Abstract
tRNA from Salmonella enterica serovar Typhimurium contains five thiolated nucleosides, 2-thiocytidine (s2C), 4-thiouridine (s4U), 5-methylaminomethyl-2-thiouridine (mnm5s2U), 5-carboxymethylaminomethyl-2-thiouridine (cmnm5s2U), and N-6-(4-hydroxyisopentenyl)-2-methylthioadenosine (ms2io6A). The levels of all of them are significantly reduced in cells with a mutated iscS gene, which encodes the cysteine desulfurase IscS, a member of the ISC machinery that is responsible for [Fe-S] cluster formation in proteins. A mutant (iscU52) was isolated that carried an amino acid substitution (S107T) in the IscU protein, which functions as a major scaffold in the formation of [Fe-S] clusters. In contrast to the iscS mutant, the iscU52 mutant showed reduced levels of only two of the thiolated nucleosides, ms2io6A (10-fold) and s2C (more than 2-fold). Deletions of the iscU, hscA, or fdx genes from the isc operon lead to a similar tRNA thiolation pattern to that seen for the iscU52 mutant. Unexpectedly, deletion of the iscA gene, coding for an alternative scaffold protein for the [Fe-S] clusters, showed a novel tRNA thiolation pattern, where the synthesis of only one thiolated nucleoside, ms2io6A, was decreased twofold. Based on our results, we suggest two principal distinct routes for thiolation of tRNA: (i) a direct sulfur transfer from IscS to the tRNA modifying enzymes ThiI and MnmA, which form s4U and the s2U moiety of (c)mnm5s2U, respectively; and (ii) an involvement of [Fe-S] proteins (an unidentified enzyme in the synthesis of s2C and MiaB in the synthesis of ms2io6A) in the transfer of sulfur to the tRNA.
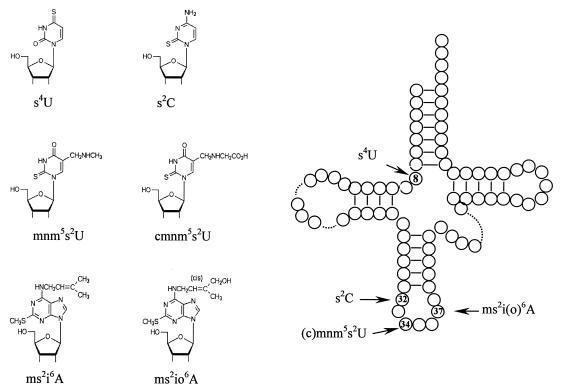
At present more than 80 different modified nucleoside derivatives of the four major nucleosides, adenosine (A), guanosine (G), uridine (U), and cytidine (C), have been characterized from tRNAs from all three domains of life (54). One subgroup of these modifications is the thiolated nucleosides (4, 39), of which 10 have been characterized so far and 5, 2-thiocytidine (s2C), 4-thiouridine (s4U), 5-methylaminomethyl-2-thiouridine (mnm5s2U), 5-carboxymethylaminomethyl-2-thiouridine (cmnm5s2U), and N-6-(4-hydroxyisopentenyl)-2-methylthioadenosine (ms2io6A), are present in tRNA from Salmonella enterica serovar Typhimurium (Fig. 1). In Escherichia coli the same thiolated nucleosides are present except for ms2io6A, which has been replaced by N-6-isopentenyl-2-methylthioadenosine (ms2i6A).
FIG. 1.
Structures and positions of thiolated nucleosides in tRNA. Abbreviations: s2C, 2-thiocytidine; s4U, 4-thiouridine; mnm5s2U, 5-methylaminomethyl-2-thiouridine; cmnm5s2U, 5-carboxymethylaminomethyl-2-thiouridine; ms2io6A, N-6-(4-hydroxyisopentenyl)-2-methylthioadenosine; ms2i6A, N-6-isopentenyl-2-methylthioadenosine. (c)mnm5s2U denotes both mnm5s2U and cmnm5s2U34; ms2i(o)6A denotes both ms2io6A and ms2i6A.
s4U, which is present in position 8 of a subpopulation of tRNAs, is the most prevalent thiolated nucleoside in tRNA from S. enterica and can act as a sensor for UV radiation, since UV exposure induces the formation of a covalent bond between s4U8 and a C13 in some tRNAs (17, 63). This structural change results in poor aminoacylation of tRNAs, thereby triggering the stringent response (52). The thio group of mnm5s2U34 is part of the recognition element for glutaminyl-tRNA synthetase (36, 60), and it also restricts the ability of the tRNA to read G-ending codons (2, 68). Although lack of the ms2 group of ms2io6A37 does not influence the growth rate (15), it does influence the reading frame maintenance (66) and the speed with which some, but not all, ternary complexes of ms2io6A37-containing tRNAs enter the A-site (35). Formation of s2C32, which is present in only four tRNAs species from S. enterica, generates an altered anticodon loop structure (5) that may result in a lower translational efficiency (discussed in reference 9). Although a mutant lacking s2C32 exhibits wild-type growth, the A-site selection rate for some of the tRNAs normally containing s2C32 is dependent on this thiolated nucleoside (24a). Thus, all thiolated nucleosides present in tRNA of S. enterica influence the activity of the tRNA in several ways and to different degrees.
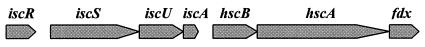
Iron-sulfur clusters constitute one of the most ancient, ubiquitous, and functionally diverse classes of biological prosthetic groups (6-8, 19, 30). Proteins containing one or more [Fe-S] clusters are commonly called [Fe-S] proteins, and they represent a large class of structurally and functionally diverse proteins that participate in many metabolic processes. The assembly of these [Fe-S] clusters into proteins is facilitated by a set of conserved proteins (IscS, IscU, IscA, HscA, HscB, and ferredoxin [Fdx]), which in many bacteria are encoded by genes organized in a single operon. In E. coli these genes constitute an operon of eight genes transcribed in the order iscR-iscS-iscU-iscA-hscB-hscA-fdx-orf3 (61) (Fig. 2). In front of this operon is a regulator gene, iscR, whose product regulates expression of the isc operon by sensing the [Fe-S] status of the cell (56). The desulfurase IscS is involved in the assembly of most [Fe-S] clusters in the cell by mobilizing the sulfur from the cysteine. The IscU functions as a scaffold for the [Fe-S] cluster assembly, and it is thought to accept sulfur from IscS and deliver it to the target apoprotein (58, 65). IscA is an alternative scaffold to IscU for IscS-directed [Fe-S] cluster assembly, and it interacts with Fdx, also encoded by the isc operon (31, 44). The HscA chaperone interacts specifically with IscU by recognizing a specific amino acid sequence (24).
FIG. 2.
Organization of the isc operon: iscR codes for the negative regulator of the operon, iscS codes for pyridoxal-phosphate-dependent cysteine desulfurase, iscU codes for the scaffold protein for [Fe-S] cluster assembly, iscA codes for the alternative scaffold protein for [Fe-S] cluster assembly, hscB codes for a J-type molecular cochaperone, hscA codes for a Hsp70-type molecular chaperone, and fdx codes for [2Fe-2S] Fdx.
