisingly, alanine concentrations were higher in glucose-grown than in succinate-grown cultures, in spite of the absence of detectable AldA enzyme activity in the former (Table 4). This finding rules out the possibility that organic acids induce aldA by elevating intracellular alanine concentrations. Alanine concentrations were much lower in cells of strain RU1327 (aldA) grown on succinate (74% lower) or glucose (34% lower) than in the wild type. The reduction in alanine concentrations in RU1327 relative to those in the wild type, when cells were grown on glucose, is consistent with the observation that aldA is transcribed (Table 3 and Northern blot analysis results [data not shown]). This suggests that AldA is present, although we could not detect it in an enzyme assay by measuring the comparatively insensitive oxidation of NADH. The higher concentration of alanine in glucose-grown cultures is likely to reflect many factors, including the concentrations of keto acids such as pyruvate, the ammonium concentration, and the redox state of the cells. It is also dependent on the transamination activity of the cultures. Clearly, AldA is only one factor regulating intracellular alanine concentrations. When aldA was overexpressed, there was a large increase in alanine concentrations, particularly in glucose-grown cells (Table 4). This finding suggests that cells grown on glucose, relative to growth on succinate, are particularly sensitive to the level of AldA, and as suggested above, this sensitivity may be due to the concentration of keto acids or the redox potential. It is noteworthy that AldA enzyme activity was detectable in glucose-grown cells in which AldA was overexpressed (Table 2). The drop in intracellular alanine concentrations when aldA was mutated and the increase when aldA was overexpressed are consistent with AldA being responsible for net alanine synthesis.
TABLE 4.
Intracellular alanine concentrations
| Strain | Carbon source | Alanine concna (mM) |
|---|---|---|
| 3841 | Glc-NH3 | 11.9 ± 0.7 |
| Succ-NH3 | 6.6 ± 1.1 | |
| RU1327 | Glc-NH3 | 7.8 ± 0.8 |
| Succ-NH3 | 1.9 ± 0.6 | |
| RU1552 | Glc-NH3 | 33.1 ± 4.2 |
| Succ-NH3 | 7.4 ± 1.3 |
Values are the means ± SEMs. All samples are averaged from at least three independent cultures. For 3841, RU1327, and RU1552, alanine concentrations are higher in glucose-grown cultures than in succinate-grown cultures (P < 0.01). Alanine concentrations are higher in strain 3841 than in RU1327 for both glucose- and succinate-grown cultures (P < 0.05) and are higher in RU1552 than in 3841 when cells are grown on glucose but not when they are grown on succinate at (P < 0.01).
DISCUSSION
The data in this paper show that aldA is induced when R. leguminosarum is grown on organic acids or alanine (Tables 2 and 3). However, alanine is a stronger inducer than organic acids, including pyruvic acid, which is the direct breakdown product of alanine. Given the importance of alanine synthesis in bacteroids and the proposal that alanine is the sole nitrogen secretion product in soybean bacteroids (36), the role of AldA in the synthesis and/or degradation of alanine is an important issue. It has already been shown that the principal pathway of alanine degradation in R. leguminosarum is via isomerization of l-alanine to d-alanine by alanine racemase (DadX) and dehydrogenation of d-alanine to pyruvate by d-alanine dehydrogenase (DadA) (1). The electron transport-linked DadA should not be confused with NADH-linked AldA. However, overexpression of aldA substituted for the Dad system in alanine catabolism (1). Thus, AldA can contribute to alanine degradation when cultures are grown on alanine as a carbon source. However, the data in this study and those of Allaway et al. (1) demonstrate that, when grown on sugars or organic acids, AldA is responsible for net alanine synthesis.
AldR was shown to positively regulate the induction of aldA and to autoregulate its own induction (Table 3). The autoregulation was discovered because aldR::gusA fusions were inactive in an aldR mutant. However, plasmids containing the aldA-aldR intergenic region also had a tendency to inactivate both aldA and aldR expression. Cultures containing aldA::gusA or aldR::gusA plasmid fusions, but which lacked GusA activity after growth under inducing conditions, also had no AldA enzyme activity. This is most likely due to the binding of AldR to the aldA-aldR intergenic regions of plasmids, preventing binding to the chromosomal region. Binding of AldR to the reporter plasmids would be abortive, since a full-length aldR gene is not present. The regulation of aldA and aldR by an autoregulatory mechanism is typical of the AsnC/Lrp class of regulators, which has been elucidated in great detail for other systems (20, 21).
