Abstract
Background and purpose
Pharmacological prophylaxis can reduce the risk of deep venous thrombosis (DVT), pulmonary embolism (PE), and death, and it is recommended 10–35 days after total hip arthroplasty (THA) and at least 10 days after total knee arthroplasty (TKA). However, early mobilization might also reduce the risk of DVT and thereby the need for prolonged prophylaxis, but this has not been considered in the previous literature. Here we report our results with short-duration pharmacological prophylaxis combined with early mobilization and reduced hospitalization.
Patients and methods
1,977 consecutive, unselected patients were operated with primary THA, TKA, or bilateral simultaneous TKA (BSTKA) in a well-described standardized fast-track set-up from 2004–2008. Patients received DVT prophylaxis with low-molecular-weight heparin starting 6–8 h after surgery until discharge. All re-admissions and deaths within 30 and 90 days were analyzed using the national health register, concentrating especially on clinical DVT (confirmed by ultrasound and elevated D-dimer), PE, or sudden death. Numbers were correlated to days of prophylaxis (LOS).
Results
The mean LOS decreased from 7.3 days in 2004 to 3.1 days in 2008. 3 deaths (0.15%) were associated with clotting episodes and overall, 11 clinical DVTs (0.56%) and 6 PEs (0.30%) were found. The vast majority of events took place within 30 days; only 1 death and 2 DVTs occurred between 30 and 90 days. During the last 2 years (854 patients), when patients were mobilized within 4 h postoperatively and the duration of DVT prophylaxis was shortest (1–4 days), the mortality was 0% (95% CI: 0–0.5). Incident cases of DVT in TKA was 0.60% (CI: 0.2–2.2), in THA it was 0.51% (CI: 0.1–1.8), and in BSTKA it was 0% (CI: 0–2.9). Incident cases of PE in TKA was 0.30% (CI: 0.1–1.7), in THA it was 0% (CI: 0–1.0), and in BSTKA it was 0% (CI: 0–2.9).
Interpretation
The risk of clinical DVT, and of fatal and non-fatal PE after THA and TKA following a fast-track set-up with early mobilization, short hospitalization, and short duration of DVT prophylaxis compares favorably with published regimens with extended prophylaxis (up to 36 days) and hospitalization up to 11 days. This calls for a reconsideration of optimal duration of chemical thromboprophylaxis.
Total hip and knee arthroplasty (THA and TKA) are associated with perioperative risks including deep venous thrombosis (DVT) and pulmonary embolism (PE)—both of which are manifestations of venous thromboembolism (VTE)—possibly leading to a post-thrombotic syndrome (PTS) or death. The mechanisms underlying VTE are not fully understood (Malone 1977, Malone and Agutter 2006). Immobility with long hospitalization after surgery may be a contributory factor (Heit et al. 2001, Seddighzadeh et al. 2007, Sharma et al. 2007).
The recent evidence-based guidelines for DVT prophylaxis according to the American College of Chest Physicians consist of at least 10 days of prophylaxis after TKA and THA, and preferably up to 35 days after THA (Geerts et al. 2008). The incidence of DVT has been found to have decreased over time, possibly as a result of increased and earlier mobilization (Xing et al. 2008). We therefore present our data on DVT, PE, and death after TKA, bilateral simultaneous TKA (BSTKA), and THA with a fast-track set-up with short DVT prophylaxis, early mobilization, and short hospitalization.
Patients and methods
The fast-track set-up was implemented in 2003 and consists of optimized logistics and evidence-based clinical features (as described in detail below) (Husted et al. 2008). Optimization of logistics includes a homogenous ward with arthroplasty patients only, regular staff, high continuity, preoperative information including intended length of stay (LOS), admission on the day of surgery, and functional discharge criteria (ability to get dressed, to get in and out of bed, to get into and up from a chair, and to walk with crutches or better).
Since then, all patients operated on with THA, TKA, bilateral simultaneous TKA (BSTKA), and revisions have been enrolled in the program. We concentrated on pain treatment and early mobilization, and our current research involves refinement of these tools for improving convalescence in order to reduce length of stay. Thus, LOS has been reduced in increments. From 2004 to 2006 the intended LOS was around 5 days, and from 2007 to 2008 the intended LOS was 3 days when local infiltration analgesia (LIA) was introduced, allowing patients to be mobilized with only moderate pain (Andersen et al. 2008a, b, c, Kerr and Kohan 2008).
The patients were consecutive and unselected, and all of them were operated using regional anesthesia—from 2004 to 2006 epidural for TKAs and spinal for THAs, but from 2007 all with spinal anesthesia with 2 mL hyperbaric bupivacaine 0.5% (TKA), 2.5 mL plain bupivacaine 0.5% (THA), and 3 mL plain bupivacaine 0.5% (BSTKA) combined with local infiltration analgesia (LIA) (Andersen et al. 2008a). Also, a standardized program in the operating theater was followed, including fluid administration (Holte et al. 2007), use of tranexamic acid (Benoni and Fredin 1996, Husted et al. 2003), small standard incisions (posterior for THA, midline skin and medial parapatellar for TKA), no drains, use of compression bandages (Andersen et al. 2008c), and cooling (Kullenberg et al. 2006). Multimodal opioid-sparing analgesia (NSAID, paracetamol, gabapentin, local analgesia) together with early mobilization has facilitated the reduction in LOS (Andersen et al. 2009).
