Abstract
Aims
A family history of premature coronary artery disease (CAD) in an apparently healthy individual conveys an increased risk of future CAD. The extent to which inducible myocardial ischaemia exists and is associated with long-term incident CAD in apparently healthy siblings of early-onset CAD patients is unknown.
Methods and results
Asymptomatic siblings (n = 1287, aged 30–59 years) of patients with onset of CAD <60 years of age underwent risk factor screening and maximal graded treadmill testing with nuclear perfusion imaging, and were followed for incident CAD events for up to 25 years. Incident CAD occurred in 15.2% of siblings (68% acute coronary syndromes); mean time to first CAD event was 8.2 ± 5.2 years. Inducible ischaemia was highly prevalent in male siblings (26.9%), and was independently associated with incident CAD. Male siblings ≥40 years of age who were low or intermediate risk by traditional risk assessment, had a prevalence of inducible ischaemia and a 10-year risk of incident CAD that were near or ≥20%. In female siblings ≥40 years of age, the presence of inducible ischaemia was also independently associated with incident CAD, but the prevalence of inducible ischaemia was markedly lower, as was the risk of incident CAD.
Conclusion
Inducible ischaemia is highly prevalent in male siblings, suggesting a previously unknown long quiescent period before the occurrence of a clinical event. While inducible ischaemia is associated with a worse prognosis, male siblings with negative tests still bear a high risk of incident disease, such that we propose that in male siblings over 40 years of age, aggressive primary prevention interventions be instituted without nuclear testing. For women, the prevalence of ischaemia was so low as to not warrant screening, but the incidence of CAD was high enough to at least warrant lifestyle interventions.
Keywords: Ischaemia, Coronary artery disease, Outcomes, Asymptomatic, Primary prevention, Risk assessment, Family, Sibling
Introduction
Over 30 years after the landmark work of Aila Rissanen identifying a sibling history of premature coronary artery disease (CAD) as a potent risk factor for CAD in previously unaffected young brothers and sisters,1,2 current primary prevention guidelines in the USA and Europe still do not provide specific preventive recommendations for individuals with a strong family history of early-onset CAD.3,4 It is now well established, however, that premature CAD clusters in families, and that siblings of individuals with early-onset CAD are at markedly increased risk.5,6 Familial clustered early-onset CAD accounts for 60% of all CAD occurring prior to 65 yeas of age.7 Similar to The Framingham Offspring Study,8 The Genetic Study of Atherosclerosis Risk (GeneSTAR) population of initially healthy asymptomatic adult siblings of early-onset CAD cases demonstrates a 52% higher risk of incident CAD than would be expected using traditional risk assessment.9–11
It is generally presumed that symptomatic myocardial ischaemia represents a late phase of preclinical CAD12 and exists primarily as a result of haemodynamically significant coronary artery stenoses.13 However, silent myocardial ischaemia may occur earlier with milder stenoses,14–17 and atherosclerotic plaque within these stenoses may be vulnerable to rupture and thrombosis. Recent position statements by the American Society of Nuclear Cardiology18 and the European Society of Cardiology Working Group on Nuclear Cardiology and Cardiac CT19 indicate that stress myocardial perfusion imaging detects silent myocardial ischaemia in some higher risk primary prevention populations, including persons with a family history of premature CAD.
Thus the aims of this study were to (i) determine the existence and duration of a silent ischaemic period preceding incident CAD in healthy siblings of patients with early-onset CAD, (ii) determine which subgroups are at higher risk of developing clinically manifest CAD, and (iii) evaluate the extent to which inducible myocardial ischaemia predicts long-term CAD outcomes and dictates a change in preventive strategies for individual siblings.
Methods
Sample and recruitment
The study population consisted of a cohort of 1296 initially asymptomatic, healthy siblings of index patients hospitalized with documented CAD prior to 60 years of age as part of an ongoing prospective study of families with early-onset CAD.9–11 Nine participants were lost to follow-up, leaving 1287 siblings (99.3% of total recruitment) for study inclusion. The study was originally known as the Johns Hopkins Sibling and Family Heart Study, and was more recently renamed GeneSTAR (Genetic Study of Atherosclerosis Risk) to reflect inclusion of a whole genome scan.20 The cohort and all protocols are identical under both study names. Briefly, index patients with documented acute myocardial infarction (MI), unstable angina with coronary artery bypass surgery (CABG) or percutaneous coronary intervention (PCI), or acute angina with angiographic evidence of a flow limiting stenosis of >50% diameter in at least one coronary artery were identified at any one of 10 Baltimore area hospitals. Of the index cases approached, 96% agreed to offer access to their unaffected healthy siblings. Siblings were eligible for the study if they were 30–60 years of age and had no known history of CAD, but were excluded if they had autoimmune disease, life-threatening co-morbidity, significant functional status limitations precluding exercise testing, or were receiving glucocorticosteroid therapy, as described previously.10 Over 92% of eligible siblings participated in the study. The study was approved by the Johns Hopkins Medicine Institutional Review Board and all participants gave informed consent.
