Abstract
Enterococcus faecalis harbors a virulence-associated surface protein encoded by the esp gene. This gene has been shown to be part of a 150-kb putative pathogenicity island. A gene similar to esp has recently been found in Enterococcus faecium isolates recovered from hospitalized patients. In the present study we analyzed the polymorphism in the esp gene of E. faecium, and we investigated the association of esp with neighboring chromosomal genes. The esp gene showed considerable sequence heterogeneity in the regions encoding the nonrepeat N- and C-terminal domains of the Esp protein as well as differences in the number of repeats. DNA sequencing of chromosomal regions flanking the esp gene of E. faecium revealed seven open reading frames, representing putative genes implicated in virulence, regulation of transcription, and antibiotic resistance. These flanking regions were invariably associated with the presence or absence of the esp gene in E. faecium, indicating that esp in E. faecium is part of a distinct genetic element. Because of the presence of virulence genes in this gene cluster, the lower G+C content relative to that of the genome, and the presence of esp in E. faecium isolates associated with nosocomial outbreaks and clinically documented infections, we conclude that this genetic element constitutes a putative pathogenicity island, the first one described in E. faecium. Except for the presence of esp and araC, this pathogenicity island is completely different from the esp-containing pathogenicity island previously disclosed in E. faecalis.
Enterococci are common inhabitants of the gastrointestinal tracts of humans and animals, and although they have been recognized as pathogens able to cause endocarditis, they were generally considered second-rate pathogens. Recent estimates, however, indicate that enterococci are now among the leading causes of nosocomial infections (57). Of all enterococcal species, Enterococcus faecalis accounted for the most infections in humans (26). However, during the past decade, the incidence of bloodstream infections caused by Enterococcus faecium increased, an increase which has been linked to the emergence of antibiotic resistance in this species (26, 40).
Little is known about virulence determinants in E. faecium (20). Recently, however, three potential virulence genes, esp, hyl, and acm, have been described for E. faecium. They were all found more frequently in clinical isolates than in fecal isolates or nonhuman isolates (13, 41, 44, 65).
Of these three putative virulence genes, only the esp gene is also found in E. faecalis (51). The Esp protein in E. faecalis is expressed as a large surface-exposed protein with a molecular mass of approximately 202 kDa. In E. faecalis, Esp is thought to be an adhesin contributing to colonization of urinary tract epithelial cells and biofilm formation (50, 59). Although detailed experimental evidence is not yet available, the higher prevalence of the E. faecium esp gene in clinical isolates suggests a role of Esp in the pathogenesis of E. faecium infections (3, 7, 12, 13, 30, 65, 68). Furthermore, the presence of the esp gene in E. faecium was also strongly associated with hospital outbreaks of vancomycin-resistant E. faecium, suggesting a role for Esp in nosocomial transmission (65).
Recently, the esp gene of E. faecium strain P61 was cloned and sequenced (13). Analysis of the sequence revealed that the enterococcal Esp (13, 51) belongs to a family of gram-positive surface-exposed proteins with repetitive structures such as the alpha C (38) and Rib (55) proteins of Streptococcus agalactiae, the R28 protein of Streptococcus pyogenes (54), and the Bap protein of Staphylococcus aureus (8), all of which are involved in virulence and in conferring protective immunity. Sequence similarity between these surface proteins is found predominantly in the repeat regions.
In E. faecalis, the esp gene is contained on a large (150-kb) genetic element (49). This element has all the characteristics of a pathogenicity island (PAI), with a GC content of 32.2%, which is significantly different from that of the rest of the E. faecalis chromosome, and the presence of genes encoding transposases, transcriptional regulators, and virulence determinants.
In this study we demonstrate considerable sequence heterogeneity among the E. faecium esp genes of various isolates. We also show that E. faecium esp is contained on a putative PAI and that the presence of this putative PAI is associated with nosocomial outbreaks of E. faecium.
(Part of this study was presented as a poster at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 27 to 30 September 2002 [abstr. B-803].)
MATERIALS AND METHODS
Bacterial strains.
E. faecium isolate E300 from hospital outbreak US-1 (11, 65) was used to clone and sequence the esp gene and the DNA region encompassing the putative PAI. E. faecium isolate E734 from hospital outbreak NL-1-1 (64, 65) and strain E470 from hospital outbreak NL-3-1 (58, 65) were used to determine sequence heterogeneity in the N- and C-terminal domains of the esp gene. Sequencing of the frameshift mutation at positions 12830 to 12832 and the stop codon at position 13719, originally found in strain E300, was performed for isolates E155 from outbreak US-2-6 (5, 65) and E734 from outbreak NL-1-1 (64, 65). Bacteria were grown on blood agar plates at 37°C for further use.
The following isolates were used to determine the presence of the putative PAI in E. faecium: isolates from hospital outbreaks Australia-1, NL-1-1, NL-2-1, NL-2-3, NL-3-1, UK-1, US-1, US-2-1, US-2-2, US-2-3, US-2-4, US-2-5, US-2-6, and US-2-7 (4, 5, 11, 28, 35, 58, 64, 65); 68 clinical isolates (44 from blood, 9 from pus, 7 from urine, 5 from peritoneal fluid, 1 from bile, 1 from lungs, and 1 from skin) (4, 11, 16, 48, 64, 67) from the SENTRY Antimicrobial Surveillance Program, originating from hospitals in 15 different countries (Australia, Austria, Belgium, France, Germany, Israel, Italy, The Netherlands, Poland, Portugal, Spain, Switzerland, Turkey, the United Kingdom, and the United States); 6 hospital surveillance isolates (feces isolates with no link to a hospital outbreak) from three different countries (France, The Netherlands, and the United Kingdom) (28, 48, 58, 64, 66); 3 community surveillance isolates from The Netherlands (feces isolates with no hospital link) (16, 62, 67); and 10 animal feces isolates from The Netherlands (2 each from cats, dogs, calves, swine, and poultry) (61-63, 66).
PCR and sequencing of the E. faecium esp gene.
The nonrepeat regions of the E. faecium esp gene were amplified and sequenced by using a combination of 17 primers based on the published E. faecalis esp sequence (GenBank/EMBL accession no. AF034779) (51) and 4 primers based on the E. faecium sequence determined in this study (Table 1). Chromosomal DNA was purified as described elsewhere (66, 67). PCR conditions for all amplification reactions were as follows: initial denaturation at 95°C for 15 min, followed by 35 cycles of 30 s at 94°C, 30 s at 52°C, and 30 s at 72°C, and a final 5-min extension at 72°C. Reactions were performed in 25-μl volumes with HotStar Taq polymerase and HotStar Master Mix buffers (Qiagen Inc., Valencia, Calif.). PCR products were purified with a PCR purification kit (Qiagen Inc.) and sequenced by using the BigDye Terminator reaction kit and an ABI PRISM 3700 DNA analyzer (both from Applied Biosystems, Foster City, Calif.).
TABLE 1.