All TKA patients were operated with a tricompartmental cemented prosthesis. Surgery was done in a bloodless field by using a femoral tourniquet (inflated to 100 mmHg above systolic blood pressure) from incision until cementation of the prosthesis was finished. 15 minutes before incision, 500 mg of intravenous tranexamic acid was administered and another 500 mg just before tourniquet release.
All THA patients were operated with an uncemented cup and either a cemented or an uncemented stem. 15 minutes before incision, 1,000 mg of intravenous tranexamic acid was administered. Immediate and full weight bearing was allowed after all operations.
Discharge criteria were strictly functional and all patients were discharged to their own homes. LOS was counted as number of nights of hospitalization after the operation.
DVT prophylaxis consisted of low-molecular-weight heparin (LMWH) (enoxaparin 40 mg subcutaneously) starting 6–8 h postoperatively and continuing once daily in the evening until discharge. No extended prophylaxis was given and no mechanical devices (including compression stockings) were used. No attempt was made to identify high-risk patients; all patients received the same regime.
Patients were mobilized on the day of operation. In the period 2004–2006, patients were mobilized in the afternoon or evening, but from 2007 the LIA technique made a more aggressive mobilization possible, usually within 2–4 h after the operation. The pain treatment consisted initially of NSAIDs and paracetamol—with opioids for breakthrough pain—but since 2007, a standardized pain treatment has been given to all patients (gabapentin, paracetamol, celecoxib with oxycodone 10 mg on request) (Andersen et al. 2008a, b).
All patients are registered in the National Health Register when they are operated and all re-admissions at any hospital in Denmark are registered. All records were scrutinized for re-admissions, and all re-admissions with DVT, PE, or death and also deaths without re-admission were analyzed and registered. All deaths were followed by autopsy except in one case, and the diagnosis was recorded and linked to the operation if a clotting episode was involved. In the case where autopsy was not performed, death was assumed to be linked to the operation. No patients were lost to follow-up, which was therefore 100%.
Statistics
The study was presented to the ethical committee for the capital region. Numbers of incident cases are the cumulated cases, i.e. the number of new cases within a specified time period divided by the size of the population initially at risk. 95% confidence intervals (CI) were calculated using the Wilson procedure without correction of continuity using the VassarStats website for statistical computation (faculty.vassar.edu/lowry/VassarStats.html). All data analyses were conducted with SPSS for Windows, version 13.0.
Results
From 2004 through 2008, 1,977 operations were performed: 947 THA, 784 TKA, and 246 BSTKA. Mean length of stay (LOS) decreased during the study period from 4.6 to 3.1 days for TKA, 6.3 to 3.9 days for THA, and 7.3 to 4.2 days for BSTKA.
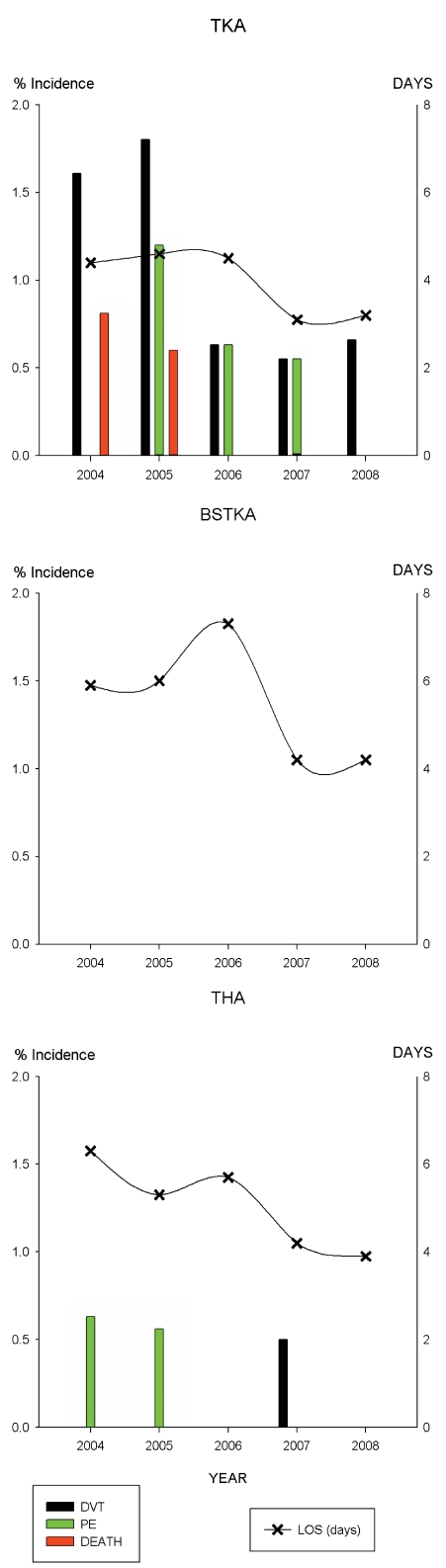
Tables 1 and 2 show distribution of operations, LOS, and corresponding numbers of incident cases of DVT, PE, and death within 90 days. The figure shows the numbers of incident cases of DVT, PE, and death for each year with corresponding mean LOS.