Participant screening
Siblings underwent a comprehensive cardiovascular risk factor screening and evaluation for exercise-induced myocardial ischaemia between 1983 and 2001. Medical history was elicited. Current cigarette smoking was assessed by self-report and verified by expired carbon monoxide levels of ≥8 p.p.m. Blood pressure was measured according to American Heart Association guidelines three times over the course of the day and the average of the three measurements was used to characterize blood pressure; hypertension was defined as an average blood pressure ≥140 mmHg systolic, or ≥90 mmHg diastolic, and/or use of an antihypertensive drug. For anthropometric measures, height in inches was determined using a fixed stadiometer and weight in pounds was measured with the subject wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. After a 12-h overnight fast, blood was drawn for measurement of lipid and glucose levels. Total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels were measured using the United States Centers for Disease Control standardized methods.21 Low-density lipoprotein (LDL) cholesterol was estimated using the Friedewald formula22 for persons with triglyceride levels up to 400 mg/dL. Glucose concentration was measured using the glucose oxidase method;23 type 2 diabetes mellitus was defined as a history of type 2 diabetes, fasting glucose level ≥126 mg/dL, and/or use of hypoglycaemic medications. We calculated the 10-year Framingham Risk Score (FRS) to categorize siblings as low risk (<10%), intermediate risk (10–20%), or high risk (≥20%) for total CAD events based on their baseline risk factor levels.24
All siblings underwent a maximal graded treadmill test using a modified Bruce protocol.10 Nuclear imaging (thallium-201) was performed immediately following the exercise treadmill test using standard methods as previously described.10 Two experienced nuclear cardiologists interpreted the images, blinded to the subject's risk factor profile and exercise test results. The presence of ischaemia was defined as a perfusion defect on the immediate post-exercise images with definitive improvement or normalization on delayed images obtained 3–4 h later.25 Ischaemia on the exercise electrocardiogram was defined as the presence of horizontal or downsloping ST-segment depression of >1 mm over baseline in three or more consecutive beats in two contiguous leads during the test or during the first 3min of recovery. The exercise electrocardiograms were read independently by two cardiologists blinded to the risk factor and perfusion image status of the individual.
Definition and adjudication of incident coronary artery disease
Siblings were followed at 5-year intervals for incident CAD events using the classification schema of The Framingham Heart Study.24 A standardized instrument was administered via telephone by a trained interviewer to elicit a history of any cardiac-related procedures or symptoms, the use of coronary disease-related medications, and any stated or inferred history of CAD. In the event of a death, the closest family member was interviewed as a proxy. All physician records, hospital records, death records, and autopsy records were reviewed for adjudication of CAD events. Total incident CAD was used as the primary event endpoint, and was defined as sudden cardiac death, hospitalization for MI documented by changes on the electrocardiogram and cardiac enzymes, unstable angina with flow limiting coronary stenoses and CABG or PCI, stable angina with flow-limiting coronary stenoses accompanied by CABG or PCI, and medically treated new onset angina with documented flow limiting stenoses. Secondary analyses were performed with acute coronary syndrome (ACS) as the endpoint defined as sudden death, acute MI, and unstable angina requiring urgent revascularization. Participants with CAD events occurring within the first 3 months of follow-up (n = 2, one MI and one new onset angina with revascularization) were censored to remove any potential screening-induced bias. Three cardiovascular experts each blinded to the assignments of the other reviewers adjudicated the coronary disease outcomes. Any discordant classification by a single member of the adjudication team resulted in review by an External Adjudication Committee consisting of at least one non-study cardiologist from Johns Hopkins and one from an extrinsic academic institution. The external review determined the final event classification using the standardized coding schema as described.24
Statistical analyses
For continuous variables, frequencies, means, and standard deviations were examined. For tests of relationships among continuous variables, the t-test and ANOVA procedures were used; contingency table arrays and the χ2 statistic were used for the examination of relationships between categorical variables. The sex-specific prevalence of inducible ischaemia was determined for each baseline age decade by the FRS risk group. A stepwise logistic regression with forward selection (P < 0.1) and backward elimination (P ≥ 0.15) with addition of known cardiovascular risk factor covariates was performed to determine significant predictors of baseline ischaemia; variables entering the model included age, sex, race, LDL and HDL cholesterol level, triglycerides, history of diabetes, hypertension, current smoking, and BMI.
