Abstract
Retinopathy, the leading cause of acquired blindness in young adults, is one of the most feared complications of diabetes, and hyperglycemia is considered as the major trigger for its development. The microvasculature of the retina is constantly bombarded by high glucose, and this insult results in many metabolic, structural and functional changes. Retinal mitochondria become dysfunctional, its DNA is damaged and proteins encoded by its DNA are decreased. The electron transport chain system becomes compromised, further producing superoxide and providing no relief to the retina from a continuous cycle of damage. Although the retina attempts to initiate repair mechanisms by inducing gene expressions of the repair enzymes, their mitochondrial accumulation remains deficient. Understanding the molecular mechanism of mitochondrial damage should help identify therapies to treat/retard this sight threatening complication of diabetes. Our hope is that if the retinal mitochondria are maintained healthy with adjunct therapies, the development and progression of diabetic retinopathy can be inhibited.
Keywords: Antioxidants, apoptosis, diabetic retinopathy, metabolic memory, mitochondria, oxidative stress
INTRODUCTION
Diabetes, a metabolic condition characterized by high blood glucose, is the result of either subnormal insulin production or the decline of insulin action. Worldwide 246 million patients have diabetes, and with the numbers growing at an alarming rate, it is considered as the epidemic of the 21st century. The sustained hyperglycemia leads the progressive development of long-term complications affecting both the macrovascular and the microvascular diseases [1–3]. Macrovascular complications include heart disease, stroke and peripheral vascular disease, and microvascular complications affect the organs that are heavily dependent on their microvasculature supply namely eyes, kidney and nerve.
Retinopathy is a potentially blinding complication of diabetes. According to the World Health Organization, approximately 5 million individuals have diabetic retinopathy, which accounts for about 5% of world blindness. In United States alone, diabetic retinopathy is responsible for about 10,000 new cases of blindness every year [4]. Since the onset of diabetic complications is directly related to the duration of diabetes and the quality of glycemic control, it is rarely detected in the first few years of the diabetes, but by 20–25 years of diabetes almost 90% of patients present some forms of retinopathy [5, 6].
PATHOGENESIS OF DIABETIC RETINOPATHY
Diabetic retinopathy results from the damage of the small vasculature of the retina, multi cellular and the light sensitive tissue at the back of the eye. It is the most vascularized tissue of the body, and its capillarity accounts for half of the diameter of this tissue. The capillaries are lined with endothelial cells responsible for maintaining the blood retinal barrier, and are surrounded by smooth muscle cells, pericytes, which provide tone to the vessels [7]. In the early stage of diabetic retinopathy, pericytes start to undergo accelerated death, and this loss of pericytes is followed by the loss of endothelial cells resulting in pericyte ghosts, acellular capillaries and microaneurysms [8–10]. Thus, before any histopathology could be observed in the retina, retinal microvascular cells (pericytes and endothelial cells) and other non capillary cells (e.g. Muller cells and glial cells), are lost selectively via apoptosis [10–12]. This accelerated apoptosis can account for the pericyte ghosts and acellular capillaries because, although histopathology of diabetic retinopathy takes over decades in humans and about one year in rats to develop, apoptosis is a rapidly consummated phenomenon, and the cell contains fragmented DNA for only a few hours [13]. Defective endothelial cell replication seen in diabetic conditions accelerates the process of retinal ischemia by exhausting the cell’s replicative capacity. Studies with rodent models have shown increased TUNEL-positive cells in retinal vasculature at 6–8 months of diabetes while histopathology appears around 10–12 months of diabetes in the same animal model [10, 11] suggesting that in diabetic retinopathy vascular cells are degenerating before other histopathology is detectable, and that the clinically silent initial phase of diabetic retinopathy consists of irreversible cellular events with late structural consequences. Capillaries begin to leak fluid into the surrounding retinal tissue resulting in microaneurysms and intra-retinal hemorrhages. Endothelial cells try to repair the damage by multiplying on the inner membrane, but this promotes occlusion of capillaries, and ischemic retina signals the release of growth factors leading to neovascularization. New vessels that are formed in the intra-retinal or into the vitreous cavity are fragile and can result in retinal detachment and, ultimately to the blindness [6]. Thus, this progressive disease is intimately regulated by the severity of hyper-glycemia and the duration of diabetes.
HYPERGLYCEMIA AND DIABETIC RETINOPATHY
Hyperglycemia is considered as the major trigger for the development of diabetic complications. Diabetes Control and Complications Trial and animal models have clearly demonstrated that the maintenance of good glycemic control slows down the onset and progression of diabetic retinopathy [2, 14–22]. However, the mechanism via which hyperglycemia manifests in diabetic retinopathy remains elusive. Diabetes is a life-long disease, and retinopathy is a duration dependent slow progressing complication, thus the retina is continually exposed to high glucose. This high glucose can damage cellular metabolism, resulting in acute changes and these acute changes, over the time, can result in long-term irreversible abnormalities in stable macromolecules. To further complicate the process, sustained hyperglycemia itself can result in irreversible changes in stable macromolecules. Animal models of diabetic retinopathy have shown that metabolic/functional alterations are observed in the retina even before any histopathology can be seen in the retina [11, 23–25]. Thus, there is a strong need to elucidate the mechanism for its development so that the progression can be halted in its path using therapies targeted to prevent dysmetabolism before it manifests into pathology.
When intracellular glucose levels are elevated polyol pathway becomes active [26], and the first rate limiting enzyme, aldose reductase, reduces glucose to sorbitol and sorbitol dehydrogenase converts sorbitol to fructose. Retinal vascular and other cells are shown to have increased polyols in diabetes, thus suggesting its role in the development of diabetic retinopathy [27]. Reaction with glucose and/or alpha-oxaloaldehydes leads to chemical modifications on the amino groups of proteins, lipids and DNA, and this can result in intra-molecular and intermolecular cross-link formation, and inhibition of AGE-mediated pathways could have potential to prevent the development and progression of diabetic retinopathy [28, 29]. Another important pathway implicated is the activation of protein kinase C. Diabetes has been shown to increase the protein kinase C activity in the retinal microvessels, the main site of lesions in diabetic retinopathy [24, 30]. Increased protein kinase C could result in many alterations that are characteristic of diabetic retinopathy, including, stimulating neovascularization and endothelial proliferation, increasing vascular permeability, stimulating apoptosis, contributing to hyperglycemia-induced oxidative stress, and abnormal blood flow [31].
Although the pathogenesis of diabetic retinopathy is not fully elucidated, oxidative stress is considered as a critical mediator of pathways implicating the development of diabetic retinopathy [32–37]. Increased prevalence of retinopathy in diabetic patients is associated with increased serum lipid hydroperoxides [38, 39]. Excessive superoxide radicals induce oxidative modification of cellular molecules activating several pathways leading to a consequent cell death [40]. Here we have summarized the recent progress toward understanding the emerging role superoxide in the development of diabetic retinopathy (Fig. 1). Our hope is that better understanding of the intracellular and molecular mechanisms of the disease, especially in the early stages of this slow progressing disease, is critical in improving the therapeutic options for the control and/or the delay of this disease.