Table 1.
Distribution of operations and length of stay (LOS)
| No. of operations | Length of stay | ||||||
|---|---|---|---|---|---|---|---|
| All | TKA | BSTKA | THA | TKA | BSTKA | THA | |
| 2004 | 311 | 124 | 28 | 159 | 4.4 | 5.9 | 6.3 |
| 2005 | 378 | 167 | 32 | 179 | 4.6 | 6.0 | 5.3 |
| 2006 | 434 | 159 | 58 | 217 | 4.5 | 7.3 | 5.7 |
| 2007 | 433 | 182 | 52 | 199 | 3.1 | 4.2 | 4.2 |
| 2008 | 421 | 152 | 76 | 193 | 3.2 | 4.2 | 3.9 |
| Total | 1,977 | 784 | 246 | 947 | |||
Table 2.
Numbers and incidences (with 95% CI) of deep venous thrombosis (DVT), pulmonary embolism (PE), and death (D) within 90 days
| Number of | Incidences (CI) | |||||
|---|---|---|---|---|---|---|
| DVT | PE | D | DVT | PE | D | |
| TKA | ||||||
| 2004 | 2 | 0 | 1 | 1.61 (0.4–5.7) | 0 (0–3.0) | 0.81 (0.1–4.4) |
| 2005 | 4 | 2 | 1 | 2.40 (0.9–6.0) | 1.20 (0.3–4.3) | 0.60 (0.1–3.3) |
| 2006 | 1 | 1 | 0 | 0.63 (0.1–3.5) | 0.63 (0.1–3.5) | 0 (0–2.4) |
| 2007 | 1 | 1 | 0 | 0.55 (0.1–3.1) | 0.55 (0.1–3.1) | 0 (0–2.1) |
| 2008 | 1 | 0 | 0 | 0.66 (0.1–3.6) | 0 (0–2.5) | 0 (0–2.5) |
| Total | 1.15 (0.6–2.2) | 0.51 (0.2–1.3) | 0.26 (0.1–1.0) | |||
| BSTKA | ||||||
| 2004 | 0 | 0 | 0 | 0 (0–12.1) | 0 (0–12.1) | 0 (0–12.1) |
| 2005 | 0 | 0 | 0 | 0 (0–10.7) | 0 (0–10.7) | 0 (0–10.7) |
| 2006 | 0 | 0 | 0 | 0 (0–6.2) | 0 (0–6.2) | 0 (0–6.2) |
| 2007 | 0 | 0 | 0 | 0 (0–6.9) | 0 (0–6.9) | 0 (0–6.9) |
| 2008 | 0 | 0 | 0 | 0 (0–4.8) | 0 (0–4.8) | 0 (0–4.8) |
| Total | 0 (0–1.5) | 0 (0–1.5) | 0 (0–1.5) | |||
| THA | ||||||
| 2004 | 0 | 1 | 1 | 0 (0–2.4) | 0.63 (0.1–3.5) | 0.63 (0.1–3.5) |
| 2005 | 0 | 1 | 0 | 0 (0–2.1) | 0.56 (0.1–3.1) | 0 (0–2.1) |
| 2006 | 0 | 0 | 0 | 0 (0–1.7) | 0 (0–1.7) | 0 (0–1.7) |
| 2007 | 1 | 0 | 0 | 0.50 (0.1–2.8) | 0 (0–1.9) | 0 (0–1.9) |
| 2008 | 1 | 0 | 0 | 0.52 (0.1–2.9) | 0 (0.1–2.0) | 0 (0.1–2.0) |
| Total | 0.21 (0.1–0.8) | 0.21 (0.1–0.8) | 0.11 (0–0.6) | |||
Deaths
From 2004 through 2008, 14 patients (0.71% (CI: 0.4–1.2)) died within 90 days of surgery: 3 in 2004 (mesenterial thrombosis (89 days), myocardial infarction (2 days), and sepsis/pneumonia (27 days)); 3 in 2005 (hepatic cirrhosis and coma (28 days), perforated colon cancer (51 days), sudden death (1 day, no autopsy)); 3 in 2006 (metastatic lung cancer (77 days), dysregulated warfarin treatment (resulting in massive bleeding) for atrial fluttering (50 days), perforated gastric ulcer (22 days)); 4 in 2007 (cardiomyopathy and failure (89 days), lung cancer (10 days), mechanical bowel obstruction (76 days), hepatic cirrhosis with esophageal bleeding (83 days)); and 1 in 2008 (aortic stenosis, pulmonary insufficiency, and cardiac failure (19 days)). Thus, 2 deaths in 2004 (1 THA and 1 TKA) and 1 in 2005 (TKA)—and none in 2006–2008—may have been related to clotting episodes, giving an overall risk of VTE-related mortality of 0.15% (CI: 0.04–0.48).