Oligonucleotides used in this study
| Name | Sequence | Strand | Start position | Target or function |
|---|---|---|---|---|
| espfs1R | 5′-ACT ATC AAC CTC TCC TGT TTT AG | − | 5616a | Sequencing of the esp gene |
| espfs2R | 5′-GAA GAG ACT TCT TCC TCT TTT C | − | 5750a | Sequencing of the esp gene |
| espfs3R | 5′-TTC GGC GCT TTT TTA TC | − | 5273a | Sequencing of the esp gene; amplification of the C-repeat region |
| espfs4F | 5′-GGA ACG CCT TGG TAT G | + | 1000a | Sequencing of the esp gene |
| espfs4R | 5′-GAA TAT GTC ACT ACA ACC GTA C | − | 3254a | Sequencing of the esp gene |
| espfs5F | 5′-GGA AAC CTG AAT TAG AAG AAG | + | 1490a | Sequencing of the esp gene |
| espfs5R | 5′-TAC TGC TAA ATC GGT CGT G | − | 2295a | Sequencing of the esp gene |
| espfs6F | 5′-ACG TGG ATG TAG AGT TTG C | + | 1973a | Sequencing of the esp gene |
| espfs6R | 5′-CCG CTT TTG GTG ATT C | − | 1798a | Sequencing of the esp gene |
| espfs7F | 5′-CGA CCG ATT TAG CAG TAA C | + | 2279a | Sequencing of the esp gene; amplification of the A-repeat region |
| espfs7R | 5′-CCG CTG CTT TCA TTT C | − | 1309a | Sequencing of the esp gene |
| espfs8F | 5′-GGT AGA GGT TGT TAT TTC TGT AGT AG | + | 5233a | Sequencing of the esp gene |
| espfs8R | 5′-TAT AGA AAT CAT CTT GAT CTG TC | − | 817a | Sequencing of the esp gene |
| espfs9F | 5′-AAA AGG TAC GGT TGT AGT GAC | + | 3228a | Sequencing of the esp gene; PCR |
| espfs9R | 5′-TTC TTC GTA TAT CCC GG | − | 305a | Sequencing of the esp gene |
| espfs10F | 5′-GAA ACA ACT GAT ACA CAA ACT G | + | 610a | Sequencing of the esp gene |
| espfs10R | 5′-CAG TTT GTG TAT CAG TTG TTT C | − | 631a | Sequencing of the esp gene |
| espfm1F | 5′-ATA ACT ATT AAG GGA GTT GAT TTG | + | 3063b | Sequencing of the esp gene |
| espfm2F | 5′-TGG TTA GCA AGA ATA ATA AGA GAG | + | 3092b | Sequencing of the esp gene |
| espfm3R | 5′-GGA CTT GCA TTA GCA AAA TC | − | 4277b | Sequencing of the esp gene |
| espfm4F | 5′-AAG TAG AGG TTA CTA TTT CTG TAG AAG | + | 8288b | Sequencing of the esp gene |
| espfm5R | 5′-CAG CTG CGC TAA CAT CTA C | − | 6769b | Amplification of the A-repeat region |
| espfm5F | 5′-AAA GAA GAT TTA CCA AAA GAT ACT AAG | + | 6706b | Amplification of the C-repeat region |
| espfm4R | 5′-AAT ACT CTC TTA TTA TTC TTG CTA ACC | − | 3119b | Inverse PCR, cloning and sequencing of PAI |
| nox1F | 5′-GTA ATT ATT GGA TCA AAC CAT TC | + | 8939b | Inverse PCR, cloning and sequencing of PAI |
| nox2R | 5′-GAA TGG TTT GAT CCA ATA ATT AC | − | 8961b | Sequencing of PAI; PCR |
| PAI-1R | 5′-ACG TTC ATG TAT GGG AAA G | − | 13814b | Sequencing of PAI |
| PAI-2F | 5′-GTA TTA GCG GTG TTC AAA ATG | + | 49b | Sequencing of PAI; PCR |
| PAI-2R | 5′-TTC CTC TGT CAA AAT AAG CTA AC | − | 755b | Sequencing of PAI |
| PAI-3R | 5′-CGA TAG GTG ACA GAA CTC ATA AC | − | 14575b | Sequencing of PAI; PCR |
| PAI-4R | 5′-GTT CCA AAA AGG CTG ATA ATC | − | 14059b | Sequencing of PAI |
| PAI-5F | 5′-CCT TGT TCC AGT CCC C | + | 14524b | Sequencing of PAI |
| PAI-5R | 5′-AAA ATC AAG CCG CCA AG | − | 13891b | Sequencing of PAI |
| PAI-6F | 5′-GAA GAA GGA ATT TGA AGT CAC | + | 2697b | Sequencing of PAI |
| PAI-6R | 5′-CTA ATG ATC GTG TAG CTA AGA AC | − | 13385b | Sequencing of PAI |
| PAI-7F | 5′-CGG ATC ATA ATA ATT ATT GTC TTT G | + | 587b | Sequencing of PAI |
| PAI-7R | 5′-GAT ATT TGT CAA TCA AAG GTT G | − | 12937b | Sequencing of PAI; PCR |
| PAI-8R | 5′-TTT AGA AGT CGC TTT GCC | − | 12492b | Sequencing of PAI |
| PAI-9R | 5′-ATC AAA GGT CTA AGA ATC CAA C | − | 11891b | Sequencing of PAI |
| PAI-10R | 5′-CAT AGG TTT TAA TTA ATT CAT TTA GC | − | 11396b | Sequencing of PAI |
| PAI-11R | 5′-CGC AGA CTC ACC AAT TTT C | − | 10987b | Sequencing of PAI |
| PAI-12R | 5′-CAG TCG TCT CGG TTC TTT C | − | 10511b | Sequencing of PAI |
| PAI-13R | 5′-CAA AGC TAA TTC TTA ATT TTA CAC G | − | 10067b | Sequencing of PAI |
| PAI-14R | 5′-CTT ATT ATT CTT GCT AAC CAT TAT TC | − | 3111b | Sequencing of PAI |
| PAI-15R | 5′-ATT GGA GTT ATC AAC ATT TTT TC | − | 2613b | Sequencing of PAI |
| PAI-16R | 5′-GTC ATA TTC ATT TAA CAC ACT ATT ATT ACC | − | 2186b | Sequencing of PAI |
| PAI-17R | 5′-CGA TTT CCT TAG TAT AAT AAA CAA TC | − | 1665b | Sequencing of PAI; PCR |
| PAI-18R | 5′-TTT GCA ATG AAT TAT AGA GTC G | − | 1170b | Sequencing of PAI |
| PAI-19R | 5′-AAT CTA TAC ACG AAT AAG AAT ATT ATC C | − | 438b | Sequencing of PAI |
| PAI-20R | 5′-GAG AAA ACA TTG ATA ATA GTC CAG | − | 10145b | Sequencing of PAI |
| PAI-21R | 5′-ATG TAT TCC ATT TTT TGA TAG TAT TTC | − | 9547b | Sequencing of PAI |
| PAI-2F-Biotin | 5′-GTA TTA GCG GTG TTC AAA ATG | + | 49b | Southern hybridization; detection of orf1 |
| espfm1R-Biotin | 5′-GTA ATT AGC ATA CCA AGG CG | − | 4068b | Southern hybridization; detection of esp |
| PAI-10F-Biotin | 5′-AAA ATA GTC ACT ACA AGT GGT ACC C | + | 9599b | Southern hybridization; detection of orf4 |
| PAI-11F-Biotin | 5′-TTG CAT CAG CAG TTA TAT TAA TG | + | 10401b | Southern hybridization; detection of orf5; PCR |
| PAI-8F-Biotin | 5′-ACC GAA AAA TAA TAC AAG TGG | + | 12760b | Southern hybridization; detection of orf6 |
| PAI-4F-Biotin | 5′-CTA GTA TGA CTA TGG CTA CAA ATG C | + | 13753b | Southern hybridization; detection of orf7 |
For sequencing of the region encompassing the A and C repeats, a slightly different approach was followed. First the A- and C-repeat regions were amplified with the primer combinations espfs6F-espfs4R and espfs9F-espfs2R, respectively, and were subsequently cloned into pCR2.1-TOPO by using the TOPO TA cloning kit (Invitrogen Life Technologies, Carlsbad, Calif.) in accordance with the manufacturer's instructions. This resulted in pJT1 and pJT2, harboring the A- and C-repeat regions, respectively. To generate subclones suitable for sequencing, overlapping deletions were constructed with the Erase-a-base system (Promega Corporation, Madison, Wis.). Subclones were sequenced by using the M13 reverse primer, the BigDye Terminator reaction kit, and an ABI PRISM 3700 DNA analyzer (all from Applied Biosystems).
The 5′ end of the esp gene was amplified by a combination of primer espfs10R and an 18-mer primer consisting of thymidines only. This PCR fragment was cloned into pCR2.1-TOPO by using the TOPO TA cloning kit (Invitrogen Life Technologies) in accordance with the manufacturer's instructions, and the resulting plasmid, designated pJT3, was sequenced using primers espfs6R, espfs10R, and espfm1F. Clone pJT2 was also used to determine the nucleotide sequence of the 3′ end of the esp gene, since primer espfs2R is located just downstream of the esp gene.
Determination of variation in the esp A and C repeats.
Two different primer combinations were used to assess repeat number variation by PCR. Primer sets espfs7F-espfm5R and espfm5F-espfs3R (Table 1) were used to amplify across the A- and C-repeat regions of the esp gene, respectively, in a set of 36 E. faecium isolates. Amplification conditions were identical to those described above. Subsequently, the amplicons were subjected to agarose gel electrophoresis (1%) in order to determine their sizes. From the sizes of the amplicons the numbers of repeats were deduced. Amplicon size differences corresponded to multiples of either 252 bp (A repeats) or 246 bp (C repeats).
Cloning and sequencing of the putative PAI.