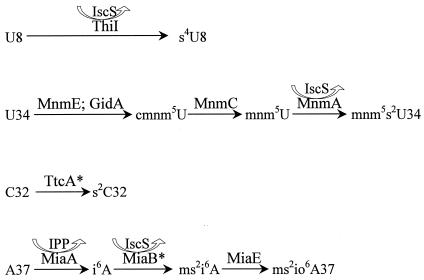
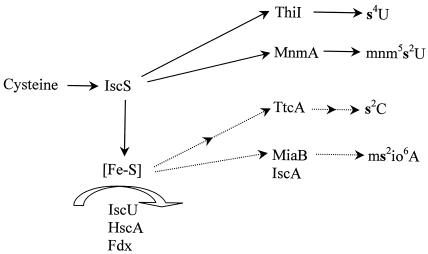
Synthesis of the thiolated nucleosides is a complex and multi-step process (Fig. 3). For many years, our knowledge about the thiolation step was limited to knowing that the sulfur originates from cysteine (3). We know now that IscS is required for the synthesis of s4U (26, 46) and, further, that IscS is involved in the synthesis of all thiolated nucleosides in tRNA of S. enterica (43) and E. coli (32). In the synthesis of s4U, the sulfur is first transferred from cysteine to IscS, thereby forming a persulfide at Cys328 in the active site of IscS. Then the persulfide sulfur from IscS is transferred to a cysteine in ThiI, which in turn transfers the sulfur to a uridine at position 8 of tRNA (26, 41, 46). Alternatively, the sulfur from ThiI may be transferred to another protein, ThiS, which transfers the sulfur to the thiazole moiety in the formation of thiamine (62, 67). Thus, the syntheses of thiamine and s4U are metabolically linked. The persulfide sulfur of IscS may also be transferred to another acceptor protein, MnmA, which in turn transfers the sulfur to a uridine in the wobble position of a subset of tRNAs forming the s2U moiety of mnm5s2U (27). The product of the miaB gene participates in the methylthiolation of A37 in a subset of tRNAs that read codons starting with U (15, 16). MiaB contains an iron-sulfur complex (49) and is a member of the Radical SAM protein superfamily, which utilizes the combination of a labile iron-sulfur cluster and S-adenosylmethionine (SAM) to initiate radical catalysis (10, 18, 59). The synthesis of s2C is poorly understood; however, it is known that the product encoded by the ttcA gene is required for its synthesis (24a). In conclusion, these results suggest that the determinants of thiolation of U at positions 2 and 4 are similar whereas the methylthiolation reaction in the synthesis of ms2io6A is different. Interestingly, studies of an iscS deletion mutant revealed that the synthesis of s2C and ms2io6A could still occur at lower rates, suggesting the existence of an alternative pathway for the mobilization of sulfur, independent of IscS (32, 43).
FIG. 3.
Biosynthetic pathways of the formation of thiolated nucleosides. U8 is thiolated at position 4 by ThiI, which receives the sulfur directly from IscS (26, 41, 46). U34 undergoes thiolation at position 2 catalyzed by MnmA, which acquires sulfur from IscS (27). The first step in the formation of the mnm5 side chain is the formation of cmnm5 group by MnmE and GidA. The cmnm5 group is then rearranged in two steps catalyzed by MnmC to synthesize the mnm5 side chain (23). The thiolation step and the formation of the mnm5 side chain occur independently, because thiolation may precede or follow the synthesis of the side chain at position 5. In the thiolation of C32 at position 2, the enzyme TtcA is involved (24a). A37 receives isopentenyl group at position 6 from isopentenyl pyrophosphate (IPP) in the reaction catalyzed by MiaA (34, 40, 53). In the later methylthiolation step at position 2 of adenosine, MiaB is involved (15, 16, 49). The MiaE protein is required for the hydroxylation of the i6 group (48). *, see the text for details.
This paper addresses the role of IscU, IscA, HscA, and Fdx in the thiolation of tRNA. We show that in contrast to the role of IscS, which is involved in the synthesis of all thiolated nucleosides, IscU, HscA, and Fdx influence only the synthesis of s2C and ms2io6A whereas IscA influences only the synthesis of ms2io6A. Based on our results, we suggest that the thiolation of tRNA occurs in two principally distinct ways—one leading to s4U and (c)mnm5s2U formation, and the other leading to s2C and ms2io6A formation.
MATERIALS AND METHODS
Bacteria and growth conditions.
The bacterial strains used were derivatives of S. enterica (Table 1). Cultures were grown in NAA complex medium (0.8% Difco nutrient broth; Difco Laboratories, Detroit, Mich.) supplemented with the aromatic amino acids, aromatic vitamins, and adenine at concentrations as described previously (12). As the defined rich liquid medium, morpholinepropanesulfonic acid (MOPS) medium, discribed by Neidhart et al. (42), was used. As the rich solid medium, TYS agar (10 g of Trypticase peptone, 5 g of yeast extract, 5 g of NaCl, and 15 g of agar per liter) was used.
TABLE 1.
Salmonella strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| LT2 | Wild type | J. Roth |
| GT2919 | hisO1242 hisD3749 proL207 zef-2502::Tn10dTc miaCl(iscU52) | 50 |
| GT3590 | hisO1242 hisD3749 zef-2516::Tn10dCm miaCl(iscU52) | This work |
| GT2422 | (TT418) glyA-540::Tn10dTc | J. Roth |
| GT6430 | hisO1224 hisC3737 zfh-2525(STM2545)::Tn10dTc | 43 |
| GT6516 | iscU52(miaCl) zee-2526::MudSacI | This work |
| GT4767 | sseA2527::Tn10dTc | This work |
| GT4768 | iscU52(miaCl) sseA2527::Tn10dTc | This work |
| DM5420 | iscA2::MudJ | 57 |
| GT6594 | ΔiscU53 | This work |
| GT6593 | ΔiscA54 | This work |
| GT6582 | ΔhscA51 | This work |
| GT6645 | Δfdx51 | This work |
| GT6595 | ΔiscA54/piscA1 | This work |
| GT6597 | ΔiscU53/piscA1 | This work |
Genetic procedures.
Transduction with phage P22 HT105/1 (int-201) (55) was performed as described previously (12). DNA sequencing was performed on either chromosomal DNA or PCR products as described in the manual for the Applied Biosystems ABI Prism cycle-sequencing BigDye Ready Reaction kit. The deletion mutants used in this study were constructed by first inserting in the gene of interest a PCR fragment coding for antibiotic resistance, which later was eliminated from the chromosome leaving an in-frame “scar” of an 84-nucleotide insertion as described previously (11). The scar in the ΔiscU53 mutant is inserted between the seventh and the seventh-to-last nucleotide, the scar in the ΔhscA51 mutant is inserted between the fifth and the fifteenth-to-last nucleotide, the scar in the ΔiscA54 mutant is inserted between the sixth and the fifth-to-last nucleotide, and the scar in the Δfdx51 mutant is inserted between the sixth and the sixth-to-last nucleotide of the respective gene. All mutations were confirmed by DNA sequencing.
Analysis of modified nucleosides in tRNA.