Pulmonary embolism
From 2004 through 2008, 6 patients had confirmed pulmonal embolism (PE) diagnosed by perfusion-ventilation lung scintigraphy, with none in the BSTKA group, 4 in the TKA group, and 2 in the THA group leading to risks from 0% to 0.63%. All PE occurred before 30 days postoperatively. No patients died from PE (except the potential one with sudden unexplained death without autopsy in 2005). During the last 2 years with the shortest LOS, the risk of PE was 0.30% (CI: 0.1–1.7) after TKA and 0% (CI: 0–1.0) after THA.
Numbers of incident cases of DVT, PE and death for each year with corresponding mean LOS.
Deep venous thrombosis
11 clinical cases of DVT were found within 90 days from 2004 through 2008 with the clinical diagnosis being confirmed by an elevated D-dimer result and a positive ultrasound examination: 9 in the TKA group (1.2%) and 2 in the THA group (0.2%). No DVT was seen in the BSTKA group. Only 2 cases of DVT were found between 30 and 90 days (1 THA, 1 TKA). None of the patients with DVT developed PE within 90 days, since all patients with PE had no clinical diagnosis of DVT. 1 of 2 DVTs in THA was proximal to the knee joint, and 3 of 9 DVTs in TKA. Combining the last 2 years with the shortest LOS, the risk of DVT in TKA was 0.6% (CI: 0.2–2.2) and 0.51% (CI: 0.1–2.1) after THA.
97 days of hospitalization for DVT/PE re-admissions were used in the TKA group (21 days, 55 days, 12 days, 8 days, and 1 day for the years 2004–2008, respectively), 0 days for the BSTKA group, and 28 days for the THA group (12 days, 4 days, 0 days, 10 days, and 2 days for 2004–2008, respectively). In addition, 80 patients were re-admitted for suspected DVT during the study period (46 TKAs, 1 BSTKA, and 33 THAs), but the diagnosis was excluded by ultrasound and D-dimer measurements.
Discussion
We found a very low risk of clinically symptomatic VTE and deaths potentially related to the operation in an unselected patient population with a consecutive set-up and 100% follow-up for 90 days. We used the unique Danish National Health Register to record all national re-admissions and the Danish Personal Register to assess mortality. In Denmark, all patients with verified DVT are re-admitted and treated in hospital, so we believe that the numbers of incident cases we found are true risks and not underestimates of the real numbers—as no patients are treated initially for DVT in an outpatient setting. Also, all charts were scrutinized for all types of re-admission in order to avoid missing cases of VTE that were miscoded as other diagnoses for re-admission.
We found an overall risk of death potentially related to the operation of 0.15%, which compares favorably with the results of a recent study where 0.31% of patients died within 90 days of an operation with THA or TKA, and following 6 weeks of 150 mg aspirine daily as the only prophylaxis (Cusick and Beverland 2009). Also, the risk of death possibly resulting from clotting episodes found in the current study was lower or similar to those in all previously published studies where postoperative mortality was validated, regardless of type (whether chemoprophylactic or mechanical) and duration of thromboprophylaxis (up to 36 days) (Mantilla et al. 2002, Blom et al. 2006, Tarity et al. 2006, Lachiewiicz and Soileau 2006, 2007, Samama et al. 2007, Eriksson et al. 2008, Aynardi et al. 2009, Lassen et al. 2009).
Rebound activation of coagulation after cessation of prophylaxis may play a role in VTE and death after surgery. Rebound hypercoagulability has been described to occur after discontinuation of chemical prophylaxis (Kontny 1997, Cundiff 2008) based on continuous generation of platelets and thrombin after cessation of the prophylactic drug. Rebound hypercoagulability is estimated to account for 2% of patients having VTE in the first 2 months after discontinuing oral anticoagulants; it is also been found, however, that an increased duration of treatment with oral anticoagulants does not reduce the overall number of adverse events (Cundiff 2008). However, the low risk of VTE in our study suggests that rebound hypercoagulability may not be a problem after a few days of thromboprophylaxis.
The evidence-based guidelines for DVT prophylaxis according to the American College of Chest Physicians involve at least 10 days of treatment after TKA and THA—and preferably up to 35 days after THA (Geerts et al. 2008). LMWH is recommended, and has been found to reduce the risk of VTE by 50%, whereas these guidelines do not support the sole use of aspirin as a prophylactic agent.
