Abstract
The RecA protein is a central component of the homologous recombination machinery and of the SOS system in most bacteria. In performing these functions, it is involved in DNA repair processes and plays an important role in natural transformation competence. This may be especially important in Helicobacter pylori, where an unusually high degree of microdiversity among strains is generated by homologous recombination. We have suggested previously that the H. pylori RecA protein is subject to posttranslational modifications that result in a slight shift in its electrophoretic mobility. Here we show that at least two genes downstream of recA are involved in this modification and that this process is dependent on genes involved in glycosylation and lipopolysaccharide biosynthesis. Site-directed mutagenesis of a putative glycosylation site results in production of an unmodified RecA protein. This posttranslational modification is not involved in membrane targeting or cell division functions but is necessary for the full function of RecA in DNA repair. Thus, it might be an adaptation to the specific requirements of H. pylori in its natural environment.
The gram-negative bacterial pathogen Helicobacter pylori is the principal cause of chronic active gastritis and peptic ulcer disease and has been implicated in the development of gastric mucosa-associated lymphoid tissue lymphoma and gastric cancer (29, 40). The very special habitat of H. pylori at the surface of gastric epithelial cells or in the mucus layer covering the epithelium suggests that this bacterium has evolved specialized features for adaptation. A comparison of the two published genome sequences shows a considerable diversity in gene content, with about 7% of all putative genes being strain specific (1). Moreover, some H. pylori strains display high mutation frequencies (3), and more diversity is created by horizontal gene transfer and free recombination between strains (41), although infections with multiple strains are not very common. Since H. pylori is naturally competent for transformation, horizontal gene transfer is supposed to occur mainly by this mechanism.
Thus, homologous recombination is an important function which helps to generate diversity, but it is also involved in maintaining genome integrity and thus species barriers (28). These functions are achieved by a complex machinery which is highly regulated and which involves many proteins (9). The RecA protein is one of the central components of this machinery. One of its main functions is the recognition of stretches of single-stranded DNA, which are subsequently complexed by helical RecA filaments. In Escherichia coli, this filamentous form of RecA is activated as a coprotease that regulates cellular functions such as the SOS response or the induction of trans-lesion DNA synthesis. Both this coprotease activity and the recombination function are thought to play major roles in the bacterial response to DNA damage. The RecA protein of H. pylori has likewise been shown to be necessary for DNA repair (36, 44), although SOS response and error-prone trans-lesion synthesis pathways do not seem to be present in H. pylori, as concluded by the absence of homologous genes (10).
Here we describe a posttranslational protein modification of the RecA protein in H. pylori, and we show that this modification is necessary for the full function of RecA in response to DNA damage.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
H. pylori strains were grown on GC agar plates (Difco) supplemented with vitamin mix (1%), horse serum (8%), vancomycin (10 mg/liter), trimethoprim (5 mg/liter), and nystatin (1 mg/liter) (serum plates) and incubated for 16 to 60 h in a microaerobic atmosphere (85% N2, 10% CO2, 5% O2) at 37°C. E. coli strains HB101 (4), GC6 (43), and DH5α (Bethesda Research Laboratories) were grown on Luria-Bertani agar plates or in Luria-Bertani liquid medium (33) supplemented with ampicillin (100 mg/liter), chloramphenicol (30 mg/liter), or kanamycin (40 mg/liter), as appropriate. Strains β2150 and β2155 (6) were grown on the same media supplemented with diaminopimelic acid (0.2 mM).
DNA manipulations.
Standard cloning and DNA analysis procedures were performed according to the methods described in reference 33. Plasmid DNA was purified from E. coli by the boiling procedure, and E. coli cells for electroporation were prepared according to the protocol recommended for the Gene Pulser (Bio-Rad). Plasmid DNA was isolated from Helicobacter strains by using Wizard minipreps (Promega) according to the protocol of the manufacturer. Amplification of DNA fragments by PCR was performed as described previously (15).
Natural transformation and bacterial conjugation.
Shuttle plasmids and suicide plasmids were introduced into H. pylori strains by conjugation or transformation as described previously (16), except that in conjugation experiments E. coli strains β2150 (for plasmids conferring kanamycin resistance) and β2155 (for plasmids conferring chloramphenicol resistance) were used as donors and β2150(pRK2013) was used as a mobilizing strain. H. pylori transformants were selected on serum plates containing 6 mg of chloramphenicol/liter or 8 mg of kanamycin/liter. For the determination of transformation rates, DNase (1 mg/ml) was added to H. pylori grown in liquid culture 1 h after the addition of plasmid DNA. The cultures were incubated for a further 6 to 12 h and then plated on selective agar plates and in parallel on nonselective agar plates to estimate the number of viable bacteria.
