Abstract
Tetrahydropapaveroline (THP), a neurotoxic tetrahydroisoquinoline alkaloid formed by condensation between dopamine and dopaldehyde, has been speculated to cause Parkinson's disease and also to contribute to alcohol dependence. Having two catechol moieties, THP may readily undergo oxidation to form an o-quinone intermediate with concomitant production of reactive oxygen species, which can cause neuronal cell death and DNA damage. This review will deal with the current knowledge of neurotoxic effects of this endogenous alkaloid and underlying biochemical mechanisms.
Keywords: tetrahydroisoquinoline, neurotoxicity, cell death, reactive oxygen species, Parkinson, alcoholism
INTRODUCTION
Tetrahydroisoquinolines (THIQs), which belong to a group of cyclized condensation adducts of biogenic amines with aldehydes, are referred to as mammalian alkaloids (Collins et al., 1979). They include salsolinol (1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline; SAL) and tetrahydropapaveroline (6,7-dihydroxy-1-(3',4'-dihydroxybenzyl)-1,2,3,4-tetrahydroisoquinoline; THP) that are derived from dopamine through condensation with acetaldehyde and dopaldehyde (3,4-dihydroxyphenylacetaldehyde), respectively (Sandler et al., 1973). THP is a putative dopaminergic neurotoxin that is implicated in the pathology of Parkinson's disease (McNaught et al., 1998; Collins 2004; Soto-Otero et al., 2006). It is known that THP is detected at a high level in the brain (Nagatsu, 1997) and the urine (Sandler et al., 1973; Cashaw, 1993) of parkinsonian patients under 3,4-dihydroxyphenylalanine (L-DOPA) therapy. In addition, significant levels of THP have also been detected in the brain after ethanol uptake (Cashaw, 1993) and/or L-DOPA treatment (Turner et al., 1974; Cashaw et al., 1987; Cashaw, 1993), and this endogenous alkaloid is considered to account for the neurobehavioral abnormalities associated with alcoholism and may act as a neurotransmitter (Sango et al., 2000). In this review, we will focus on the neurochemical/neuropharmacological properties of THP and related THIQs with regards to their possible implications in the pathology of some neuronal disorders including Parkinsonism and alcohol addiction.
PARKINSON'S DISEASE AND THP
Parkinson's disease is the second-commonest neurodegenerative disease and first described by James Parkinson in 1817. The symptoms of the disease involve bradykinesia, resting tremor, and muscular rigidity. The most prominent neuropathology in PD is the progressive loss of dopamine neurons in the substantia nigra pars compacta (SNc). Dopaminergic neurons in the SNc project to the dorsolateral striatum and upon stimuli, they release an important neurotransmitter, dopamine. Therefore, the loss of dopamine neurons of SNc results in reduction of striatal DA level, and further results in symptoms of Parkinson's disease. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a well known exogenous dopaminergic neurotoxin and is converted by monoamine oxidase (MAO) to the 1-methyl-4-phenyl-pyridinium ion (MPP+), that selectively kills the nigrostriatal dopamine neurons (Collins et al., 1979; Nagatsu, 1997; McNaught et al., 1998; Storch et al., 2002). THIQs and their N-methylated metabolites may function in a manner similar to MPTP or MPP+ and has been demonstrated that they can provoke parkinsonian-like symptoms in monkeys (Nagatsu and Yoshida, 1988). Since THP bears two catechol moieties, the compound may readily undergo redox cycling to produce reactive oxygen species (ROS) as well as toxic quinoids (Surh, 1999).
Although L-DOPA, the natural precursor of dopamine, is the most effective and frequently prescribed drug for controlling symptoms of Parkinson's disease (Marsden, 1994), the dopaminergic neurons continue to die in parkinsonians during the L-DOPA therapy (Ogawa, 1994). L-DOPA treatment may even hasten the underlying neurodegenerative process (Fahn and Cohen, 1992) and also provokes some side effects (Morris, 1978), a phenomenon referred as "L-DOPA paradox". The dopaminergic neurotoxicity and other adverse effects of L-DOPA may be due to the elevated levels of THP (Sandler et al., 1973; Matsubara et al., 1992; Cashaw, 1993; McNaught et al., 1998; Collins, 2004). Accumulation of dopamine after L-DOPA treatment, increases the formation of THP in parkinsonian patients and experimental rats (Turner et al., 1974; Cashaw et al., 1987; Cashaw, 1993). In addition, treatment of PC12 cells with L-DOPA induced apoptosis by generating ROS, which was aggravated by THP cotreatment (Lee et al., 2003).
Therefore, parkinsonian patients treated with L-DOPA for long-term need to be monitored for the relationship between plasma concentrations of THP and any plausible symptoms of neurotoxicity (Kim et al., 2006). Moreover, it would also be worthwhile determining any differences in the levels of THP among untreated parkinsonian subjects, those on L-DOPA medication and normal individuals.