The antiplatelet effect of aspirin is, nevertheless, the reason for the use of this drug in many hospitals, in the USA especially but also in Europe. Cusick and Beverland (2009) advocated prophylaxis with aspirin (150 mg daily for 6 weeks) only for the standard patient, except for patients with a prior history of PE. They concluded that: “…with modern surgical practice, elective hip and knee replacement should no longer be considered high-risk procedures.” Others have supported this belief. Thus, some propose differentiated DVT prophylaxis by identifying low- and high-risk patients after THA and TKA, balancing the need for prophylaxis against the risk of bleeding (Lachiewicz and Soileau 2006, 2007, Dorr et al. 2007). Such regimens have led to incidences of 0.25% for (non-fatal) PE after THA and TKA (Dorr et al. 2007), and 0.35% for PE after TKA and 0.7% after THA (Lachiewicz and Soileau 2006 and 2007). These very low incidences are similar to our results on PE, although the patients were followed up for different periods of time (1–6 months vs. 90 days).
Others have found incidences of PE after THA in the range 0.1–0.7% with different regimens, with prophylactic treatment from 10–14 days and up to 36 days of rivaroxoban or enoxaparin and up to 6 weeks of aspirin (Mantilla et al. 2002, Lachiewicz and Soileau 2006, 2007, Eriksson et al. 2008, Lassen et al. 2008, Cusick and Beverland 2009). The rate of PE we found is similar to these incidences, although our patients were treated with enoxaparin for only a few days. The standardization in the use of regional analgesia and very early mobilization combined with short hospitalization may be important in achieving such low incidences of PE (Rodgers et al. 2000).
DVT can lead to a post-thrombotic syndrome (PTS) with swelling, varicose veins, pain and discomfort, and hyperpigmentation. However, PTS following TKA has been found to have a similar incidence with or without DVT, and with no major sequelae after ultrasonographically proven DVT (McAndrew et al. 2010); nor was there any substantial increase in risk of PTS after asymptomatic proximal or distal DVT following TKA or THA (Lonner et al. 2006). Also, the largest study on the possibility of PTS after THA failed to find any predictors of PTS—including the infrequent occurrence of DVT (Mant et al. 2008).
We found risks of clinically proven and symptomatic DVT of 0.21% (THA) and 1.15% (TKA) during the 5-year period. Again, these figures compare favorably with studies with longer treatment. Thus, 36 days of treatment with rivaroxaban or enoxaparin after THA resulted in 0.3% and 0.5% risk of DVT, respectively (Eriksson et al. 2008), although LOS or mobilization was not accounted for. Treatment with the same chemoprophylaxis for 10–14 days after TKA led to 1.1% and 2.2% risk of symptomatic DVT with a follow-up of 49 days at most (Lassen et al. 2008), combined with a mean LOS of 6.2 and 6.3 days (the type of mobilization was not mentioned). A multicenter study with prolonged treatment with various types of chemoprophylaxis for 36 ± 9.8 days (59% of patients also used compression stockings) found 1.3% and 2.3% risk of clinically symptomatic DVT after THA and TKA, with a follow-up of 90 days (Samama et al. 2007). This study also found that lack of ambulation was predictive of VTE, as did a study on early mobilization where mobilization within 24 h from the operation resulted in a 30-fold reduction in the risk of postoperative DVT (Pearse et al. 2007). The very early ambulation of our patients supported by our pain protocol with LIA (Andersen et al. 2008 a,b,c, 2009) may thus play an important role in reducing VTE.
Another study involving 6,639 THAs and 8,326 TKAs found cumulative incidences of VTE of 1.8% and 2.3% (Warwick et al. 2007). The data from 100 hospitals in 13 countries were pooled, with varying degrees of chemical and mechanical prophylaxis. Median LOS varied from 3 to 11 days; more than one-third of the patients were discharged to rehabilitation facilities, and more than one-third of the patients had general anesthesia, reflecting the different approaches between departments. However, that study found a lack of compliance with the recommendations regarding the duration of prophylaxis, and found that the risk of VTE extended well beyond LOS in hospital with mean occurrence of VTE of 21.5 and 9.7 days after surgery for THA and TKA patients. Although that study had a very different set-up from our standardized fast-track scenario with prophylaxis only during hospitalization, we found much smaller risks of DVT. We believe that the uniform approach with spinal anesthesia and multimodal analgesia facilitating early mobilization may have been responsible for this difference.
We found only 2 cases of DVT after day 30 (and within 90 days), which coincides with the finding that on average symptomatic DVT occurs 27 days and 17 days after THA and TKA (Dahl et al. 2000). Also, we found 1 of 2 cases of DVT in the proximal veins (proximal to the knee joint) in THA and 3 of 9 cases of DVT in TKA, which corresponds with the findings of Dahl et al.