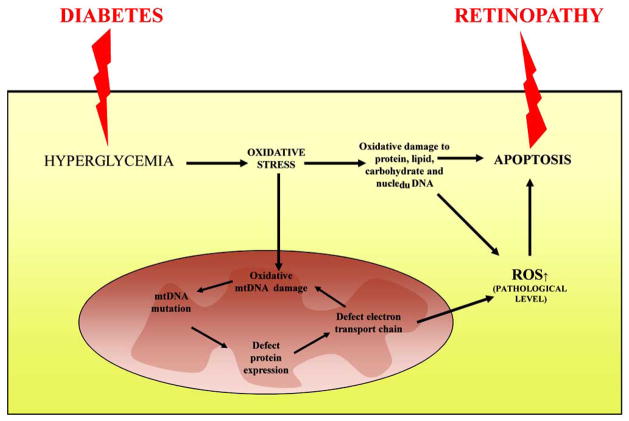
Fig. 1.
Schematic representation of the molecular mechanisms for the development of diabetic retinopathy through oxidative stress. Diabetes-induced overproduction of reactive oxygen species (ROS) not only damage protein, lipid, and carbohydrate, it also damages mitochondrial DNA. This causes deficiency in respiratory chain subunits and increased superoxide production, resulting in a vicious cycle. Apoptosis is increased leading to the development of retinopathy.
DIABETES AND INCREASED OXIDATIVE STRESS
Clinical and experimental studies have clearly demonstrated that hyperglycemia is the first trigger in the pathogenesis of diabetic complications. It activates many metabolic and hemodynamic pathways and their downstream effectors, and increases oxidative stress by a number of different mechanisms including autooxidation of glucose, increased superoxide production/decreased scavenging, activation of polyol pathway and protein kinase C, and increased formation of advanced glycation end products [41]. Glucose, by reacting with hydrogen peroxide in the presence of metals (eg., Fe2+ and Cu2+), can form highly reactive hydroxyl radicals, which can compromise mitochondrial electron transport system and damage DNA, further increasing superoxide accumulation. Since aldose reductase of the polyol pathway uses NADPH to reduce glucose to sorbitol, the availability of NADPH for glutathione reductase, an important antioxidant defense enzyme, is decreased [26, 42, 43]. Hyperglycemia increases the formation of advanced glycation end products by increasing non enzymatic glycation, and binding of glycation end products to their receptor increases the generation of intracellular reactive oxygen species (ROS) via NADPH oxidase [41]. Furthermore, activation of protein kinase C, via diacylglycerol formation (which is increased in diabetes), results in subsequent ROS production via NADPH oxidase [44].
In the development of diabetic retinopathy, aldose reductase is activated and sorbitol levels are elevated in the retina [45], and activation of protein kinase C increases vascular permeability, alterations in blood flow and neovascularization [24, 46–49]. In addition, increased accumulation of advanced glycation is observed within retinal capillary cells [28]. Our studies have shown that these diabetes-induced abnormalities in the retina are inter-related, and can be prevented by antioxidant administration [24, 50], suggesting their regulation by free radicals. Thus, sustained high glucose has many avenues to increase oxidative stress in the retina.
MITOCHONDRIA AND THE GENERATION REACTIVE OXYGEN SPECIES
As stated above, free radicals play an important role in the development of diabetic retinopathy. Mitochondria are the power house of the cell [51], and use approximately 90% of the consumed O2 for oxidative phosphorylation and ATP synthesis. However, mitochondrial electron transport chain generates superoxide as an unavoidable by-product by transferring the electrons from reducing substrates to molecular oxygen via electron transport chain complexes I–IV, and this makes mitochondria as the targets for damaging effect of oxidants. Complex I releases superoxide into the matrix and complex III releases superoxide to both sides of the inner membrane [52]. These free radicals, the molecules or molecular fragments that contain one or more unpaired electrons in molecular orbital, have a high degree of reactivity. Thus, the oxygen that sustains aerobic life is not only fundamentally essential for energy metabolism and respiration; it can further interact with other molecules to generate ‘secondary’ free radicals as hydroxyl, peroxyl, alkoxyl and hydrogen peroxide. Although it is well established that these radicals are toxic and lead to many diseases, they have physiological functions and are part of numerous cellular signaling pathways triggering complex cellular events. Increased superoxide can induce sustained detrimental effects by damaging mitochondrial DNA and proteins, resulting in altered electron transport chain subunits, which could further produce increased amount of superoxide [53, 54]. Further, NO can react with superoxide and generate peroxynitrite, a powerful oxidative agent with a long mean life cycle, peroxynitrite directly can induce lipid peroxidation, inactivation on enzymes and activate cascades of events resulting in DNA damage and viability of the cell [55–57]. Diabetes has been shown to increase mitochondrial superoxide and peroxynitrite levels in the retina and its cells, and increased oxidative stress is implicated in the development of diabetic retinopathy [25, 58–64]. Mitochondria become dysfunctional and their membrane potentials are impaired, and the activity of complex III of the electron transport chain system is impaired in the retina and its capillary cells [22, 35, 65]. Anti-oxidants supplementations inhibit oxidative stress and the development of retinopathy in animal models of diabetic retinopathy [25, 61, 66].
Mammalian cells are equipped with an excellent antioxidant defense system to neutralize free radicals and preserve the cellular redox homeostasis. This antioxidant defense system is comprised of several cellular and mitochondrial enzymes, including superoxide scavenging enzymes, glutathione peroxidase and reductase. Superoxide radicals do not readily cross membranes, but manganese superoxide dismutase (MnSOD), a superoxide scavenging enzyme, converts intramitochondrial superoxide to hydrogen peroxide, which can diffuse out of mitochondria. In addition, intracellular antioxidants, such as glutathione, vitamin E and ascorbic acid to help neutralize the detrimental effect of free radicals. In diabetes, retina experiences compromised antioxidant defense system; the activities of antioxidant defense enzymes-MnSOD, glutathione peroxidase and catalase are decreased, and the levels of glutathione are subnormal [25, 58]. The expression of thioredoxin-interacting protein, an endogenous inhibitor of thioredoxin, is decreased [67]. Thus, these studies have further strengthened the role of oxidative damage/defense in the development of diabetic retinopathy. However, it should be acknowledged that the cell can produce ROS from non mitochondrial sources, including NADPH oxidase and from fatty acid metabolism [68], and diabetes-induced activation of NADPH oxidase has been shown to mediate abnormalities associated with the development of diabetic retinopathy, including blood retinal barrier breakdown [69, 70].
MITOCHONDRIAL SUPEROXIDE DISMUTASE AND DIABETIC RETINOPATHY
As stated above, MnSOD acts as one of the major mitochondrial scavengers, and diabetes inhibits its activity in the retina [34, 58, 63]. However, how diabetes compromises retinal MnSOD remains to be elucidated. One of the possibilities is via alteration in its epigenetic regulation. MnSOD gene, Sod2, has 5 exons and 4 introns, and a proximal promoter region and a distinct intron 2 enhancer region regulating the expression of sod2. Redox sensitive nuclear transcription factor (NFkB) p65/p50 is shown to activate the transcription of sod2, and in contrast, NFkB p50/p50 represses sod2 [71, 72]. Our studies have shown that diabetes-induced oxidative stress activates NFkB in the retina and its capillary cells [18, 73], and our initial studies have shown that NFkB p50/p50 is elevated at the enhancer of retinal sod2 in diabetes [Zhong and Kowluru, unpublished observations], and this could suppress MnSOD transcription. In addition, gene sod2 is located in chromatin which can be modulated by histone modifications. While histone acetylation opens the chromatin and activates gene expression, histone methylation represses gene expression [74]. Our preliminary results have shown that trimethylated histone H4 lysine 20 is increased at the promoter and enhancer region of retinal sod2, suggesting that epigenetic modifications of histone could compact sod2 and make it less accessible to other transcription factors [Zhong and Kowluru, unpublished observations]. Another possibility could be its post-translational modifications, the decreased stability of MnSOD mRNA and the oxidized modification by peroxynitrite protein [19, 75] may account for the decreased activity of MnSOD in retina.