Plasmid constructions.
Plasmid pDH38, which was used for the complementation of an H. pylori recA deletion mutant, was described earlier (36). For the construction of the enolase mutants, an EcoRI/BamHI deletion derivative of plasmid pWS48, which contains the recA-eno region of H. pylori strain P1 (36), was constructed. The enolase gene was disrupted by replacing an internal 260-bp SphI fragment with an aphA-3 resistance gene cassette, resulting in plasmid pWS55, or with a terminatorless cat resistance gene cassette, resulting in plasmid pGAH3. Plasmid pWS106 was constructed by cloning the recA gene amplified with primers WS67 (5′-GAAGATCTTATTCCATTTCTTCTAAAG-3′) and WS68 (5′-CGGAATTCGCAATAGATGAAGACAAAC-3′) into the EcoRI and BamHI sites of the expression vector pEV40 (31). The recA-eno downstream region comprising genes hp155 to hp158 was amplified with primers ET10 (5′-CGCGGATCCAAGAGTTGTTTAAGCATGGC-3′) and ET11 (5′-TAATGCACTGCAGCCCACAATACGACAAAATC-3′) from chromosomal DNA of strain 26695 and cloned into the PstI and BamHI sites of pBluescript II KS (Stratagene). From there, the recA-eno downstream region was subcloned into the BglII and XhoI sites of plasmid pMin1 (22), resulting in plasmid pWS124. The site-specific mutation was introduced into the recA gene as follows. A 5′ portion of the recA gene of strain P1 was amplified by PCR with primers RH147 (5′-AGCTGGGTCGACTTTCTTAACGCGTGGCTC-3′) and DH13 (5′-GGGGTACCAAGCTTATCGCGCTCACATC-3′) and cloned into the KpnI and SalI sites of vector pIC20R1 (26), resulting in plasmid pGAH4. A 3′ portion of the recA gene containing the site-specific mutation was amplified with primers WS86 (5′-GCTCTAGACTGCAGAGATCAAAGGATCTTCTT-3′) and WS87 (5′-AACGGACGCGTTAAGAAAAATCACCGGTGTTTTGCACAAAATGAATACTATG-3′) and cloned into the MluI and BglII sites of plasmid pGAH4. The mutagenized recA gene was excised from the resulting plasmid pWS126 and subcloned into the KpnI and BglII sites of the shuttle plasmid pHel3 (16) to yield plasmid pWS127.
Transposon shuttle mutagenesis.
TnMax5 transposon mutagenesis of plasmid pWS124 was performed as described previously (22).
Production of anti-RecA antiserum and immunoblotting.
A His6-tagged RecA fusion protein derived from strain P1 was overproduced in E. coli 2136 from plasmid pWS106 and purified from inclusion bodies according to the method described in reference 39. The purified fusion protein was used to raise the polyclonal rabbit antiserum AK263. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting were performed as described previously (36). For the development of Western blots, nitrocellulose filters were blocked with 3% bovine serum albumin in TBS (50 mM Tris-HCl [pH 7.5], 150 mM NaCl) and incubated with AK263 at a dilution of 1:3,000. Protein A-conjugated alkaline phosphatase was used to visualize bound antibody.
Membrane preparations.
H. pylori cells were grown on solid or in liquid media for 24 to 48 h, then harvested, washed, and resuspended in preparation buffer (10 mM Tris-HCl, pH 8.0). Bacteria were lysed by sonication, centrifuged for 10 min at 7,000 × g to remove unbroken cells and cell debris. The supernatant was collected and separated by ultracentrifugation (45 min, 230,000 × g) into cytoplasmic and total membrane fractions. Proteins in the cytoplasmic fractions were concentrated by chloroform-methanol precipitation (46), and the membrane fractions were washed with preparation buffer and resuspended in SDS-PAGE sample solution.
UV and metronidazole sensitivity measurements.
The susceptibility of H. pylori strains to UV radiation was determined as described previously (36). For the determination of metronidazole sensitivity, E-test strips (AB Biodisk, Solna, Sweden) were placed on serum agar plates inoculated with standard H. pylori suspensions according to the method described in reference 44. MICs were scored after 5 days of growth.
Nucleoid staining and microscopy.