The DNA region adjacent to esp was cloned by an inverse-PCR strategy. Approximately 10 μg of chromosomal DNA was digested with EcoRI or BclI, and the resulting fragments were self-ligated. Ligated DNA was amplified with primer espfm4R, located in the 5′ end of the esp gene, and primer nox1F, located just downstream of the esp gene, by using the Expand Long Template PCR system (Roche Diagnostics Nederland B.V., Almere, The Netherlands). Six-kilobase BclI and 7.9-kb EcoRI inverse-PCR products were cloned into pCR2.1-TOPO by using the TOPO TA cloning kit (Invitrogen Life Technologies) in accordance with the manufacturer's instructions, producing plasmids pJT4 (EcoRI digest) and pJT5 (BclI digest). Overlapping deletions of pJT4 and pJT5 were constructed with the Erase-a-base system (Promega) to generate subclones suitable for sequencing. One strand of the pJT4 and pJT5 subclones was sequenced with the M13 forward primer in combination with the BigDye Terminator reaction kit by using an ABI PRISM 3700 DNA analyzer (all from Applied Biosystems). Gaps in the DNA sequence of the first strand and sequence information of the second strand were obtained by direct sequencing of PCR products with primers based on the emerging nucleotide sequence of the first strand. Primers that were used for PCR and sequencing of this DNA region are listed in Table 1. PCR conditions were the same as those described above.
Detection of the putative PAI in E. faecium isolates.
Southern hybridization was used to determine the presence of six open reading frames (ORFs) contained in the putative PAI in a set of 105 E. faecium isolates. For this purpose, chromosomal DNA preparations were digested with HaeIII, separated by agarose gel electrophoresis (0.7% agarose gels), transferred onto a Hybond N+ nylon membrane (Nycomed Amersham plc, Little Chalfont, Buckingham, United Kingdom), and subsequently hybridized to six biotin-labeled oligonucleotide probes specific for the six ORFs according to the protocol developed by Schouls and coworkers (47a). The oligonucleotides used as probes for hybridization are listed in Table 1.
Nucleotide sequence accession numbers.
The DNA sequences reported in this article have been deposited in the GenBank/EMBL/DDBJ nucleotide sequence databases under accession no. AY322150 (E. faecium E300 putative PAI), AY322497 (E. faecium E155 hypothetical phage gene), AY322498 (E. faecium E734 permease gene), AY322499 (E. faecium E734 esp 5′ end), AY322501 (E. faecium E734 esp 3′ end), AY322500 (E. faecium E470 esp 5′ end), and AY322502 (E. faecium E470 esp 3′ end).
RESULTS
Sequence analysis of the E. faecium esp gene.
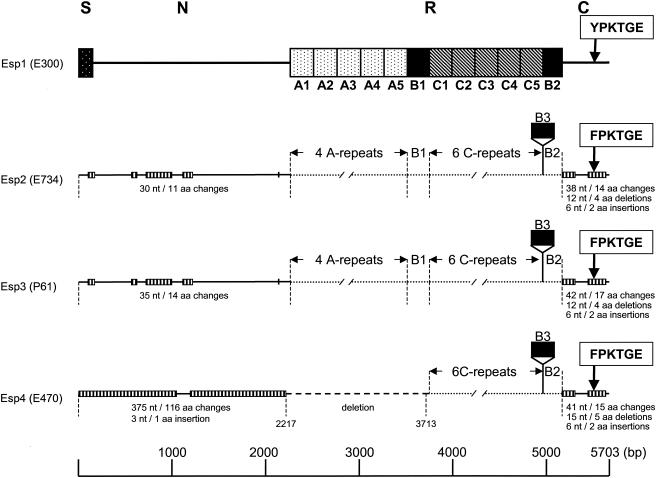
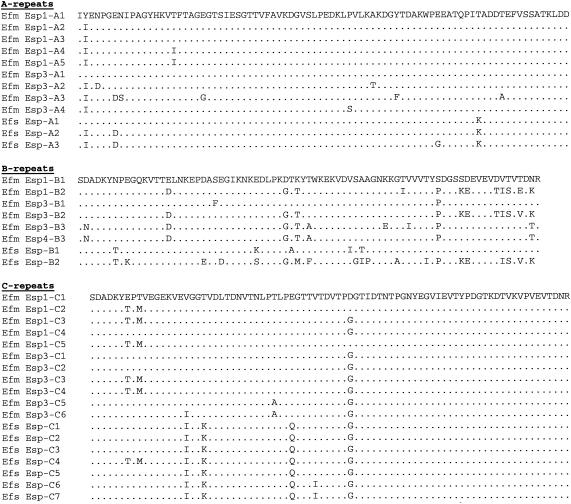
In an attempt to obtain the DNA sequence of the esp gene in E. faecium, a DNA region of strain E300, which was recovered during a hospital outbreak (11, 65), was amplified by PCR using primers based on the E. faecalis esp sequences (Table 1), followed either by direct sequencing of the PCR products or by making overlapping deletions of a cloned amplicon followed by sequencing of the deletion mutants. Sequence analysis revealed one ORF of 5,703 nucleotides that is predicted to encode a polypeptide of 1,900 amino acid residues with a calculated molecular mass of ∼205 kDa. The deduced amino acid sequence of the E. faecium Esp protein revealed a high degree of similarity to, but appeared not to be identical with, the recently described Esp of E. faecium strain P61 and the E. faecalis Esp protein (51). The E. faecium E300 Esp is predicted to be synthesized as a precursor with a 49-amino-acid signal peptide that precedes an N-terminal region of 706 amino acids, a central repeat region, and a C-terminal domain (Fig. 1). The N-terminal domain has 99 and 91% amino acid sequence identities with E. faecium P61 Esp and E. faecalis Esp, respectively. Remarkably, the first 23 amino acid residues of the processed protein of E300 are highly different from those of E. faecalis Esp. The central repeat region of the variant E300 Esp protein contains five A repeats of 84 amino acids, followed by one B1 repeat (79 amino acids), five C repeats (82 amino acids), and one B2 repeat (68 amino acids) (Fig. 1). The beginning and end of the B and C repeats were chosen slightly differently from those published by Shankar et al. (51) and Eaton and Gasson (13), so that only complete instead of truncated copies of C repeats are present in the central part of the esp gene (Fig. 2). The repeats in E. faecium E300 are highly similar to those of E. faecium P61 Esp and E. faecalis Esp, with amino acid identities of 98 to 99% for the A repeats, 96 to 98% for the B1 repeat, 97 to 98% for the C repeats, and 87 to 99% for the B2 repeat. E. faecium E300 Esp lacked the third B repeat (B3) that was reported for the P61 Esp (Fig. 1 and 2).
FIG. 1.
Schematic representation of the inferred E. faecium Esp protein and comparison of four E. faecium Esp variants. Esp1, deduced sequence of the Esp protein of strain E300, comprising the signal sequence (S) (solid box with white dots), N-terminal region (N), repeat region (R), and C-terminal region (C). The A-, B-, and C-repeat units are indicated (dotted, solid, and crosshatched boxes, respectively). YPKTGE and FPKTGE, anchor motifs in the C-terminal region. Solid lines in Esp2 (accession no. AF444000, AY322499, and AY322501), Esp3 (accession no. AJ487981), and Esp4 (accession no. AY322500 and AY322502) represent regions for which the DNA and amino acid sequences were compared to those of Esp1 (accession no. AF443999 and AY322150), while dotted lines represent regions that were not compared. Striped boxes in Esp2, -3, and -4 indicate locations of nucleotide (nt) and amino acid (aa) changes, with numbers of nucleotide and amino acid changes, insertions, and deletions relative to Esp1 indicated below. The dashed line in Esp4 represents the deletion in the esp gene of E470. The start and end points of this deletion, positions 2217 and 3713, respectively, relative to the E300 esp sequence, are indicated.
FIG. 2.
Comparison of the primary structures of the A, B, and C repeats of E. faecium Esp1 (E300), E. faecium Esp3 (P61) (13), and E. faecalis Esp (51) and the B3 repeat of E. faecium Esp4 (E470). Dots indicate identical amino acid residues. Only those amino acid residues of Esp3, Esp4, and E. faecalis Esp that differ from the repeats of E. faecium Esp1 are represented by letters. Efs, E. faecalis; Efm, E. faecium.
The C-terminal domain of 167 amino acid residues contains a membrane-spanning hydrophobic region, the YPKTGE cell wall anchor motif, and a charged tail presumably extending into the cytoplasm, ending with a glutamic acid. This domain is also highly similar to those of E. faecium P61 Esp and E. faecalis Esp, with 87 and 84% amino acid identities, respectively. The overall similarities of E. faecium E300 Esp with the E. faecium P61 and E. faecalis Esp proteins, disregarding the number of repeats, are 96 and 92%, respectively.
Sequence heterogeneity in the E. faecium esp gene.
