Abstract
Aims
Cardiac hybrid imaging by fusing single-photon emission computed tomography (SPECT) myocardial perfusion imaging with coronary computed tomography angiography (CCTA) provides important complementary diagnostic information for coronary artery disease (CAD) assessment. We aimed at assessing the impact of cardiac hybrid imaging on the choice of treatment strategy selection for CAD.
Methods and results
Three hundred and eighteen consecutive patients underwent a 1 day stress/rest 99mTc-tetrofosmin SPECT and a CCTA on a separate scanner for evaluation of CAD. Patients were divided into one of the following three groups according to findings in the hybrid images obtained by fusing SPECT and CCTA: (i) matched finding of stenosis by CCTA and corresponding reversible SPECT defect; (ii) unmatched CCTA and SPECT finding; (iii) normal finding by both CCTA and SPECT. Follow-up was confined to the first 60 days after hybrid imaging as this allows best to assess treatment strategy decisions including the revascularization procedure triggered by its findings. Hybrid images revealed matched, unmatched, and normal findings in 51, 74, and 193 patients. The revascularization rate within 60 days was 41, 11, and 0% for matched, unmatched, and normal findings, respectively (P< 0.001 for all inter-group comparisons).
Conclusion
Cardiac hybrid imaging with SPECT and CCTA provides an added clinical value for decision making with regard to treatment strategy for CAD.
Keywords: Coronary artery disease, SPECT/CT fusion imaging, Coronary CT angiography, Myocardial perfusion imaging, Treatment strategy, Clinical decision making
Introduction
Although coronary angiography has remained the gold standard for the diagnosis of coronary artery disease (CAD), the assessment of functional lesion relevance cannot be based on purely morphologic criteria. Indeed, many factors other than lumen size which cannot be comprehensively evaluated by coronary angiography alone determine whether or not a lesion induces myocardial ischaemia.1 Nevertheless, in clinical practice, a luminal narrowing greater than 50% is widely used as a cut-off to define the presence of relevant CAD. For prognostically relevant target vessel revascularization comprehensive evaluation including proof of ischaemia is mandatory, because interventions on non-flow limiting stenoses confer no prognostic or symptomatic benefit to the patients, but are still associated with the risk of the intervention.2,3 As a consequence, non-invasive functional assessment with single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) has been suggested as gatekeeper for coronary angiography.4,5 Up until recently, complimentary information on coronary artery anatomy and the degree and site of myocardial ischaemia has been integrated mentally by combining the information obtained from invasive coronary angiography and SPECT-MPI, respectively. Unfortunately, however, standard myocardial distribution territories correspond in only 50–60% to the real anatomic tree.6 Although several attempts of software-based hybrid imaging integrating invasive coronary angiography and SPECT have been made to better match the coronary arteries with the corresponding perfused myocardial territories, this was not widely implemented into clinical practice, as its invasiveness does not allow pre-interventional non-invasive decision making.6–9 With the introduction of coronary computed tomography angiography (CCTA), a non-invasive method for accurate visualization of coronary anatomy is now available.10 This has paved the way for purely non-invasive SPECT/CT hybrid imaging,11 directly relating individual myocardial perfusion territories to the subtending coronary artery. Although the integration of SPECT or PET devices with multislice CT scanners into hybrid devices has increased the interest in cardiac fusion imaging12,13 and recent results confirm its diagnostic strengths,14,15 the added clinical value of cardiac hybrid imaging in the decision making for treatment strategy selection has not yet been documented. The aim of the present study was to evaluate the impact of fused SPECT and CCTA cardiac imaging on the subsequent treatment strategy of CAD.