MnSOD protein has to be translocated into mitochondria depending on the N terminal mitochondrial targeting sequence and one nucleotide polymorphism (SNP) Ala16/Val is shown to affect its translocation [76, 77]. The change of cytosine to thymine (C to T) results in valine (GTT) replacement of analine (GCT) at codon 16 amino acid of MnSOD protein. Ana16/Val disrupts the helix structure of MnSOD and affects its mitochondrial transportation, and Val16/Val genotype has less enzyme activity than Ala16/Ala counterpart [77]. Diabetic retinopathy is shown to be associated with the homozygous Val16/Val and hemizygous Ala/Val [78, 79]. Thus, in order to prevent/inhibit the development of diabetic retinopathy it is clear that we need to fully understand how diabetes regulates retinal MnSOD.
REACTIVE OXYGEN SPECIES AND MITOCHONDRIA DNA
Mitochondria, the major source of ROS, contain their own DNA, and this DNA is very small and circular with only 16.2kb. Nuclear DNA is packaged into nucleosomes, but mtDNA lacks histones and is packed as nucleoid-like structures [80, 81]. This ‘naked’ DNA, due to its close proximity to the ROS-generating electron transport chain, is particularly vulnerable to damage from insults generated by the electron transport [81, 82]. Diabetes damages DNA in the retinal mitochondria; although the retina tries to overcome damage to its mitochondrial DNA by inducing DNA repair enzymes, they remain deficient in the mitochondria [22]. Mitochondrial DNA encodes only 13 subunits of the electron transport system: seven from complex I (ND1, ND2, ND3, ND4, ND4L, ND5, and ND6), one from complex III (cytochrome b), three from complex IV (COI, COII and COIII), and two from complex V (subunits 6 and 8) [83]. The expression of cytochrome b in the retina is compromised in diabetes, and the activity of complex III becomes subnormal [22, 37]. The transcription of rest of the subunits of electron transport chain complexes requires mitochondrial transcription factors (Tfam, Tfb1m and Tfb2m), which are encoded by the nucleus and are transported to the mitochondria. Mitochondrial transcription factors are regulated by nuclear respiratory factors -1 (NRF1) and -2 (NRF2 or GA-binding protein), and these NRFs also regulate the transcription of several of key mitochondrial proteins [84]. Acute oxidative stress appears to signal the transcription of NRFs and mitochondria transcription factors resulting in an increase of mitochondria biogenesis and mitochondria density [85]. The expression of NRF1 and Tfam is increased in diabetic brain, and is decreased in skeletal muscle [86]. How mitochondrial transcriptional factors regulate the development of diabetic retinopathy is currently being under investigation.
Diabetes increases oxidative damage in the mtDNA, increased levels of oxidatively modified DNA are observed in the retina and its capillary cells [22, 37]. Amplification of mitochondrial DNA is reduced suggesting reduced progression of polymerase along the DNA template. This is accompanied by subnormal DNA repair system in the mitochondria; although diabetes increases the transcript abundance of retinal DNA glycosylases, their protein expressions remain subnormal in the mitochondria. Since complex I and complex III are the major sources of superoxide production and complex III transfers electrons from reduced ubiquinone to cytochrome c, the reduced activity of complex III in the retina in diabetes [22, 37], further exacerbates the situation resulting in a vicious cycle in which the decreased synthesis of mitochondrial DNA-encoded subunits impairs the electron transport system and further augments the generation of superoxide promoting damage to mitochondrial DNA. To make the situation worse, the antioxidant defense system in the retinal mitochondria also becomes subnormal in diabetes, MnSOD activity is impaired and mitochondrial glutathione levels are decreased [22, 37]. Thus, the damaged mitochondrial DNA and impaired DNA repair system appear to be important in the pathogenesis of diabetic retinopathy.
LINK BETWEEN OXIDATIVE STRESS AND OTHER DIABETES-INDUCED RETINAL METABOLIC ABNORMALITIES
Convincing results have shown that overproduction of superoxide by mitochondria acts as a common promoter of the major biochemical abnormalities associated with the development of diabetic retinopathy [87–89]. Superoxide radicals, via inactivating glyceraldehyde-3-phosphate dehydrogenase, increase the levels of the glycolytic metabolite glyceraldehyde-3-phosphate, and this activates protein kinase C via increasing diacylglycerol, hexosamine pathway via increasing fructose-6 phosphate, and increases the formation of advanced glycation end products via increasing methyl-glyoxal formation [89]. Mitochondrial ROS, via activating poly (ADP-ribose) polymerase, can break strands of nuclear DNA, which, via modifying glyceraldehyde-3-phosphate, reduces its activity. In the pathogenesis of diabetic retinopathy glyceraldehyde-3-phosphate activity is decreased in the retina and its capillary cells, and the enzymes becomes covalently modified [21, 90]. Furthermore, the translocation of retinal glyceraldehyde-3-phosphate from cytosol to the nucleus is facilitated because of its increased hydrophobicity, shifting the enzyme from being just glycolytic to being an apoptotic moiety, and apoptosis of retinal capillary cells precedes the development of diabetic retinopathy [10, 11]. Our studies have shown that overexpression of glyceraldehyde-3-phosphate prevents glucose-induced inhibition of its activity, nuclear translocation, apoptosis, and activation of protein kinase C and hexosamine pathways [90]. Thus, the possibility that increased mitochondrial superoxide provide a link between increased mitochondrial dysfunction and other metabolic abnormalities associated with the development of diabetic retinopathy appears to be very strong.
OXIDATIVE STRESS AND APOPTOSIS
Mitochondria dysfunction can initiate an intrinsic apoptotic pathway and damage its DNA [91]. ROS, by opening the mitochondrial permeability transition pores, alter the mitochondrial membrane potential, and this allows cytochrome c and apoptotic induced factor to release in the cytosol and Bax to translocate to the mitochondria. These events subsequently activate apoptosis machinery [92–94]. In the pathogenesis of diabetic retinopathy, retinal capillary cells and other non capillary cells undergo accelerated apoptosis before clinical manifestations of retinopathy are observed [10, 12, 20, 33, 61, 95]. Apoptosis of pericytes and endothelial cells can serve as a predictor of histopathology characteristic of diabetic retinopathy [11, 33, 61], and mitochondrial dysfunction is considered as one of the underlying pathways [33, 35]. Retina and its capillary cells, in diabetic conditions present increased mitochondrial superoxide, mitochondria permeability is increased and they start to leak cytochrome C into the cytosol and Bax translocates to the mitochondria [33, 35]. Our recent studies have shown that the damage of retinal mitochondria in diabetes is mediated via activation of matrix metalloproteinases, especially MMP-2 and MMP-9, and overexpression of the enzyme responsible for quenching mitochondrial superoxide, in addition to preventing diabetes-induced superoxide accumulation and activation of MMPs, inhibits capillary cell apoptosis and the degeneration of retinal capillaries in diabetic mice [64, 65].