In a previous study, sequence heterogeneity was identified in an internal fragment of the E. faecium esp gene (65). To determine sequence heterogeneity in E. faecium esp genes in more detail, the DNA regions encoding the N- and C-terminal domains of two additional esp genes from two outbreak-related vancomycin-resistant E. faecium isolates (E734 and E470) were amplified and sequenced, and the DNA sequences were compared to the corresponding E300 and P61 esp sequences. These comparisons revealed considerable polymorphism in the DNA regions encoding the N- and C-terminal domains, resulting in four different copies of the E. faecium esp gene, designated esp1 to esp4, tentatively encoding four different Esp proteins, Esp1 to Esp4; esp1 is the sequenced esp gene of strain E300, and esp3 is the sequenced esp gene of E. faecium P61 (13) (Fig. 1). The esp2 gene was found in strain E734 from outbreak NL-1 and harbored 70 nucleotide differences from esp1, resulting in 26 amino acid changes. Furthermore, a 4-amino-acid deletion and a 2-amino-acid insertion, relative to the Esp1 protein, were found in the C-terminal domain of Esp2, as well as a third copy of the B3 repeat. Also, the FPKTGE cell wall anchor motif in the C-terminal domain of Esp2 was different from that in Esp1 but identical to the anchor motif found in the P61 Esp3 protein (13). In general, the esp2 gene closely resembled the P61 esp3 gene: the sequenced regions of esp2 differed by only 11 nucleotides from esp3. The esp4 gene, found in strain E470 from outbreak NL-3, contained 416 nucleotide differences in the regions encoding the N- and C-terminal domains relative to esp1, resulting in 131 amino acid changes, with most of the differences found in the region encoding the N-terminal domain. In addition to nucleotide changes, the esp4 gene contained a large deletion in the region encoding the N-terminal domain, which also included the entire A-repeat region.
In addition to the observed nucleotide differences, the repeat regions of Esp appeared to be highly polymorphous, with variations in the numbers of A, B, and C repeats. This is not unexpected, since polymorphisms in these regions have been reported before in E. faecalis and E. faecium (13, 51). Thirty-six E. faecium isolates were analyzed for the numbers of A and C repeats. The number of A repeats varied from 0 to 6, while the number of C-repeats varied from 4 to 7, resulting in 10 different esp repeat profiles (Table 2). All strains originating from a single outbreak had identical repeat regions. Ten of the isolates, from outbreak NL-1-1, were collected during a 2-year period between April 2000 and April 2003, and they were all indistinguishable with respect to the number of repeats. In addition, the Esp repeat profile of these isolates was identical to that of the two isolates from outbreak NL-2-1, which previously had been shown to be epidemiologically linked to outbreak NL-1-1 (35). This Esp repeat profile of the Dutch outbreak strains was also found in epidemiologically unrelated clinical isolates from Greece, Italy, and France. This finding suggests that Esp repeat profiles are relatively stable, at least among strains associated with a single outbreak.
TABLE 2.
Variations in A, B, and C repeats in esp analyzed for 36 E. faecium isolates
| Source | No. of strains analyzed | Countrya | No. of repeats
|
esp repeat profile | ||
|---|---|---|---|---|---|---|
| A | C | B3 | ||||
| Clinical isolate | 1 | PO | 6 | 5 | 1 | 1 |
| Clinical isolate | 1 | AU | 6 | 5 | 1 | 1 |
| Clinical isolate | 1 | GR | 4 | 6 | 1 | 2 |
| Clinical isolate | 1 | GR | 5 | 6 | 1 | 3 |
| Clinical isolate | 1 | UK | 6 | 6 | 1 | 4 |
| Clinical isolate | 1 | IT | 4 | 6 | 1 | 2 |
| Clinical isolate | 1 | FR | 4 | 6 | 1 | 2 |
| Clinical isolate | 1 | UK | 5 | 6 | 1 | 3 |
| Clinical isolate | 1 | AU | 5 | 4 | 1 | 5 |
| Hospital outbreak NL-1-1 | 10 | NL | 4 | 6 | 1 | 2 |
| Hospital outbreak NL-2-1 | 2 | NL | 4 | 6 | 1 | 2 |
| Hospital outbreak NL-3 | 1 | NL | 0 | 6 | 1 | 6 |
| Hospital outbreak UK-1 | 4 | UK | 6 | 6 | 1 | 4 |
| Hospital outbreak US-1 | 2 | US | 3 | 5 | 0 | 7 |
| Hospital outbreak US-2-1 | 1 | US | 0 | 6 | 1 | 6 |
| Hospital outbreak US-2-2 | 1 | US | 0 | 6 | 1 | 6 |
| Hospital outbreak US-2-3 | 1 | US | 0 | 6 | 1 | 6 |
| Hospital outbreak US-2-4 | 1 | US | 3 | 6 | 0 | 8 |
| Hospital outbreak US-2-5 | 1 | US | 3 | 6 | 1 | 9 |
| Hospital outbreak US-2-6 | 1 | US | 3 | 6 | 1 | 9 |
| Hospital outbreak US-2-7 | 1 | US | 3 | 6 | 1 | 9 |
| Hospital survey | 1 | UK | 6 | 7 | 1 | 10 |
PO, Poland; AU, Austria; GR, Greece; IT, Italy; FR, France; NL, The Netherlands; UK, United Kingdom; US, United States.
A cluster of genes adjacent to the E. faecium esp gene.
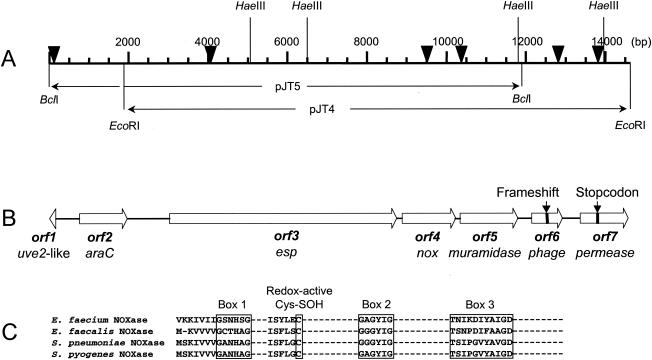
Recently, it was reported that the esp gene of E. faecalis was part of a large (150-kb) PAI (49). To examine whether the esp gene in E. faecium was also present on a PAI, an inverse-PCR strategy was used on BclI- and EcoRI-digested chromosomal DNA to obtain sequence information for a 14-kb DNA fragment. Sequencing of this DNA fragment revealed seven ORFs including the esp gene (Fig. 3). A search for homology using the GenBank/EMBL database showed that the predicted amino acid sequence of ORF1 (41 amino acids) had 42% similarity to the N-terminal part of the Uve2 protein encoded by the vanE gene cluster (Table 3). From this similarity it was also clear that only a part of this putative gene was cloned and sequenced. The uve2 gene contained in the vanE gene cluster is 26% identical to the sigma factor SpoIIG of Bacillus subtilis (6). The orf2 gene is predicted to encode a 401-amino-acid protein. This putative gene exhibited similarity with the araC gene found in the E. faecalis PAI (Table 3).
FIG. 3.
Schematic representation of the putative PAI in E. faecium and alignment of NADH oxidase regions. (A) Genetic map of the PAI. Numbers correspond to base pair positions relative to accession no. AY322150. Only restriction enzyme recognition sites relevant for this study are shown. The locations of the two clones that were constructed to derive the entire sequence are indicated. Arrowheads indicate the positions of the oligonucleotide probes used in the Southern hybridization. (B) Physical map of the PAI. Large open arrows with proposed names below indicate sizes, locations, and orientations of predicted ORFs. The positions of the frameshift and stop codon in the PAI of strain E300 are indicated. (C) Comparison of sequence fingerprints of the FAD binding region (boxes 1 and 3), the NADH contact region (box 2), and the cysteine-sulfenic acid redox center of the NADH oxidases (NOXase) of E. faecium (this study) with three previously identified homologues: E. faecalis (GenBank accession no. X68847) (45), S. pneumoniae (GenBank accession no. AF014458) (2), and S. pyogenes (GenBank accession no. AF101442) (19).
TABLE 3.
GC contents of the 7 ORFs contained in the putative E. faecium PAI and maximum predicted amino acid similarities
| Locus | GC content (%) | Homologue | Maximum amino acid similarity of predicted protein to homologue (%) |
|---|---|---|---|
| orf1 | 32.5 | E. faecalis Uve2 | 42 |
| orf2 | 27.9 | E. faecalis AraC | 61 |
| orf3 | 38.1 | E. faecalis Esp | 92 |
| orf4 | 28.9 | E. faecalis NADH oxidase | 35 |
| orf5 | 35.6 | S. pyogenes muramidase | 27 |
| orf6 | 32.8 | L. monocytogenes bacteriophage protein | 33 |
| orf7 | 43.6 | L. lactis MDR protein | 45 |
The esp1 gene, the third ORF in this gene cluster, which is described in detail above, is present downstream of the araC gene (Fig. 3). The orf4 gene is predicted to encode a 447-amino-acid protein. This putative gene is located just downstream of esp and displays the highest similarity with the nox gene of E. faecalis, encoding an NADH oxidase. Although the overall similarities with homologous proteins in Streptococcus pneumoniae, E. faecalis, and S. pyogenes are relatively low (29.3, 34.8, and 33.8%, respectively), the three sequence motifs representing the flavin adenine dinucleotide (FAD) binding region, the NADH contact region, and a cysteine residue essential for redox activity are conserved in the putative E. faecium NADH oxidase present in this gene cluster (2, 19, 45) (Fig. 3C).