Methods
Patients
We included 318 consecutive patients who were referred for the evaluation of known or suspected CAD by SPECT-MPI and CCTA within 1 ± 3 days. The results of the fused SPECT-MPI and CCTA images were reported to the referring physician who took into account the hybrid imaging test result, as well as the clinical history and the symptoms to make a decision towards invasive coronary angiography or conservative management. Patients were followed for revascularization procedures, including percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG) after completing cardiac hybrid imaging. Follow-up was confined to the first 60 days after hybrid imaging as this allows best to assess the treatment strategy decisions including the revascularization procedure directly triggered by its findings, while later interventions are not directly triggered by the hybrid imaging results but rather reflect events of the natural course of the disease process. The study protocol was approved by the institutional review board (local ethics committee) and written informed consent was obtained from each patient before enrolment. The study population was in part shared with the recently reported registry on long-term outcome prediction by hybrid SPECT/CT imaging.16 The pre-test likelihood of CAD was determined using the Diamond and Forrester method, with a risk threshold of <13.4% for low risk, between 13.4 and 87.2% for intermediate risk, and >87.2% for high risk, as previously reported.17
Hybrid imaging
All patients underwent a 1-day stress/rest SPECT-MPI protocol with standard adenosine stress (0.14 mg/kg/min over 6 min)18 on a dual-head gamma camera (Millenium VG and Hawkeye or Ventri, both GE Healthcare, Milwaukee, Wisconsin) as previously reported.19 On a stand-alone 64-slice CT scanner (LightSpeed VCT, GE Healthcare), a low-dose CT was performed for attenuation correction of SPECT-MPI.20 In addition, all patients underwent contrast-enhanced CCTA with helical scanning (n= 248) or prospective ECG triggering (n= 70) as previously described in detail.21–23 In order to achieve a target heart rate <65 bpm, intravenous metoprolol (5–20 mg) was administered prior to the CCTA examination if necessary. Patients with atrial fibrillation were not referred for CCTA. Furthermore, all patients received 2.5 mg sublingual isosorbiddinitrate 2 min prior to the scan.
Images from SPECT-MPI and CCTA were fused on a dedicated workstation (Advantage Workstation 4.3, GE Healthcare) using the CardIQ Fusion software package (GE Healthcare) as previously described in detail.24 Briefly, an optimized alignment tool allows projection of the SPECT image on the left ventricular epicardial surface obtained from the CCTA. The 3D volume rendered fusion images allow a panoramic view of the coronary artery tree projected onto the left ventricular myocardial perfusion territories. Then images can be displayed in freely selectable angles and displayed in standard anterior, posterior, lateral, and apical view for standardized documentation and reporting (Figure 1). Radiation dose for SPECT-MPI was calculated as 99mTc-tetrofosmin activity times 7.9 mSv/GBq, while effective radiation dose for CCTA was estimated as dose-length product times a conversion coefficient for the chest k = 0.014 mSv/(mGy·cm).