Bacterial strains were grown in NAA medium at 37°C to about 4 × 108 to 6 × 108 cells/ml (100 to 150 Klett units). The cells were lysed, and total RNA was prepared (13), dissolved in R200 buffer (10 mM Tris-H3PO4 [pH 6.3], 15% ethanol, 200 mM KCl), and applied to a Nucleobond column equilibrated with the same buffer. tRNA was eluted with the same buffer, except that the KCl concentration was raised to 600 mM. The tRNA was precipitated with 2.5 volumes of cold ethanol containing 1% of potassium acetate, washed twice with 70% ethanol, and dried. It was then dissolved in water, and a 100-μg sample was degraded to nucleosides with nuclease P1 followed by treatment with bacterial alkaline phosphatase (22). The resulting hydrolysate was analyzed by high-performance liquid chromatography (HPLC) (21). The chromatograms were scanned at specific wavelengths to optimize the quantification of each of the four thiolated nucleosides. The levels of the various thiolated nucleosides at the specific wavelengths were normalized to that of t6A at 254 nm. The values for ms2i(o)6A represent those for ms2io6A and ms2i6A taken together; similarly, the values for (c)mnm5s2U represent those for mnm5s2U and cmnm5s2U taken together.
RESULTS
Strain GT2919 has a reduced growth rate and is deficient in both s2C and ms2io6A.
Strains with a + 1 frameshift mutation, hisD3749, are dependent on added histidine for growth. A defective  (encoded by the proL gene) allows
(encoded by the proL gene) allows 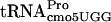 (encoded by the proM gene) to suppress the hisD3749 mutation, resulting in the His+ phenotype (51). By using localized mutagenesis in the proL region, strain GT2919 (proL207 hisD3749) was isolated (50), which, besides being His+, had a reduced growth rate and a changed tRNA modification pattern. tRNA from strain GT2919 had reduced levels of two modified nucleosides: s2C and ms2io6A (Fig. 4). Note, however, that those two modified nucleosides are not present in
(encoded by the proM gene) to suppress the hisD3749 mutation, resulting in the His+ phenotype (51). By using localized mutagenesis in the proL region, strain GT2919 (proL207 hisD3749) was isolated (50), which, besides being His+, had a reduced growth rate and a changed tRNA modification pattern. tRNA from strain GT2919 had reduced levels of two modified nucleosides: s2C and ms2io6A (Fig. 4). Note, however, that those two modified nucleosides are not present in 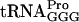 . Genetic studies revealed that the proL207 mutation, which caused the His+ phenotype, was linked neither to the decreased levels of s2C and ms2io6A nor to the reduced growth rate. A new mutation causing the last two phenotypes was temporarily called miaC1, since it was the third gene identified to influence the synthesis of ms2io6A.
. Genetic studies revealed that the proL207 mutation, which caused the His+ phenotype, was linked neither to the decreased levels of s2C and ms2io6A nor to the reduced growth rate. A new mutation causing the last two phenotypes was temporarily called miaC1, since it was the third gene identified to influence the synthesis of ms2io6A.
FIG. 4.
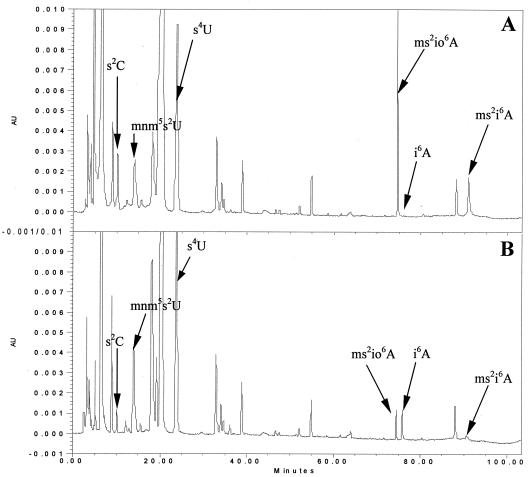
HPLC chromatograms of tRNA hydrolysates from wild-type (A) and iscU52 mutant (B) strains. The nucleosides were monitored at 295 nm to maximize the detection of all thiolated nucleosides. mnm5s2U, s4U, ms2io6A, and i6A were identified by comparing UV spectra with published spectra (21); for s2C, the molecular weight of the protonated form was determined by mass spectrometry (24a). AU, absorbance units.
The miaC1 mutation is located within the isc operon.
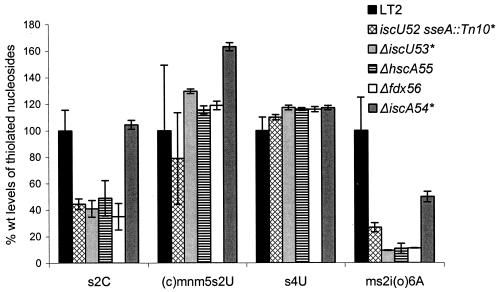
To localize the miaC1 mutation, a random pool of Tn10dTc insertions in the wild-type strain LT2 was introduced into the slow-growing GT3590 mutant (miaC1) and fast-growing colonies on rich medium plates at 30°C were monitored. The Tn10dTc insertion from one of the fast-growing transductants was found to be 28% linked to the miaC1 mutation, as demonstrated by backcrosses to strain GT3590 (data not shown). The miaC1 mutation was transferred to the wild-type strain LT2 by P22 transduction. tRNA was prepared from 10 slow-growing and 10 fast-growing transductants for HPLC analysis of their modification patterns. All slow-growing transductants had reduced levels of s2C and ms2io6A in their tRNAs, whereas the fast-growing ones showed the wild-type tRNA modification pattern (Fig. 4; for quantifications, see Fig. 5). One fast- and one slow-growing transductant were saved as congenic strains GT4767 (miaC+) and GT4768 (miaC1).
FIG. 5.
Mutations in the iscU, hscA, and fdx genes affect the levels of s2C and ms2io6A, whereas mutation in iscA reduces only the level of ms2io6A. *, The iscU and iscA mutations were complemented by a plasmid harboring the iscU and iscA genes in the high-copy-number pGEM vector (data not shown).
The chromosomal region on each side of the Tn10dTc transposon in strain GT4768 was sequenced with primers specific for the ends of the Tn10dTc transposon. The results showed that Tn10dTc was inserted into the sseA gene. The miaC1 mutation causing slow growth and a deficiency in s2C and ms2io6A in the tRNA was localized to the region between the sseA and STM2545 genes by transductional mapping using strains carrying different markers located in the vicinity of the sseA gene (glyA-540::Tn10dTc, zee-2526::MudSacI, and STM2545::Tn10dTc) (data not shown). The cotransduction frequency (28%) between the miaC1 mutation and sseA2527::Tn10dTc, together with the mapping data, suggested that the mutation was within the isc operon.
The miaC1 mutation is located in the iscU gene.
To locate the miaC1 mutation more precisely, we sequenced the entire isc operon in strain GT4768. The only mutation that we found was in the iscU gene, resulting in a substitution of Thr for Ser at position 107 of the IscU protein. Therefore, we renamed miaC1 to iscU52. A plasmid that contains iscU and iscA genes complemented the slow growth and modification deficiency of the iscU52 mutant GT4768 (see the discussion of Fig. 5). These results demonstrate that the slow growth and reduced levels of s2C and ms2io6A in tRNA are caused by the iscU52 mutation.