None of the patients developed PE from DVT within 90 days, which is in accordance with the 2 studies by Kim et al. (2002, 2003) in which proximal thrombi after THA, TKA, and BSTKA were not found to be associated with pulmonary embolism, even without treatment. Dahl et al. (2003) also found only 6 of 50 patients with confirmed PE to have DVT. This may depend on genetic and prothrombotic factors (Kim and Kim 2007).
It should be emphasized that only clinically relevant DVT and PE requiring re-admission were included in our clinical study, as no routine ultrasound examinations were performed. This would lead to an underestimation of the total number of DVTs, as the clinically silent ones are not included. However, quite a few patients were re-admitted suspected of having DVT by their general practitioner. For every one with a positive ultrasound, 8 were negative, as we found only 11 DVTs during the 5 years. As all deaths are accounted for—and as PTS is of little clinical relevance—we have found that clinically symptomatic DVT is a relevant outcome parameter.
We encountered no VTE-related deaths, no PEs, and no DVTs in our study population of 123 patients operated with 246 BSTKAs. The data on bilateral simultaneous total knee arthroplasty suggest that it is safe and has few complications, low mortality, and few VTEs in a fast-track setting with careful selection of the patients (excluding every form of ischemic heart disease) (Kim et al. 2009).
It is both interesting and reassuring from our point of view that we have had no deaths due to VTEs during the last few years in this consecutive, unselected population, and that there is no inverse correlation between risk of VTEs and LOS, where the continuous reduction in LOS during the study period was associated with a shorter duration of prophylaxis.
Our study has several strengths: (1) there was no selection bias since the patients were included in a continuous way based upon referral by the general practioners in the uptake area of the hospital; (2) The patients operated at our hospital are representative of THA and TKA patients; age, sex, co-morbidities etc. are typical of all patients and have been described in detail (Husted et al. 2008); (3) there was 100% follow-up; and (4) the results of our study can be expected to be the same in other departments, as our patients are typical unselected arthroplasty patients—provided that our fast-track protocol is copied. However, in our study only historical comparisons are given, as there have been no randomized controlled trials (RCTs) on fast-track surgery with early mobilization compared to a more conventional approach without early mobilization, despite the fact that early mobilization appears to be associated with lower rates of DVT (Pearse et al. 2007, Xing et al. 2008).
In conclusion, our fast-track THA and TKA regimen with early mobilization and short hospitalization—and subsequently a very short duration of LMWH chemoprophylaxis—resulted in very low risks of clotting-related death, PE, and DVT. This study was not designed (i.e. regarding the set-up and statistical power) to identify these parameters as the sole determinants of low risk of VTE, as the underlying reasons are indeed multifactorial. Rodgers et al. (2000), Pearse et al. (2007), and Xing et al. (2008) have suggested that earlier mobilization has an important role in reduction of VTE and our study appears to confirm this. A large, properly designed RCT with only the timing (and therefore the amount) of mobilization after surgery varying between groups is needed to determine the true importance of mobilization (but it would require several thousand patients).
As the risks of VTE in the present study were lower or comparable to the reported incidences in the literature from studies based on the recommended duration of prophylaxis of at least 10 days for both THA and TKA, or up to 35 days for THA, we suggest that the principles of chemical thromboprophylaxis should be reconsidered, taking into account the clinical set-up, the time of first mobilization, and the length of hospital stay.
Acknowledgments
HH and HK designed the study. HH, KSO, BBK, TØ, and CW gathered the data. HH analyzed the data. HH wrote the initial draft and HH, KSO, BBK, TØ, CW, and HK revised it.
An unrestricted research grant has been received from the Lundbeck Foundation for research in fast-track total hip and knee arthroplasty. None of the authors have received or will receive any personal financial benefit from this work.