Figure 1.
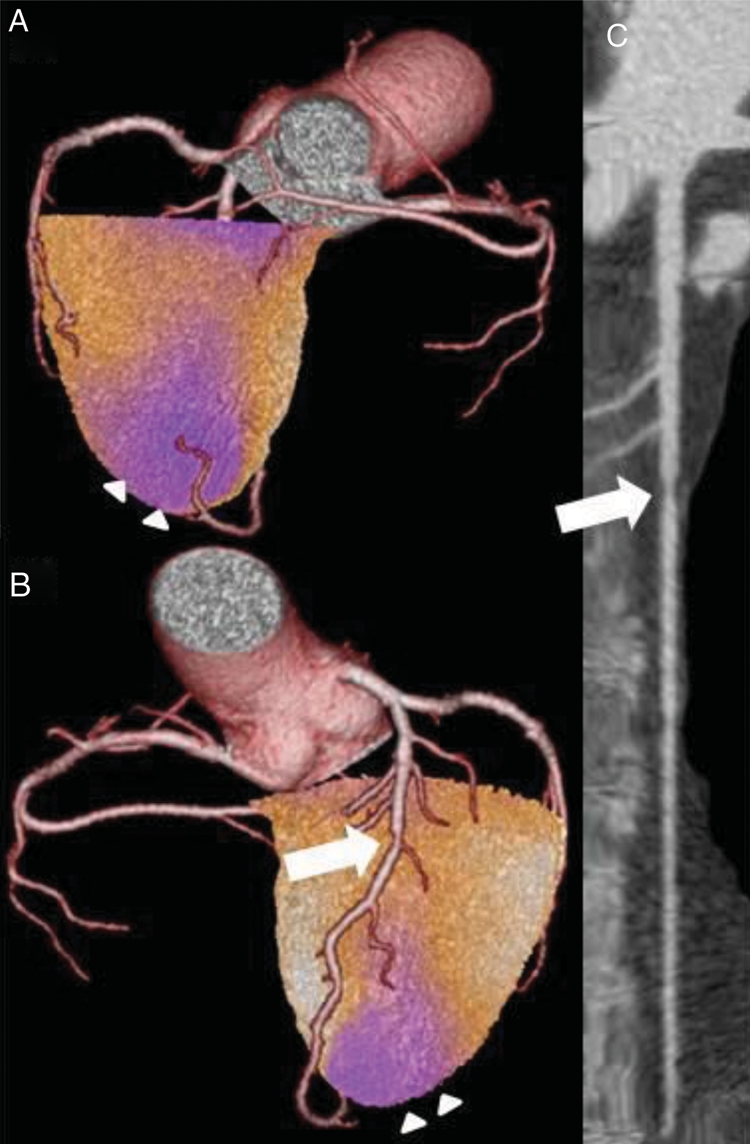
Matched hybrid finding in single-photon emission computed tomography/computed tomography showing in the posterior (A) and anterior (B) view an ischaemia of the apex (arrowheads) in a territory subtended by a stenotic LAD (arrow) as further documented in the multiplanar coronary computed tomography angiography reconstruction (C).
Hybrid image interpretation
Two experienced nuclear cardiologists analysed the fused SPECT and CCTA images by consensus with regard to functionally relevant coronary stenoses. For each patient, myocardial tomograms were divided into 20 segments. Following 5-point system was used to score the segments: 0 = normal, 1 = equivocal, 2 = moderate, 3 = severe reduction of radioisotope uptake, 4 = absence of detectable tracer in a segment. An abnormal scan was defined as one in which two or more segments had stress scores ≥2. A scan was classified as reversible perfusion defect if a stress defect was associated with a rest score ≤1 or a stress defect score of 4 with a rest score of 2. As ischaemia-driven patient management is most evidence-based only reversible defects were considered for further analysis.
A matched SPECT/CT hybrid imaging finding was defined as a reversible SPECT-MPI defect in a territory subtended by a stenotic coronary artery (defined as narrowing of the coronary luminal diameter ≥50%). All other combinations of pathologic findings were classified as unmatched. Consequently, in order to assess the clinical value of hybrid images on decision making for treatment strategy selection, all patients were assigned to one of the following three categories of findings: (i) matched: CCTA and matched (reversible) SPECT findings as defined above; (ii) unmatched: any unmatched pathologic finding from CCTA and/or SPECT; (iii) normal: no pathologic finding, i.e. no stenosis by CCTA and no (fixed or reversible) defect by SPECT.
Statistical analysis
SPSS software (SPSS 15.0, SPSS Inc.) was used for statistical testing. Quantitative variables were expressed as mean ± standard deviation and categorical variables as frequencies or percentages. The Chi-square test was used to compare the revascularization rates between the different patient groups. P-values from two-sided tests of less than 0.05 were considered statistically significant.
Results
Patient characteristics
Coronary computed tomography angiography and SPECT were successfully performed in 318 patients. Baseline characteristics of the study population are given in Table 1.
Table 1.
Baseline characteristics
| n | 318 |
| Age (years) | 61 ± 11 |
| Males, n (%) | 213 (67) |
| Risk factors, n (%) | |
| Hypertension | 177 (56) |
| Dyslipidaemia | 143 (45) |
| Diabetes | 44 (14) |
| Smoking | 92 (29) |
| Positive family history | 87 (27) |
| Reason referral, n (%) | |
| Pre-operative evaluation, equivocal/abnormal stress test | 122 (38) |
| Non-anginal chest pain | 58 (18) |
| Atypical angina | 60 (20) |
| Typical angina | 46 (14) |
| Dyspnoea | 32 (10) |
| Pre-test likelihood of CAD, n (%) | |
| Low | 24 (10) |
| Intermediate | 182 (73) |
| High | 44 (17) |
| Known CAD, n (%) | 68 (21) |
| Previous MI | 19 (6) |
CAD, coronary artery disease; MI, myocardial infarction.