The orf5 product is predicted to be synthesized as a 483-amino-acid precursor with an amino-terminal signal sequence of 27 amino acid residues and shows similarity with peptidoglycan hydrolases, N-acetylmuramidases, and autolysins of E. faecalis, Enterococcus hirae, Lactococcus lactis, and S. pyogenes (Table 3; Fig. 3). Alignment of the E. faecium putative muramidase polypeptide with the muramidase-2 gene product of E. hirae and the E. faecalis autolysin reveals that similarity is restricted to the N-terminal enzymatically active domain and that the E. faecium putative muramidase protein lacks the C-terminal peptidoglycan anchor domain (29). These findings make it unclear whether this gene encodes a functional muramidase or autolysin. In addition, the putative muramidase also contains the S144SKK, S178GN, D258/E282, and K354TG motifs found in serine β-lactamases and penicillin-binding proteins (18). This could mean that this E. faecium protein also displays penicillin binding properties comparable to those of the muramidase-2 protein of E. hirae (10, 29).
Downstream of the putative muramidase gene are two small ORFs displaying similarity with phage-associated hypothetical proteins of Lactobacillus spp., Listeria monocytogenes, S. pyogenes, and Pseudomonas aeruginosa. Detailed examination of the sequence at positions 12830 to 12832 suggests the presence of a frameshift in isolate E300. Sequencing of this region in the epidemic E. faecium isolate E155 (US-2-6) (65) showed the presence of an extra nucleotide (“A”) at position 12832 and confirmed that in this isolate the two ORFs in fact belong to one single ORF, orf6, which is predicted to encode a 256-amino-acid protein with a calculated molecular weight of 29,369. The deduced amino acid sequence of ORF6 revealed the highest similarity, 33%, with an unknown bacteriophage protein of L. monocytogenes strain EGD-e (Table 3; Fig. 3) (21).
The last ORF, orf7, exhibited amino acid sequence identity with multidrug resistance permeases of Clostridium perfringens and L. lactis, suggesting that the orf7 gene may encode a multidrug resistance efflux pump (Table 3; Fig. 3). In E300, orf7 was interrupted by a stop codon at position 13719. Again, repeated sequencing of this region in E. faecium isolate E734, which belonged to hospital outbreak NL-1-1 (65), demonstrated that in this isolate the sequence TTA, encoding a leucine, was present instead of the TAA stop codon observed in strain E300. The fact that no stop codon was found in the uninterrupted orf7 gene suggests that only a part of this gene is present in the cloned and sequenced copy of the E. faecium esp gene cluster.
The E. faecium esp gene cluster is part of a putative PAI.
To investigate whether there was a physical link between esp and the other ORFs in this gene cluster, E. faecium isolates carrying esp and esp-deficient strains were analyzed for the presence of the other six ORFs by Southern hybridization. Chromosomal DNAs of 105 E. faecium isolates were digested with HaeIII and hybridized to six oligonucleotide probes derived from internal parts of, and specific for, orf1 and orf3 to orf7 (Fig. 3). The selection included 50 vancomycin-susceptible and 55 vancomycin-resistant (vanA-positive) isolates (Table 4). All 26 isolates that were esp positive also reacted with all six oligonucleotide probes, while all 79 esp-negative isolates failed to react with any of the six oligonucleotides. This shows that in this set of isolates, the entire esp gene cluster was either present or absent. The 26 isolates carrying this gene cluster included epidemic and clinical isolates, while this cluster was absent in all surveillance and animal isolates. Furthermore, the hybridization results showed that orf1 and orf3 were located on identical-sized DNA fragments, as were orf4 and orf5, and orf6 and orf7 (data not shown). To further examine whether ORFs 1 to 7 are located in proximity to each other, PCRs were performed with forward and reverse primers specific for different ORFs. PCRs with the primer combinations PAI2F (orf1)-PAI17R (orf2), espfs9F (orf3)-nox2R (orf4), PAI11F (orf5)-PAI7R (orf6), and PAI11F (orf5)-PAI3R (orf7) (Table 1) demonstrated that ORFs 1 and 2, ORFs 3 and 4, ORFs 5 and 6, and ORFs 6 and 7 are located adjacent to each other (data not shown).
TABLE 4.
Presence of a putative PAI among vancomycin-susceptible and -resistant E. faecium isolates from different sources
| Strain origin | No. of isolates
|
|||
|---|---|---|---|---|
| PAI−
|
PAI+
|
|||
| VSEFa | VREFb | VSEF | VREF | |
| Hospital outbreak isolates | ||||
| AU-1 | 1 | |||
| NL-1-1 | 1 | |||
| NL-2-1 | 2 | |||
| NL-2-3 | 1 | |||
| NL-3-1 | 1 | |||
| UK-1 | 2 | |||
| US-1 | 3 | |||
| US-2-1 | 1 | |||
| US-2-2 | 1 | |||
| US-2-3 | 1 | |||
| US-2-4 | 1 | |||
| US-2-5 | 1 | |||
| US-2-6 | 1 | |||
| US-2-7 | 1 | |||
| Clinical isolates | 42 | 17 | 8 | 1 |
| Hospital surveillance isolates | 6 | |||
| Community surveillance isolates | 3 | |||
| Pet isolates (dogs, cats) | 4 | |||
| Swine isolates | 2 | |||
| Poultry isolates | 2 | |||
| Calf isolates | 2 | |||
| Total | 42 | 37 | 8 | 18 |
Vancomycin-susceptible E. faecium.
Vancomycin-resistant E. faecium.
The observation that all six of the putative genes are either present or absent and are physically linked on the genome strongly suggests that this cluster of genes is part of a PAI. This is also supported by the fact that several of the putative genes in this gene cluster have a deviant GC content, ranging from 27.9 to 43.6%, compared to the average GC content of 37.8% in E. faecium (Table 3; http://www.jgi.doe.gov/JGI_microbial/html/enterococcus/enterococcus_homepage.html).
DISCUSSION
In this study we have identified a cluster of six genes in E. faecium that are potentially involved in regulation, virulence, and antibiotic resistance and are linked to the esp virulence gene. We have designated this gene cluster a putative PAI because of (i) the Southern blot analysis of esp positive and -negative E. faecium isolates, which demonstrated the presence or absence of the entire gene cluster and a physical link among the seven putative genes in this cluster; (ii) the deviant GC content of this gene cluster (34.4%) compared to the GC content of the E. faecium strain DO chromosome (37.8%); (iii) the presence of putative virulence genes; and (iv) the fact that this island is absent in all human surveillance and animal isolates but present in epidemic and clinical isolates. The large variation in GC content of the seven ORFs present in the putative PAI, ranging from 27.9 up to 43.6%, suggests that this island was generated not as a result of one single event but as a result of a complex evolution involving multiple steps in different bacterial ancestors, and that it was finally acquired by E. faecium through horizontal gene transfer.
The presence of the putative PAI seems to be associated with epidemicity, since 13 of the 14 clones analyzed from different hospital outbreaks contained this PAI. This finding is in line with previous findings that suggested the existence of an epidemic E. faecium subpopulation with specific genetic characteristics (65). The fact that such a subpopulation is characterized not only by the presence of esp but also by the acquisition of a large genomic island may improve rapid detection of potential epidemic E. faecium strains, thus facilitating rapid implementation of infection control strategies. Furthermore, proteins encoded by the putative PAI may be potential targets for specific therapies, for example, to eradicate or prevent gastrointestinal colonization by potentially epidemic E. faecium.