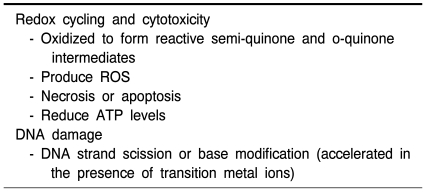
Despite lack of direct evidence supporting the role for THP in neuronal death in Parkinson's diease, accumulating data from cell culture and animal studies suggest that this endogenous THIQ may act as a dopaminergic neurotoxin (Nagatsu, 1997; McNaught et al., 1998; Collins, 2004) and the neurotoxic potential of THP is summarized in Table 1.
Table 1.
Neurotoxic potential of THP
ALCOHOL DEPENDENCE AND THP
THIQs have been also postulated to play a role in the pathogenesis of chronic alcoholism (Collins et al., 1979; Nace, 1986). Collins proposed that oxidative metabolites of endogenous THIQs may be responsible for neuronal damage associated with chronic alcoholism (Collins et al., 1979).
Possible implications of THIQs in alcohol dependence were inferred from the observation that rats which normally rejected alcohol would, following direct delivery of THP, drink ethyl alcohol in increasingly excessive amounts (Myers and Melchior, 1977). When rats were injected with THP, the preference for alcohol lasted up to 10 months (Duncan and Deitrich, 1980; Myers and Privette, 1989). Likewise, microinjection of THP into the ventral tegmental area of anesthetized high-ethanol-preferring (HEP) rats directly altered the function of the mesolimbic pathway and the dopaminergic system. Such a perturbation could account for the induction of alcohol preference (Myers and Robinson, 1999).
Since (S)-THP can serve as an intermediate in de novo synthesis of more complex alkaloids including morphine and codeine in mammals (Haber et al., 1997; Sango et al., 2000), overproduction of THP during alcohol consumption may increase synthesis of morphine and codeine and this may be associated with alcohol addiction (Kirby, 1967; Davis and Walsh, 1970; Nace, 1986). Although the physiologic roles of endogenous morphine and codeine are unknown, they are known to have analgesic effect and to invoke physical dependence (Weitz et al., 1986; Donnerer et al., 1987). The complete morphine and codeine biosynthetic pathways in mammalian species are still unclear, but THP has been suggested as a possible precursor as in the case of plants (Davis and Walsh, 1970; Matsubara et al., 1992). If THP is an intermediate for the morphine and codeine biosynthesis in mammals, the levels of all these endogenous substances in L-DOPA-treated parkinsonian patients should be higher than those in normal control subjects. When, Matsubara and colleagues measured the urinary levels of morphine and codeine as well as THP in parkinsonian patients under L-DOPA therapy, they were found to be significantly higher than those in healthy non-drinker controls (Matsubara et al., 1992). It is also noteworthy that there were very low levels of THP, morphine and codeine in the urine of abstinent alcoholics. Thus, these narcotic substances, endogenously formed from THP as an intermediate, might contribute to alcohol dependence (Davis and Walsh, 1970; Collins, 2004).
GENERATION OF THP
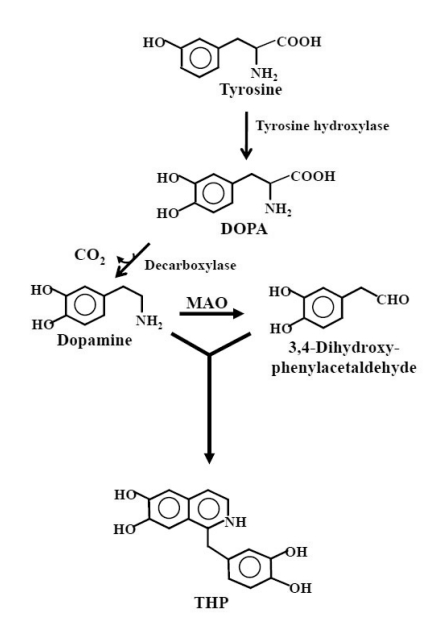
THP is considered to be formed spontaneously by non-enzymatic Pictet-Spengler condensation of dopamine with its aldehyde metabolite (3,4-dihydroxyphenylacetaldehyde) produced by monoamine oxidase (MAO) (Fig. 1) (Holtz et al., 1964; Walsh et al., 1970). In parkinsonian patients receiving L-DOPA treatment, L-DOPA is converted into dopamine by DOPA decarboxylase and the excess dopamine may inhibit aldehyde dehydrogenase (Turan et al., 1989), thereby blocking the conversion of 3,4-dihydroxyphenylacetaldehyde into 3,4-dihydroxyphenylacetic acid. The accumulation of a relatively high concentration of 3,4-dihydroxyphenylacetaldehyde, together with the high levels of dopamine derived from L-DOPA administration, may favor the formation of THP (Soto-Otero et al., 2006).
Fig. 1.
Biosynthesis of THP. Tyrosine is converted to DOPA by tyrosine hydroxylase and further to dopamine. Although the Pictet-Spengler condensation reaction of dopamine with its aldehyde metabolite (3,4-dihydroxyphenylacetaldehyde) produced by monoamine oxidase (MAO) yields racemic THP, (S)-enantiomer is predominantly found in the brain of man, suggesting the occurrence of enzymatic synthesis.