Single-photon emission computed tomography and coronary computed tomography angiography findings
Single-photon emission computed tomography revealed normal perfusion in 248 patients (78%) and an abnormal perfusion in 70 patients (22%). A normal CCTA examination was observed in 209 patients (66%), while CCTA identified a significant stenosis in 109 patients (34%). Matched pathologic hybrid findings (CCTA stenosis with corresponding reversible MPI defect) were observed in 51 patients (16%). Unmatched findings were present in 74 patients (23%), while the remaining patients were normal by both imaging methods (n= 193, 61%).
The effective radiation dose for stress/rest SPECT-MPI was 10.1 ± 0.9 mSv, while the estimated radiation dose for the CCTA was 17.9 ± 5.8 mSv when helical scanning was used (n= 248). After introducing prospective triggering for CCTA, the effective radiation dose was systematically recorded and resulted in 1.9 ± 0.5 mSv (n= 70).
Impact on patient management
During follow-up, of the patients with matched findings (n= 51) by hybrid cardiac imaging, 31 (61%) were further evaluated by invasive coronary angiography of whom 21 underwent a revascularization procedure (PCI: n= 20; CABG: n= 1), thus resulting in a revascularization rate of 41%. In 15 of the 20 patients not further evaluated by invasive angiography, the reason for not being referred to invasive angiography was the limited extent of the ischaemia, which was significantly smaller (2.7 ± 5.0%) than in those patients referred to angiography (9.0 ± 9.1%, P< 0.001). In the remaining five patients, other reasons for conservative decision were observed such as for example difficult lesion accessibility.
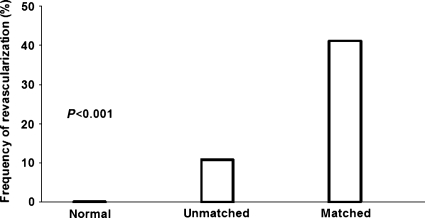
In the patient group with unmatched findings (n= 74), 15 (20%) underwent invasive coronary angiography, of whom in 8 patients revascularization was performed (PCI: n= 6; CABG: n= 2). This resulted in a revascularization rate of 11% which was substantially lower than in the group with matched findings (i.e. 41%; P< 0.001). In contrast, in the normal group, no invasive coronary angiography was performed (Figure 2).
Figure 2.
The frequency of revascularization was significantly different among the three patient groups, i.e. that with normal, unmatched, and matched hybrid findings (P< 0.001 by Chi-square test).
Of note, in the matched groups, the yield of CAD per angiography was 90% with a PCI rate per angiography of 68%, compared with 80% and 53% in the unmatched group.
Discussion
The present study shows that cardiac hybrid imaging with fused SPECT/CT images provides an added clinical value in the decision-making process leading to adequate treatment strategy.
Despite the fact that invasive coronary angiography is costly and associated with a small, but significant rate of procedural and in hospital morbidity and mortality,25,26 so far, invasive coronary angiography has remained the gold standard for the detection of CAD and decision-making for or against a revascularization procedure. Nevertheless, it has been suggested that for best clinical practice, myocardial perfusion should also be assessed in addition to the anatomical information provided by angiography. Indeed, only under these conditions may an appropriate and evidence-based clinical decision-making process ensue. This has been recently confirmed by the prospective randomized FAME trial using coronary pressure measurements during angiography.2 Myocardial perfusion imaging with SPECT is a well-established non-invasive method for the assessment of myocardial ischaemia. A recently introduced non-invasive method for assessing coronary anatomy is CCTA. Although CCTA is accurate and helpful in risk stratification,10,27 it has been recognized that CCTA alone may not replace conventional invasive CA as it essentially also only provides anatomical information. However, the latest advance in non-invasive cardiac imaging allows to obtain hybrid images from separately acquired CCTA and SPECT-MPI images.28 This has been shown to be helpful in clinical routine, as it allows non-invasive and comprehensive assessment of CAD based on anatomical and functional information and thus may contribute to avoid unnecessary invasive angiographies.5 A previous study documented that hybrid imaging may indeed provide added diagnostic information.28 However, that study evaluated patients already selected for invasive coronary