Cox proportional hazards analysis of total incident CAD events was performed with adjustment for baseline traditional CAD risk factors and intrafamilial clustering using the General Estimating Equation (GEE) of Zeger and Liang.26 The final and best fitting model allowed for different underlying hazards according to sex and race. Hazard ratios, 95% confidence intervals, and statistical significance for each variable in the models were determined. In the final model, hazard ratios were standardized for inducible ischaemia and selected modifiable risk factors, and reflect the increased risk above the sex- and race-specific baseline hazards. A similar analysis was performed for the secondary endpoint of ACS using a competitive risk model.27 Since the occurrence of non-ACS cardiac events with subsequent treatment competes with the risk of ACS, to estimate the ACS-specific relative hazards, we used the modelling for cause-specific hazards of competing events as described by Lau et al.27 In these models the time to each of the competing events is censored when the other event occurs, but covariate adjustment for both ACS and non-ACS events is done in the same model. The 10-year total CAD event risk was analysed separately for siblings without diabetes and an FRS <20%, by sex, age decade at baseline, and the absence or presence of inducible ischaemia and also for siblings with diabetes and/or FRS ≥20% by sex and the absence or presence of inducible ischaemia.
Results
Baseline characteristics
The 1287 siblings were identified from 699 families (one index case per family; 1.8 ± 1.1 siblings per index case). Index cases were 67% male, with a mean age of 46.5 ± 7.3 years for the first CAD event, and had the following diagnoses: MI (48.2%), unstable angina with revascularization (31.8%), and stable angina with revascularization or managed medically (19.8%). The sibling sample was 54.7% female; 53.0% Americans of European ancestry, 47.0% Americans of African ancestry. All were healthy and without any chest pain or angina-equivalent symptoms at baseline screening. No subject had a silent MI on the baseline resting electrocardiogram.
Baseline population characteristics are shown in Table 1 by the absence or presence of silent myocardial ischaemia at baseline. Baseline myocardial ischaemia was significantly associated with older age, male sex, European American ancestry, hypertension, higher levels of LDL cholesterol and triglycerides, and lower levels of HDL cholesterol but not with diabetes, current smoking, or BMI. During follow-up, 28% of siblings with inducible ischaemia developed clinically manifest CAD compared with 12% of siblings without ischaemia, P < 0.0001. Incident CAD was significantly associated with traditional risk factors in both ischaemic and non-ischaemic siblings.
Table 1.
Population characteristics by the absence or presence of inducible silent ischaemia (n = 1287)
| Characteristics | Overall population, n = 1287 | Ischaemia absent, n = 1051 | Ischaemia present, n = 236 | P-value |
|---|---|---|---|---|
| Age, yearsa | 46.4 ± 7.0 | 46.0 ± 7.0 | 48.4 ± 6.8 | <0.0001 |
| Male sex, % | 45.3 | 40.5 | 66.5 | <0.0001 |
| African American, % | 47.0 | 48.3 | 41.1 | 0.04 |
| Hypertensive, % | 50.4 | 47.8 | 58.1 | 0.004 |
| Diabetic, % | 9.6 | 9.3 | 10.6 | 0.55 |
| Current smoking, % | 31.7 | 31.8 | 31.4 | 0.90 |
| Low-density lipoprotein cholesterol, mmol/La | 3.73 ± 1.15 | 3.67 ± 1.14 | 3.98 ± 1.18 | 0.0004 |
| High-density lipoprotein cholesterol, mmol/La | 1.34 ± 0.42 | 1.36 ± 0.42 | 1.26 ± 0.38 | 0.0009 |
| Triglycerides, mmol/La | 1.61 ± 1.32 | 1.56 ± 1.20 | 1.84 ± 1.73 | 0.02 |
| Body-mass index, kg/m2a | 29.2 ± 6.2 | 29.3 ± 6.5 | 29.1 ± 5.1 | 0.67 |
Overview of baseline characteristics and cardiovascular risk factors of the study population.
aMean (1 SD).
Inducible ischaemia
Exercise-induced ischaemia was present at baseline in 236 siblings (18.3%) and was strongly associated with age in both sexes. Males had more than double the prevalence of ischaemia compared with females (26.9 vs. 11.2%, P < 0.0001). Exercise electrocardiographic abnormalities alone were observed in 5.4% of males and 6.6% of females. Ischaemia defined by a reversible perfusion defect but without ST-segment abnormalities occurred in 16.4% of males and 2.8% of females. The presence of both an abnormal exercise electrocardiogram and reversible ischaemia was observed in 5.1% of males and 1.8% of females.