Activation of MMP-9 is considered to be under the control of H-Ras, a small molecular weight G-protein, and its downstream signaling pathway [96]. Our studies with animal models of diabetic retinopathy, and also in in vitro models, have clearly demonstrated that diabetes activates H-ras and MMP-9 in retina and its microvasculature contributing to the accelerated apoptosis of retinal capillary cells [97], a phenomenon which predicts the development of pathology associated with retinopathy. H-Ras serves as a “molecular switch” converting signals from cell membrane to nucleus and serves as a key regulator of the signaling cascade triggered by oxidative stress [98]. H-Ras exists as either inactive GDP-bound form or an active GTP-bound form [99], and guanine nucleotide exchange factor in ERK and JNK pathway activates Ras by converting Ras-GDP to Ras-GTP [100]. This activated Ras stimulates the production of super-oxide radicals by NADPH oxidase-like activity [101], and also increase in intracellular ROS triggers H-Ras activation, which further stimulates ROS level by activating NADPH oxidase [98, 101]. Activated H-Ras stimulates the translocation of Raf-1 to plasma membrane, where it phosphorlyates the tyrosine kinase/serine kinases of the mitogen-activated protein kinase [102, 103]. Hyperglycemia has been shown to markedly stimulate the activation of ERK 1/2, c-Jun NH2-terminal kinase, stress activated protein kinase and p38MAPK in retinal cells [104]. Thus, clear elucidation of the signaling pathway should help us identify the molecular targets to prevent the development of diabetic retinopathy.
Furthermore, recent studies have demonstrated that mice overexpression of anti-apoptotic protein Bcl-2 in the vascular endothelium inhibits degeneration of retinal capillaries in diabetic mice [105]. These observations have implicated the role of mitochondrial dysfunction in the pathogenesis of diabetic retinopathy. However, fission and fusion are also necessary to maintain mitochondrial integrity, and this machinery is reported to be disintegrated during apoptosis [106]. How fusion-fission regulates apoptosis of retinal capillary cells in the development of diabetic retinopathy remains to be explored.
SUPEROXIDE DAMAGE AND METABOLIC MEMORY PHENOMENON
As stated above, hyperglycemia is the main instigator of diabetes complications, including retinopathy. Two longitudinal studies have demonstrated that good glycemic control reduces the risk of the development of the retinopathy, and the duration of poor glycemic control before initiation of good glycemic control dictates the benefits of good glycemic control. Good glycemic control initiated at the early stage of diabetes has the most profound long-term impact [2, 107]. These studies have suggested that poor glycemic control in early stages of the disease leaves an imprint in the tissues that are the targets of micro and macro vascular complications, suggesting a ‘metabolic memory’ phenomenon. This memory phenomenon associated with the continued progression of diabetic retinopathy is also observed in the animal models of diabetic retinopathy-studies using chemically-induced diabetic dogs and rodents have demonstrated that diabetic retinopathy resists arrest after hyperglycemic insult is terminated [16, 17, 21, 108]. We have shown that oxidative stress is an important contributor to this metabolic memory phenomenon-retina and its capillary cells continue to experience increased oxidative stress, and the enzyme responsible for scavenging mitochondrial superoxide, glutathione levels and the overall antioxidant capacity of the retina continues to be subnormal, and the microvasculature of the retina remains to show increased peroxynitrite accumulation [17–21, 109]. Mitochondrial dysfunction itself can lead to increased production of ROS, which can increase oxidative stress if the defense mechanisms of the cell are overwhelmed [110]. Our recent studies have demonstrated that diabetes-induced retinal mitochondrial DNA damage also continues even after hyperglycemia is terminated, and the mtDNA-encoded genes remain compromised producing a continuous cycle of superoxide production [22, 37], and apoptosis of capillary cells continues [20]. Thus, increased superoxide production in diabetes not only contributes to the development of diabetic retinopathy, it also plays an important part in its continued progression even after hyperglycemic insult is eliminated.
ANTIOXIDANTS AND DIABETIC RETINOPATHY
From the discussion presented here, diabetic retinopathy is a multifactorial slow progressing blinding disease which presents many challenges to treat. Good glycemic control remains one of the best options, but maintenance of such control for this lifelong disease presents many difficult challenges, including the risk of hypoglycemia and weight gain. Diabetes increases oxidative stress in the retina and its capillary cells, and mitochondrial dysfunction has an important role in the development of diabetic retinopathy. Thus, strategies targeting towards decreasing oxidative stress and keeping the mitochondria homeostasis offer an interesting avenue to prevent or to control the development of diabetic retinopathy. However, repair of the specific dysfunction portion of the mitochondria to increase its overall antioxidant defense could be challenging.
The direct activation of MnSOD, impaired in diabetic retinopathy, is a good strategy to prevent mitochondria dysfunction since it is the main superoxide scavenger in this organelle (Fig. (2)). Administration of lipoic acid, a co-factor for some of the enzymes including MnSOD, have been shown by our laboratory to prevent the apoptosis of retinal capillary cells and the development of retinopathy in diabetic rats and abrogate mitochondrial dysfunction [34, 61]. Others have shown that lipoic acid delays retinal blood barrier breakdown, a hallmark of diabetic retinopathy [111]. A synthetic mimetic of MnSOD, MnTBAP, prevents hyperglycemia-induced superoxide generation and apoptosis in retinal endothelial cells, and overexpression of MnSOD in mice prevents the development of retinopathy in diabetic mice [35, 64]. These studies have strongly suggested that regulation of MnSOD by pharmacologic or genetic means have potential to inhibit the development of retinopathy in diabetic patients.
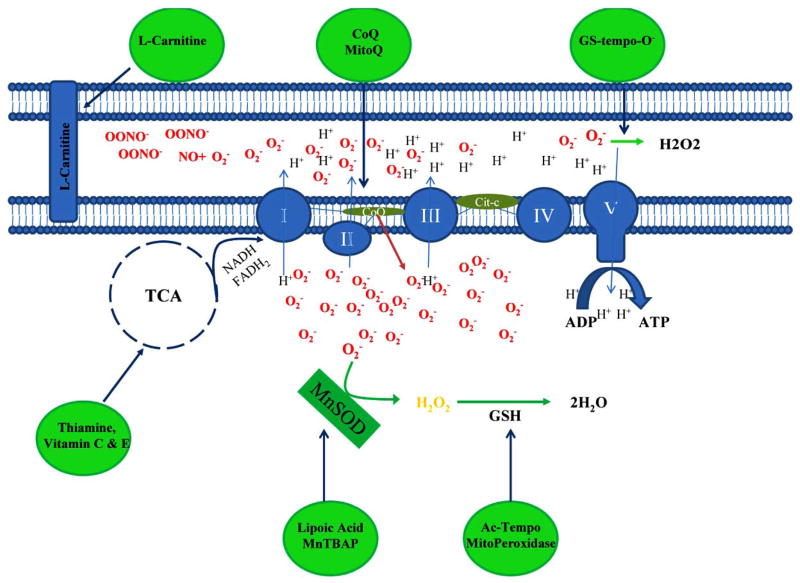
Fig. 2.
Schematic representation of the modes of action of the antioxidants. L-Carnitine transports fatty acid into mitochondria; vitamin C, vitamin E and thiamine are coenzyme factors for tricarboxylic acid cycles; lipoic acid, MnTBAP and GS-tempo-O decrease superoxide levels; Mito peroxidase and AC-TEMPO preserve glutathione to neutralizes hydrogen peroxide to water; and Coenzyme Q10 (CoQ) and MitoQ prevent complexes of the electron transport chain system.