Bacteria grown in liquid culture were harvested by centrifugation, washed twice, and resuspended in phosphate-buffered saline (PBS). The suspension was centrifuged onto coverslips (5 min, 1,500 × g) and fixed with 3.7% paraformaldehyde in PBS for 30 min at room temperature. After two washes with PBS, coverslips were incubated for 30 min in PBS containing 1 μg of 4′,6′-diamidino-2-phenylindole (DAPI)/ml, washed twice with PBS, and placed upon a slide containing a single drop of Fluoprep (BioMérieux). Cells were photographed with a Leica DM fluorescence microscope equipped with a Diagnostic Instruments SP401-220 digital camera.
RESULTS
Size variation of the H. pylori RecA protein.
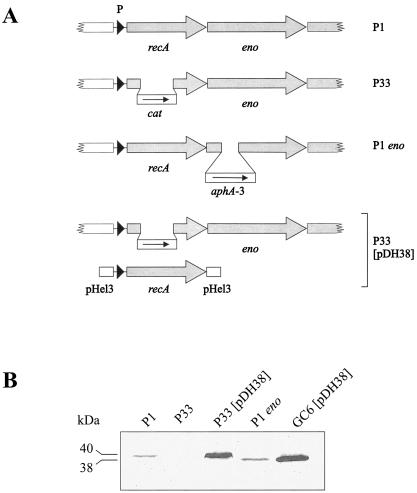
The recA gene in H. pylori encodes a protein with a calculated size of 37.6 kDa. During previously published complementation studies (36), it was noticed that H. pylori RecA migrates at an apparent size of 38 kDa when expressed from the shuttle plasmid pDH38 in E. coli but at a size of 40 kDa when the same shuttle plasmid was used in H. pylori (Fig. 1). Thus, we suspected that H. pylori RecA might be subject to a posttranslational modification. RecA is a highly conserved protein among bacterial species, and in many cases, bacterial recA genes are capable of complementing an E. coli recA phenotype (for examples, see references 12 and 23). Since the H. pylori recA gene was not able to do so (despite a protein sequence similarity of 74% and identity of 62%), we also suspected that this posttranslational modification might be necessary for the function of the H. pylori RecA protein (36).
FIG. 1.
Construction and complementation of H. pylori recA and eno mutants. (A) The isogenic recA mutant in strain P1 (P33) was constructed by replacing a central part of the recA gene by a terminatorless chloramphenicol resistance (cat) cassette, and the eno mutant was constructed by insertion of an aphA-3 gene in an internal SphI site. P33 was complemented by using the recA gene including its promoter cloned on the shuttle vector pHel3 (pDH38). (B) Total cell lysates of H. pylori strains P1, P33, P33(pDH38) and P1 eno and of E. coli strain GC6(pDH38) were applied to an SDS 6 to 12% gradient gel, blotted, and reacted with antiserum AK263. The size difference between the RecA proteins produced in P33(pDH38) and GC6(pDH38) suggests that RecA is subject to posttranslational modification in H. pylori.
Production of an unmodified RecA protein in H. pylori.
The recA gene in H. pylori is followed downstream by a gene encoding an enolase homologue (eno). The two genes are cotranscribed, and mutations in the eno gene have been shown to result in a slightly increased sensitivity of the bacteria to DNA-damaging agents, which was attributed to an effect on recA expression (44). Therefore, we constructed an enolase mutant by inserting a kanamycin resistance cassette into the eno gene of strain P1 (Fig. 1A) to detect effects on RecA production and/or size. The P1 eno strain did not produce lower amounts of RecA protein, as the wild-type P1 strain did, as estimated by immunoblotting (Fig. 1B). But the RecA protein seems to be unmodified in the P1 eno strain; its electrophoretic mobility is higher than in the wild-type and seems to be the same as in the E. coli strain GC6(pDH38) expressing H. pylori recA. Since mutations in the enolase gene have been reported to confer enhanced sensitivity to DNA-damaging agents (44), we were interested in the phenotypes of the enolase mutant. We were, however, unable to detect a difference between the wild type and the enolase mutant regarding transformation competence or sensitivity to UV radiation (data not shown). The only obvious phenotype of the enolase mutant was its growth in an elongated cell shape, which is presumably due to a cell division defect (see below).
Involvement of other genes in the recA locus.