A homologue of E. faecium esp contained in this putative PAI was first described in E. faecalis, where it was found in a high proportion of clinical strains (51). Recently it was shown that the E. faecalis esp gene is part of a large (150-kb) PAI (49). The E. faecalis esp gene encodes a surface-exposed protein and is thought to be involved in colonization of the urinary tract (50) and biofilm formation (59). In E. faecium, the esp gene was initially found in vancomycin-resistant outbreak-related isolates (65); later, it was also found in vancomycin-susceptible clinical isolates (3, 12, 13, 68). Recently, the E. faecium gene from a clinical isolate, P61, was cloned and sequenced by Eaton and Gasson (13). It displayed 89% similarity with the E. faecalis esp gene. It also exhibited global structural similarity to the S. agalactiae Rib and alpha C proteins, the R28 protein of S. pyogenes, and the biofilm-associated protein (Bap) of S. aureus, all of which are known virulence factors conferring protective immunity (8, 33, 37, 54, 55). All these proteins contain a repeat region in which amino acid similarities are most prominent (13, 51). The E. faecium esp genes analyzed in this study were highly similar but not identical to the P61 esp gene. In addition to variations in the numbers of A, B, and C repeats, extensive polymorphism was found in the N- and C-terminal nonrepeat regions. This may suggest that the esp gene was not acquired recently by E. faecium or that the esp gene is a relatively “ancient” gene acquired by E. faecium during multiple occasions. In addition, heterogeneity, especially in the surface-exposed N-terminal region, may correspond to different functions or specificities of different Esp variants. Differences in repeat numbers in esp, both in E. faecalis and in E. faecium, have been reported previously (13, 51). It is questionable whether this heterogeneity in repeat numbers can be used as an epidemiological tool. Comparison of the esp repeat profiles of epidemiologically linked and unrelated strains suggests that esp repeat profiling may be used to study local outbreaks but probably does not discriminate sufficiently to serve as a major tool for global epidemiology unless it is used in combination with genotyping schemes such as multilocus sequence typing or pulsed-field gel electrophoresis.
The presence of the esp gene in isolates from epidemiologically distinct sources seems to differ between E. faecalis and E. faecium. While the presence of the esp gene in E. faecium is confined to clinical and epidemic isolates, in E. faecalis the esp gene is also found in isolates from farm animals and food (12, 17, 24). This could be related to differences in the frequency of horizontal transmission of the esp gene in E. faecalis and E. faecium.
In addition to esp, two other putative virulence genes were found on this genetic island: the nox and muramidase genes, encoding a NADH oxidase and muramidase or autolysin, respectively. NADH oxidases are enzymes that can catalyze the four-electron reduction of O2 to H2O and are considered to perform normal household functions. In E. faecalis, NADH oxidase is involved in glycolytic metabolism (47). However, similar enzymes in S. pyogenes, Streptococcus mutans, and S. pneumoniae are considered virulence factors involved in adaptive responses to O2, enabling these bacteria to grow in O2-rich environments (2, 19, 25, 69). Furthermore, the NADH oxidase of S. pneumoniae is also involved in natural competence for genetic exchange (2, 14). It is not yet known whether the NADH oxidase found on the E. faecium putative PAI is involved in virulence. One can speculate that E. faecium isolates harboring this enzyme are better equipped to leave the anaerobic conditions in the gut and grow in more oxygen rich niches such as the urinary tract or the bloodstream.
The muramidase gene is predicted to encode an enzyme with important physiological functions during cell growth and division (52, 53, 56). Most of these enzymes have a domain structure (42). The E. faecium muramidase encoded by the putative PAI displayed similarity only with the N-terminal enzymatically active domain of the E. hirae muramidase-2 and seems to lack the C-terminal peptidoglycan binding domain. It was shown previously that the muramidase-2 enzyme of E. hirae covalently binds penicillin (10). It is not known whether the E. faecium muramidase described here is able to bind β-lactam antibiotics, but the characteristic motifs present in serine β-lactamases and penicillin-binding proteins are also conserved in this protein. In addition to basic cell functions, some bacterial peptidoglycan hydrolases, muramidase or autolysin, have been implicated in virulence by contributing to primary adhesion, biofilm formation, or other, yet unknown processes (1, 22, 27, 31, 36, 39, 46). Some other murein hydrolases, such as the lysostaphin of Staphylococcus simulans, may also act as bacteriocins (70). The production of bacteriocins may provide a competitive advantage in specific niches, thus promoting intestinal colonization. Furthermore, a peptidoglycan hydrolase gene of Neisseria gonorrhoeae, atlA, was also found on a PAI (9), and it was demonstrated that this atlA gene was required for DNA secretion during growth. This suggests that peptidoglycan hydrolases may also play a role in DNA transfer events. Further characterization of the peptidoglycan hydrolase encoded by the muramidase-like gene on the putative PAI is needed to establish a potential role in penicillin binding, pathogenesis, or intestinal colonization.
The first two ORFs of this putative PAI encode putative transcriptional regulators. orf1, which was cloned and sequenced only partially, may encode a sigma-like factor, while orf2 most likely encodes a protein that belongs to the AraC family of global regulators. Both AraC and alternate sigma factors are often found on PAIs (reviewed by Hacker and Kaper [23] and Egan [15]). Interestingly, an araC-like gene was also found on the recently described E. faecalis PAI, downstream of esp, while the E. faecium araC is located upstream of esp (49). Transcriptional regulators contained on PAIs may regulate virulence genes located on the same island or genes located outside the PAI. At this moment the role of these regulators in E. faecium is the subject of ongoing research.
The last two ORFs were disrupted in isolate E300 but were found intact in other isolates. They may encode a hypothetical bacteriophage protein and a multidrug resistance efflux pump. Bacteriophages have been implicated in the mobilization of PAIs, and several PAIs contain sequences with homology to bacteriophage integrase genes (reviewed by Hacker and Kaper [23]). The exact function of this putative phage protein remains to be elucidated. Sequence analysis and alignment of the last ORF suggested that orf7 may encode a putative multidrug resistance efflux pump that was only partially present on the cloned and sequenced copy of the putative PAI. Although virulence and antibiotic resistance may often be linked (34), antibiotic resistance genes are rarely found on PAIs. Recently, a PAI carrying a resistance locus conferring resistance to streptomycin, ampicillin, chloramphenicol, and tetracycline was found in Shigella flexneri (32, 60).
Comparison of the putative E. faecium PAI and the recently published E. faecalis PAI revealed that these two enterococcal PAIs are different, although they share at least two genes: araC and esp (49). It is intriguing that these two related enterococcal species, which are often found in the same niche, carry different PAIs. On the other hand, the epidemiology of the two species seems to be different. While E. faecalis is more frequently encountered among clinical isolates, E. faecium, mainly ampicillin- and vancomycin-resistant isolates, is more often associated with epidemic spread in hospitals (26, 40, 43). It is possible that differences in PAI sequences between E. faecium and E. faecalis, in addition to differences in antibiotic susceptibility, may account for these epidemiological differences.
Since PAIs may provide a rapid and flexible means of evolution of virulence by generating new pathogenic variants, it is not unlikely that the acquisition of a PAI by E. faecium has played an important role in the rapid emergence of E. faecium as a nosocomial pathogen.
Acknowledgments
We thank Michael Dunne, Jr., J. Zoe Jordens, Christina Vandenbroucke-Grauls, David Tribe, Ellen Mascini, Ad Fluit, Ellen Stobberingh, and Dik Mevius for providing isolates. We also thank Han de Neeling for helpful discussions and critical reading of the manuscript.
This work was supported by a Marie Curie Fellowship of the European Community program “Quality of Life and Management of Living Resources” under contract QLK2-CT-2001-50991.