It was postulated that excess acetaldehyde, secondary to ethanol metabolism, would competitively inhibit the breakdown of dopaldehyde by aldehyde dehydrogenase (Davis et al., 1970). The resultant accumulation of dopaldehyde would lead to an additional condensation reaction with dopamine to augment THP formation (Davis et al., 1970; Nace, 1986). THP was detected in specific brain regions of the rat after acute ethanol administration (3 g/kg body weight), but not in the same regions of untreated animals. Most brain regions had detectable levels of THP 100 minutes after the animals received ethanol, and the striatum contained the highest concentration of the alkaloid (Cashaw, 1993). THP can be detected in the brain of rats not only after acute ethanol administration (Cashaw, 1993), but also when animals were subjected to alcohol consumption ad libitum for a longer period (Haber et al., 1997). In the latter study, alcohol administration for 18 months induced formation of the (S) enantiomer of THP only in the striatum of the rat brain.
The stereo-chemically specific THP has also been identified in human brains in a later study (Sango et al., 2000) and interestingly, only the (S) enantiomer of THP was detected, suggesting that this endogenous dicatechol isoquinoline is presumably synthesized in the brain by an enzyme-catalyzed reaction. It is reported that some edible plants adopt the distinct enzymatic pathways for the synthesis of (S)-THP (Rueffer et al., 1983), and exogenous THP consumed by man is anticipated to cross the blood-brain barrier (Cashaw and Geraghty, 1991). Thus, it is possible that the stereo-chemically specific exogenous or peripheral origin of THP is detected in the brain. It is interesting to note that dietary sources of SAL contribute to its detection in biological samples (Smythe and Duncan, 1985), and this may also be the case for THP.
NEUROTOXIC EFFECTS AND MECHANISMS
As mentioned previously, THP has been speculated to be implicated in the etiology of several human neurological, behavioral, and psychiatric disorders, such as parkinsonism and alcohol addiction (Collins et al., 1979; Collins, 2004). In this part of review, we will address the possible neurotoxic mechanisms of THP.
Inhibition of tyrosine hydroxylase and dopamine uptake
It is reported that THP significantly decreased the intracellular dopamine content in PC12 cells (Kim et al., 2006). THP also reduced the activity of tyrosine hydroxylase, the rate-determining enzyme for the production of DOPA, and had an inhibitory effect on bovine adrenal tyrosine hydroxylase. Since the reduction of dopamine content by THP in PC12 cells was inversed by the antioxidant N-acetyl-L-cysteine (NAC), it is believed that the reduction of the basal dopamine content by THP is caused by oxidative stress (Kim et al., 2006).
When Okada et al., examined the Ki values of THP and three synthetic derivatives for inhibition of dopamine uptake, they were almost similar to that of MPP+. These results suggest that THP and its derivatives might be transported through DAT and be involved in Parkinson's disease (Okada et al., 1998).
Inhibition of mitochondrial respiration and serotonin production
MPTP-like toxins, THIQs, have been speculated to induce Parkinson's disease. Since the neurodegeneration in MPTP-induced parkinsonism is thought to be caused by the inhibition of mitochondrial respiration, the effects THP on the mitochondrial respiration were investigated (Suzuki et al., 1990; Morikawa et al., 1996; Nagatsu, 1997). THP significantly inhibited the state 3 and 4 respiration and reduced the respiratory control ratio. Toxic properties of THP on mitochondrial respiration were quite similar to those of MPP+, supporting the hypothesis that MPTP- or MPP+-like substances may be responsible for the dopamine neuron degeneration (Nagatsu, 1997; McNaught et al., 1998; Collins, 2004).
THP is also known to decrease serotonin content in a concentration-dependent manner in serotonin-producing mastocytoma cells (Kim et al., 2003). Furthermore, the activity of tryptophan hydroxylase was also inhibited when exposed to THP, suggesting that THP treatment leads to a decrease in serotonin content by inhibiting tryptophan hydroxylase activity (Kim et al., 2003).