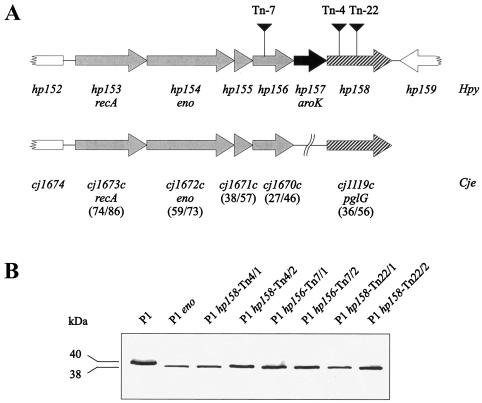
In the two published H. pylori genome sequences, the recA gene is the first gene of a putative operon containing, in addition to eno, a shikimate kinase gene homologue (aroK) and three open reading frames with unknown function (Fig. 2A). Two of these open reading frames (hp155 and hp156) have homologues in Campylobacter jejuni (cj1671c and cj1670c, respectively), which are also located downstream of the recA gene there (Fig. 2A). The third open reading frame (hp158) displays significant homology to a C. jejuni gene termed pglG, which is part of a gene locus involved in protein glycosylation in C. jejuni (42). We reasoned that in H. pylori the putative operon structure of the recA locus might reflect a functional correlation between the corresponding gene products. Therefore, we decided to examine the role of mutations in recA-eno downstream genes on the production or function of RecA. The recA-eno downstream region was amplified by PCR and cloned into the minimal vector pMin1, resulting in plasmid pWS124. Using this plasmid, the recA-eno downstream region was subjected to TnMax5 transposon mutagenesis. Transposon insertions were mapped to the reading frames hp156 and hp158 (Fig. 2A), and corresponding H. pylori P1 mutants were generated by natural transformation. Immunoblotting experiments with antiserum AK263 showed that in all of these insertion mutants the RecA protein was obviously unmodified. Since insertion of the kanamycin resistance cassette in the eno gene may have a polar effect on the expression of downstream genes such as hp158, we sought to check whether the eno gene itself is involved in modification. Therefore, we constructed a P1 mutant with a terminatorless chloramphenicol resistance cassette (11) inserted into the eno gene. This mutant produced an unmodified RecA protein as well (data not shown), suggesting that both eno and hp158 (and possibly other genes in the locus) are involved in RecA modification.
FIG. 2.
Presence of a putative operon at the recA locus in H. pylori and effect of downstream genes on RecA size. (A) Downstream of the enolase gene, there are four further genes which are putatively cotranscribed with recA and eno. Homologues to the first four genes are arranged identically in the C. jejuni recA locus. The last gene of the putative operon displays a significant homology to the C. jejuni gene pglG, whose product is involved in protein glycosylation. Numbers in parentheses are percents identity/similarity between the H. pylori and C. jejuni proteins. (B) TnMax5 transposon insertions in the genes hp156 and hp158 in strain P1 result in the production of unmodified RecA proteins.
Glycosylation as a possible modification.
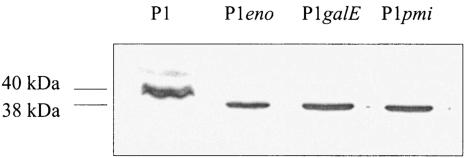
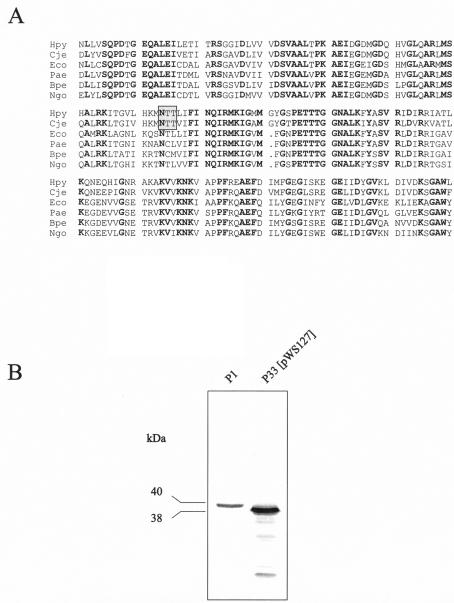
The C. jejuni general glycosylation locus consists of 10 pgl genes (pglA to pglJ) that have been shown to be involved in protein glycosylation (25, 42), but it also contains the galE gene, which is usually associated with lipopolysaccharide (LPS) biosynthesis. Although protein glycosylation is not a common feature of cytoplasmic proteins, we considered the possibility of a RecA glycosylation in H. pylori. Genes involved in early steps of LPS biosynthesis may provide the substrates for glycosylation reactions. Therefore, we constructed isogenic H. pylori P1 mutants defective in the galE gene (hp360) encoding a UDP-glucose-4-epimerase, or the pmi gene (also called rfbM or hp43) encoding a bifunctional mannose-6-phosphate isomerase/GDP-mannose pyrophosphorylase, both of which are involved in LPS biosynthesis (8). The galE gene of strain P1 was disrupted with plasmid pGH26 (24), and the pmi gene was disrupted with plasmid pDHO25::TnMax5-3 (18). Both mutations resulted in the production of a RecA protein without an apparent modification (Fig. 3), suggesting