REFERENCES
- 1.Allignet, J., S. Aubert, K. G. Dyke, and N. El Solh. 2001. Staphylococcus caprae strains carry determinants known to be involved in pathogenicity: a gene encoding an autolysin-binding fibronectin and the ica operon involved in biofilm formation. Infect. Immun. 69:712-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auzat, I., S. Chapuy-Regaud, G. Le Bras, D. Dos Santos, A. D. Ogunniyi, I. Le Thomas, J. R. Garel, J. C. Paton, and M. C. Trombe. 1999. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol. Microbiol. 34:1018-1028. [DOI] [PubMed] [Google Scholar]
- 3.Baldassarri, L., L. Bertuccini, M. G. Ammendolia, G. Gherardi, and R. Creti. 2001. Variant esp gene in vancomycin-sensitive Enterococcus faecium. Lancet 357:1802. [DOI] [PubMed] [Google Scholar]
- 4.Bell, J. M., J. C. Paton, and J. Turnidge. 1998. Emergence of vancomycin-resistant enterococci in Australia: phenotypic and genotypic characteristics of isolates. J. Clin. Microbiol. 36:2187-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonten, M. J. M., M. K. Hayden, C. Nathan, T. W. Rice, and R. A. Weinstein. 1998. Stability of vancomycin-resistant enterococcal genotypes isolated from long-term-colonized patients. J. Infect. Dis. 177:378-382. [DOI] [PubMed] [Google Scholar]
- 6.Boyd, D. A., T. Cabral, P. Van Caeseele, J. Wylie, and M. R. Mulvey. 2002. Molecular characterization of the vanE gene cluster in vancomycin-resistant Enterococcus faecalis N00-410 isolated in Canada. Antimicrob. Agents Chemother. 46:1977-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coque, T. M., R. Willems, R. Canton, R. Del Campo, and F. Baquero. 2002. High occurrence of esp among ampicillin-resistant and vancomycin-susceptible Enterococcus faecium clones from hospitalized patients. J. Antimicrob. Chemother. 50:1035-1038. [DOI] [PubMed] [Google Scholar]
- 8.Cucarella, C., C. Solano, J. Valle, B. Amorena, I. Lasa, and J. R. Penades. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dillard, J. P., and H. S. Seifert. 2001. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol. Microbiol. 41:263-277. [DOI] [PubMed] [Google Scholar]
- 10.Dolinger, D. L., L. Daneo Moore, and G. D. Shockman. 1989. The second peptidoglycan hydrolase of Streptococcus faecium ATCC 9790 covalently binds penicillin. J. Bacteriol. 171:4355-4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunne, W. M., and W. Wang. 1997. Clonal dissemination and colony morphotype variation of vancomycin-resistant Enterococcus faecium isolates in metropolitan Detroit, Michigan. J. Clin. Microbiol. 35:388-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton, T. J., and M. J. Gasson. 2001. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl. Environ. Microbiol. 67:1628-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton, T. J., and M. J. Gasson. 2002. A variant enterococcal surface protein Espfm in Enterococcus faecium; distribution among food, commensal, medical, and environmental isolates. FEMS Microbiol. Lett. 216:269-275. [DOI] [PubMed] [Google Scholar]
- 14.Echenique, J. R., S. Chapuy-Regaud, and M. C. Trombe. 2000. Competence regulation by oxygen in Streptococcus pneumoniae: involvement of ciaRH and comCDE. Mol. Microbiol. 36:688-696. [DOI] [PubMed] [Google Scholar]
- 15.Egan, S. M. 2002. Growing repertoire of AraC/XylS activators. J. Bacteriol. 184:5529-5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endtz, H., N. van den Braak, A. van Belkum, J. A. J. W. Kluytmans, J. G. M. Koeleman, L. Spanjaard, A. Voss, A. J. L. Weersink, C. M. J. E. Vandenbroucke-Grauls, A. G. M. Buiting, A. van Duin, and H. A. Verbrugh. 1997. Fecal carriage of vancomycin-resistant enterococci in hospitalized patients and those living in the community in The Netherlands. J. Clin. Microbiol. 35:3026-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franz, C. M. A. P., A. B. Muscholl Silberhorn, N. M. K. Yousif, M. Vancanneyt, J. Swings, and W. H. Holzapfel. 2001. Incidence of virulence factors and antibiotic resistance among enterococci isolated from food. Appl. Environ. Microbiol. 67:4385-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghuysen, J. M. 1991. Serine beta-lactamases and penicillin-binding proteins. Annu. Rev. Microbiol. 45:37-67. [DOI] [PubMed] [Google Scholar]
- 19.Gibson, C. M., T. C. Mallett, A. Claiborne, and M. G. Caparon. 2000. Contribution of NADH oxidase to aerobic metabolism of Streptococcus pyogenes. J. Bacteriol. 182:448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilmore, M. S., P. S. Coburn, S. R. Nallapareddy, and B. E. Murray. 2002. Enterococcal virulence, p. 301-354. In M. S. Gilmore, D. B. Clewell, P. Courvalin, G. M. Dunny, B. E. Murray, and L. B. Rice (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. American Society for Microbiology, Washington, D.C.
- 21.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K.-D. Entian, H. Fsihi, F. G.-D. Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L.-M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J.-C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J.-A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 22.Gotz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 23.Hacker, J., and J. B. Kaper. 2000. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54:641-679. [DOI] [PubMed] [Google Scholar]
- 24.Hammerum, A. M., and L. B. Jensen. 2002. Prevalence of esp, encoding the enterococcal surface protein, in Enterococcus faecalis and Enterococcus faecium isolates from hospital patients, poultry, and pigs in Denmark. J. Clin. Microbiol. 40:4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higuchi, M. 1984. The effect of oxygen on the growth and mannitol fermentation of Streptococcus mutans. J. Gen. Microbiol. 130:1819-1826. [DOI] [PubMed] [Google Scholar]
- 26.Iwen, P. C., D. M. Kelly, J. Linder, S. H. Hinrichs, E. A. Dominguez, M. E. Rupp, and K. D. Patil. 1997. Change in prevalence and antibiotic resistance of Enterococcus species isolated from blood cultures over an 8-year period. Antimicrob. Agents Chemother. 41:494-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jedrzejas, M. J. 2001. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65:187-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordens, J. Z., J. Bates, and D. T. Griffiths. 1994. Faecal carriage and nosocomial spread of vancomycin-resistant Enterococcus faecium. J. Antimicrob. Chemother. 34:515-528. [DOI] [PubMed] [Google Scholar]
- 29.Joris, B., S. Englebert, C. P. Chu, R. Kariyama, L. Daneo Moore, G. D. Shockman, and J. M. Ghuysen. 1992. Modular design of the Enterococcus hirae muramidase-2 and Streptococcus faecalis autolysin. FEMS Microbiol. Lett. 70:257-264. [DOI] [PubMed] [Google Scholar]
- 30.Leavis, H. L., R. J. L. Willems, J. Top, E. Spalburg, E. M. Mascini, A. C. Fluit, A. Hoepelman, A. J. de Neeling, and M. J. M. Bonten. 2003. Evolutionary insights in the emergence of epidemic and non-epidemic multi-resistant Enterococcus faecium. Emerg. Infect. Dis. 9:1108-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loessner, M. J., K. Kramer, F. Ebel, and S. Scherer. 2002. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol. Microbiol. 44:335-349. [DOI] [PubMed] [Google Scholar]
- 32.Luck, S. N., S. A. Turner, K. Rajakumar, H. Sakellaris, and B. Adler. 2001. Ferric dicitrate transport system (Fec) of Shigella flexneri 2a YSH6000 is encoded on a novel pathogenicity island carrying multiple antibiotic resistance genes. Infect. Immun. 69:6012-6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madoff, L. C., J. L. Michel, E. W. Gong, D. E. Kling, and D. L. Kasper. 1996. Group B streptococci escape host immunity by deletion of tandem repeat elements of the alpha C protein. Proc. Natl. Acad. Sci. USA 93:4131-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez, J. L., and F. Baquero. 2002. Interactions among strategies associated with bacterial infection: pathogenicity, epidemicity, and antibiotic resistance. Clin. Microbiol. Rev. 15:647-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mascini, E. M., A. C. Gigengack-Baars, R. J. Hene, T. E. Kamp-Hopmans, A. J. Weersink, and M. J. Bonten. 2000. Epidemiologic increase of various genotypes of vancomycin-resistant Enterococcus faecium in a university hospital. Ned. Tijdschr. Geneeskd. 144:2572-2576. (In Dutch.) [PubMed] [Google Scholar]
- 36.Mercier, C., C. Durrieu, R. Briandet, E. Domakova, J. Tremblay, G. Buist, and S. Kulakauskas. 2002. Positive role of peptidoglycan breaks in lactococcal biofilm formation. Mol. Microbiol. 46:235-243. [DOI] [PubMed] [Google Scholar]