To verify the role of IscU in the formation of the two modified nucleosides, s2C and ms2io6A, in tRNA, we deleted the iscU gene. The ΔiscU53 mutation had a similar effect on the modification of tRNA to that of the iscU52 point mutation: it caused a 60% reduction in the level of s2C and more than a 10-fold reduction in the level of ms2io6A (Fig. 5).
Lack of the HscA chaperone results in s2C and ms2io6A deficiency in tRNA.
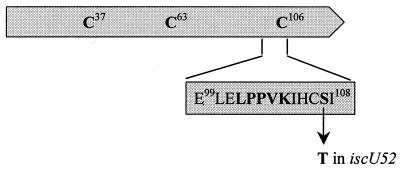
Three conserved cysteine residues at positions 37, 63, and 106 are all essential for the function of IscU in vivo by providing a scaffold for the sequential assembly of [Fe-S] clusters (1, 28, 58, 69). At residues 99 and 103 of IscU, the motif LPPVK is found, which is required for the interaction with the molecular chaperone HscA (24). Sequence alignment demonstrated that this motif is invariant in all of the IscU homologs identified to date. The amino acid substitution S107T in IscU52 is only 3 amino acids away from this conserved region (Fig. 6) and might prevent the IscU-HscA interaction by altering the structure of the HscA recognition domain. This hypothesis suggests a role of HscA in the modification of tRNA. Therefore, a strain (GT6582) with a nonpolar deletion of the hscA gene was constructed. Evidently, tRNA from the ΔhscA51 mutant showed a similar decrease in the levels of s2C and ms2io6A to that of tRNA from the ΔiscU53 mutant (Fig. 5), consistent with the view that HscA and IscU interact during the synthesis of these two thiolated nucleosides.
FIG. 6.
Schematic presentation of the IscU protein with conserved cysteines indicated. C63 forms a disulfide bridge with C328 of IscS (28). In the expanded region, the HscA binding site, LPPVK (24), and S107, which is altered to T107 by the iscU52 mutation, are depicted.
IscA influences only the level of ms2io6A.
Since IscA and IscU have a similar function in [Fe-S] cluster assembly (31, 44), we decided to investigate the involvement of IscA in tRNA thiolation. Strain DM5420 (iscA2::MudJ) contains a MudJ transposon insertion disrupting the iscA gene and most probably decreasing the expression of the downstream genes hscB, hscA, and fdx due to polarity effects (57). Analysis of the modification pattern of the tRNA from the strain DM5420 showed that the levels of s2C and ms2io6A were both decreased to levels similar to those reached in the hscA mutant (Table 2), suggesting that the effect we observed in DM5420 strain might be caused by the decrease in the synthesis of HscA. To establish which of the two proteins, IscA or HscA, is required for the synthesis of these two thiolated nucleosides, a strain (GT6593) with a nonpolar deletion of the iscA gene was constructed. Unexpectedly, analysis of the total tRNA purified from that strain revealed that the presence of IscA is critical only for the synthesis of ms2io6A, since its level was decreased twofold (Fig. 5). The levels of the other three thiolated nucleosides were similar (s2C) or increased [(c)mnm5s2U and s4U] compared to the levels observed in the wild-type strain.
TABLE 2.
Levels of thiolated nucleosides in tRNA from the different mutants of iscA, and iscU grown in NAA rich medium
| Genotype | Relative level (%) ± SDa
|
|||
|---|---|---|---|---|
| s2C (247 nm) | (c)mnm5s2U (274 nm) | s4U (330 nm) | ms2i(o)6A (242 nm) | |
| Wild type | 100 ± 15.6 (0.59) | 100 ± 49.3 (0.38) | 100 ± 10.1 (5.28) | 100 ± 25.0 (1.10) |
| iscA::MudJ | 60.6 ± 28.4 | 123.7 ± 5.9 | 141.2 ± 0.2 | 6.3 ± 2.2 |
| ΔiscA54 | 104.5 ± 3.4 | 163.1 ± 3.1 | 117.1 ± 1.6 | 49.8 ± 4.0 |
| ΔiscU53 | 41.0 ± 6.3 | 129.6 ± 1.7 | 117.2 ± 1.7 | 9.3 ± 0.2 |
| ΔiscA54/piscA1 | 112.8 | 127.4 | 112.4 | 110.6 |
| ΔiscU53/piscA1 | 40.5 | 128.2 | 116.6 | 10.4 |
Results are percentages of the wild-type levels. The numbers in parentheses are the levels of the various thiolated nucleosides at the indicated wavelength relative to the level of t6A at 254 nm. SD, standard deviation.
Increased levels of the IscA cannot substitute for the function of the IscU.
Since both IscU and IscA can serve as scaffolds for [Fe-S] assembly, we tested whether IscA provided at higher levels could substitute for the activity of IscU. Therefore, a plasmid (piscA1) carrying the iscA+ gene (57) was introduced into the strains GT6594 (ΔiscU53) and GT 6593 (ΔiscA54). Analysis of the tRNA modification pattern revealed that the low levels of ms2io6A in the ΔiscA54 mutant were restored to wild-type levels when IscA was provided on the plasmid (Table 2). However, tRNA originating from the ΔiscU53/piscA1 strain still had the thiolation pattern characteristic of the ΔiscU53 mutant. We conclude that IscA cannot substitute for IscU in the synthesis of s2C or of ms2io6A.
Fdx influences the synthesis of two thiolated nucleosides, s2C and ms2io6A.
IscA was shown to form a complex with and transfer iron and sulfide to Fdx (Fdx is another member of the isc operon) to form [2Fe-2S] holoferredoxin (44). We tested if a lack of Fdx would give a similar phenotype to that resulting from a lack of IscA. A Δfdx51 mutant (strain GT6645) was constructed, and its tRNA thiolation pattern was analyzed. In contrast to the ΔiscA54 tRNA, which was affected only in the levels of ms2io6A, the tRNA from the Δfdx51 mutant had reduced levels of two thiolated nucleosides, s2C (35% of the wild-type level remaining) and ms2io6A (11% of the wild-type level remaining) (Fig. 5), similar to the reduction observed in the ΔiscU53 and ΔhscA51 strains.
Growth characteristics of the mutants defective in the isc operon.
The iscU52 mutant forms small colonies on rich-medium agar plates. Therefore, the colony sizes of all the mutants used in this study were determined by measuring the diameters of the colonies grown on rich TYS agar plates at 30°C (the reduction in growth was more pronounced at 30°C than at 37°C) for 24 h. A general reduction in colony sizes to 64 to 77% of the size of the wild-type colonies was observed (Table 3).
TABLE 3.
Growth characteristics of different mutants mutated in the isc operon
| Genotype | % of the wild-type growth rate in rich medium (growth constant k[h−1] ± SD)a | Relative size of the colonies (%)b |
|---|---|---|
| Wild type | 100 (1.54 ± 0.03) | 100 |
| iscU+ sseA::Tn10dTc | 101 (1.55 ± 0.02) | 95 |
| iscU52 sseA::Tn10dTc | 70 (1.07 ± 0.00) | 64 |
| ΔiscU53c | 81 (1.25 ± 0.13) | 69 |
| ΔhscA51 | 85 (1.31 ± 0.16) | 69 |
| ΔiscA54 | 89 (1.37 ± 0.11) | 77 |
| Δfdx51 | 70 (1.08 ± 0.04) | 66 |
Growth rate in rich MOPS medium (42) is expressed as the specific growth rate constant k, which is ln 2/mass doubling time in hours. SD, standard deviation.