that the RecA modification may indeed be a glycosylation. A sequence comparison of RecA proteins from different organisms reveals the presence of an asparagine glycosylation site in the RecA proteins of H. pylori and C. jejuni but not in the RecA proteins of most other bacteria (Fig. 4A). Although enzymatic deglycosylation of the H. pylori RecA protein with N-glycosidase F was not successful (data not shown), we wondered whether the putative asparagine glycosylation site is important for the RecA modification. Therefore, we decided to introduce a site-specific mutation into the glycosylation site. By a PCR-based mutagenesis procedure, we replaced the codon ACT for the second threonine in the motif with an ATG codon, resulting in a threonine-to-methionine (T189M) mutation. A methionine is present at this position in the RecA protein of Bordetella pertussis and thus should be tolerated. The mutated recA gene was introduced into H. pylori P33 on the pHel3 shuttle vector (pWS127). Western blot analysis of the corresponding H. pylori strain demonstrates the overproduction of a RecA protein which indeed seems to be unmodified (Fig. 4B).
FIG. 3.
Effect of mutations in the galE and pmi genes. Total cell lysates of wild-type strain P1 and the isogenic mutants eno, galE, and pmi were separated by SDS-PAGE, immunoblotted, and reacted with antiserum AK263.
FIG. 4.
(A) Sequence comparison of RecA proteins from different bacteria. A putative N-glycosylation site (boxed) is present in the RecA proteins of H. pylori and C. jejuni but not in RecA proteins of other bacteria. Hpy, H. pylori; Cje, C. jejuni; Eco, E. coli; Pae, Pseudomonas aeruginosa; Bpe, B. pertussis; Ngo, N. gonorrhoeae. (B) Size difference between the wild-type RecA protein and the RecA protein with a site-specific mutation (T189M).
Membrane association of the H. pylori RecA protein.
One possible function of posttranslational modifications of a cytoplasmic protein might be membrane targeting. Localization of the H. pylori RecA protein has not been examined so far, but RecA has been shown to be targeted to the cytoplasmic membrane during transformation in the naturally competent bacteria Streptococcus pneumoniae (27). H. pylori cells were separated into cytoplasmic and total membrane fractions, and the RecA content of these fractions was determined by immunoblotting (Fig. 5). Considerable amounts of the protein were associated with the total membrane fractions, but RecA was also present in the cytoplasmic fractions. Membrane-bound RecA could be removed by treatment with 1 M NaCl or with 0.1 M sodium carbonate buffer (pH 11), which indicates a peripheral membrane association (data not shown). The distribution between the cytoplasm and the total membrane fraction was, however, independent of the size of the RecA protein, suggesting that the putative modification is not involved in membrane targeting. This is also supported by the RecA (T189M)-producing mutant, which displays the same distribution (data not shown). Interestingly, however, the modification seems to be removed in the cytoplasmic form of RecA during cell fractionation but not in the membrane-bound form. Since we detect only the modified form in a total cell lysate, we would conclude that an activity which removes the modification is released during fractionation which does not affect the membrane-bound form.
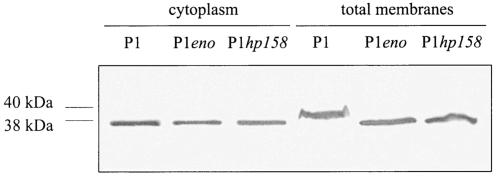
FIG. 5.
Association of H. pylori RecA with the bacterial membrane. H. pylori wild-type strain P1 and the isogenic eno and hp158 mutants were separated into total membrane fractions and cytoplasmic fractions. Immunoblotting with AK263 reveals not only a RecA association with the membranes but also a localization in the cytoplasm. Equal amounts of membrane fractions and cytoplasmic fractions were loaded in each lane.
Phenotypic effects of RecA modification.