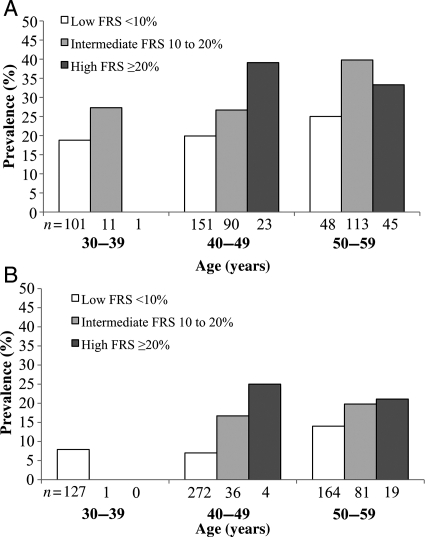
In males, the prevalence of ischaemia increased with age decade (P = 0.004) and FRS group (P = 0.001), except in the oldest age group (Figure 1A). In females, the prevalence of ischaemia, although notably lower, also increased with age decade (P = 0.005) and FRS group (P = 0.007). Almost all females had a low FRS and a very low prevalence of ischaemia in the youngest age group (Figure 1B).
Figure 1.
Sex-specific prevalence of inducible ischaemia by age decade and Framingham Risk Score group (n = 1287). (A) Male; P = 0.004 across age decades; P = 0.001 across FRS categories. (B) Female; P = 0.005 across age decades; P = 0.007 across Framingham Risk Score categories.
A stepwise multivariate regression analysis with addition of risk factors and ischaemia as covariates showed that age (P < 0.0001), sex (P < 0.0001), and LDL cholesterol (P = 0.002) were independently associated with inducible ischaemia.
Incident coronary artery disease outcomes
The median follow-up time was 10.0 years (range 3 months to 25 years). Follow-up was conducted in 5-year intervals with censoring at the time of occurrence of an incident CAD event. For those without events, 38.3% were followed for a maximum of 5 years, 34.8% for 10 years, 15.9% for 15 years, 7.9% for 20 years, and 3.1% for 25 years. During the overall follow-up, there were 195 people with newly documented CAD events, including 131 males (22.5%) and 64 females (9.0%). Incident ACSs occurred in 132 siblings, 90 males (15.4%) and 42 females (6.0%). The time to event following baseline screening ranged from 3.5 months to 23.8 years, with a mean of 8.2 ± 5.2 years. Acute coronary syndromes, including sudden death (7%), acute MI (32%), or unstable angina with revascularization (29%) accounted for 68% of all events. Stable new onset symptomatic CAD, including angina with revascularization and medically treated angina with significant angiographic flow-limiting coronary stenoses accounted for the remainder of events.
Cox proportional hazard analyses of incident coronary artery disease outcomes
A Cox proportional hazard analysis of incident total CAD events was performed, as shown in Table 2. The model allows for different underlying hazards by sex and race strata, is adjusted for age, and for modifiable risk factors including hypertension, LDL cholesterol, diabetes, and current smoking, as well as the presence of ischaemia at baseline. As expected, all traditional risk factors were strong independent predictors of CAD events. Additionally, baseline ischaemia was a significant independent predictor of incident total CAD with a hazard ratio of 1.53 (95% CI: 1.11–2.11). A separate Cox proportional hazard analysis of incident ACS was performed using a competitive risk model; the hazard ratio was 1.73 (95% CI: 1.17–2.55) for ischaemia.
Table 2.
Predictors of incident coronary artery disease at 5–25 years of follow-up: Cox proportional hazard model (n = 1248)
| Variable | Hazard ratio† | 95% Confidence interval | P-value |
|---|---|---|---|
| Hypertension | 1.81 | 1.30–2.51 | <0.001 |
| LDL-C ≥3.4 mmol/L | 1.52 | 1.10–2.09 | 0.01 |
| Diabetes | 1.88 | 1.21–2.92 | 0.005 |
| Current smoking | 1.75 | 1.27–2.40 | <0.001 |
| Inducible silent ischaemia | 1.53 | 1.11–2.11 | 0.009 |
†Cox proportional hazard analysis adjusted for age as a continuous variable and familial clustering under the assumption of different underlying hazards for sex and race.