References
- Andersen LØ, Husted H, Otte KS, Kristensen BB, Kehlet H. High-volume infiltration in total knee arthroplasty: a randomized, double-blind placebo-controlled trial. Acta Anaesthesiol Scand. 2008a;52((10)):1331–5. doi: 10.1111/j.1399-6576.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- Andersen LØ, Kristensen BB, Husted H, Otte KS, Kehlet H. Local anaesthetics after total knee arthroplasty: intraarticular or extraarticular administration? A randomized, double-blind, placebo-controlled study. Acta Orthop. 2008b;79((6)):800–5. doi: 10.1080/17453670810016885. [DOI] [PubMed] [Google Scholar]
- Andersen LØ, Husted H, Otte KS, Kristensen BB, Kehlet H. A compression bandage improves local infiltration analgesia in total knee arthroplasty. Acta Orthop. 2008c;79((6)):806–11. doi: 10.1080/17453670810016894. [DOI] [PubMed] [Google Scholar]
- Andersen LØ, Gaarn-Larsen L, Kristensen BB, Husted H, Otte KS, Kehlet H. Subacute pain and function after fast-track hip and knee arthroplasty. Anaesthesia. 2009;64((5)):508–13. doi: 10.1111/j.1365-2044.2008.05831.x. [DOI] [PubMed] [Google Scholar]
- Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop. 2009;(467)((1)):213–8. doi: 10.1007/s11999-008-0528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomized, double-blind study of 86 patients. J Bone Joint Surg (Br) 1996;78((3)):434–40. [PubMed] [Google Scholar]
- Blom A, Pattison G, Whitehouse S, Taylor A, Bannistar G. Early death following primary total hip arthroplasty: 1,727 procedures with mechanical thrombo-prophylaxis. Acta Orthop. 2006;77((3)):345–6. doi: 10.1080/17453670610046244. [DOI] [PubMed] [Google Scholar]
- Cundiff DK. Clinical evidence for rebound hypercoagulability after discontinuing oral anticoagulants for venous thromboembolism. Medscape J Med. 2008;10((11)):258. [PMC free article] [PubMed] [Google Scholar]
- Cusick LA, Beverland DE. The incidence of fatal pulmonary embolism after primary hip and knee replacement in a consecutive series of 4253 patients. J Bone Joint Surg (Br) 2009;91((5)):645–8. doi: 10.1302/0301-620X.91B5.21939. [DOI] [PubMed] [Google Scholar]
- Dahl OE, Gudmundsen TE, Haukeland L. Late occurring clinical deep vein thrombosis in joint-operated patients. Acta Orthop Scand. 2000;71((1)):47–50. doi: 10.1080/00016470052943883. [DOI] [PubMed] [Google Scholar]
- Dahl OE, Gudmundsen TE, Bjørnarå BT, Solheim DM. Risk of clinical pulmonary embolism after joint surgery in patients receiving low-molecular-weight heparin prophylaxis in hospital: a 10-year prospective register of 3,954 patients. Acta Orthop Scand. 2003;74((3)):299–304. doi: 10.1080/00016470310014229. [DOI] [PubMed] [Google Scholar]
- Dorr LD, Gendelman V, Maheshwari AV, Boutary M, Wan Z, Long WT. Multimodal thromboprophylaxis for total hip and knee arthroplasty based on risk assessment. J Bone Joint Surg (Am) 2007;89((12)):2648–57. doi: 10.2106/JBJS.F.00235. [DOI] [PubMed] [Google Scholar]
- Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W, RECORD1 study group N Engl J Med. 2008;358((26)):2765–75. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW, American College of Chest Physicians Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest (6 suppl) 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- Heit JA, Melton LJ, 3rd, Lohse CM, Petterson TM, Silverstein MD, Mohr DN, O'Fallon WM. Incidence of venous thromboembolism in hospitalized patients vs community residents. Mayo Clin Proc. 2001;76((11)):1102–10. doi: 10.4065/76.11.1102. [DOI] [PubMed] [Google Scholar]
- Holte K, Kristensen BB, Valentiner L, Foss NB, Husted H, Kehlet H. Liberal versus restrictive fluid management in knee arthroplasty: a randomized, double-blind study. Anesth Analg. 2007;105((2)):465–74. doi: 10.1213/01.ane.0000263268.08222.19. [DOI] [PubMed] [Google Scholar]
- Husted H, Blønd L, Sonne-Holm S, Holm G, Jacobsen TW, Gebuhr P. Tranexamic acid reduces blood loss and blood transfusions in primary total hip arthroplasty. Acta Orthop Scand. 2003;74:665–9. doi: 10.1080/00016470310018171. [DOI] [PubMed] [Google Scholar]
- Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79((2)):168–73. doi: 10.1080/17453670710014941. [DOI] [PubMed] [Google Scholar]
- Kerr DR, Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop. 2008;79((2)):174–83. doi: 10.1080/17453670710014950. [DOI] [PubMed] [Google Scholar]
- Kim YH, Kim JS. Incidence and natural history of deep-vein thrombosis after total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg (Br) 2002;84((4)):566–70. doi: 10.1302/0301-620x.84b4.12330. [DOI] [PubMed] [Google Scholar]
- Kim YH, Oh SH, Kim JS. Incidence and natural history of deep-vein thrombosis after total hip arthroplasty. A prospective and randomised clinical study. J Bone Joint Surg (Br) 2003;85((5)):661–5. [PubMed] [Google Scholar]