angiography. Thus, it remained unclear whether and how the findings obtained by hybrid imaging may have impacted on the clinical decision process. Moreover, that study used fixed and reversible defects on hybrid images to assess the clinical value of hybrid imaging. In contrast, the current study used only reversible defects because ischaemia in territories subtended by stenotic coronaries constitutes the primary indication for revascularization, while scar tissue does not. This may have contributed to the fact that hybrid findings had a major impact on clinical decision making in patients evaluated for CAD. The substantial difference in intervention frequencies between matched and unmatched findings reflects the difficulty in determining the appropriate therapy for patients with chronic stable CAD as, for example, seen in the COURAGE trial.29 This is further evidenced by the fact that even in the group with matched findings, not all lesions were revascularized as optimal medical treatment offers an excellent alternative in many patients. Hybrid imaging provides a solid ground on which decision making towards revascularization can be based. The correct identification of patients who qualify for invasive coronary angiography and potentially for intervention by hybrid imaging may help saving the cost and the risk of unnecessary diagnostic angiography and ineffective revascularization. In fact, the yield of CAD by invasive angiography after initial evaluation by hybrid imaging was 90%, comparing favourably with the low yield of 39% recently reported in a large registry of elective diagnostic angiographies.30 Consequently, the improved non-invasive evaluation by hybrid imaging increased the intervention rate per diagnostic angiography to 68% in the group with matched findings, a proportion which is substantially higher than figures reported in registries of most European countries. For example, in the German registry, a PCI or a bypass was only performed in 36% of the patients undergoing an elective invasive angiography.31
We acknowledge the following limitations to our study: Although patients were included consecutively, there was no randomization to different treatment strategies as in this prospective observational study the impact of cardiac hybrid imaging on clinical decision-making in daily clinical routine was assessed. Further prospective clinical studies are necessary to define whether this hybrid SPECT/CT-guided approach has a positive effect on long-term outcome. In parallel, this would offer an assessment of cost efficacy. From the present data, we cannot prove that without non-invasive imaging, all patients would have been referred for invasive angiography. Nevertheless, the fact that 90% of the present study population represent an intermediate to high-risk population seems to support the notion that in 80% of the patients of the present study with unmatched findings an invasive angiography was avoided, although no data on the clinical benefit were obtained. Finally, the additive radiation burden from combined nuclear and CT scanning is a limitation of this approach as values of up to 41 mSv have been initially reported.32 However, the mean radiation dose in the first 248 patients was substantially lower, namely 28 mSv. Furthermore, radiation dose can be reduced significantly by implementing dose reduction techniques for SPECT33–35 and by using prospective ECG-triggering for CCTA.22,23,36 Of note, the latter has been used for the last 70 patients in the present study, resulting in an effective radiation dose of 1.9 ± 0.5 mSv for CCTA reducing the cumulative effective dose of the entire SPECT/CT study to 12 mSv. Combining optimized SPECT and CT will allow to reach values below 5 mSv34 and in optimal cases below 3 mSv for such hybrid examinations.37
Thus, in summary, cardiac hybrid imaging with SPECT and CCTA is a feasible tool providing an added clinical value for decision making with regard to treatment strategy for CAD.
Conflict of interest: none declared.
Funding
The study was supported by a grant from the Swiss National Science Foundation. Funding to pay the Open Access publication charges for this article was provided by the Swiss National Science Foundation.
Acknowledgements
We are grateful to Patrick von Schulthess, Ennio Mueller, Edlira Loga, Mirjam De Bloeme, Josephine Trinckauf, Sabine Knöfel, and Désirée Beutel for their excellent technical support.