The colonies were grown on rich medium plates at 30°C for 24 h. The sizes of the colonies are given relative to the size of the colonies of the wild-type strain. The size of the wild-type colony was 1.05 ± 0.06 mm.
A similar reduced growth rate for an iscU deletion mutant in E. coli has been reported (64).
The steady-state growth rates in a defined rich medium at 37°C were also reduced in the various mutants compared to that of the wild type (Table 3). In the iscU52 and Δfdx51 mutants, the reduction was 30%, and in the ΔiscU53, ΔiscA54, and ΔhscA51 mutants, it was somewhat lower (10 to 20%). We noticed that the iscU52, ΔiscU53, ΔhscA51, and Δfdx51 mutants were unable to form dense cultures, since they never grew to to a cell density of more that 2.4 to 2.7 optical density at 420 nm (OD420) units, whereas the wild-type strain reached a cell density of 5.5 to 5.7 OD420 units. The ΔiscA54 mutant had an intermediate final cell density of 3.8 to 4.0 OD420 units.
DISCUSSION
In this study we showed that different [Fe-S] proteins encoded in the isc operon differentially affect the synthesis of the five thiolated nucleosides present in tRNA of S. enterica. Whereas mutation in the iscS gene reduces the levels of all the thiolated nucleosides in tRNA [s2C, s4C, (c)mnm5s2U, and ms2io6A] (32, 43), mutation in the iscU, hscA, or fdx gene reduced the synthesis of only two of them, s2C and ms2io6A. Mutation in the iscA gene reduced the level of only one thiolated nucleoside, ms2io6A (Fig. 5).
In the synthesis of s4U and the s2U moiety of (c)mnm5s2U, the sulfur is delivered from IscS to ThiI and MnmA, respectively; they, in turn, transfer it to tRNA (26, 27, 33, 41, 46). ThiI and MnmA share a weak sequence homology and carry conserved cysteine residues, but neither of them is an [Fe-S] protein. On the other hand, MiaB, which is involved in the synthesis of ms2io6A, possesses an oxygen-sensitive [Fe-S] cluster, whose presence is essential for successful methylthiolation of the adenosine of tRNA in vivo (49). Synthesis of s2C is dependent on the TtcA protein (24a), and its amino acid sequence does not reveal any obvious [Fe-S] cluster motif. However, it contains seven Cys residues, of which four are clustered in two conserved C-X1-X2-C motifs that could have the potential for [Fe-S] cluster formation.
The lack of IscU, HscA, or Fdx reduces the activities of [Fe-S] enzymes 5- to 10-fold in E. coli, most probably due to the absence of [Fe-S] clusters in these enzymes (64). Assuming that the homologous proteins encoded by the isc operon of S. enterica have similar effects, we expected that the activity of the [Fe-S] cluster protein MiaB should be reduced in the ΔiscU53, ΔhscA51, and Δfdx51 mutants. Indeed, this was observed, since the level of ms2io6A in tRNA was reduced 10-fold compared to the level in the wild type (Fig. 5). The levels of s2C were reduced two- to threefold, further suggesting that TtcA contains an [Fe-S] cluster of its own or that there are other [Fe-S] cluster-containing proteins upstream or/and downstream of TtcA in the s2C synthetic pathway. However, the activity of those unknown [Fe-S] proteins is not absolutely required, since low levels of thiolation are still produced in the various isc operon mutants. It is also possible that small amounts of the correct clusters originate from alternative [Fe-S] cluster-forming machinery, such as SufABCDSE.
Based on these results, we suggest that there are two principal distinct routes for the biosynthesis of the thiolated nucleosides (Fig. 7). Following the action of IscS, which affects the formation of all thiolated nucleosides in tRNA, the synthesis diverges into (i) the syntheses of s4U and (c)mnm5s2U, where the sulfur is directly transferred from IscS to the tRNA-modifying enzymes and where apparently no [Fe-S] protein participates, and (ii) the biosynthetic pathways leading to the synthesis of s2C and ms2io6A, which need, besides IscS, other constituents of the ISC machinery since they comprise proteins containing [Fe-S] clusters. Also in support of the presence of two separate pathways is the recent observation that synthesis of s4U and s2U is completely dependent on IscS as the sulfur donor, whereas an inefficient synthesis of s2C and ms2io6A occurs in an IscS-independent way (32, 43).
FIG. 7.
Working model for sulfur trafficking in tRNA thiolation. Solid lines represent experimentally verified pathways, and dashed lines represent hypothetical productive interactions between the proteins. See the text for further detail.
IscU and IscA are both scaffold proteins, presumably with similar functions in the [Fe-S] cluster assembly (1, 44). Since most bacteria seem to have both these scaffold proteins, their function might be not completely overlapping. It is generally thought that IscU is the key player in the assembly process. This view is supported by the fact that deletion of the two IscU homologues in yeast is lethal whereas deletion of both IscA homologs is not (20, 25, 29, 47). We therefore expected that lack of IscA would result in no detectable phenotype if IscU was epistatic to IscA and the target apoproteins were the same for the two scaffold proteins. Alternatively, if these two scaffold proteins had an additive effect, we would expect the lack of IscA to have an effect on the synthesis of s2C and ms2io6A, since lack of IscU reduced the synthesis of both these nucleosides. Surprisingly, only the synthesis of ms2io6A was affected by the deletion of the iscA gene (Fig. 5). This result could indicate that, for optimal activity, MiaB requires the assistance of the scaffold protein IscA or, more probably, could reflect the possible role of IscA in the restoration of the [Fe-S] cluster in MiaB. MiaB has an oxygen-labile cluster, which is a common feature of proteins belonging to the Radical SAM family. Such clusters are more sensitive to oxidative damage and require more efficient repair. This could explain, why the absence of IscA affected only the synthesis of ms2io6A, but not that of s2C, provided that the protein(s) working in the latter pathway has a more stabile [Fe-S] cluster.
Since IscA and IscU have similar functions in the [Fe-S] cluster assembly, it can be assumed that overproduction of one of them may suppress the lack of the other. However, introduction of a plasmid encoding IscA did not suppress the phenotype of the ΔiscU53 mutant, since it still had decreased levels of s2C and ms2io6A (Table 2). Hence, IscA cannot substitute for IscU in the assembly of [Fe-S] clusters in MiaB or in the protein(s) participating in the synthesis of s2C.
Biotin synthase (BioB) is an [Fe-S] enzyme and catalyzes the last step of biotin biosynthesis (38). BioB and MiaB have functional similarity since they both catalyze a C-H to C-S bond conversion and are members of the same family of Radical SAM enzymes (37, 59). Recently, it was shown that [Fe-S] cluster assembly occurred in BioB in vitro when the transient cluster was provided by IscA (45). However, our experiments on the suppression of the ΔiscU53 mutant by the piscA1 plasmid could not confirm cluster assembly in MiaB by IscA when we tested MiaB for tRNA-modifying activity (Table 2). This could be due to differences in the conditions as we monitored the processes inside the cell, which is difficult to reproduce in the experiments done in vitro, or could be due to the fact that MiaB, in contrast to BioB, needs a cluster provided exclusively by IscU.