Since the enolase mutant displayed a morphology that suggested a cell division defect and since RecA has an effect on cell division in Bacillus subtilis (37), we first examined the influence of RecA modification on bacterial cell morphology. Whereas the wild-type strain P1 grown in liquid culture exhibited a curved morphology with a certain degree of clumping (Fig. 6A), the hp156 (data not shown) and the hp158 mutants (Fig. 6B) seemed to be shorter and more spiral shaped. An influence on cell division, however, was only visible in the enolase mutant (Fig. 6C), as it displays a considerable number of elongated cells. This cell division defect becomes especially apparent after nucleoid staining with DAPI (Fig. 6D). Since only the enolase mutant but not mutants defective in hp156 or hp158 display this phenotype, it is probably independent of the RecA modification but dependent on the presence or absence of the enolase.
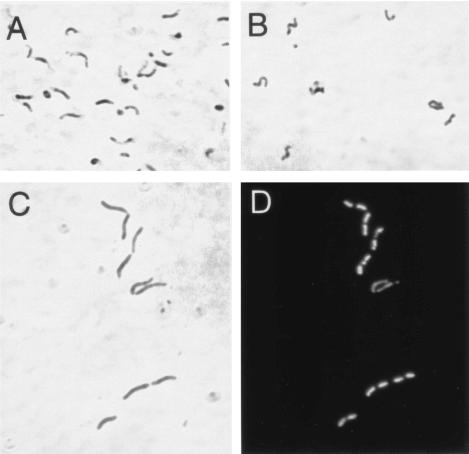
FIG. 6.
Effect of mutations in the recA locus on bacterial morphology. H. pylori wild-type strain P1 (A) and the isogenic mutants P1hp158 (B) and P1eno (C and D) were grown in liquid culture and prepared for microscopy during exponential growth. Panels A to C are phase-contrast micrographs, and panel D shows the same view as in panel C but with DAPI fluorescence.
The H. pylori RecA protein is necessary for natural transformation competence (36), for resistance against DNA damage caused by UV radiation, chemical mutagens, or antimicrobial agents such as metronidazole, and for survival at low pH (44). To determine the influence of RecA modification on these phenotypes, we performed transformation competence measurements and UV radiation, low pH, and metronidazole sensitivity assays. Surprisingly, we did not find any difference between the wild-type strain P1, the isogenic mutants in the enolase, hp156, or hp158 genes, and the RecA (T189M)-producing strain with respect to transformation competence, UV, or acid resistance (data not shown). When we compared, however, the sensitivity against metronidazole, the wild-type and mutant bacteria behaved differently (Fig. 7). In comparison to the recA mutant, both the eno and hp158 mutants displayed an increased resistance to metronidazole, but it was significantly lower than in the wild type. The metronidazole sensitivity of the recA mutant could be complemented with wild-type RecA produced from the shuttle plasmid pDH38, whereas it was only incompletely complemented with RecA (T189M) produced from plasmid pWS127. These results suggest that the modification is needed for the full function of the RecA protein in H. pylori.
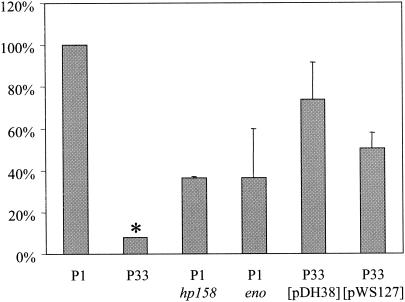
FIG. 7.
Influence of RecA modification on metronidazole sensitivity. H. pylori wild-type strain P1, the isogenic recA (P33), eno, and hp158 mutants, and P33 complemented with wild-type RecA (pDH38) or with RecA (T189M) (pWS127) were assayed for metronidazole sensitivity with an E-test. Due to variations between individual experiments, results were calculated as ratios of MICs for the mutants in comparison to those for the wild-type for each experiment. The MIC for the P1 wild-type strain was on average 0.25 μg/ml. All results are expressed as means of the results from at least three independent experiments. *, The MIC for strain P33 was 0.016 μg/ml in only one of five experiments, otherwise it was <0.016 μg/ml.
DISCUSSION
Posttranslational modification, such as glycosylation, phosphorylation, or acylation, is a common means for modulating structural and functional properties of proteins. In eucaryotic cells, for instance, most of the cell surface and secreted proteins are glycosylated. Such modifications are less abundant in bacteria, but more and more posttranslational modifications of surface-exposed proteins are being described. In particular, the glycosylation of bacterial surface proteins is now recognized as a common posttranslational modification (2, 32). For example, the pilin subunits of Neisseria meningitidis (45) and the flagellins of C. jejuni (7, 14), Helicobacter felis (20), and H. pylori (21, 35) are modified by glycosylation. We describe here a putative modification of the H. pylori RecA protein, which as a cytoplasmic protein, is more unusual and has not been described for any bacterial RecA protein so far.