References
- 1.Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, Maccarthy PA, Van't Veer M, Pijls NH. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 2.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 3.Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van't Veer M, Bar F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–2111. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 4.Hoilund-Carlsen PF, Johansen A, Christensen HW, Vach W, Moldrup M, Bartram P, Veje A, Haghfelt T, Eval MIL. Potential impact of myocardial perfusion scintigraphy as gatekeeper for invasive examination and treatment in patients with stable angina pectoris: observational study without post-test referral bias. Eur Heart J. 2006;27:29–34. doi: 10.1093/eurheartj/ehi503. [DOI] [PubMed] [Google Scholar]
- 5.Gaemperli O, Husmann L, Schepis T, Koepfli P, Valenta I, Jenni W, Alkadhi H, Luscher TF, Kaufmann PA. Coronary CT angiography and myocardial perfusion imaging to detect flow-limiting stenoses: a potential gatekeeper for coronary revascularization? Eur Heart J. 2009;30:2921–2929. doi: 10.1093/eurheartj/ehp304. [DOI] [PubMed] [Google Scholar]
- 6.Schindler TH, Magosaki N, Jeserich M, Oser U, Krause T, Fischer R, Moser E, Nitzsche E, Zehender M, Just H, Solzbach U. Fusion imaging: combined visualization of 3D reconstructed coronary artery tree and 3D myocardial scintigraphic image in coronary artery disease. Int J Card Imaging. 1999;15:357–368. doi: 10.1023/a:1006232407637. discussion 369–370. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura Y, Fukuchi K, Katafuchi T, Sagou M, Oka H, Ishida Y, Murase K. Superimposed display of coronary artery on gated myocardial perfusion scintigraphy. J Nucl Med. 2004;45:1444–1449. [PubMed] [Google Scholar]
- 8.Peifer JW, Ezquerra NF, Cooke CD, Mullick R, Klein L, Hyche ME, Garcia EV. Visualization of multimodality cardiac imagery. IEEE Trans Biomed Eng. 1990;37:744–756. doi: 10.1109/10.102790. [DOI] [PubMed] [Google Scholar]
- 9.Faber TL, Santana CA, Garcia EV, Candell-Riera J, Folks RD, Peifer JW, Hopper A, Aguade S, Angel J, Klein JL. Three-dimensional fusion of coronary arteries with myocardial perfusion distributions: clinical validation. J Nucl Med. 2004;45:745–753. [PubMed] [Google Scholar]
- 10.Schroeder S, Achenbach S, Bengel F, Burgstahler C, Cademartiri F, de Feyter P, George R, Kaufmann P, Kopp AF, Knuuti J, Ropers D, Schuijf J, Tops LF, Bax JJ. Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J. 2008;29:531–556. doi: 10.1093/eurheartj/ehm544. [DOI] [PubMed] [Google Scholar]
- 11.Flotats A, Knuuti J, Gutberlet M, Marcassa C, Bengel FM, Kaufmann PA, Rees MR, Hesse B. Hybrid cardiac imaging: SPECT/CT and PET/CT. A joint position statement by the European Association of Nuclear Medicine (EANM), the European Society of Cardiac Radiology (ESCR) and the European Council of Nuclear Cardiology (ECNC) Eur J Nucl Med Mol Imaging. 2011;38:201–212. doi: 10.1007/s00259-010-1586-y. [DOI] [PubMed] [Google Scholar]
- 12.Santana CA, Garcia EV, Faber TL, Sirineni GK, Esteves FP, Sanyal R, Halkar R, Ornelas M, Verdes L, Lerakis S, Ramos JJ, Aguade-Bruix S, Cuellar H, Candell-Riera J, Raggi P. Diagnostic performance of fusion of myocardial perfusion imaging (MPI) and computed tomography coronary angiography. J Nucl Cardiol. 2009;16:201–211. doi: 10.1007/s12350-008-9019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato A, Nozato T, Hikita H, Miyazaki S, Takahashi Y, Kuwahara T, Takahashi A, Hiroe M, Aonuma K. Incremental value of combining 64-slice computed tomography angiography with stress nuclear myocardial perfusion imaging to improve noninvasive detection of coronary artery disease. J Nucl Cardiol. 2010;17:19–26. doi: 10.1007/s12350-009-9150-5. [DOI] [PubMed] [Google Scholar]