While all the mutants analyzed had a decreased synthesis of s2C and ms2io6A, the levels of the other two thiolated nucleosides, s4U and (c)mnm5s2U, were slightly increased (17 to 29% compared to the wild-type levels [Fig. 5]). It is known that the transcriptional repressor of the isc operon, IscR, needs a functional [Fe-S] cluster for its activity. In iscS and hscA mutants (56) as well as in iscU and fdx mutants (Fig. 5; Table 3), the assembly of [Fe-S] clusters is significantly reduced and therefore IscR loses its repressing abilities, resulting in increased expression of the isc operon. Since IscS is directly transferring the sulfur to tRNA-modifying enzymes, the increased levels of s4U and (c)mnm5s2U in tRNA may reflect an increased level of IscS. Such an explanation would require a slight undermodification of the tRNA under the growth conditions used; i.e., some tRNAs would not have a molar content of s4U and (c)mnm5s2U. This may be true, since thiolation of tRNA varies with the growth rate (14). The observed increased levels of s4U and (c)mnm5s2U in various mutants (Fig. 5) suggest that deficiency in any of the [Fe-S] assembly proteins, IscU, IscA, HscA, or Fdx, results in more efficient transfer of sulfur to uridines of tRNA. The very large increase in the level of (c)mnm5s2U in the ΔiscA54 mutant is more difficult to reconcile with such a suggestion, since it would require that about 39% of the possible (c)mnm5s2U sites in the tRNA not be thiolated when cells are growing logarithmically in a rich medium.
Observed defects in the growth rate of strains harboring various mutations in the isc operon can be due either to a reduction in the activities of some [Fe-S] enzymes critical for cell growth or to the lack of thiolated nucleosides in the tRNA. Since lack of s2C (24a) and lack of the methylthio group of ms2io6A (15) in the tRNA does not influence the growth rate, we suggest that the reduced growth rate in ΔiscU53, ΔhscA51, or Δfdx51 mutants is caused by the deficiencies of [Fe-S] clusters in a protein(s) critical to obtain a maximal growth rate but not specifically for the synthesis of s2C and ms2io6A.
Acknowledgments
This work was supported by grants from the Swedish Cancer Foundation (project 680) and Swedish Science Research council (project BU-2930).
We are grateful for the generous gift of strain DM5420 and plasmid piscA1 from Diana Downs, University of Wisconsin, Madison. We thank Kerstin Jacobsson for skillful analysis of modified nucleosides by HPLC and Mike Pollard, Tord Hagervall, Mikael Wikström, and Arunas Leipus for critical reading of the manuscript.
REFERENCES
- 1.Agar, J. N., C. Krebs, J. Frazzon, B. H. Huynh, D. R. Dean, and M. K. Johnson. 2000. IscU as a scaffold for iron-sulfur cluster biosynthesis: sequential assembly of [2Fe-2S] and [4Fe-4S] clusters in IscU. Biochemistry 39:7856-7862. [DOI] [PubMed] [Google Scholar]
- 2.Agris, P., H. Sierzputowska-Gracz, W. Smith, A. Malkiewicz, E. Sochacka, and B. Nawrot. 1992. Thiolation of uridine carbon-2 restricts the motional dynamics of the transfer RNA wobble position nucleoside. J. Am. Chem. Soc. 114:2652-2656. [Google Scholar]
- 3.Ajitkumar, P., and J. D. Cherayil. 1988. Thionucleosides in transfer ribonucleic acid: diversity, structure, biosynthesis, and function. Microbiol. Rev. 52:103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auffinger P., and E. Westhof. 1998. Location and distribution of modified nucleotides in tRNA, p. 569-576. In H. Grosjean and R. Benne (ed.), Modification and editing of RNA. ASM Press, Washington, D.C.
- 5.Baumann, U., W. Fischer, and M. Sprinzl. 1985. Analysis of modification-dependent structural alterations in the anticodon loop of Escherichia coli tRNAArg and their effects on the translation of MS2 RNA. Eur. J. Biochem. 152:645-649. [DOI] [PubMed] [Google Scholar]
- 6.Beinert, H. 2000. Iron-sulfur proteins: ancient structures, still full of surprises. J. Biol. Inorg. Chem. 5:2-15. [DOI] [PubMed] [Google Scholar]
- 7.Beinert, H., R. H. Holm, and E. Münck. 1997. Iron-sulfur clusters: nature's modular, multipurpose structures. Science 277:653-659. [DOI] [PubMed] [Google Scholar]
- 8.Beinert, H., and P. J. Kiley. 1999. Fe-S proteins in sensing and regulatory functions. Curr. Opin. Chem. Biol. 3:152-157. [DOI] [PubMed] [Google Scholar]
- 9.Björk G. R. 1992. The role of modified nucleosides in tRNA interactions, p. 23-85. In D. L. Hatfield, B. J. Lee, and R. M. Pirtle (ed.), Transfer RNA in protein synthesis. CRC Press, Inc., Boca Raton, Fla.
- 10.Cheek, J., and J. B. Broderick. 2001. Adenosylmethionine-dependent iron-sulfur enzymes: versatile clusters in a radical new role. J. Biol. Inorg. Chem. 6:209-226. [DOI] [PubMed] [Google Scholar]
- 11.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis W., D. Botstein, and J. R. Roth. 1980. A manual for genetic engineering: advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 13.Emilsson, V., and C. G. Kurland. 1990. Growth rate dependence of transfer RNA abundance in Escherichia coli. EMBO J. 9:4359-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emilsson, V., A. K., Näslund, and C. G. Kurland. 1992. Thiolation of transfer RNA in Escherichia coli varies with growth rate. Nucleic Acids Res. 20:4499-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esberg, B., and G. R. Björk. 1995. The methylthio group (ms2) of N6-(4-hydroxyisopentenyl)-2-methylthioadenosine (ms2io6A) present next to the anticodon contributes to the decoding efficiency of the tRNA. J. Bacteriol. 177:1967-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esberg, B., H. C. Leung, H. C. Tsui, G. R. Björk, M. E. Winkler. 1999. Identification of the miaB gene, involved in methylthiolation of isopentenylated A37 derivatives in the tRNA of Salmonella typhimurium and Escherichia coli. J. Bacteriol. 181:7256-7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favre, A., M. Yaniv, and A. M. Michelson. 1969. The photochemistry of 4-thiouridine in Escherichia coli t-RNAVal1. Biochem. Biophys. Res. Commun. 37:266-271. [DOI] [PubMed] [Google Scholar]
- 18.Fontecave, M., E. Mulliez, and S. Ollagnier-de-Choudens. 2001. Adenosylmethionine as a source of 5′-deoxyadenosyl radicals. Curr. Opin. Chem. Biol. 5:506-511. [DOI] [PubMed] [Google Scholar]
- 19.Frazzon, J., J. R. Fick, and D. R. Dean. 2002. Biosynthesis of iron-sulphur clusters is a complex and highly conserved process. Biochem. Soc. Trans. 30:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Garland, S. A., K. Hoff, L. E. Vickery, and V. C. Culotta. 1999. Saccharomyces cerevisiae ISU1 and ISU2: members of a well-conserved gene family for iron-sulfur cluster assembly. J. Mol. Biol. 294:897-907. [DOI] [PubMed] [Google Scholar]
- 21.Gehrke, C. W., and K. C. Kuo. 1990. Ribonucleoside analysis by reversed-phase high performance liquid chromatography, p. A3-A71. In C. W. Gehrke and K. C. T. Kuo (ed.), Chromatography and modification of nucleosides. A. Analytical methods for major and modified nucleosides. Journal of Chromatography library. Elsevier, Amsterdam, The Netherlands.