MECHANISMS UNDERLYING THP-INDUCED NEUROTOXICITY
Redox cycling and cytotoxicity
The oxidation chemistry of THIQ analogs was extensively studied by electrochemical approaches. Catechol-bearing THIQs can undergo auto-oxidation or enzymatic oxidation (Zhang and Dryhurst, 2001; De Marco et al., 2002) and subsequent generation of reactive quinones via semiquinones may cause the degeneration of dopaminergic neurons and other injuries. The catechol-quinone redox cycling, that is, the one-electron reduction of quinone to semiquinone, and the auto-oxidation of semiquinone to the quinone, is able to release large quantities of superoxide anion which, in turn, can spontaneously or by superoxide dismutase (SOD) action be transformed into hydrogen peroxide (H2O2). H2O2, via the Fenton reaction, can readily be decomposed to give rise to hydroxyl radical, which represents extremely reactive species with devastating action on practically every cell components and organelles (De Marco et al., 2002). Although quinoidal forms of THIQs are anticipated to be reactive per se, they can be converted back to the parent catechol molecules, with concomitant production of ROS capable of damaging critical cellular molecules, such as DNA, RNA, protein and membrane lipid. According to previous studies in our laboratory, redox cycling of SAL (Jung and Surh, 2001; Kim et al., 2001) and THP (Soh et al., 2003; Shin et al., 2004) was facilitated in the presence of certain transition metal ions such as Cu2+ leading to ROS overproduction and subsequently oxidative cell death and DNA damage. Therefore, ROS overproduction is likely to contribute to the mechanisms underlying deleterious effects of THP and other THIQs (McNaught et al., 1998; Soto-Otero et al., 2006). The presence of ascorbate enhances this process by establishing a redox cycle, which regenerates THP from its quinoidal form. Ascorbate-promoted THP auto-oxidation caused an increase in protein carbonyl content, which was increased when the autoxidation occurs in the presence of iron (McNaught et al., 1998).
Dopamine-derived catechol-bearing THIQs are neurotoxic to some extent and might be implicated in the pathogenesis of Parkinson's disease. THP predominantly caused necrosis whereas papaveroline and N-methyl-papaveroline induced apoptosis in SH-SY5Y cells when evidenced by typical features of condensed and fragmented nuclei (Maruyama et al., 2000). The cell death type appeared to depend on adenosine triphosphate (ATP) level, since THP treatment reduced the ATP level whereas papaverolines did not (Maruyama et al., 2000). When the effect of SAL and THP on the viability of melanoma cell lines was investigated, THP appeared to be more cytotoxic than SAL and DOPA (De Marco et al., 2002). THP-induced cytotoxicity was partially protected by exogenous catalase and SOD, and completely abolished by reduced glutathione (GSH) and NAC, suggesting that THP toxicity was likely due to increased oxidative stress (De Marco et al., 2002; Shin et al., 2004). Interestingly, THP induced apoptosis in the human leukemia cell line (HL-60), but did not in its hydrogen peroxide (H2O2)-resistant clone HP100, which also supports the involvement of ROS in THP induced cytotoxicity (Kobayashi et al., 2006). In addition, THP exerted toxicity toward the mitochondrial respiratory chain (Suzuki et al., 1990; Morikawa et al., 1996). Mitochondrial metabolism in terms of α-ketoglutarate dehydrogenase activity was inhibited in murine and human melanoma cells and human epithelial keratinocytes in the presence of THP (De Marco et al., 2002; Foppoli et al., 2005).
The effects of THP and SAL on human primary melanocytes were re-evaluated by treating the cells with variable concentrations of each THIQ (Perluigi et al., 2003). When the cytotoxicity was compared, THP became overtly toxic in lower concentrations while SAL showed no cytotoxic effect up to 100µ M. In contrast to SAL, THP strongly caused dramatic decrease of α-ketoglutarate dehydrogenase activity (Perluigi et al., 2003).
THP-induced cytotoxicity appeared to be mediated by c-Jun-terminal kinase and p38 mitogen-activated protein kinase (MAPK), as evidenced by results that pretreatment with inhibitors of these kinases rescued the glioma cells from THP-induced cytotoxicity. THP-treated PC12 cells exhibited increased intracellular accumulation of ROS and underwent apoptosis as determined by poly(ADP-ribose) polymerase cleavage, an increased ratio of Bax to Bcl-xL, positive terminal transferase-mediated dUTP nick end labeling (TUNEL), and nuclear fragmentation/condensation (Shin et al., 2004). Furthermore, exposure of PC12 cells to THP combined with L-DOPA elicited synergistic effects, increasing the proportion of TUNEL-positive apoptotic cells.
Taking all the aforementioned findings together, it is evident that THP provokes cytotoxicity in many different cell lines through induction of oxidative stress. However, the molecular milieu mediating THP-induced oxidative cell death needs to be uncovered.
DNA damage
In the presence of cupric ion, THP caused phiX174 supercoiled DNA or calf thymus DNA damage determined by strand scission or formation of 8-oxo-7,8-dihydro-2'-deoxyguanosine. The DNA damage in the presence of THP and copper was ameliorated by some ROS scavengers/antioxidants and catalase (Soh et al., 2003).
In the presence of Fe(III)EDTA, THP caused DNA damage at every nucleotide whereas in the presence of Cu(II), THP caused the damage at T and G of 5'-TG-3' sequence (Kobayashi et al., 2006). The DNA damage was attenuated by catalase and the metal chelators (Kobayashi et al., 2006). Table 2 highlights the biochemical basis of THP-induced neurotoxicity.
Table 2.