Coronary artery disease event outcomes at 10 years
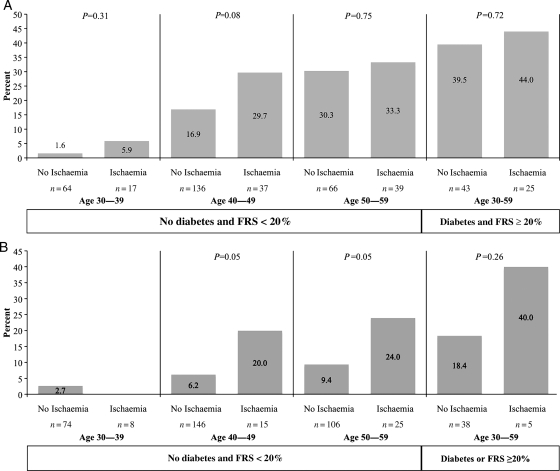
The sex-specific 10-year incidence of CAD by the presence of baseline inducible ischaemia in siblings (n = 733) without CAD risk equivalence (type 2 diabetes or a calculated FRS ≥20% excluded), and with at least 10 years of follow-up, is shown in Figure 2 by age decade at baseline. Data for siblings considered having a CAD risk equivalents are shown for comparison. Among low- or intermediate risk males, the 10-year incidence of CAD events increased with age decade at screening, and with the presence of ischaemia (P = 0.0001 and P = 0.008, respectively). The incidence of CAD events was ≥20% in males with baseline ischaemia beginning at 40 years of age and close to 20% in those without ischaemia (Figure 2A). Rates of CAD were well >20% in men with and without ischaemia over 50 years of age. Men with CAD risk equivalents also had notably >20% risk with and without ischaemia.
Figure 2.
Incident coronary artery disease at 10 years of follow-up by the absence or presence of inducible silent ischaemia by age decade in siblings considered low or intermediate risk by traditional risk factor assessment and in siblings considered having a coronary artery disease risk equivalent. Coronary artery disease equivalent defined as diabetes and/or 10-Year Framingham Risk Score ≥20%. (A) Male (n=427) and (B) Female (n=417).
Among non-diabetic intermediate and low-risk FRS females, the 10-year incidence of CAD events was considerably lower than in males, and increased with baseline age decade. Coronary artery disease risk was 20% in females ≥40 years of age and higher after 50 years of age among those with ischaemia (Figure 2B). The prevalence of ischaemia was low until age 50. In females with CAD risk equivalents (n = 43) the overall 10-year CAD event rate was just >20% in the small number who had ischaemia, and nearly 20% in those without ischaemia.
Discussion
There remains a paucity of data regarding the presence of a quiescent period of coronary disease sufficient to cause inducible ischaemia in persons with a family history of premature coronary disease. Although a small number of studies in apparently healthy males 40–70 years of age found the prevalence to be between 2.5 and 14%,28,29 we found a considerably higher prevalence of inducible ischaemia in healthy asymptomatic siblings of persons with early onset CAD. The length of time from the identification of ischaemia to the occurrence of a clinically manifest CAD outcome was quite long, a mean of 8 years. While it is known that latent atherosclerotic disease is present for long periods of time, this long period of inducible ischaemia in siblings prior to a clinical CAD event is a novel finding that provides a stimulus for reconsidering the importance of family history in establishing therapeutic prevention thresholds and goal levels of risk factors, particularly among male siblings. As anticipated, CAD risk factors were highly prevalent and were significantly associated with both inducible ischaemia and incident CAD events.
In our study, excluding all siblings who would already be identified as a CAD risk equivalent because of diabetes or a FRS of ≥20%, the prevalence of inducible ischaemia was high among males ≥40 years of age. Similarly, high rates of incident CAD events were observed beginning at 40 years of age, even among men without ischaemia. These findings are similar to those observed in diabetics of comparable age30,31 and on that basis alone appear to warrant similar thresholds for the initiation of aggressive preventive interventions according to published guidelines in the USA32 and in Europe.33 Thus, brothers of persons with early onset CAD have both a high prevalence of early inducible ischaemia and also bear a sufficiently high enough risk of CAD to warrant aggressive preventive therapies without undergoing stress perfusion imaging. Additionally, risk factor levels are high enough to suggest a potential benefit.
The issue is far less clear among women and presents a conundrum with regard to recommendations. Rates of incident CAD were 20% or higher only among those with inducible ischaemia, but beginning as early as 40 years of age. It would be tempting to recommend that stress perfusion imaging be considered for more precise risk stratification, but given the low prevalence of inducible ischaemia in women, one would have to consider the cost to the medical care system and the possible effects of additional radiation exposure. In context, for every 100 female siblings aged 50–59 years undergoing stress perfusion imaging to identify inducible ischaemia, only 4.6 individuals would have ischaemia and go on to have an event over the subsequent 10 years. Thus, given the overall pro-health benefits of therapeutic lifestyle interventions, at a minimum, adopting those recommendations in women with a family history or premature CAD appears to be justified. Beyond that, more aggressive risk modification would need to be tailored to the unique risk profiles of women with a family history. Clinicians may consider nuclear stress testing in sisters of CAD patients where family history is particularly strong and/or unique risk factor profiles are present that warrant attention. The yield of exercise testing without nuclear perfusion imaging is very low in both sexes.