Therapies with vitamins and supplements have been used to treat diseases associated with mitochondrial dysfunction. In diabetic animals, administration of a diet complemented with antioxidant vitamins C and E, inhibits the impairment of antioxidant defense system [58]. However, the inhibition is better if this vitamins C+E mixture is complemented with Trolox, N-acetyl cystein, beta-carotene and selenium [25], or with beta-carotene, zinc and copper [66]. This AREDS-based micronutrients, which is being used for other ocular disease-age-related macular degeneration [112], also prevents diabetes-induced inhibition of retinal MnSOD and mitochondrial electron transport complex III [66], and are being used for other ocular disease-age-related macular degeneration [112]. Thus, there is a need to test these micronutrients in clinical settings. It should be acknowledged that the clinical studies, though very limited in number, have demonstrated some beneficial effects against oxidative stress, but the data are inconsistent and have focused on the outcomes related to complications other than retinopathy [113, 114].
Thiamine (vitamin B1), an essential cofactor for several enzymes in the Krebs cycle, is a protective agent against metabolic damage induced by diabetes [115]. Studies have demonstrated that thiamine and benfotiamine (a lipophilic form of thiamine) protect retinal pericyte against apoptosis induced by mitochondrial dysfunction [116], and also retinal histopathology and other metabolic pathways associated with the development of diabetic retinopathy [115].
L-carnitine, which facilitates the transport of long chain fatty acids into mitochondria, has been show to attenuate mitochondrial dysfunction and inhibit cell apoptosis during diabetes [117]. In other ocular diseases, eg., cataract and glaucoma, L-carnitine is shown to provide beneficial effect and improve insulin sensitivity in insulin-resistant diabetic patients, suggesting a possible beneficial effect on the progression of diabetic retinopathy [118].
Taurine, a non essential amino acid, is the most abundant amino acid in several tissues including the retina and its deficiency is involved in retinal degenerations. Taurine supplementation has been shown to protect mitochondrial oxidative stress in liver and myocardium cells, and in diabetes taurine supplementation attenuates histopathology associated with diabetic retinopathy [119]. However the use of taurine as a therapy to prevent mitochondria dysfunction in diabetic retinopathy needs further investigation.
Mitochondria inner membrane is highly impermeable, and this could decrease the efficacy of the supplements since they cannot cross the membrane and reach the targets. In order to facilitate the diffusion of exogenous antioxidant, researchers are actively taking the advantage of mitochondrial membrane’s negative potential required for the electron transport chain function to develop new strategies to deliver antioxidants into the organelle. Administration of coenzyme Q10 (CoQ), an indispensable component for the activity of Complex I, II and III as shown in Fig. (2) of mitochondrial electron transport chain, has been shown to attenuate complications triggered by mitochondria dysfunction in neurodegenerative disease [120]. CoQ supplementation in diabetic patients is shown to decrease oxidative stress by attenuating the impairment in electron transport chain complex [121]. Mito Q, a mixture of ubiquinol (mitoquinol) and ubiquinone (mitoquinone) mimics the activity of CoQ antioxidant coenzyme, and administration of Mito Q decreases cardiac ischemia-reperfusion injury in rats [122]. In addition, CoQ and idebenone, a short-chain quinine have also been used for various mitochondrial diseases. Due to the unique mitochondrial localization of enzymes that catalyze the release of drugs from prodrugs, MitoPeroxidase, glutathione peroxidase like action, is shown to decrease caspase-3 mediated apoptosis [123]. In addition, an alternative class of free-radical scavenging agents, Ac-TEMPO and GS-TEMPO, is shown to preserve mitochondrial glutathione [124]. How these therapies will affect the development of retinopathy in diabetic patients remains to be explored.
In conclusion, therapies which prevent superoxide accumulation, maintain mitochondrial homeostasis and protect its DNA appear to be the most likely strategies to prevent the development of diabetic retinopathy. Thus, therapies that could target multiple steps of oxidative stress and mitochondrial damage should provide a hope for the prevention of this multifactorial blinding complication of diabetes.
References
- 1.Engerman RL, Kern TS. Diabetic retinopathy: is it a consequence of hyperglycemia. Diabet Med. 1985;2(3):200–203. doi: 10.1111/j.1464-5491.1985.tb00636.x. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287(19):2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26(2):77–82. [Google Scholar]
- 5.Porta M, Bandello F. Diabetic retinopathy: A clinical update. Diabetologia. 2002;45(12):1617–1634. doi: 10.1007/s00125-002-0990-7. [DOI] [PubMed] [Google Scholar]
- 6.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350(1):48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 7.Kuwabara T, Cogan DG. Retinal vascular patterns. VI. Mural cells of retinal capillaries. Arch Ophthalmol. 1963;69:492–502. doi: 10.1001/archopht.1963.00960040498013. [DOI] [PubMed] [Google Scholar]
- 8.Bresnick GH, Davis MD, Myers FL, de Venecia G. Clinico-pathologic correlations in diabetic retinopathy. II. Clinical and histologic appearances of retinal capillary microaneurysms. Arch Ophthalmol. 1977;95(7):1215–1220. doi: 10.1001/archopht.1977.04450070113010. [DOI] [PubMed] [Google Scholar]
- 9.Engerman RL. Pathogenesis of diabetic retinopathy. Diabetes. 1989;38(10):1203–1206. doi: 10.2337/diab.38.10.1203. [DOI] [PubMed] [Google Scholar]
- 10.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97(12):2883–2890. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kern TS, Tang J, Mizutani M, Kowluru R, Nagraj R, Lorenzi M. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: Comparison of diabetes and galactosemia. Invest Ophthalmol Vis Sci. 2000;41(12):3972–3978. [PubMed] [Google Scholar]
- 12.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102(4):783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993;262(5134):695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- 14.Diabetes Control and Complications Trial Research Group. Early worsening of diabetic retinopathy in the diabetes control and complication trial. Arch Ophthalmol. 1998;116(11):874–886. doi: 10.1001/archopht.116.7.874. [DOI] [PubMed] [Google Scholar]
- 15.UKPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 16.Engerman RL, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987;36(7):808–812. doi: 10.2337/diab.36.7.808. [DOI] [PubMed] [Google Scholar]
- 17.Kowluru RA. Effect of re-institution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes. 2003;52(3):818–823. doi: 10.2337/diabetes.52.3.818. [DOI] [PubMed] [Google Scholar]
- 18.Kowluru RA, Chakrabarti S, Chen S. Re-Institution of good metabolic control in diabetic rats on the activation of caspase-3 and nuclear transcriptional factor (NF-kB) in the retina. Acta Diabetol. 2004;44(4):194–199. doi: 10.1007/s00592-004-0165-8. [DOI] [PubMed] [Google Scholar]