The important questions for rhizobial AldA expression are not so much those about the mechanistic details of AsnC regulation, but rather those about what the regulation means for nitrogen fixation in legume nodules. Both aldR and aldA were expressed in the II/III interzone and in zone III of pea nodules, confirming widespread expression in the nodule (Fig. 1). However, a key difference between soybean and pea bacteroids is the relative expression levels, which are around 0.3 to 1 versus 0.08 μmol min−1 mg of protein−1, respectively (1, 36). Some of the differences between the reported behaviors of soybean and pea bacteroids in alanine secretion may therefore relate to AldA activity. To test this hypothesis, AldA activity was increased by expression of aldA on a stable multicopy plasmid (Table 2). The effect was to dramatically lower the symbiotic performance of pea plants, as measured by dry weight. However, the total nitrogenase activity, as measured by acetylene reduction, was unaltered. The normal activity of nitrogenase suggests that the negative impact of aldA overexpression on plant growth is unlikely to be due to aldA being generally deleterious to bacteroids. Instead, artificially altering de novo alanine synthesis appears to perturb the acquisition of nitrogen by the plant. Perhaps locking up too much of the ammonium fixed by bacteroids, as alanine does, may be detrimental to nitrogen transfer to the plant. Elevating the de novo synthesis of glutamate in bean bacteroids by overexpression of glutamate dehydrogenase also lowers symbiotic performance (23). In light of the recent proposal that amino acid cycling is essential to nitrogen fixation in pea nodules, the results presented here are highly significant. It was proposed that amino acid cycling is driven by transamination and not by de novo amino acid synthesis, as catalyzed by AldA (19). Amino acid cycling requires ammonium and an amino acid to be secreted to the plant. However, it is apparent that de novo amino acid synthesis by AldA will alter the balance of ammonium, pyruvate, and alanine and that overexpression of aldA may perturb amino acid cycling.
Overall, the role of AldA in peas seems consistent with balancing organic acid, ammonium, and amino acid levels in the cell but not with alanine being synthesized as the sole secretion product of N2 reduction by pea bacteroids. The levels of pyruvate and alanine are crucial to the function of malic enzyme and pyruvate dehydrogenase, both of which are essential for functioning of the tricarboxylic acid cycle and nitrogen fixation (7, 11). Our study showing that aldA mutants can still fix nitrogen is also in accord with this conclusion (1). However, the absence of a strong symbiotic phenotype in an aldA mutant could mean that there is a totally plastic partitioning between alanine and ammonium. Thus, alanine could still be the principal nitrogen secretion product in planta, but ammonium could replace it if alanine synthesis were prevented. The data in this paper make this possibility very unlikely, since moderate overexpression of aldA in the bacteroid is harmful to nitrogen acquisition by the plant. Finally, the complex induction of aldA, in cells grown either on organic acids or on alanine, suggests that it may have a subtle role in balancing their intracellular levels.
Acknowledgments
This work was funded by a grant from the BBSRC (to P.P.) and by an exchange program of the British Council (P.P. and U.P).
REFERENCES
- 1.Allaway, D., E. Lodwig, L. A. Crompton, M. Wood, T. R. Parsons, T. Wheeler, and P. S. Poole. 2000. Identification of alanine dehydrogenase and its role in mixed secretion of ammonium and alanine by pea bacteroids. Mol. Microbiol. 36:508-515. [DOI] [PubMed] [Google Scholar]
- 2.Bergersen, F. J., and G. L. Turner. 1967. Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim. Biophys. Acta 141:507-515. [DOI] [PubMed] [Google Scholar]
- 3.Beringer, J. E. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 84:188-198. [DOI] [PubMed] [Google Scholar]