- Kim YH, Kim JS. The 2007 John Charnley Award. Factors leading to low prevalence of DVT and pulmonary embolism after THA: analysis of genetic and prothrombotic factors. Clin Orthop. 2007;((465)):33–9. doi: 10.1097/BLO.0b013e318156bfac. [DOI] [PubMed] [Google Scholar]
- Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg (Br) 2009;91((1)):64–8. doi: 10.1302/0301-620X.91B1.21320. [DOI] [PubMed] [Google Scholar]
- Kontny F. Reactivation of the coagulation system: rationale for long-term antithrombotic treatment. Am J Cardiol. 1997;80((5A)):55E–60E. doi: 10.1016/s0002-9149(97)00492-x. [DOI] [PubMed] [Google Scholar]
- Kullenberg B, Ylipää S, Söderlund K, Resch S. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21((8)):1175–9. doi: 10.1016/j.arth.2006.02.159. [DOI] [PubMed] [Google Scholar]
- Lachiewicz PF, Soileau ES. Multimodal prophylaxis for THA with mechanical compression. Clin Orthop. 2006;453:225–30. doi: 10.1097/01.blo.0000238861.84733.9d. [DOI] [PubMed] [Google Scholar]
- Lachiewicz PF, Soileau ES. Mechanical calf compression and aspirin prophylaxis for total knee arthroplasty. Clin Orthop. 2007;((464)):61–4. doi: 10.1097/BLO.0b013e3181468951. [DOI] [PubMed] [Google Scholar]
- Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosenecher N, Bandel TJ, Misselwitz F, Turpie AG, RECORD3 investigators Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358((26)):2776–86. doi: 10.1056/NEJMoa076016. [DOI] [PubMed] [Google Scholar]
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604. doi: 10.1056/NEJMoa0810773. [DOI] [PubMed] [Google Scholar]
- Lonner JH, Frank J, McGuire K, Lotke PA. Postthrombotic syndrome after asymptomatic deep vein thrombosis following total knee and hip arthroplasty. Am J Orthop. 2006;35((10)):469–72. [PubMed] [Google Scholar]
- Malone PC. A hypothesis concerning the aetiology of venous thrombosis. Med Hypotheses. 1977;3((5)):189–201. doi: 10.1016/0306-9877(77)90005-6. [DOI] [PubMed] [Google Scholar]
- Malone PC, Agutter PS. The aetiology of deep venous thrombosis. QJM. 2006;99:581–93. doi: 10.1093/qjmed/hcl070. [DOI] [PubMed] [Google Scholar]
- Mant MJ, Eurich DT, Russell DB, Majumdar SR. Post-thrombotic syndrome after total hip arthroplasty is uncommon. Acta Orthop. 2008;79((6)):794–9. doi: 10.1080/17453670810016876. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anaesthesiology. 2002;96((5)):1140–6. doi: 10.1097/00000542-200205000-00017. [DOI] [PubMed] [Google Scholar]
- McAndrew CM, Fitzgerald SJ, Kraay MJ, Goldberg VM. Incidence of postthrombotic syndrome in patients undergoing primary total knee arthroplasty for osteoarthritis. Clin Orthop. 2010;468((1)):178–81. doi: 10.1007/s11999-009-0929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse EO, Caldwell BF, Lockwood RJ, Hollard J. Early mobilisation after conventional knee replacement may reduce the risk of postoperative venous thromboembolism. J Bone Joint Surg (Br) 2007;89:316–22. doi: 10.1302/0301-620X.89B3.18196. [DOI] [PubMed] [Google Scholar]
- Rodgers A, Walker N, Schug S, McKee A, Kehlet H, van Zundert A, Sage D, Futter M, Saville G, Clark T, MacMahon S. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321:1493. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samama CM, Raynaud P, Parent F, Barré J, Mertl P, Mismetti P. Epidemiology of venous thromboembolism after lower limb arthroplasty: the FOTO study. J Thromb Haemost. 2007;5((12)):2360–7. doi: 10.1111/j.1538-7836.2007.02779.x. [DOI] [PubMed] [Google Scholar]
- Seddighzadeh A, Zurawska U, Shetty R, Goldhaber SZ. Venous thromboembolism in patients undergoing surgery: low rates of prophylaxis and high rates of filter insertion. Thromb Haemost. 2007;98((6)):1220–5. doi: 10.1160/th07-06-0405. [DOI] [PubMed] [Google Scholar]
- Sharma OP, Oswanski MF, Joseph RJ, Tonui P, Westrick L, Raj SS, Tatchell T, Waite PJ, Gandaio A. Venous thromboembolism in trauma patients. Am Surg. 2007;73((11)):1173–80. doi: 10.1177/000313480707301121. [DOI] [PubMed] [Google Scholar]
- Tarity TD, Herz AL, Parvizi J, Rothman RH. Ninety-day mortality after hip arthroplasty: a comparison between unilateral and simultaneous bilateral procedures. J Arthroplasty (Suppl 2) 2006;21((6)):60–4. doi: 10.1016/j.arth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Warwick D, Friedman RJ, Agnelli G, Gil-Garay E, Johnson K, FitzGerald G, Turibio FM. Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events. J Bone Joint Surg (Br) 2007;89:799–807. doi: 10.1302/0301-620X.89B6.18844. [DOI] [PubMed] [Google Scholar]
- Xing KH, Morrison G, Lim W, Douketis J, Odueyungbo A, Crowther M. Has the incidence of deep vein thrombosis in patients undergoing total hip/knee arthroplasty changed over time? A systematic review of randomized controlled trials. Thromb Res. 2008;123((1)):24–34. doi: 10.1016/j.thromres.2008.05.005. [DOI] [PubMed] [Google Scholar]