Biochemical mechanisms underlying THP-induced neurotoxicity
CELLULAR PROTECTIVE RESPONSE AGAINST THP-INDUCED INJURIES
As THP has two catechol moieties, it undergoes auto-oxidation or enzymatic oxidation to produce ROS, which may contribute to the THP-induced cytotoxicity. Although overproduction of ROS is cytotoxic, the initial accumulation of moderate amounts of ROS may provoke defense responses of cells. The body's antioxidant defense system serves to protect the cells from excess ROS production and is comprised of both endogenous and exogenous entities (Fisher-Wellman and Bloomer, 2009). The endogenous antioxidant defense include radical scavengers (e.g., GSH, thioredoxin, bilirubin, uric acid, etc.), and antioxidant enzymes, such as SOD, catalase, glutathione peroxidase, glutathione S-transferase, glutamate-cysteine ligase, NAD(P)H:quinone oxidoreductase 1, etc. The exogenous antioxidants (e.g., carotenoids, tocopherols, ascorbate, bioflavonoids, etc.) are provided as diet, especially fruits and vegetables.
Keratinocytes of the human epidermis, a tissue particularly exposed to oxidant stimuli, possess a wide range of antioxidant and detoxifying mechanisms aimed to avoid oxidative damage of the tissue. It is demonstrated that THP and L-DOPA upregulated expression of intracellular antioxidant enzymes to a different extent in normal keratinocytes of human epidermal origin when compared to transformed ones (Foppoli et al., 2005). Normal diploid keratinocytes adequately scavenge toxic substances through the coordinated activation of several concurrent antioxidant pathways. Conversely, in transformed cells, the whole oxidative burden must be neutralized by the limited set of conserved pathways that, accordingly, has to be highly activated (Foppoli et al., 2005).
THP treatment activated the redox-sensitive nuclear factor κB (NF-κB). Preincubation of PC12 cells with NF-κB inhibitors, such as L-1-tosylamido-2-phenylethyl chloromethyl ketone and parthenolide, aggravated THP-induced cytotoxicity (Shin et al., 2004). In addition, THP treatment altered survival or death signals including not only MAPK but also Akt/protein kinase B.
The redox-sensitive transcription factor Nrf2 is known to regulate expression of detoxifying and antioxidant enzymes and other defensive proteins against oxidative stress and other noxious conditions (Motohashi and Yamamoto, 2004). THP treatment elevated nuclear translocation of Nrf2 and its subsequent binding to antioxidant response element. An important enzyme that plays an essential role in cellular survival response to a wide variety of stress is heme oxygenase-1 (HO-1). Treatment of PC12 cells with THP increased expression of HO-1, and THP-induced cytotoxicity was attenuated by the HO-1 inducer (SnCl2). In addition, pharmacologic inhibition of HO-1 activity exacerbated THP induced cell death (Park et al., 2007). When PC12 cells were transfected with dominant-negative Nrf2 after THP treatment, the cytotoxicity was increased and HO-1 expression was decreased. These results suggest that cells try to protect themselves from THP-mediated cell death by activating Nrf2 and inducing antioxidant/cytoprotective enzymes, particularly HO-1.
CONCLUDING REMARKS
Over the past three decades, THP and other structurally related THIQ alkaloids have been studied for neurotoxic potential in parkinsonism and alcoholism. Additional studies are necessary to uncover the molecular targets of this neurotoxin, especially those involved in cell death or cell defense intracellular signaling cascades. In addition, the adverse effects of THP on cellular functions other than neurotoxic activity merit further investigation.
ACKNOWLEDGEMENTS
This work was supported by the Research Institute of Pharmaceutical Sciences, Seoul National University.
References
- 1.Cashaw JL. Determination of tetrahydropapaveroline in the urine of parkinsonian patients receiving L-dopa-carbidopa (Sinemet) therapy by high-performance liquid chromatography. J Chromatogr. 1993;613:267–273. doi: 10.1016/0378-4347(93)80141-p. [DOI] [PubMed] [Google Scholar]
- 2.Cashaw JL. Tetrahydropapaveroline in brain regions of rats after acute ethanol administration. Alcohol. 1993;10:133–138. doi: 10.1016/0741-8329(93)90092-3. [DOI] [PubMed] [Google Scholar]