- 22.Gehrke, C. W., K. C. Kuo, R. A. McCune, K. O. Gerhardt, and P. F. Agris. 1982. Quantitative enzymatic hydrolysis of tRNAs: reversed-phase high-performance liquid chromatography of tRNA nucleosides. J. Chromatogr. 230:297-308. [PubMed] [Google Scholar]
- 23.Hagervall, T. G., C. G. Edmonds, J. A. McCloskey, and G. R. Björk. 1987. Transfer RNA(5-methylaminomethyl-2-thiouridine)-methyltransferase from Escherichia coli K-12 has two enzymatic activities. J. Biol. Chem. 262:8488-8495. [PubMed] [Google Scholar]
- 24.Hoff, K. G., D. T. Ta, T. L. Tapley, J. J. Silberg, and L. E. Vickery. 2002. Hsc66 substrate specificity is directed toward a discrete region of the iron-sulfur cluster template protein IscU. J. Biol. Chem. 277:27353-27359. [DOI] [PubMed] [Google Scholar]
- 24a.Jager, G., R. Leipuviene, M. G. Pollard, Q. Qian, and G. R. Björk. 2004. The conserved Cys-X1-X2-Cys motif present in the TtcA protein is required for the thiolation of cytidine in position 32 of tRNA from Salmonella enterica serovar Typhimurium. J. Bacteriol. 186:750-757. [DOI] [PMC free article] [PubMed]
- 25.Jensen, L. T., and V. C. Culotta. 2000. Role of Saccharomyces cerevisiae ISA1 and ISA2 in iron homeostasis. Mol. Cell. Biol. 20:3918-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kambampati, R., and C. T. Lauhon. 2000. Evidence for the transfer of sulfane sulfur from IscS to ThiI during the in vitro biosynthesis of 4-thiouridine in Escherichia coli tRNA. J. Biol. Chem. 275:10727-10730. [DOI] [PubMed] [Google Scholar]
- 27.Kambampati, R., and C. T. Lauhon. 2003. MnmA and IscS are required for in vitro 2-thiouridine biosynthesis in Escherichia coli. Biochemistry 42:1109-1117. [DOI] [PubMed] [Google Scholar]
- 28.Kato, S., H. Mihara, T. Kurihara, Y. Takahashi, U. Tokumoto, T. Yoshimura, and N. Esaki. 2002. Cys-328 of IscS and Cys-63 of IscU are the sites of disulfide bridge formation in a covalently bound IscS/IscU complex: implications for the mechanism of iron-sulfur cluster assembly. Proc. Natl. Acad. Sci. USA 99:5948-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaut, A., H. Lange, K. Diekert, G. Kispal, and R. Lill. 2000. Isa1p is a component of the mitochondrial machinery for maturation of cellular iron-sulfur proteins and requires conserved cysteine residues for function. J. Biol. Chem. 275:15955-15961. [DOI] [PubMed] [Google Scholar]
- 30.Kiley, P. J., and H. Beinert. 2003. The role of Fe-S proteins in sensing and regulation in bacteria. Curr. Opin. Microbiol. 6:181-185. [DOI] [PubMed] [Google Scholar]
- 31.Krebs, C., J. N. Agar, A. D. Smith, J. Frazzon, D. R. Dean, B. H. Huynh, and M. K. Johnson. 2001. IscA, an alternate scaffold for Fe-S cluster biosynthesis. Biochemistry 40:14069-14080. [DOI] [PubMed] [Google Scholar]
- 32.Lauhon, C. T. 2002. Requirement for IscS in biosynthesis of all thionucleosides in Escherichia coli. J. Bacteriol. 184:6820-6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lauhon, C. T., and R. Kambampati. 2000. The iscS gene in Escherichia coli is required for the biosynthesis of 4-thiouridine, thiamine, and NAD. J. Biol. Chem. 275:20096-20103. [DOI] [PubMed] [Google Scholar]
- 34.Leung, H. C. E., Y. Q. Chen, and M. E. Winkler. 1997. Regulation of substrate recognition by the MiaA tRNA prenyltransferase modification enzyme of Escherichia coli K-12. J. Biol. Chem. 272:13073-13083. [DOI] [PubMed] [Google Scholar]
- 35.Li, J. N., B. Esberg, J. F. Curran, and G. R. Björk. 1997. Three modified nucleosides present in the anticodon stem and loop influence the in vivo aa-tRNA selection in a tRNA-dependent manner. J. Mol. Biol. 271:209-221. [DOI] [PubMed] [Google Scholar]
- 36.Madore, E., C. Florentz, R. Giege, S. Sekine, S. Yokoyama, and J. Lapointe. 1999. Effect of modified nucleotides on Escherichia coli tRNA(Glu) structure and on its aminoacylation by glutamyl-tRNA synthetase—predominant and distinct roles of the mnm(5) and s(2) modifications of U34. Eur. J. Biochem. 266:1128-1135. [DOI] [PubMed] [Google Scholar]
- 37.Marquet, A. 2001. Enzymology of carbon-sulfur bond formation. Curr. Opin. Chem. Biol. 5:541-549. [DOI] [PubMed] [Google Scholar]
- 38.Marquet, A., B. T. Bui, and D. Florentin. 2001. Biosynthesis of biotin and lipoic acid. Vitam. Horm. 61:51-101. [DOI] [PubMed] [Google Scholar]
- 39.McCloskey, J. A., D. E. Graham, S. Zhou, P. F. Crain, M. Ibba, J. Konisky, D. Söll, and G. J. Olsen. 2001. Post-transcriptional modification in archaeal tRNAs: identities and phylogenetic relations of nucleotides from mesophilic and hyperthermophilic Methanococcales. Nucleic Acids Res. 29:4699-4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore, J. A., and C. D. Poulter. 1997. Escherichia coli dimethylallyl diphosphate: tRNA dimethylallyltransferase: a binding mechanism for recombinant enzyme. Biochemistry 36:604-614. [DOI] [PubMed] [Google Scholar]
- 41.Mueller, E. G., P. M. Palenchar, and C. J. Buck. 2001. The role of the cysteine residues of ThiI in the generation of 4-thiouridine in tRNA. J. Biol. Chem. 276:33588-33595. [DOI] [PubMed] [Google Scholar]
- 42.Neidhardt, F. C., P. L. Bloch, S. Pedersen, and S. Reeh. 1977. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J. Bacteriol. 129:378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilsson, K., H. K. Lundgren, T. G. Hagervall, and G. R. Björk. 2002. The cysteine desulfurase IscS is required for synthesis of all five thiolated nucleosides present in tRNA from Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:6830-6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ollagnier-de-Choudens, S., T. Mattioli, Y. Takahashi, and M. Fontecave. 2001. Iron-sulfur cluster assembly: characterization of IscA and evidence for a specific and functional complex with ferredoxin. J. Biol. Chem. 276:22604-22607. [DOI] [PubMed] [Google Scholar]