Concerns about the negative sequelae of broadly applied aggressive preventive interventions in male siblings need to be balanced with potential benefits. Aspirin is associated with intracranial34 and gastrointestinal bleeding35 but its use for primary prevention in men is highly beneficial, demonstrating a significant risk reduction in cardiovascular events.36 Based on the risk/benefit ratio, the US Preventive Services Task Force recommends aspirin use in men with a 10-year risk of cardiovascular events >6%, unless contraindicated.37 By this standard, most male siblings ≥40 years of age from high-risk families would fall into this category where the benefits are expected to outweigh the risks. In Europe, aspirin prophylaxis for primary prevention is more controversial38 with recommended use in asymptomatic individuals only if their 10-year risk of total CAD mortality is >10% and blood pressure is controlled as closely as possible to goal.4 Under these contingencies, based on our findings, most male siblings 40 years of age or older, would be eligible for aspirin chemoprophylaxis.
Statin therapy used for primary prevention of CAD events was recently shown to be beneficial in a large scale meta-analysis of randomized trials.39 In males without known CAD but with a 10-year risk for MI or stroke of ≥20%, the number needed to treat to prevent one event over 5 years is significantly less than the number associated with acute renal failure, cataracts, liver dysfunction, or myopathy.40 Guidelines released in the USA41 and in Europe4 recommend a goal LDL cholesterol level <2.5 mmol/L in asymptomatic people at high risk of developing cardiovascular events. Few siblings in our study were close to that goal level at screening. Low-density lipoprotein cholesterol levels were high and associated with both the ischaemia substrate for CAD and with CAD events.
While there clearly is significant predictive value of stress perfusion testing in our study, men over 40 years of age had very high rates of incident CAD, even in the absence of ischaemia. This creates a primary prevention scenario whereby the testing has few to no therapeutic implications in asymptomatic individuals. In women, the likelihood of finding inducible ischaemia is simply too low to warrant such testing on a routine basis.
Conclusion
The presence of a long period of asymptomatic inducible ischaemia in male siblings of persons with premature CAD offers an opportunity for aggressive preventive therapies to ameliorate the high risk of subsequent CAD. Aggressive preventive therapies are already recommended for other subpopulations with risks in the same range as observed in brothers of premature CAD patients. We suggest that the risk benefit ratio of the therapies currently available is likely to be in favour of earlier preventive interventions, using lower thresholds of risk factors for treatment, and lower therapeutic goal levels. In female siblings, therapeutic lifestyle interventions and therapies tailored to their individual risk factor profiles appear to be justified.
Funding
This work was supported by grants from The Johns Hopkins General Clinical Research Center (Grant M01-RR000052 from the National Center for Research Resources, National Institutes of Health), the National Institute of Nursing Research (Grant RO1 NR08153), and the National Heart, Lung, and Blood Institute (Grants R01 HL59684 and R01 HL071025).
Conflict of interest: none declared.
References
- 1.Rissanen AM, Nikkila EA. Coronary artery disease and its risk factors in families of young men with angina pectoris and in controls. Br Heart J. 1977;39:875–883. doi: 10.1136/hrt.39.8.875. doi:10.1136/hrt.39.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rissanen AM, Nikkila EA. Identification of the high-risk groups in familial coronary heart disease. Atherosclerosis. 1984;53:37–46. doi: 10.1016/0021-9150(84)90103-5. doi:10.1016/0021-9150(84)90103-5. [DOI] [PubMed] [Google Scholar]
- 3.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, Hong Y, Miller NH, Lauer RM, Ockene IS, Sacco RL, Sallis JF, Jr, Smith SC, Jr, Stone NJ, Taubert KA. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. doi:10.1161/01.CIR.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 4.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De Backer G, Ebrahim S, Gjelsvik B, Herrmann-Lingen C, Hoes A, Humphries S, Knapton M, Perk J, Priori SG, Pyorala K, Reiner Z, Ruilope L, Sans-Menendez S, Scholte op Reimer W, Weissberg P, Wood D, Yarnell J, Zamorano JL, Walma E, Fitzgerald T, Cooney MT, Dudina A. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts) Eur Heart J. 2007;28:2375–2414. doi: 10.1093/eurheartj/ehm316. doi:10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 5.Silberberg JS, Wlodarczyk J, Fryer J, Robertson R, Hensley MJ. Risk associated with various definitions of family history of coronary heart disease. The Newcastle Family History Study II. Am J Epidemiol. 1998;147:1133–1139. doi: 10.1093/oxfordjournals.aje.a009411. [DOI] [PubMed] [Google Scholar]