- 14.Namdar M, Hany TF, Koepfli P, Siegrist PT, Burger C, Wyss CA, Luscher TF, von Schulthess GK, Kaufmann PA. Integrated PET/CT for the assessment of coronary artery disease: a feasibility study. J Nucl Med. 2005;46:930–935. [PubMed] [Google Scholar]
- 15.Kajander S, Joutsiniemi E, Saraste M, Pietila M, Ukkonen H, Saraste A, Sipila HT, Teras M, Maki M, Airaksinen J, Hartiala J, Knuuti J. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation. 2010;122:603–613. doi: 10.1161/CIRCULATIONAHA.109.915009. [DOI] [PubMed] [Google Scholar]
- 16.Pazhenkottil AP, Nkoulou RN, Ghadri JR, Herzog BA, Buechel RR, Kuest SM, Wolfrum M, Fiechter M, Husmann L, Gaemperli O, Kaufmann PA. Prognostic value of cardiac hybrid imaging integrating single-photon emission computed tomography with coronary computed tomography angiography. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr047. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–1358. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 18.Hesse B, Tagil K, Cuocolo A, Anagnostopoulos C, Bardies M, Bax J, Bengel F, Busemann Sokole E, Davies G, Dondi M, Edenbrandt L, Franken P, Kjaer A, Knuuti J, Lassmann M, Ljungberg M, Marcassa C, Marie PY, McKiddie F, O'Connor M, Prvulovich E, Underwood R, van Eck-Smit B. EANM/ESC procedural guidelines for myocardial perfusion imaging in nuclear cardiology. Eur J Nucl Med Mol Imaging. 2005;32:855–897. doi: 10.1007/s00259-005-1779-y. [DOI] [PubMed] [Google Scholar]
- 19.Fleischmann S, Koepfli P, Namdar M, Wyss CA, Jenni R, Kaufmann PA. Gated (99m)Tc-tetrofosmin SPECT for discriminating infarct from artifact in fixed myocardial perfusion defects. J Nucl Med. 2004;45:754–759. [PubMed] [Google Scholar]
- 20.Schepis T, Gaemperli O, Koepfli P, Ruegg C, Burger C, Leschka S, Desbiolles L, Husmann L, Alkadhi H, Kaufmann PA. Use of coronary calcium score scans from stand-alone multislice computed tomography for attenuation correction of myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging. 2007;34:11–19. doi: 10.1007/s00259-006-0173-8. [DOI] [PubMed] [Google Scholar]
- 21.Gaemperli O, Schepis T, Valenta I, Koepfli P, Husmann L, Scheffel H, Leschka S, Eberli FR, Luscher TF, Alkadhi H, Kaufmann PA. Functionally relevant coronary artery disease: comparison of 64-section CT angiography with myocardial perfusion SPECT. Radiology. 2008;248:414–423. doi: 10.1148/radiol.2482071307. [DOI] [PubMed] [Google Scholar]
- 22.Husmann L, Valenta I, Gaemperli O, Adda O, Treyer V, Wyss CA, Veit-Haibach P, Tatsugami F, von Schulthess GK, Kaufmann PA. Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J. 2008;29:191–197. doi: 10.1093/eurheartj/ehm613. [DOI] [PubMed] [Google Scholar]
- 23.Herzog BA, Husmann L, Burkhard N, Gaemperli O, Valenta I, Tatsugami F, Wyss CA, Landmesser U, Kaufmann PA. Accuracy of low-dose computed tomography coronary angiography using prospective electrocardiogram-triggering: first clinical experience. Eur Heart J. 2008;29:3037–3042. doi: 10.1093/eurheartj/ehn485. [DOI] [PubMed] [Google Scholar]
- 24.Gaemperli O, Schepis T, Kalff V, Namdar M, Valenta I, Stefani L, Desbiolles L, Leschka S, Husmann L, Alkadhi H, Kaufmann PA. Validation of a new cardiac image fusion software for three-dimensional integration of myocardial perfusion SPECT and stand-alone 64-slice CT angiography. Eur J Nucl Med Mol Imaging. 2007;34:1097–1106. doi: 10.1007/s00259-006-0342-9. [DOI] [PubMed] [Google Scholar]