- 4.Boesten, B., J. Batut, and P. Boistard. 1998. DctBD-dependent and independent expression of the Sinorhizobium (Rhizobium) meliloti C4-dicarboxylate transport gene (dctA) during symbiosis. Mol. Plant-Microbe Interact. 11:878-886. [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Brown, C. M., and M. J. Dilworth. 1975. Ammonia assimilation by Rhizobium cultures and bacteroids. J. Gen. Microbiol. 86:39-48. [DOI] [PubMed] [Google Scholar]
- 7.Cabanes, D., P. Boistard, and J. Batut. 2000. Symbiotic induction of pyruvate dehydrogenase genes from Sinorhizobium meliloti. Mol. Plant-Microbe Interact. 13:483-493. [DOI] [PubMed] [Google Scholar]
- 8.Cullimore, J. V., and M. J. Bennett. 1991. Nitrogen assimilation in the legume root nodule: current status of the molecular biology of the plant enzymes. Can. J. Microbiol. 38:461-466. [Google Scholar]
- 9.DelVecchio, V. G., V. Kapatral, R. J. Redkar, G. Patra, C. Mujer, T. Los, N. Ivanova, I. Anderson, A. Bhattacharyya, A. Lykidis, G. Reznik, L. Jablonski, N. Larsen, M. D'Souza, A. Bernal, M. Mazur, E. Goltsman, E. Selkov, P. H. Elzer, S. Hagius, D. O'Callaghan, J.-J. Letesson, R. Haselkorn, N. Kyrpides, and R. Overbeek. 2002. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA 99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dilworth, M. J., and A. R. Glenn. 1982. Movements of ammonia in Rhizobium leguminosarum. J. Gen. Microbiol. 128:29-37. [Google Scholar]
- 11.Driscoll, B. T., and T. M. Finan. 1993. NAD+-dependent malic enzyme of Rhizobium meliloti is required for symbiotic nitrogen fixation. Mol. Microbiol. 7:865-873. [DOI] [PubMed] [Google Scholar]
- 12.Galibert, F., T. M. Finan, S. R. Long, A. Puhler, P. Abola, F. Ampe, F. Barloy-Hubler, M. J. Barnett, A. Becker, P. Boistard, G. Bothe, M. Boutry, L. Bowser, J. Buhrmester, E. Cadieu, D. Capela, P. Chain, A. Cowie, R. W. Davis, S. Dreano, N. A. Federspiel, R. F. Fisher, S. Gloux, T. Godrie, A. Goffeau, B. Golding, J. Gouzy, M. Gurjal, I. Hernandez-Lucas, A. Hong, L. Huizar, R. W. Hyman, T. Jones, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, E. Kiss, C. Komp, V. Lalaure, D. Masuy, C. Palm, M. C. Peck, T. M. Pohl, D. Portetelle, B. Purnelle, U. Ramsperger, R. Surzycki, P. Thebault, M. Vandenbol, F. J. Vorholter, S. Weidner, D. H. Wells, K. Wong, K. C. Yeh, and J. Batut. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 13.Goodner, B., G. Hinkle, S. Gattung, N. Miller, M. Blanchard, B. Qurollo, B. S. Goldman, Y. Cao, M. Askenazi, C. Halling, L. Mullin, K. Houmiel, J. Gordon, M. Vaudin, O. Iartchouk, A. Epp, F. Liu, C. Wollam, M. Allinger, D. Doughty, C. Scott, C. Lappas, B. Markelz, C. Flanagan, C. Crowell, J. Gurson, C. Lomo, C. Sear, G. Strub, C. Cielo, and S. Slater. 2001. Genome sequence of the plant pathogen and biotechnology agent Agrobacterium tumefaciens C58. Science 294:2323-2328. [DOI] [PubMed] [Google Scholar]
- 14.Gottfert, M., S. Rothlisberger, C. Kundig, C. Beck, R. Marty, and H. Hennecke. 2001. Potential symbiosis-specific genes uncovered by sequencing a 410-kilobase DNA region of the Bradyrhizobium japonicum chromosome. J. Bacteriol. 183:1405-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston, A. W. B., and J. E. Beringer. 1975. Identification of the Rhizobium strains in pea root nodules using genetic markers. J. Gen. Microbiol. 87:343-350. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7:331-338. [DOI] [PubMed] [Google Scholar]
- 17.Kurz, W. G. W., D. A. Rokosh, and T. A. LaRue. 1975. Enzymes of ammonia assimilation in Rhizobium leguminosarum bacteroids. Can. J. Microbiol. 21:1009-1012. [DOI] [PubMed] [Google Scholar]
- 18.Li, Y., R. Parsons, D. A. Day, and F. J. Bergersen. 2002. Reassessment of major products of N2 fixation by bacteroids from soybean root nodules. Microbiology 148:1959-1966. [DOI] [PubMed] [Google Scholar]
- 19.Lodwig, E. M., A. H. F. Hosie, A. Bourdes, K. Findlay, D. Allaway, R. Karunakaran, J. A. Downie, and P. S. Poole. 2003. Amino-acid cycling drives nitrogen fixation in the legume-Rhizobium symbiosis. Nature 422:722-726. [DOI] [PubMed] [Google Scholar]