- 19.Kowluru RA, Kanwar M, Kennedy A. Metabolic memory phenomenon and accumulation of peroxynitrite in retinal capillaries. Exp Diabetes Res. 2007;2007:2196. doi: 10.1155/2007/21976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowluru RA, Chan PS. Metabolic memory in diabetes - from in vitro oddity to in vivo problem: Role of Apoptosis. Brain Res Bull. 2010;81(2–3):297–302. doi: 10.1016/j.brainresbull.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanwar M, Kowluru R. Role of glyceraldehyde 3-phosphate dehydrogenase in the development and progression of diabetic retinopathy. Diabetes. 2009;58(1):227–234. doi: 10.2337/db08-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madsen-Bouterse SA, Mohammad G, Kanwar M, Kowluru RA. Role of mitochondrial DNA damage in the development of diabetic retinopathy, and the metabolic memory phenomenon associated with its progression. Antioxid Redox Signal. 2010;13(6):797–805. doi: 10.1089/ars.2009.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kowluru R, Kern TS, Engerman RL. Abnormalities of retinal metabolism in diabetes or galactosemia II. Comparison of gamma-glutamyl transpeptidase in retina and cerebral cortex, and effects of antioxidant therapy. Curr Eye Res. 1994;13(12):891–896. doi: 10.3109/02713689409015092. [DOI] [PubMed] [Google Scholar]
- 24.Kowluru RA, Jirousek MR, Stramm LE, Farid NA, Engerman RL, Kern TS. Abnormalities of retinal metabolism in diabetes or experimental galactosemia. V. Relationship between protein kinase C and ATPases. Diabetes. 1998;47(3):464–469. doi: 10.2337/diabetes.47.3.464. [DOI] [PubMed] [Google Scholar]
- 25.Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50(8):1938–1942. doi: 10.2337/diabetes.50.8.1938. [DOI] [PubMed] [Google Scholar]
- 26.Oates PJ, Mylari BL. Aldose reductase inhibitors: therapeutic implications for diabetic complications. Expert Opin Investig Drugs. 1999;8(12):2095–2119. doi: 10.1517/13543784.8.12.2095. [DOI] [PubMed] [Google Scholar]
- 27.Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res. 2007;2007:61038. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stitt AW. The role of advanced glycation in the pathogenesis of diabetic retinopathy. Exp Mol Pathol. 2003;75(1):95–108. doi: 10.1016/s0014-4800(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 29.Stitt AW, Hughes SJ, Canning P, Lynch O, Cox O, Frizzell N, Thorpe SR, Cotter TG, Curtis TM, Gardiner TA. Substrates modified by advanced glycation end-products cause dysfunction and death in retinal pericytes by reducing survival signals mediated by platelet-derived growth factor. Diabetologia. 2004;47(10):1735–1746. doi: 10.1007/s00125-004-1523-3. [DOI] [PubMed] [Google Scholar]
- 30.Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC β inhibitor. Science. 1996;272(5262):728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 31.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106(8):1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy S, Lorenzi M. Early biosynthetic changes in the diabetic-like retinopathy of galactosefed rats. Diabetologia. 1996;39(6):735–738. doi: 10.1007/BF00418547. [DOI] [PubMed] [Google Scholar]
- 33.Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Invest Ophthalmol Vis Sci. 2003;44(12):5327–5334. doi: 10.1167/iovs.03-0353. [DOI] [PubMed] [Google Scholar]
- 34.Kowluru RA, Atasi L, Ho YS. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2006;47(4):1594–1599. doi: 10.1167/iovs.05-1276. [DOI] [PubMed] [Google Scholar]
- 35.Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48(8):3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 36.Al-Shabrawey M, Rojas M, Sanders T, Behzadian A, El-Remessy A, Bartoli M, Parpia AK, Liou G, Caldwell RB. Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci. 2008;49(7):3239–3244. doi: 10.1167/iovs.08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madsen-Bouterse S, Zhong Q, Mohammad G, Ho YS, Kowluru RA. Oxidative damage of mitochondrial DNA in diabetes, and its protection by manganese superoxide dismutase. Free Radic Res. 2010;44(3):313–321. doi: 10.3109/10715760903494168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baynes JW, Thrope SR. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes. 1999;48(1):1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Yanoff M, Jian B, He Z. Altered mRNA levels of anti-oxidant enzymes in pre-apoptotic pericytes from human diabetic retinas. Cell Mol Biol. 1999;45(1):59–66. [PubMed] [Google Scholar]
- 40.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48(6):749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamada Y, Fujii H, Fukagawa M. Role of oxidative stress in diabetic bone disorder. Bone. 2009;45(1):S35–S38. doi: 10.1016/j.bone.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Naruse K, Nakamura J, Hamada Y, Nakayama M, Chaya S, Komori T, Kato K, Kasuya Y, Miwa K, Hotta N. Aldose reductase inhibition prevents glucose-induced apoptosis in cultured bovine retinal microvascular pericytes. Exp Eye Res. 2000;71(3):309–315. doi: 10.1006/exer.2000.0882. [DOI] [PubMed] [Google Scholar]
- 43.Miwa K, Nakamura J, Hamada Y, Naruse K, Nakashima E, Kato K, Kasuya Y, Yasuda Y, Kamiya H, Hotta N. The role of polyol pathway in glucose-induced apoptosis of cultured retinal pericytes. Diabetes Res Clin Pract. 2003;60(1):1–9. doi: 10.1016/s0168-8227(02)00248-6. [DOI] [PubMed] [Google Scholar]
- 44.Yang J, Lane PH, Pollock JS, Carmines PK. Protein kinase C-dependent NAD(P)H oxidase activation induced by type 1 diabetes in renal medullary thick ascending limb. Hypertension. 2010;55(2):468–473. doi: 10.1161/HYPERTENSIONAHA.109.145714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun W, Oates PJ, Coutcher JB, Gerhardinger C, Lorenzi MA. A selective aldose reductase inhibitor of a new structural class prevents or reverses early retinal abnormalities in experimental diabetic retinopathy. Diabetes. 2006;55(10):2757–2762. doi: 10.2337/db06-0138. [DOI] [PubMed] [Google Scholar]
- 46.Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, King GL. Characterization of the mechanism for the chronic activation of DAG-PKC pathway in diabetes hypergalactosemia. Diabetes. 1994;43(9):1122–1129. doi: 10.2337/diab.43.9.1122. [DOI] [PubMed] [Google Scholar]
- 47.Aiello LP, Brusell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, Smith LE, King GL. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta isoform-selective inhibitor. Diabetes. 1997;46(9):1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 48.Park JY, Takahara N, Gabriele A, Chou E, Naruse K, Suzuma K, Yamauchi T, Ha SW, Meier M, Rhodes CJ, King GL. Induction of endothelin-1 expression by glucose: an effect of protein kinase C activation. Diabetes. 2000;49(7):1239–1248. doi: 10.2337/diabetes.49.7.1239. [DOI] [PubMed] [Google Scholar]
- 49.Yokota T, Ma RC, Park JY, Isshiki K, Sotiropoulos KB, Rauniyar RK, Bornfeldt KE, King GL. Role of protein kinase C on the expression of platelet-derived growth factor endothelin-1 in the retina of diabetic rats cultured retinal capillary pericytes. Diabetes. 2003;52(3):838–845. doi: 10.2337/diabetes.52.3.838. [DOI] [PubMed] [Google Scholar]
- 50.Kowluru RA. Diabetes-induced elevations in retinal oxidative stress, protein kinase C and nitric oxide are inter-related. Acta Diabetol. 2001;38(4):179–185. doi: 10.1007/s592-001-8076-6. [DOI] [PubMed] [Google Scholar]
- 51.Green DR, Amarante-Mendes GP. The point of no return: mitochondria, caspases, and the commitment to cell death. Results Probl Cell Differ. 1998;24:45–61. doi: 10.1007/978-3-540-69185-3_3. [DOI] [PubMed] [Google Scholar]