The RecA protein is a ubiquitous protein which combines many functions on a relatively short polypeptide chain. In E. coli, it has different DNA-binding sites, an ATP-binding site, and coprotease activities for the LexA repressor, the UmuD protein, and various phage repressors. Accordingly, there are various mechanisms involving RecA for repairing damaged DNA. Since a LexA homolog is not present in the H. pylori genome and since an SOS response pathway also seems to be absent in H. pylori, a coprotease activity may be dispensable for the H. pylori RecA protein. However, the RecA protein of S. pneumoniae, where a lexA homologue is also lacking, is nevertheless able to cleave the E. coli LexA protein (38). In E. coli, one of the main functions of RecA in response to DNA-damaging agents is to help in repairing stalled replication forks. There are two mechanisms for this situation: error-free replication restarts catalyzed by polymerase II (PolB), probably in conjunction with the restart primosome containing (among other proteins) PriA (34), and error-prone trans-lesion synthesis catalyzed by polymerase V (UmuD′2C) (13). Both mechanisms involve RecA for strand invasion during D-loop formation or for formation of an activated nucleoprotein filament (RecA*) ahead of the stalled replication fork, respectively (13). It has been suggested that the role of RecA in these processes is not its recombinational activity but possibly only its DNA-pairing activity (5). H. pylori does not seem to possess homologues to either polB, umuC, or umuD, but there are homologues to priA, the restart primosome components dnaB and dnaG, and to the recR gene (10), whose product is thought to be involved in loading RecA to single-stranded DNA. It is not clear what the absence of polymerases II and V (and also DinB or polymerase IV) means for the importance of repairing stalled replication forks in H. pylori. However, it may be possible that the posttranslational modification of RecA is an adaptation to this different situation. The fact that the absence of the modification has only a slight influence on DNA repair is no contradiction to such a putative role: in E. coli, polB mutants also do not have any phenotype with respect to UV sensitivity, probably because polymerases II and V can complement each other, although acting at different time points after the induction of damage (30).
One of the functions of RecA-mediated recombination in H. pylori is in natural transformation, where incoming DNA has to be recombined into the chromosome. DNA transport across the bacterial membranes is accomplished by a type IV transport system (17), and it may be speculated that there is a direct contact of incoming DNA with membrane-associated RecA. In S. pneumoniae, RecA has been shown to be membrane-associated during phases of competence (27). Membrane association in this organism is mediated by the accessory protein CinA (colligrin or competence- and damage-inducible protein). The cinA gene is cotranscribed with the recA gene, and expression is induced during competent phases. Genes with homology to cinA can be found in a number of species, but in many cases, homology extends only to the 3′ region. This is also the case in H. pylori, where the hp952 gene displays a 3′ cinA homology. Since the C-terminal part of CinA is probably the RecA-binding domain, this suggests that HP952 would not be able to mediate a membrane association. The RecA protein in H. pylori is indeed membrane associated, but this association is not dependent on the posttranslational modification. Our observation that the modification is removed in the cytoplasmic form of RecA but retained in the membrane-associated form, suggests some kind of function for membrane targeting. Defined transformation-competent states or phases have not been described for H. pylori, although competence is highest during the early logarithmic phase (19). However, the modification status of RecA does not change during growth phases (data not shown), which suggests that it is not critical for competence.
The recA locus in H. pylori consists of a putative operon of seemingly unrelated genes, one of which encodes a glycolytic enzyme and another which encodes an enzyme involved in biosynthesis of aromatic amino acids. We show here that at least two of these genes are necessary for RecA modification. It is currently unclear what the contribution of the enolase as a metabolic enzyme might be. Interestingly enough, the closely related organism C. jejuni has a recA locus with a similar gene arrangement. The recA gene is also the first gene of a putative operon, followed by an enolase gene, two genes with homology to hp155 and hp156, respectively, and a putative DNA ligase gene (genes cj1673c to cj1669c). The product of the hp156 homolog cj1670c has recently been termed Campylobacter glycoprotein A (CgpA) due to its lectin-binding activity, and it has been shown to be posttranslationally modified by a glycan containing N-acetylgalactosamine residues (25). More recently, the chemical structure of the glycan was determined and shown to be present on at least 21 further C. jejuni proteins (47). Since HP156 has a signal sequence, we would predict that it is not involved in the modification process but might rather be a glycosylated surface protein in H. pylori as well.