- 37.Michel, J. L., L. C. Madoff, D. E. Kling, D. L. Kasper, and F. M. Ausubel. 1991. Cloned alpha and beta C-protein antigens of group B streptococci elicit protective immunity. Infect. Immun. 59:2023-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michel, J. L., L. C. Madoff, K. Olson, D. E. Kling, D. L. Kasper, and F. M. Ausubel. 1992. Large, identical, tandem repeating units in the C protein alpha antigen gene, bca, of group B streptococci. Proc. Natl. Acad. Sci. USA 89:10060-10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milohanic, E., R. Jonquieres, P. Cossart, P. Berche, and J. L. Gaillard. 2001. The autolysin Ami contributes to the adhesion of Listeria monocytogenes to eukaryotic cells via its cell wall anchor. Mol. Microbiol. 39:1212-1224. [DOI] [PubMed] [Google Scholar]
- 40.Murdoch, D. R., S. Mirrett, L. J. Harrell, J. S. Monahan, and L. B. Reller. 2002. Sequential emergence of antibiotic resistance in enterococcal bloodstream isolates over 25 years. Antimicrob. Agents Chemother. 46:3676-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nallapareddy, S. R., G. M. Weinstock, and B. E. Murray. 2003. Clinical isolates of Enterococcus faecium exhibit strain-specific collagen binding mediated by Acm, a new member of the MSCRAMM family. Mol. Microbiol. 47:1733-1747. [DOI] [PubMed] [Google Scholar]
- 42.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rice, L. B. 2001. Emergence of vancomycin-resistant enterococci. Emerg. Infect. Dis. 7:183-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rice, L. B., L. Carias, S. Rudin, C. Vael, H. Goossens, C. Konstabel, I. Klare, S. R. Nallapareddy, W. Huang, and B. E. Murray. 2003. A potential virulence gene, hylEfm, predominates in Enterococcus faecium of clinical origin. J. Infect. Dis. 187:508-512. [DOI] [PubMed] [Google Scholar]
- 45.Ross, R. P., and A. Claiborne. 1992. Molecular cloning and analysis of the gene encoding the NADH oxidase from Streptococcus faecalis 10C1. Comparison with NADH peroxidase and the flavoprotein disulfide reductases. J. Mol. Biol. 227:658-671. [DOI] [PubMed] [Google Scholar]
- 46.Rupp, M. E., P. D. Fey, C. Heilmann, and F. Gotz. 2001. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. J. Infect. Dis. 183:1038-1042. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt, H. L., W. Stocklein, J. Danzer, P. Kirch, and B. Limbach. 1986. Isolation and properties of an H2O-forming NADH oxidase from Streptococcus faecalis. Eur. J. Biochem. 156:149-155. [DOI] [PubMed] [Google Scholar]
- 47a.Schouls, L. M., C. S. Schot, and J. A. Jacobs. 2003. Horizontal transfer of segments of the 16S rRNA genes between species of the Streptococcus anginosus group. J. Bacteriol. 185:7241-7246. [DOI] [PMC free article] [PubMed]
- 48.Schouten, M. A., R. J. Willems, W. A. Kraak, J. Top, J. A. Hoogkamp-Korstanje, and A. Voss. 2001. Molecular analysis of Tn1546-like elements in vancomycin-resistant enterococci isolated from patients in Europe shows geographic transposon type clustering. Antimicrob. Agents Chemother. 45:986-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shankar, N., A. S. Baghdayan, and M. S. Gilmore. 2002. Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis. Nature 417:746-750. [DOI] [PubMed] [Google Scholar]
- 50.Shankar, N., C. V. Lockatell, A. S. Baghdayan, C. Drachenberg, M. S. Gilmore, and D. E. Johnson. 2001. Role of Enterococcus faecalis surface protein Esp in the pathogenesis of ascending urinary tract infection. Infect. Immun. 69:4366-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shankar, V., A. S. Baghdayan, M. M. Huycke, G. Lindahl, and M. S. Gilmore. 1999. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect. Immun. 67:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shockman, G. D. 1992. The autolytic (′suicidase') system of Enterococcus hirae: from lysine depletion autolysis to biochemical and molecular studies of the two muramidases of Enterococcus hirae ATCC 9790. FEMS Microbiol. Lett. 79:261-267. [DOI] [PubMed] [Google Scholar]
- 53.Shockman, G. D., L. Daneo-Moore, R. Kariyama, and O. Massidda. 1996. Bacterial walls, peptidoglycan hydrolases, autolysins, and autolysis. Microb. Drug Resist. 2:95-98. [DOI] [PubMed] [Google Scholar]
- 54.Stalhammar-Carlemalm, M., T. Areschoug, C. Larsson, and G. Lindahl. 1999. The R28 protein of Streptococcus pyogenes is related to several group B streptococcal surface proteins, confers protective immunity and promotes binding to human epithelial cells. Mol. Microbiol. 33:208-219. [DOI] [PubMed] [Google Scholar]
- 55.Stalhammar-Carlemalm, M., L. Stenberg, and G. Lindahl. 1993. Protein Rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J. Exp. Med. 177:1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi, J., H. Komatsuzawa, S. Yamada, T. Nishida, H. Labischinski, T. Fujiwara, M. Ohara, J. Yamagishi, and M. Sugai. 2002. Molecular characterization of an atl null mutant of Staphylococcus aureus. Microbiol. Immunol. 46:601-612. [DOI] [PubMed] [Google Scholar]
- 57.Tannock, G. W., and G. Cook. 2002. Enterococci as members of the intestinal microflora of humans, p. 101-132. In M. S. Gilmore, D. B. Clewell, P. Courvalin, G. M. Dunny, B. E. Murray, and L. B. Rice (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. American Society for Microbiology, Washington, D.C.
- 58.Timmers, G. J., W. C. van Der Zwet, I. M. Simoons-Smit, P. H. Savelkoul, H. H. Meester, C. M. Vandenbroucke-Grauls, and P. C. Huijgens. 2002. Outbreak of vancomycin-resistant Enterococcus faecium in a haematology unit: risk factor assessment and successful control of the epidemic. Br. J. Haematol. 116:826-833. [DOI] [PubMed] [Google Scholar]
- 59.Toledo Arana, A., J. Valle, C. Solano, M. J. Arrizubieta, C. Cucarella, M. Lamata, B. Amorena, J. Leiva, J. R. Penades, and I. Lasa. 2001. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 67:4538-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turner, S. A., S. N. Luck, H. Sakellaris, K. Rajakumar, and B. Adler. 2003. Molecular epidemiology of the SRL pathogenicity island. Antimicrob. Agents Chemother. 47:727-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Belkum, A., N. van den Braak, R. Thomassen, H. Verbrugh, and H. Endtz. 1996. Vancomycin-resistant enterococci in cats and dogs. Lancet 348:1038-1039. [DOI] [PubMed] [Google Scholar]
- 62.van den Bogaard, A. E., P. Mertens, N. H. London, and E. E. Stobberingh. 1997. High prevalence of colonization with vancomycin- and pristinamycin-resistant enterococci in healthy humans and pigs in The Netherlands: is the addition of antibiotics to animal feeds to blame? J. Antimicrob. Chemother. 40:454-456. [DOI] [PubMed] [Google Scholar]
- 63.van den Bogaard, A. E., R. Willems, N. London, J. Top, and E. E. Stobberingh. 2002. Antibiotic resistance of faecal enterococci in poultry, poultry farmers and poultry slaughterers. J. Antimicrob. Chemother. 49:497-505. [DOI] [PubMed] [Google Scholar]
- 64.van der Steen, L. F., M. J. Bonten, E. van Kregten, J. J. Harssema-Poot, R. Willems, and C. A. Gaillard. 2000. Vancomycin-resistant Enterococcus faecium outbreak in a nephrology ward. Ned. Tijdschr. Geneeskd. 144:2568-2572. (In Dutch.) [PubMed] [Google Scholar]
- 65.Willems, R. J., W. Homan, J. Top, M. van Santen-Verheuvel, D. Tribe, X. Manzioros, C. Gaillard, C. M. Vandenbroucke-Grauls, E. M. Mascini, E. van Kregten, J. D. van Embden, and M. J. Bonten. 2001. Variant esp gene as a marker of a distinct genetic lineage of vancomycin-resistant Enterococcus faecium spreading in hospitals. Lancet 357:853-855. [DOI] [PubMed] [Google Scholar]
- 66.Willems, R. J. L., J. Top, N. van den Braak, A. van Belkum, H. Endtz, D. Mevius, E. Stobberingh, A. van den Bogaard, and J. D. A. van Embden. 2000. Host specificity of vancomycin-resistant Enterococcus faecium. J. Infect. Dis. 182:816-823. [DOI] [PubMed] [Google Scholar]
- 67.Willems, R. J. L., J. Top, N. van den Braak, A. van Belkum, D. J. Mevius, G. Hendriks, M. Van Santen Verheuvel, and J. D. A. van Embden. 1999. Molecular diversity and evolutionary relationships of Tn1546-like elements in enterococci from humans and animals. Antimicrob. Agents Chemother. 43:483-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woodford, N., M. Soltani, and K. J. Hardy. 2001. Frequency of esp in Enterococcus faecium isolates. Lancet 358:584. [DOI] [PubMed] [Google Scholar]
- 69.Yu, J., A. P. Bryant, A. Marra, M. A. Lonetto, K. A. Ingraham, A. F. Chalker, D. J. Holmes, D. Holden, M. Rosenberg, and D. McDevitt. 2001. Characterization of the Streptococcus pneumoniae NADH oxidase that is required for infection. Microbiology 147:431-438. [DOI] [PubMed] [Google Scholar]
- 70.Zygmunt, W. A., H. P. Browder, and P. A. Tavormina. 1967. Lytic action of lysostaphin on susceptible and resistant strains of Staphylococcus aureus. Can. J. Microbiol. 13:845-853. [DOI] [PubMed] [Google Scholar]