- 3.Cashaw JL, Geraghty CA. Tetrahydropapaveroline and the blood-brain barrier in rats. Alcohol. 1991;8:317–319. doi: 10.1016/0741-8329(91)90481-b. [DOI] [PubMed] [Google Scholar]
- 4.Cashaw JL, Geraghty CA, McLaughlin BR, Davis VE. A method for determination of subpicomole concentrations of tetrahydropapaveroline in rat brain by high-performance liquid chromatography with electrochemical detection. Anal Biochem. 1987;162:274–282. doi: 10.1016/0003-2697(87)90037-6. [DOI] [PubMed] [Google Scholar]
- 5.Collins MA. Tetrahydropapaveroline in Parkinson's disease and alcoholism: a look back in honor of Merton Sandler. Neurotoxicology. 2004;25:117–120. doi: 10.1016/S0161-813X(03)00145-1. [DOI] [PubMed] [Google Scholar]
- 6.Collins MA, Nijm WP, Borge GF, Teas G, Goldfarb C. Dopamine-related tetrahydroisoquinolines: significant urinary excretion by alcoholics after alcohol consumption. Science. 1979;206:1184–1186. doi: 10.1126/science.505002. [DOI] [PubMed] [Google Scholar]
- 7.Davis VE, Walsh MJ. Alcohol, amines, and alkaloids: a possible biochemical basis for alcohol addiction. Science. 1970;167:1005–1007. doi: 10.1126/science.167.3920.1005. [DOI] [PubMed] [Google Scholar]
- 8.Davis VE, Walsh MJ, Yamanaka Y. Augmentation of alkaloid formation from dopamine by alcohol and acetaldehyde in vitro. J Pharmacol Exp Ther. 1970;174:401–412. [PubMed] [Google Scholar]
- 9.De Marco F, Perluigi M, Marcante ML, Coccia R, Foppoli C, Blarzino C, Rosei MA. Cytotoxicity of dopamine-derived tetrahydroisoquinolines on melanoma cells. Biochem Pharmacol. 2002;64:1503–1512. doi: 10.1016/s0006-2952(02)01353-9. [DOI] [PubMed] [Google Scholar]
- 10.Donnerer J, Cardinale G, Coffey J, Lisek CA, Jardine I, Spector S. Chemical characterization and regulation of endogenous morphine and codeine in the rat. J Pharmacol Exp Ther. 1987;242:583–587. [PubMed] [Google Scholar]
- 11.Duncan C, Deitrich RA. A critical evaluation of tetrahydroisoquinoline induced ethanol preference in rats. Pharmacol Biochem Behav. 1980;13:265–281. doi: 10.1016/0091-3057(80)90083-0. [DOI] [PubMed] [Google Scholar]
- 12.Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann Neurol. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- 13.Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dyn Med. 2009;8:1. doi: 10.1186/1476-5918-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foppoli C, De Marco F, Blarzino C, Perluigi M, Cini C, Coccia R. Biological response of human diploid keratinocytes to quinone-producing compounds: role of NAD(P)H:quinone oxidoreductase 1. Int J Biochem Cell Biol. 2005;37:852–863. doi: 10.1016/j.biocel.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Haber H, Roske I, Rottmann M, Georgi M, Melzig MF. Alcohol induces formation of morphine precursors in the striatum of rats. Life Sci. 1997;60:79–89. doi: 10.1016/s0024-3205(96)00597-8. [DOI] [PubMed] [Google Scholar]
- 16.Holtz P, Stock K, Westernmann E. Formation of Tetrahydropapaveroline from Dopamine in vitro. Nature. 1964;203:656–658. doi: 10.1038/203656b0. [DOI] [PubMed] [Google Scholar]
- 17.Jung Y, Surh Y. Oxidative DNA damage and cytotoxicity induced by copper-stimulated redox cycling of salsolinol, a neurotoxic tetrahydroisoquinoline alkaloid. Free Radic Biol Med. 2001;30:1407–1417. doi: 10.1016/s0891-5849(01)00548-2. [DOI] [PubMed] [Google Scholar]
- 18.Kim EI, Yin S, Kang MH, Hong JT, Oh KW, Lee MK. Reduction of serotonin content by tetrahydropapaveroline in murine mastocytoma P815 cells. Neurosci Lett. 2003;339:131–134. doi: 10.1016/s0304-3940(03)00004-1. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Soh Y, Jang JH, Lee JS, Oh YJ, Surh YJ. Differential cell death induced by salsolinol with and without copper: possible role of reactive oxygen species. Mol Pharmacol. 2001;60:440–449. [PubMed] [Google Scholar]
- 20.Kim YM, Reed W, Wu W, Bromberg PA, Graves LM, Samet JM. Zn2+-induced IL-8 expression involves AP-1, JNK, and ERK activities in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1028–L1035. doi: 10.1152/ajplung.00479.2005. [DOI] [PubMed] [Google Scholar]