- 6.Bertuzzi M, Negri E, Tavani A, La Vecchia C. Family history of ischemic heart disease and risk of acute myocardial infarction. Prev Med. 2003;37:183–187. doi: 10.1016/s0091-7435(03)00094-x. doi:10.1016/S0091-7435(03)00094-X. [DOI] [PubMed] [Google Scholar]
- 7.Williams RR. Understanding genetic and environmental risk factors in susceptible persons. West J Med. 1984;141:799–806. [PMC free article] [PubMed] [Google Scholar]
- 8.Murabito JM, Pencina MJ, Nam BH, D'Agostino RB, Sr, Wang TJ, Lloyd-Jones D, Wilson PW, O'Donnell CJ. Sibling cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults. JAMA. 2005;294:3117–3123. doi: 10.1001/jama.294.24.3117. doi:10.1001/jama.294.24.3117. [DOI] [PubMed] [Google Scholar]
- 9.Becker DM, Yook RM, Moy TF, Blumenthal RS, Becker LC. Markedly high prevalence of coronary risk factors in apparently healthy African-American and white siblings of persons with premature coronary heart disease. Am J Cardiol. 1998;82:1046–1051. doi: 10.1016/s0002-9149(98)00553-0. doi:10.1016/S0002-9149(98)00553-0. [DOI] [PubMed] [Google Scholar]
- 10.Blumenthal RS, Becker DM, Moy TF, Coresh J, Wilder LB, Becker LC. Exercise thallium tomography predicts future clinically manifest coronary heart disease in a high-risk asymptomatic population. Circulation. 1996;93:915–923. doi: 10.1161/01.cir.93.5.915. [DOI] [PubMed] [Google Scholar]
- 11.Vaidya D, Yanek LR, Moy TF, Pearson TA, Becker LC, Becker DM. Incidence of coronary artery disease in siblings of patients with premature coronary artery disease: 10 years of follow-up. Am J Cardiol. 2007;100:1410–1415. doi: 10.1016/j.amjcard.2007.06.031. doi:10.1016/j.amjcard.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerne A, Kranjec I. Atherosclerotic burden in coronary and peripheral arteries in patients with first clinical manifestation of coronary artery disease. Heart Vessels. 2002;16:217–226. doi: 10.1007/s003800200028. doi:10.1007/s003800200028. [DOI] [PubMed] [Google Scholar]
- 13.Iskandrian AS, Hakki AH, Kane-Marsch S. Prognostic implications of exercise thallium-201 scintigraphy in patients with suspected or known coronary artery disease. Am Heart J. 1985;110:135–143. doi: 10.1016/0002-8703(85)90527-7. doi:10.1016/0002-8703(85)90527-7. [DOI] [PubMed] [Google Scholar]
- 14.Schachinger V, Britten MB, Elsner M, Walter DH, Scharrer I, Zeiher AM. A positive family history of premature coronary artery disease is associated with impaired endothelium-dependent coronary blood flow regulation. Circulation. 1999;100:1502–1508. doi: 10.1161/01.cir.100.14.1502. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Jerosch-Herold M, Jacobs DR, Jr, Shahar E, Folsom AR. Coronary risk factors and myocardial perfusion in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;47:565–572. doi: 10.1016/j.jacc.2005.09.036. doi:10.1016/j.jacc.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 16.Kuo L, Davis MJ, Cannon MS, Chilian WM. Pathophysiological consequences of atherosclerosis extend into the coronary microcirculation. Restoration of endothelium-dependent responses by L-arginine. Circ Res. 1992;70:465–476. doi: 10.1161/01.res.70.3.465. [DOI] [PubMed] [Google Scholar]
- 17.Zeiher AM, Krause T, Schachinger V, Minners J, Moser E. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circulation. 1995;91:2345–2352. doi: 10.1161/01.cir.91.9.2345. [DOI] [PubMed] [Google Scholar]
- 18.Hendel RC, Abbott BG, Bateman TM, Blankstein R, Calnon DA, Leppo JA, Maddahi J, Schumaecker MM, Shaw LJ, Ward RP, Wolinsky DG. The role of radionuclide myocardial perfusion imaging for asymptomatic individuals. J Nucl Cardiol. 2011;18:3–15. doi: 10.1007/s12350-010-9320-5. [DOI] [PubMed] [Google Scholar]
- 19.Perrone-Filardi P, Achenbach S, Mohlenkamp S, Reiner Z, Sambuceti G, Schuijf JD, Van der Wall E, Kaufmann PA, Knuuti J, Schroeder S, Zellweger MJ. Cardiac computed tomography and myocardial perfusion scintigraphy for risk stratification in asymptomatic individuals without known cardiovascular disease: a position statement of the Working Group on Nuclear Cardiology and Cardiac CT of the European Society of Cardiology. Eur Heart J. 2010 doi: 10.1093/eurheartj/ehq235. ehq235v1-ehq235. Published online ahead of print 14 July 2010. [DOI] [PubMed] [Google Scholar]