- 25.Pepine CJ, Allen HD, Bashore TM, Brinker JA, Cohn LH, Dillon JC, Hillis LD, Klocke FJ, Parmley WW, Ports TA. ACC/AHA guidelines for cardiac catheterization and cardiac catheterization laboratories. American College of Cardiology/American Heart Association Ad Hoc Task Force on Cardiac Catheterization. Circulation. 1991;84:2213–2247. doi: 10.1161/01.cir.84.5.2213. [DOI] [PubMed] [Google Scholar]
- 26.Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, Legako RD, Leon DF, Murray JA, Nissen SE, Pepine CJ, Watson RM, Ritchie JL, Gibbons RJ, Cheitlin MD, Gardner TJ, Garson A, Jr, Russell RO, Jr, Ryan TJ, Smith SC., Jr. ACC/AHA guidelines for coronary angiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation. 1999;99:2345–2357. doi: 10.1161/01.cir.99.17.2345. [DOI] [PubMed] [Google Scholar]
- 27.Rubinshtein R, Halon DA, Gaspar T, Peled N, Lewis BS. Cardiac computed tomographic angiography for risk stratification and prediction of late cardiovascular outcome events in patients with a chest pain syndrome. Int J Cardiol. 2009;137:108–115. doi: 10.1016/j.ijcard.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 28.Gaemperli O, Schepis T, Valenta I, Husmann L, Scheffel H, Duerst V, Eberli FR, Luscher TF, Alkadhi H, Kaufmann PA. Cardiac image fusion from stand-alone SPECT and CT: clinical experience. J Nucl Med. 2007;48:696–703. doi: 10.2967/jnumed.106.037606. [DOI] [PubMed] [Google Scholar]
- 29.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 30.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Buuren F, Horstkotte D. 24. Bericht über die Leistungszahlen der Herzkatheterlabore in der Bundesrepublik Deutschland. Der Kardiologe. 2009;3:512–518. [Google Scholar]
- 32.Rispler S, Keidar Z, Ghersin E, Roguin A, Soil A, Dragu R, Litmanovich D, Frenkel A, Aronson D, Engel A, Beyar R, Israel O. Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol. 2007;49:1059–1067. doi: 10.1016/j.jacc.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 33.Husmann L, Herzog BA, Gaemperli O, Tatsugami F, Burkhard N, Valenta I, Veit-Haibach P, Wyss CA, Landmesser U, Kaufmann PA. Diagnostic accuracy of computed tomography coronary angiography and evaluation of stress-only single-photon emission computed tomography/computed tomography hybrid imaging: comparison of prospective electrocardiogram-triggering vs. retrospective gating. Eur Heart J. 2009;30:600–607. doi: 10.1093/eurheartj/ehn536. [DOI] [PubMed] [Google Scholar]
- 34.Pazhenkottil AP, Herzog BA, Husmann L, Buechel RR, Burger IA, Valenta I, Landmesser U, Wyss CA, Kaufmann PA. Non-invasive assessment of coronary artery disease with CT coronary angiography and SPECT: a novel dose-saving fast-track algorithm. Eur J Nucl Med Mol Imaging. 2010;37:522–527. doi: 10.1007/s00259-009-1273-z. [DOI] [PubMed] [Google Scholar]
- 35.Valenta I, Treyer V, Husmann L, Gaemperli O, Schindler MJ, Herzog BA, Veit-Heibach P, Buechel RR, Nkoulou R, Pazhenkottil AP, Kaufmann PA. New reconstruction algorithm allows shortened acquisition time for myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging. 2010;37:750–757. doi: 10.1007/s00259-009-1300-0. [DOI] [PubMed] [Google Scholar]
- 36.Achenbach S, Marwan M, Ropers D, Schepis T, Pflederer T, Anders K, Kuettner A, Daniel WG, Uder M, Lell MM. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J. 2010;31:340–346. doi: 10.1093/eurheartj/ehp470. [DOI] [PubMed] [Google Scholar]
- 37.Herzog BA, Husmann L, Landmesser U, Kaufmann PA. Low-dose computed tomography coronary angiography and myocardial perfusion imaging: cardiac hybrid imaging below 3 mSv. Eur Heart J. 2009;30:644. doi: 10.1093/eurheartj/ehn490. [DOI] [PubMed] [Google Scholar]