- 45.Ollagnier-de Choudens, S., L. Nachin, Y. Sanakis, L. Loiseau, F. Barras, and M. Fontecave. 2003. SufA from Erwinia chrysanthemi. Characterization of a scaffold protein required for iron-sulfur cluster assembly. J. Biol. Chem. 278:17993-18001. [DOI] [PubMed] [Google Scholar]
- 46.Palenchar, P. M., C. J. Buck, H. Cheng, T. J. Larson, and E. G. Mueller. 2000. Evidence that ThiI, an enzyme shared between thiamine and 4-thiouridine biosynthesis, may be a sulfurtransferase that proceeds through a persulfide intermediate. J. Biol. Chem. 275:8283-8286. [DOI] [PubMed] [Google Scholar]
- 47.Pelzer, W., U. Mühlenhoff, K. Diekert, K. Siegmund, G. Kispal, and R. Lill. 2000. Mitochondrial Isa2p plays a crucial role in the maturation of cellular iron-sulfur proteins. FEBS Lett. 476:134-139. [DOI] [PubMed] [Google Scholar]
- 48.Persson, B. C., and G. R. Björk. 1993. Isolation of the gene (miaE) encoding the hydroxylase involved in the synthesis of 2-methylthio-cis-ribozeatin in tRNA of Salmonella typhimurium and characterization of mutants. J. Bacteriol. 175:7776-7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pierrel, F., G. R. Björk, M. Fontecave, and M. Atta. 2002. Enzymatic modification of tRNAs: MiaB is an iron-sulfur protein. J. Biol. Chem. 277:13367-13370. [DOI] [PubMed] [Google Scholar]
- 50.Qian, Q., and G. R. Björk. 1997. Structural requirements for the formation of 1-methylguanosine in vivo in tRNAProGGG of Salmonella typhimurium. J. Mol. Biol. 266:283-296. [DOI] [PubMed] [Google Scholar]
- 51.Qian, Q., J. N. Li, H. Zhao, T. G. Hagervall, P. J. Farabaugh, and G. R. Björk. 1998. A new model for phenotypic suppression of frameshift mutations by mutant tRNAs. Mol. Cell 1:471-482. [DOI] [PubMed] [Google Scholar]
- 52.Ramabhadran, T. V., and J. Jagger. 1976. Mechanism of growth delay induced in Escherichia coli by near ultraviolet radiation. Proc. Natl. Acad. Sci. USA 73:59-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenbaum, N., and M. L. Gefter. 1972. Delta 2-isopentenylpyrophosphate: transfer ribonucleic acid 2-isopentenyltransferase from Escherichia coli. Purification and properties of the enzyme. J. Biol. Chem. 247:5675-5680. [PubMed] [Google Scholar]
- 54.Rozenski, J., P. F. Crain, and J. A. McCloskey. 1999. The RNA modification database: 1999 update. Nucleic Acids Res. 27:196-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmieger, H. 1972. Phage P22-mutants with increased or decreased transduction abilities. Mol. Gen. Genet. 119:75-88. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz, C. J., J. L. Giel, T. Patschkowski, C. Luther, F. J. Ruzicka, H. Beinert, and P. J. Kiley. 2001. IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins. Proc. Natl. Acad. Sci. USA 98:14895-14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skovran, E., and D. M. Downs. 2000. Metabolic defects caused by mutations in the isc gene cluster in Salmonella enterica serovar Typhimurium: Implications for thiamine synthesis. J. Bacteriol. 182:3896-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith, A. D., J. N. Agar, K. A. Johnson, J. Frazzon, I. J. Amster, D. R. Dean, and M. K. Johnson. 2001. Sulfur transfer from IscS to IscU: the first step in iron-sulfur cluster biosynthesis. J. Am. Chem. Soc. 123:11103-11104. [DOI] [PubMed] [Google Scholar]
- 59.Sofia, H. J., G. Chen, B. G. Hetzler, J. F. ReyesSpindola, and N. E. Miller. 2001. Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: functional characterization using new analysis and information visualization methods. Nucleic Acids Res. 29:1097-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sylvers, L. A., K. C. Rogers, M. Shimizu, E. Ohtsuka, and D. Söll. 1993. A 2-thiouridine derivative in tRNAGlu is a positive determinant for aminoacylation by Escherichia coli glutamyl-tRNA synthetase. Biochemistry 32:3836-3841. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi, Y., and M. Nakamura. 1999. Functional assignment of the ORF2-iscS-iscU-iscA-hscB-hscA-fdx-ORF3 gene cluster involved in the assembly of Fe-S clusters in Escherichia coli. J. Biochem. (Tokyo) 126:917-926. [DOI] [PubMed] [Google Scholar]
- 62.Taylor, S. V., N. L. Kelleher, C. Kinsland, H. J. Chiu, C. K. Costello, A. D. Backstrom, F. W. McLafferty, and T. P. Begley. 1998. Thiamin biosynthesis in Escherichia coli—identification of this thiocarboxylate as the immediate sulfur donor in the thiazole formation. J. Biol. Chem. 273:16555-16560. [DOI] [PubMed] [Google Scholar]
- 63.Thomas, G., and A. Favre. 1975. 4-Thiouridine as the target for near-ultraviolet light induced growth delay in Escherichia coli. Biochem. Biophys. Res. Commun. 66:1454-1461. [DOI] [PubMed] [Google Scholar]
- 64.Tokumoto, U., and Y. Takahashi. 2001. Genetic analysis of the isc operon in Escherichia coli involved in the biogenesis of cellular iron-sulfur proteins. J. Biochem. (Tokyo) 130:63-71. [DOI] [PubMed] [Google Scholar]
- 65.Urbina, H. D., J. J. Silberg, K. G. Hoff, and L. E. Vickery. 2001. Transfer of sulfur from IscS to IscU during Fe/S cluster assembly. J. Biol. Chem. 276:44521-44526. [DOI] [PubMed] [Google Scholar]
- 66.Urbonavicius, J., Q. Qian, J. M. Durand, T. G. Hagervall, and G. R. Björk. 2001. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 20:4863-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Webb, E., K. Claas, and D. M. Downs. 1997. Characterization of thiI, a new gene involved in thiazole biosynthesis in Salmonella typhimurium. J. Bacteriol. 179:4399-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yokoyama, S., T. Watanabe, K. Murao, H. Ishikura, Z. Yamaizumi, S. Nishimura, and T. Miyazawa. 1985. Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc. Natl. Acad. Sci. USA 82:4905-4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuvaniyama, P., J. N. Agar, V. L. Cash, M. K. Johnson, and D. R. Dean. 2000. NifS-directed assembly of a transient [2Fe-2S] cluster within the NifU protein. Proc. Natl. Acad. Sci. USA 97:599-604. [DOI] [PMC free article] [PubMed] [Google Scholar]