- 20.Johnson AD, Yanek LR, Chen MH, Faraday N, Larson MG, Tofler G, Lin SJ, Kraja AT, Province MA, Yang Q, Becker DM, O'Donnell CJ, Becker LC. Genome-wide meta-analyses identifies seven loci associated with platelet aggregation in response to agonists. Nat Genet. 2010;42:608–613. doi: 10.1038/ng.604. doi:10.1038/ng.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers GL, Kimberly MM, Waymack PP, Smith SJ, Cooper GR, Sampson EJ. A reference method laboratory network for cholesterol: a model for standardization and improvement of clinical laboratory measurements. Clin Chem. 2000;46:1762–1772. [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23.Pesce AJ, Kaplan LA. Methods in Clinical Chemistry. St. Louis: Mosby; 1987. [Google Scholar]
- 24.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 25.Hansen CL, Goldstein RA, Akinboboye OO, Berman DS, Botvinick EH, Churchwell KB, Cooke CD, Corbett JR, Cullom SJ, Dahlberg ST, Druz RS, Ficaro EP, Galt JR, Garg RK, Germano G, Heller GV, Henzlova MJ, Hyun MC, Johnson LL, Mann A, McCallister BD, Jr, Quaife RA, Ruddy TD, Sundaram SN, Taillefer R, Ward RP, Mahmarian JJ. Myocardial perfusion and function: single photon emission computed tomography. J Nucl Cardiol. 2007;14:e39–e60. doi: 10.1016/j.nuclcard.2007.09.023. doi:10.1016/j.nuclcard.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. doi:10.2307/2531248. [PubMed] [Google Scholar]
- 27.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. doi:10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleg JL, Gerstenblith G, Zonderman AB, Becker LC, Weisfeldt ML, Costa PT, Jr, Lakatta EG. Prevalence and prognostic significance of exercise-induced silent myocardial ischemia detected by thallium scintigraphy and electrocardiography in asymptomatic volunteers. Circulation. 1990;81:428–436. doi: 10.1161/01.cir.81.2.428. doi:10.1161/01.CIR.81.2.428. [DOI] [PubMed] [Google Scholar]
- 29.Laukkanen JA, Kurl S, Lakka TA, Tuomainen TP, Rauramaa R, Salonen R, Eranen J, Salonen JT. Exercise-induced silent myocardial ischemia and coronary morbidity and mortality in middle-aged men. J Am Coll Cardiol. 2001;38:72–79. doi: 10.1016/s0735-1097(01)01311-0. doi:10.1016/S0735-1097(01)01311-0. [DOI] [PubMed] [Google Scholar]
- 30.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. doi:10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 31.Wackers FJ, Young LH, Inzucchi SE, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Wittlin SD, Heller GV, Filipchuk N, Engel S, Ratner RE, Iskandrian AE. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27:1954–1961. doi: 10.2337/diacare.27.8.1954. doi:10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 32.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. doi:10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 33.Ryden L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jonsson B, Laakso M, Malmberg K, Priori S, Ostergren J, Tuomilehto J, Thrainsdottir I, Vanhorebeek I, Stramba-Badiale M, Lindgren P, Qiao Q, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Deckers JW, Bertrand M, Charbonnel B, Erdmann E, Ferrannini E, Flyvbjerg A, Gohlke H, Juanatey JR, Graham I, Monteiro PF, Parhofer K, Pyorala K, Raz I, Schernthaner G, Volpe M, Wood D. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 34.He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: a meta-analysis of randomized controlled trials. JAMA. 1998;280:1930–1935. doi: 10.1001/jama.280.22.1930. doi:10.1001/jama.280.22.1930. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez-Diaz S, Garcia Rodriguez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22. doi: 10.1186/1741-7015-4-22. doi:10.1186/1741-7015-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306–313. doi: 10.1001/jama.295.3.306. doi:10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 37.Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 38.Barnett H, Burrill P, Iheanacho I. Don't use aspirin for primary prevention of cardiovascular disease. BMJ. 2010;340:c1805. doi: 10.1136/bmj.c1805. doi:10.1136/bmj.c1805. [DOI] [PubMed] [Google Scholar]
- 39.Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, de Craen AJ, Knopp RH, Nakamura H, Ridker P, van Domburg R, Deckers JW. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376. doi: 10.1136/bmj.b2376. doi:10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197. doi: 10.1136/bmj.c2197. doi:10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]