- 21.Kirby GW. Biosynthesis of the morphine alkaloids. Science. 1967;155:170–173. doi: 10.1126/science.155.3759.170. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H, Oikawa S, Kawanishi S. Mechanism of DNA damage and apoptosis induced by tetrahydropapaveroline, a metabolite of dopamine. Neurochem Res. 2006;31:523–532. doi: 10.1007/s11064-006-9044-8. [DOI] [PubMed] [Google Scholar]
- 23.Lee JJ, Kim YM, Yin SY, Park HD, Kang MH, Hong JT, Lee MK. Aggravation of L-DOPA-induced neurotoxicity by tetrahydropapaveroline in PC12 cells. Biochem Pharmacol. 2003;66:1787–1795. doi: 10.1016/s0006-2952(03)00421-0. [DOI] [PubMed] [Google Scholar]
- 24.Marsden CD. Parkinson's disease. J Neurol Neurosurg Psychiatry. 1994;57:672–681. doi: 10.1136/jnnp.57.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maruyama W, Sango K, Iwasa K, Minami C, Dostert P, Kawai M, Moriyasu M, Naoi M. Dopaminergic neurotoxins, 6,7-dihydroxy-1-(3',4'-dihydroxybenzyl)-isoquinolines, cause different types of cell death in SH-SY5Y cells: apoptosis was induced by oxidized papaverolines and necrosis by reduced tetrahydropapaverolines. Neurosci Lett. 2000;291:89–92. doi: 10.1016/s0304-3940(00)01381-1. [DOI] [PubMed] [Google Scholar]
- 26.Matsubara K, Fukushima S, Akane A, Kobayashi S, Shiono H. Increased urinary morphine, codeine and tetrahydropapaveroline in parkinsonian patient undergoing L-3,4-dihydroxyphenylalanine therapy: a possible biosynthetic pathway of morphine from L-3,4-dihydroxyphenylalanine in humans. J Pharmacol Exp Ther. 1992;260:974–978. [PubMed] [Google Scholar]
- 27.McNaught KS, Carrupt PA, Altomare C, Cellamare S, Carotti A, Testa B, Jenner P, Marsden CD. Isoquinoline derivatives as endogenous neurotoxins in the aetiology of Parkinson's disease. Biochem Pharmacol. 1998;56:921–933. doi: 10.1016/s0006-2952(98)00142-7. [DOI] [PubMed] [Google Scholar]
- 28.Morikawa N, Nakagawa-Hattori Y, Mizuno Y. Effect of dopamine, dimethoxyphenylethylamine, papaverine, and related compounds on mitochondrial respiration and complex I activity. J Neurochem. 1996;66:1174–1181. doi: 10.1046/j.1471-4159.1996.66031174.x. [DOI] [PubMed] [Google Scholar]
- 29.Morris JG. A review of some aspects of the pharmacology of levodopa. Clin Exp Neurol. 1978;15:24–50. [PubMed] [Google Scholar]
- 30.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Myers RD, Melchior CL. Alcohol drinking: abnormal intake caused by tetrahydropapaveroline in brain. Science. 1977;196:554–556. doi: 10.1126/science.557839. [DOI] [PubMed] [Google Scholar]
- 32.Myers RD, Privette TH. A neuroanatomical substrate for alcohol drinking: identification of tetrahydropapaveroline (THP)-reactive sites in the rat brain. Brain Res Bull. 1989;22:899–911. doi: 10.1016/0361-9230(89)90035-x. [DOI] [PubMed] [Google Scholar]
- 33.Myers RD, Robinson DE. Tetrahydropapaveroline injected in the ventral tegmental area shifts dopamine efflux differentially in the shell and core of nucleus accumbens in high-ethanol-preferring (HEP) rats. Alcohol. 1999;18:83–90. doi: 10.1016/s0741-8329(99)00008-7. [DOI] [PubMed] [Google Scholar]
- 34.Nace EP. Alcoholism: epidemiology, diagnosis, and biological aspects. Alcohol. 1986;3:83–87. doi: 10.1016/0741-8329(86)90014-5. [DOI] [PubMed] [Google Scholar]
- 35.Nagatsu T. Isoquinoline neurotoxins in the brain and Parkinson's disease. Neurosci Res. 1997;29:99–111. doi: 10.1016/s0168-0102(97)00083-7. [DOI] [PubMed] [Google Scholar]
- 36.Nagatsu T, Yoshida M. An endogenous substance of the brain, tetrahydroisoquinoline, produces parkinsonism in primates with decreased dopamine, tyrosine hydroxylase and biopterin in the nigrostriatal regions. Neurosci Lett. 1988;87:178–182. doi: 10.1016/0304-3940(88)90166-8. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa N. Levodopa and dopamine agonists in the treatment of Parkinson's disease: advantages and disadvantages. Eur Neurol. 1994;34(Suppl. 3):20–28. doi: 10.1159/000119538. [DOI] [PubMed] [Google Scholar]
- 38.Okada T, Shimada S, Sato K, Kotake Y, Kawai H, Ohta S, Tohyama M, Nishimura T. Tetrahydropapaveroline and its derivatives inhibit dopamine uptake through dopamine transporter expressed in HEK293 cells. Neurosci Res. 1998;30:87–90. doi: 10.1016/s0168-0102(97)00121-1. [DOI] [PubMed] [Google Scholar]