- 52.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279(47):49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 53.Van Houten B, Woshner V, Santos JH. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair. 2005;5(2):145–152. doi: 10.1016/j.dnarep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Stuart JA, Brown MF. Mitochondrial DNA maintenance and bioenergetics. Biochim Biophys Acta. 2006;1757(2):79–89. doi: 10.1016/j.bbabio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Radi R, Cassina A, Hodara R. Nitric oxide and peroxynitrite interactions with mitochondria. Biol Chem. 2002;383(3–4):401–409. doi: 10.1515/BC.2002.044. [DOI] [PubMed] [Google Scholar]
- 56.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7(7):517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 57.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kowluru RA, Kern TS, Engerman RL. Abnormalities of retinal metabolism in diabetes or experimental galactosemia. IV. Anti-oxidant defense system. Free Radic Biol Med. 1997;22(4):587–592. doi: 10.1016/s0891-5849(96)00347-4. [DOI] [PubMed] [Google Scholar]
- 59.Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med. 2003;35(11):1491–1499. doi: 10.1016/j.freeradbiomed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 60.Kowluru RA, Koppolu P, Chakrabarti S, Chen S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic Res. 2003;37(11):1169–1180. doi: 10.1080/10715760310001604189. [DOI] [PubMed] [Google Scholar]
- 61.Kowluru RA, Odenbach S. Effect of long-term administration of alpha lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004;53(12):3233–3238. doi: 10.2337/diabetes.53.12.3233. [DOI] [PubMed] [Google Scholar]
- 62.Kowluru RA. Diabetic retinopathy: Mitochondrial dysfunction and retinal capillary cell death. Antioxid Redox Signal. 2005;7(11–12):1581–1587. doi: 10.1089/ars.2005.7.1581. [DOI] [PubMed] [Google Scholar]
- 63.Kowluru RA, Kowluru V, Ho YS, Xiong Y. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic Biol Med. 2006;41(8):1191–1196. doi: 10.1016/j.freeradbiomed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 64.Kowluru RA, Kanwar M. Oxidative stress and the development of diabetic retinopathy: Contributory role of matrix metalloproteinase-2. Free Radic Biol Med. 2009;46(12):1677–1685. doi: 10.1016/j.freeradbiomed.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohammad G, Kowluru RA. Matrix metalloproteinase-2 in the development of diabetic retinopathy and mitochondrial dysfunction. Lab Invest. 2010 doi: 10.1038/labinvest.2010.89. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kowluru RA, Kanwar M, Chan PS, Zhang JP. Inhibition of retinopathy and retinal metabolic abnormalities in diabetic rats with AREDS-based micronutrients. Arch Ophthalmol. 2008;126(9):1266–1272. doi: 10.1001/archopht.126.9.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perrone L, Devi TS, Hosoya KI, Terasaki T, Singh LP. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol. 2009;221(1):262–272. doi: 10.1002/jcp.21852. [DOI] [PubMed] [Google Scholar]
- 68.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78(2):547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 69.Li J, Wang JJ, Yu Q, Chen K, Mahadev K, Zhang SX. Inhibition of reactive oxygen species by lovastatin down-regulates VEGF expression and ameliorates blood-retinal barrier breakdown in db/db mice: role of NADPH oxidase 4. Diabetes. 2010;59(6):1528–1538. doi: 10.2337/db09-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caldwell RB, Zhang W, Romero MJ, Caldwell RW. Vascular dysfunction in retinopathy-an emerging role for arginase. Brain Res Bull. 2010;81(2–3):303–309. doi: 10.1016/j.brainresbull.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miao L, St Clair DK. Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med. 2009;47(5):344–356. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo Z, Boekhoudt GH, Boss JM. Role of the intronic enhancer in tumor necrosis factor-mediated induction of manganous superoxide dismutase. J Biol Chem. 2003;278(26):23570–23578. doi: 10.1074/jbc.M303431200. [DOI] [PubMed] [Google Scholar]
- 73.Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002;51(7):2241–2248. doi: 10.2337/diabetes.51.7.2241. [DOI] [PubMed] [Google Scholar]
- 74.Fuchs J, Demidov D, Houben A, Schubert I. Chromosomal histone modification patterns--from conservation to diversity. Trends Plant Sci. 2006;11(4):199–208. doi: 10.1016/j.tplants.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 75.Muscoli C, Mollace V, Wheatley J, Masini E, Ndengele M, Wang ZQ, Salvemini D. Superoxide-mediated nitration of spinal manganese superoxide dismutase: a novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain. 2004;111(1–2):96–103. doi: 10.1016/j.pain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 76.Rosenblum JS, Gilula NB, Lerner RA. On signal sequence polymorphisms and diseases of distribution. Proc Natl Acad Sci USA. 1996;93(9):4471–4473. doi: 10.1073/pnas.93.9.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sutton A, Khoury H, Prip-Buus C, Cepanec C, Pessayre D, Degoul F. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics. 2003;13(3):145–157. doi: 10.1097/01.fpc.0000054067.64000.8f. [DOI] [PubMed] [Google Scholar]
- 78.Hovnik T, Dolzan V, Bratina NU, Podkrajsek KT, Battelino T. Genetic polymorphisms in genes encoding antioxidant enzymes are associated with diabetic retinopathy in type 1 diabetes. Diabetes Care. 2009;32(12):2258–2262. doi: 10.2337/dc09-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petrovic MG, Cilensek I, Petrovic D. Manganese superoxide dismutase gene polymorphism (V16A) is associated with diabetic retinopathy in Slovene (Caucasians) type 2 diabetes patients. Dis Markers. 2008;24(1):59–64. doi: 10.1155/2008/940703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen XJ, Butow RA. The organization and inheritance of the mitochondrial genome. Nat Rev Gene. 2008;6(11):815–825. doi: 10.1038/nrg1708. [DOI] [PubMed] [Google Scholar]
- 81.Kucej M, Kucejova B, Subramanian R, Chen XJ, ABR Mitochondrial nucleoids undergo remodeling in response to metabolic cues. J Cell Sci. 2008;121(Pt 11):1861–1868. doi: 10.1242/jcs.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wanrooij S, Falkenberg M. The human mitochondrial replication fork in health and disease. Biochim Biophys Acta. 2010;1797(8):1378–1388. doi: 10.1016/j.bbabio.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 83.Johannsen DL, Ravussin E. The role of mitochondria in health and disease. Curr Opin Pharmacol. 2009;9(6):780–786. doi: 10.1016/j.coph.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scarpulla RC. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann NY Acad Sci. 2008;1147:321–334. doi: 10.1196/annals.1427.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yin W, Signore AP, Iwai M, Cao G, Gao Y, Chen J. Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury. Stroke. 2008;39(11):3057–3063. doi: 10.1161/STROKEAHA.108.520114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bugger H, Chen D, Riehle C, Soto J, Theobald HA, Hu XX, Ganesan B, Weimer BC, Abel ED. Tissue-specific remodeling of the mitochondrial proteome in type 1 diabetic akita mice. Diabetes. 2009;58(9):1986–1997. doi: 10.2337/db09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kowluru RA, Menon B, Gierhart D. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rat. Invest Ophthalmol Vis Sci. 2008;49(4):1645–1651. doi: 10.1167/iovs.07-0764. [DOI] [PubMed] [Google Scholar]
- 88.Nishikawa T, Edelstein D, Brownlee M. The missing link: a single unifying mechanism for diabetic complications. Kidney Int Suppl. 2000;77:S26–S30. doi: 10.1046/j.1523-1755.2000.07705.x. [DOI] [PubMed] [Google Scholar]