- 20.Madhusudhan, K. T., K. L. Hester, V. Friend, and J. R. Sokatch. 1997. Transcriptional activation of the bkd operon of Pseudomonas putida by BkdR. J. Bacteriol. 179:1992-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madhusudhan, K. T., J. Luo, and J. R. Sokatch. 1999. In vitro transcriptional studies of the bkd operon of Pseudomonas putida: l-branched-chain amino acids and d-leucine are the inducers. J. Bacteriol. 181:2889-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathew, E., J. Zhi, and M. Freundlich. 1996. Lrp is a direct repressor of the dad operon in Escherichia coli. J. Bacteriol. 178:7234-7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendoza, A., B. Valderrama, A. Leija, and J. Mora. 1998. NifA-dependent expression of glutamate dehydrogenase in Rhizobium etli modifies nitrogen partitioning during symbiosis. Mol. Plant-Microbe Interact. 11:83-90. [DOI] [PubMed] [Google Scholar]
- 24.Poole, P. S., A. Blyth, C. J. Reid, and K. Walters. 1994. myo-Inositol catabolism and catabolite regulation in Rhizobium leguminosarum bv. viciae. Microbiology 140:2787-2795. [Google Scholar]
- 25.Poole, P. S., N. A. Schofield, C. J. Reid, E. M. Drew, and D. L. Walshaw. 1994. Identification of chromosomal genes located downstream of dctD that affect the requirement for calcium and the lipopolysaccharide layer of Rhizobium leguminosarum. Microbiology 140:2797-2809. [DOI] [PubMed] [Google Scholar]
- 26.Prell, J., B. Boesten, P. Poole, and U. B. Priefer. 2002. The Rhizobium leguminosarum bv. viciae VF39 gamma aminobutyrate (GABA) aminotransferase gene (gabT) is induced by GABA and highly expressed in bacteroids. Microbiology 148:615-623. [DOI] [PubMed] [Google Scholar]
- 27.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 28.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Schafer, A., A. Tauch, W. Jager, J. Kalinowski, G. Thierbach, and A. Puhler. 1994. Small mobilizable multipurpose cloning vectors derived from the Escherichia coli plasmids pk18 and pk19—selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 31.Scharff, A. M., H. Egsgaard, P. E. Hansen, and L. Rosendahl. 2003. Exploring symbiotic nitrogen fixation and assimilation in pea root nodules by in vivo 15N nuclear magnetic resonance spectroscopy and liquid chromatography-mass spectrometry. Plant Physiol. 131:367-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, M. T., and D. W. Emerich. 1993. Alanine dehydrogenase from soybean nodule bacteroids—kinetic mechanism and pH studies. J. Biol. Chem. 268:10746-10753. [PubMed] [Google Scholar]
- 33.Smith, M. T., and D. W. Emerich. 1993. Alanine dehydrogenase from soybean nodule bacteroids: purification and properties. Arch. Biochem. Biophys. 304:379-385. [DOI] [PubMed] [Google Scholar]
- 34.Streeter, J. G. 1989. Estimation of ammonium concentration in the cytosol of soybean nodules. Plant Physiol. 90:779-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Temple, S. J., C. P. Vance, and J. S. Gantt. 1998. Glutamate synthase and nitrogen assimilation. Trends Plant Sci. 3:51-56. [Google Scholar]
- 36.Waters, J. K., B. L. Hughes, L. C. Purcell, K. O. Gerhardt, T. P. Mawhinney, and D. W. Emerich. 1998. Alanine, not ammonia, is excreted from N2-fixing soybean nodule bacteroids. Proc. Natl. Acad. Sci. USA 95:12038-12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson, R. J., and V. K. Rastogi. 1993. Cloning and nucleotide sequencing of Rhizobium meliloti aminotransferase genes: an aspartate aminotransferase required for symbiotic nitrogen fixation is atypical. J. Bacteriol. 175:1919-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinstein, M., R. C. Roberts, and D. R. Helinski. 1992. A region of the broad-host-range plasmid RK2 causes stable in planta inheritance of plasmids in Rhizobium meliloti cells isolated from alfalfa root nodules. J. Bacteriol. 174:7486-7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wild, J., and T. Klopotowski. 1981. d-Amino acid dehydrogenase of Escherichia coli K12: positive selection of mutants defective in the enzyme activity and localization of the structural gene. Mol. Gen. Genet. 181:373-378. [DOI] [PubMed] [Google Scholar]
- 40.Willins, D. A., C. Ryan, J. V. Platko, and J. M. Calvo. 1991. Characterization of Lrp, an Escherichia coli regulatory protein that mediates a global response to leucine. J. Biol. Chem. 266:10768-10774. [PubMed] [Google Scholar]