- 39.Park SH, Jang JH, Li MH, Na HK, Cha YN, Surh YJ. Nrf2-mediated heme oxygenase-1 induction confers adaptive survival response to tetrahydropapaveroline-induced oxidative PC12 cell death. Antioxid Redox Signal. 2007;9:2075–2086. doi: 10.1089/ars.2007.1828. [DOI] [PubMed] [Google Scholar]
- 40.Perluigi M, De Marco F, Foppoli C, Coccia R, Blarzino C, Marcante ML, Cini C. Tyrosinase protects human melanocytes from ROS-generating compounds. Biochem Biophys Res Commun. 2003;305:250–256. doi: 10.1016/s0006-291x(03)00751-4. [DOI] [PubMed] [Google Scholar]
- 41.Rueffer M, Nagakura N, Zenk MH. Partial Purification and Properties of S-Adenosylmethionine: (R), (S)-Norlaudanosoline-6-O-Methyltransferase from Argemone platyceras Cell Cultures. Planta Med. 1983;49:131–137. doi: 10.1055/s-2007-969833. [DOI] [PubMed] [Google Scholar]
- 42.Sandler M, Carter SB, Hunter KR, Stern GM. Tetrahydroisoquinoline alkaloids: in vivo metabolites of L-dopa in man. Nature. 1973;241:439–443. doi: 10.1038/241439a0. [DOI] [PubMed] [Google Scholar]
- 43.Sango K, Maruyama W, Matsubara K, Dostert P, Minami C, Kawai M, Naoi M. Enantio-selective occurrence of (S)-tetrahydropapaveroline in human brain. Neurosci Lett. 2000;283:224–226. doi: 10.1016/s0304-3940(00)00963-0. [DOI] [PubMed] [Google Scholar]
- 44.Shin MH, Jang JH, Surh YJ. Potential roles of NF-kappaB and ERK1/2 in cytoprotection against oxidative cell death induced by tetrahydropapaveroline. Free Radic Biol Med. 2004;36:1185–1194. doi: 10.1016/j.freeradbiomed.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 45.Smythe GA, Duncan MW. Precise GC/MS assays for salsolinol and tetrahydropapaveroline: the question of artifacts and dietary sources and the influence of alcohol. Prog Clin Biol Res. 1985;183:77–84. [PubMed] [Google Scholar]
- 46.Soh Y, Shin MH, Lee JS, Jang JH, Kim OH, Kang H, Surh YJ. Oxidative DNA damage and glioma cell death induced by tetrahydropapaveroline. Mutat Res. 2003;544:129–142. doi: 10.1016/j.mrrev.2003.06.023. [DOI] [PubMed] [Google Scholar]
- 47.Soto-Otero R, Sanmartin-Suárez C, Sánchez-Iglesias S, Hermida-Ameijeiras A, Sánchez-Sellero I, Méndez-Alvarez E. Study on the ability of 1,2,3,4-tetrahydropapaveroline to cause oxidative stress: Mechanisms and potential implications in relation to parkinson's disease. J Biochem Mol Toxicol. 2006;20:209–220. doi: 10.1002/jbt.20138. [DOI] [PubMed] [Google Scholar]
- 48.Storch A, Ott S, Hwang YI, Ortmann R, Hein A, Frenzel S, Matsubara K, Ohta S, Wolf HU, Schwarz J. Selective dopaminergic neurotoxicity of isoquinoline derivatives related to Parkinson's disease: studies using heterologous expression systems of the dopamine transporter. Biochem Pharmacol. 2002;63:909–920. doi: 10.1016/s0006-2952(01)00922-4. [DOI] [PubMed] [Google Scholar]
- 49.Surh Y. Tetrahydropapaveroline, a dopamine-derived isoquinoline alkaloid, undergoes oxidation: implications for DNA damage and neuronal cell death. Eur J Clin Invest. 1999;29:650–651. doi: 10.1046/j.1365-2362.1999.00511.x. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki K, Mizuno Y, Yoshida M. Inhibition of mitochondrial respiration by 1,2,3,4-tetrahydroisoquinolinelike endogenous alkaloids in mouse brain. Neurochem Res. 1990;15:705–710. doi: 10.1007/BF00973651. [DOI] [PubMed] [Google Scholar]
- 51.Turan SC, Shah P, Pietruszko R. Inactivation of aldehyde dehydrogenase in intact rat liver mitochondria by dopamine. Alcohol. 1989;6:455–460. doi: 10.1016/0741-8329(89)90051-7. [DOI] [PubMed] [Google Scholar]
- 52.Turner AJ, Baker KM, Algeri S, Erigerio A, Garattini S. Tetrahydropapaveroline: formation in vivo and in vitro in rat brain. Life Sci. 1974;14:2247–2257. doi: 10.1016/0024-3205(74)90106-4. [DOI] [PubMed] [Google Scholar]
- 53.Walsh MJ, Davis VE, Yamanaka Y. Tetrahydropapaveroline: an alkaloid metabolite of dopamine in vitro. J Pharmacol Exp Ther. 1970;174:388–400. [PubMed] [Google Scholar]
- 54.Weitz CJ, Lowney LI, Faull KF, Feistner G, Goldstein A. Morphine and codeine from mammalian brain. Proc Natl Acad Sci U S A. 1986;83:9784–9788. doi: 10.1073/pnas.83.24.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang F, Dryhurst G. Chromatographic separation and spectrometric identification of the oxidation products from a tetrahydro-isoquinoline alkaloid. J Pharm Biomed Anal. 2001;25:181–189. doi: 10.1016/s0731-7085(00)00472-6. [DOI] [PubMed] [Google Scholar]