- 89.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 90.Madsen-Bouterse S, Mohammad G, Kowluru RA. Glyceraldehyde 3 phosphate dehydrogenase in retinal microvasculature: Implications for the development and progression of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51(3):1765–1772. doi: 10.1167/iovs.09-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu CY, Lee CF, Wei YH. Role of reactive oxygen species-elicited apoptosis in the pathophysiology of mitochondrial and neurodegenerative diseases associated with mitochondrial DNA mutations. J Formos Med Assoc. 2009;108(8):599–611. doi: 10.1016/s0929-6646(09)60380-6. [DOI] [PubMed] [Google Scholar]
- 92.Anuradha CD, Kanno S, Hirano S. Oxidative damage to mitochondria is a preliminary step to caspase-3 activation in fluoride-induced apoptosis in HL-60 cells. Free Radic Biol Med. 2001;31(3):367–373. doi: 10.1016/s0891-5849(01)00591-3. [DOI] [PubMed] [Google Scholar]
- 93.Karpinich NO, Tafani M, Rothman RJ, Russo MA, Farber JL. The course of etoposide-induced apoptosis from damage to DNA and p53 activation to mitochondrial release of cytochrome c. J Biol Chem. 2002;277(19):16547–16562. doi: 10.1074/jbc.M110629200. [DOI] [PubMed] [Google Scholar]
- 94.Armstrong JS. Mitochondria: a target for cancer therapy. Br J Pharmacol. 2006;147(3):239–248. doi: 10.1038/sj.bjp.0706556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mizutani M, Gerhardinger C, Lorenzi M. Muller cell changes in human diabetic retinopathy. Diabetes. 1998;47(3):455–459. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- 96.Lee KW, Kim MS, Kang NJ, Kim DH, Surh YJ, Lee HJ, Moon A. H-Ras selectively up-regulates MMP-9 and COX-2 through activation of ERK1/2 and NF-kappaB: an implication for invasive phenotype in rat liver epithelial cells. Int J Cancer. 2006;119(8):1767–1775. doi: 10.1002/ijc.22056. [DOI] [PubMed] [Google Scholar]
- 97.Kowluru RA. Role of matrix metalloproteinase-9 in the development of diabetic retinopathy and its regulation by H-Ras. Invest Ophthalmol Vis Sci. 2010;51(8):4320–4326. doi: 10.1167/iovs.09-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cuda G, Paterno R, Ceravolo R, Candigliota M, Perrotti N, Perticone F, Faniello MC, Schepis F, Ruocco A, Mele E, Cassano S, Bifulco M, Santillo M, Avvedimento EV. Protection of human endothelial cells from oxidative stress: role of Ras-ERK1/2 signaling. Circulation. 2002;105(8):968–974. doi: 10.1161/hc0802.104324. [DOI] [PubMed] [Google Scholar]
- 99.Raepple D, von Lintig F, Zemojtel T, Duchniewicz M, Jung A, Lübbert M, Boss GR, Scheele JS. Determination of Ras-GTP Ras-GDP in patients with acute myelogenous leukemia (AML) myeloproliferative syndrome (MPS) juvenile myelomonocytic leukemia (JMML) acute lymphocytic leukemia (ALL) and malignant lymphoma: assessment of mutational indirect activation. Ann Hematol. 2009;88(4):319–324. doi: 10.1007/s00277-008-0593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chardin P, Camonis JH, Gale NW, van Aelst L, Schlessinger J, Wigler MH, Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993;260(5112):1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- 101.Irani K, Xia Y, Zweier JL. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275(5306):1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 102.Allen RG, Maria T. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28(3):463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 103.Kowluru RA, Kanwar M. Translocation of H-Ras and its implications in the development of diabetic retinopathy. Biochem Biophys Res Commun. 2009;387(3):461–476. doi: 10.1016/j.bbrc.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roth S, Shaikh AR, Hennelly MM, Li Q, Bindokas V, Graham CE. Mitogen-activated protein kinases and retinal ischemia. Invest Ophthalmol Vis Sci. 2003;44(12):5383–5395. doi: 10.1167/iovs.03-0451. [DOI] [PubMed] [Google Scholar]
- 105.Kern TS, Du Y, Miller CM, Hatala DA, Levin LA. Overexpression of Bcl-2 in vascular endothelium inhibits the microvascular lesions of diabetic retinopathy. Am J Pathol. 2010;176(5):2550–2558. doi: 10.2353/ajpath.2010.091062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22(12):1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342(18):381–389. [Google Scholar]
- 108.Hammes HP, Klinzing I, Wiegand S, Bretzel RG, Cohen AM, Federlin K. Islet transplantation inhibits diabetic retinopathy in the sucrose-fed diabetic Cohen diabetic rat. Invest Ophthalmol Vis Sci. 1993;34(6):2092–2096. [PubMed] [Google Scholar]
- 109.Kowluru RA, Zhong Q, Kanwar M. Metabolic memory and diabetic retinopathy: role of inflammatory mediators in retinal pericytes. Exp Eye Res. 2010;90(5):617–623. doi: 10.1016/j.exer.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jezek P, Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int J Biochem Cell Biol. 2005;37(12):2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 111.Berkowitz BA, Roberts R, Stemmler A, Luan H, Gradianu M. Impaired apparent ion demand in experimental diabetic retinopathy: correction by lipoic Acid. Invest Ophthalmol Vis Sci. 2007;48(10):4753–4758. doi: 10.1167/iovs.07-0433. [DOI] [PubMed] [Google Scholar]
- 112.Age Related Eye Disease Study Research Group. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS. Report No. 20. Arch Ophthalmol. 2007;125(5):671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- 113.Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25(4):612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- 114.Guerrero-Romero F, Rodríguez-Morán M. Complementary therapies for diabetes: the case for chromium, magnesium, and antioxidants. Arch Med Res. 2005;36(3):250–257. doi: 10.1016/j.arcmed.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 115.Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9(3):294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 116.Berrone E, Beltramo E, Solimine C, Ape AU, Porta M. Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J Biol Chem. 2006;281(14):9307–9313. doi: 10.1074/jbc.M600418200. [DOI] [PubMed] [Google Scholar]
- 117.Hao J, Shen W, Tian C, Liu Z, Ren J, Luo C, Long J, Sharman E, Liu J. Mitochondrial nutrients improve immune dysfunction in the type 2 diabetic Goto-Kakizaki rats. J Cell Mol Med. 2009;13(4):701–711. doi: 10.1111/j.1582-4934.2008.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pescosolido N, Imperatrice B, Karavitis P. Ocular disorders secondary to systemic disease and the potential role of carnitines. Drugs R D. 2008;1:15–22. doi: 10.2165/0126839-200809001-00003. [DOI] [PubMed] [Google Scholar]
- 119.Parvez S, Tabassum H, Banerjee BD, Raisuddin S. Taurine prevents tamoxifen-induced mitochondrial oxidative damage in mice. Basic Clin Pharmacol Toxicol. 2008;102(4):382–387. doi: 10.1111/j.1742-7843.2008.00208.x. [DOI] [PubMed] [Google Scholar]
- 120.Yong-Kee CJ, Salomonczyk D, Nash JE. Development and validation of a screening assay for the evaluation of putative neuro-protective agents in the treatment of parkinson’s disease. Neurotox Res. 2010 doi: 10.1007/s12640-010-9174-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 121.Hamilton SJ, Chew GT, Watts GF. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care. 2009;32(5):810–812. doi: 10.2337/dc08-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19(9):1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 123.Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: A new therapeutic direction. Biochim Biophys Acta. 2006;1762(2):256–265. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 124.Borisenko GG, Martin I, Zhao Q, Amoscato AA, Tyurina YY, Kagan VE. Glutathione propagates oxidative stress triggered by myeloperoxidase in HL-60 cells. Evidence for glutathionyl radical-induced peroxidation of phospholipids and cytotoxicity. J Biol Chem. 2004;279(22):23453–23462. doi: 10.1074/jbc.M400119200. [DOI] [PubMed] [Google Scholar]