Although we were so far unable to prove this conclusion directly, several lines of evidence suggest that H. pylori RecA is posttranslationally modified by glycosylation. (i) It contains a putative asparagine glycosylation motif in contrast to most other RecA proteins. This motif is rather uncommon in bacterial sequences. Of 114 RecA sequences (InterPro entry IPR001553), only 9 contain a putative asparagine glycosylation motif. (ii) Modification is dependent on a gene whose homologue is involved in protein glycosylation in C. jejuni. (iii) Site-specific mutagenesis of the glycosylation motif results in production of an unmodified RecA protein, which also has a functional defect. However, a direct detection and molecular characterization of the putative glycosylation remains to be established in further studies.
Acknowledgments
We are grateful to C. Förster for excellent technical assistance and to G. Poplutz for plasmid constructions.
This work was supported by the Deutsche Forschungsgemeinschaft (grant HA2697/3-3 to R.H.).
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Benz, I., and M. A. Schmidt. 2002. Never say never again: protein glycosylation in pathogenic bacteria. Mol. Microbiol. 45:267-276. [DOI] [PubMed] [Google Scholar]
- 3.Björkholm, B., M. Sjölund, P. G. Falk, O. G. Berg, L. Engstrand, and D. I. Andersson. 2001. Mutation frequency and biological cost of antibiotic resistance in Helicobacter pylori. Proc. Natl. Acad. Sci. USA 98:14607-14612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer, H. W., and D. Roulland-Dussoix. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41:459-472. [DOI] [PubMed] [Google Scholar]
- 5.Courcelle, J., A. K. Ganesan, and P. C. Hanawalt. 2001. Therefore, what are recombination proteins there for? Bioessays 23:463-470. [DOI] [PubMed] [Google Scholar]
- 6.Dehio, C., and M. Meyer. 1997. Maintenance of broad-host-range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal plasmid transfer from Escherichia coli. J. Bacteriol. 179:538-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doig, P., N. Kinsella, P. Guerry, and T. J. Trust. 1996. Characterization of a post-translational modification of Campylobacter flagellin: identification of a sero-specific glycosyl moiety. Mol. Microbiol. 19:379-387. [DOI] [PubMed] [Google Scholar]
- 8.Edwards, N. J., M. A. Monteiro, G. Faller, E. J. Walsh, A. P. Moran, I. S. Roberts, and N. J. High. 2000. Lewis X structures in the O antigen side-chain promote adhesion of Helicobacter pylori to the gastric epithelium. Mol. Microbiol. 35:1530-1539. [DOI] [PubMed] [Google Scholar]
- 9.Eggleston, A. K., and S. C. West. 1996. Exchanging partners: recombination in E. coli. Trends Genet. 12:20-26. [DOI] [PubMed] [Google Scholar]
- 10.Fischer, W., D. Hofreuter, and R. Haas. 2001. Natural transformation, recombination and repair, p. 249-257. In H. L. Mobley, G. L. Mendz, and S. L. Hazell (ed.), Helicobacter pylori: physiology and genetics. ASM Press, Washington, D.C. [PubMed]
- 11.Fischer, W., J. Püls, R. Buhrdorf, B. Gebert, S. Odenbreit, and R. Haas. 2001. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol. Microbiol. 42:1337-1348. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg, I., and J. J. Mekalanos. 1986. Cloning of the Vibrio cholerae recA gene and construction of a Vibrio cholerae recA mutant. J. Bacteriol. 165:715-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman, M. F. 2000. Coping with replication ‘train wrecks’ in Escherichia coli using Pol V, Pol II and RecA proteins. Trends Biochem. Sci. 25:189-195. [DOI] [PubMed] [Google Scholar]
- 14.Guerry, P., P. Doig, R. A. Alm, D. H. Burr, N. Kinsella, and T. J. Trust. 1996. Identification and characterization of genes required for post-translational modification of Campylobacter coli VC167 flagellin. Mol. Microbiol. 19:369-378. [DOI] [PubMed] [Google Scholar]
- 15.Haas, R., T. F. Meyer, and J. P. M. van Putten. 1993. Aflagellated mutants of Helicobacter pylori generated by genetic transformation of naturally competent strains using transposon shuttle mutagenesis. Mol. Microbiol. 8:753-760. [DOI] [PubMed] [Google Scholar]
- 16.Heuermann, D., and R. Haas. 1998. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 257:519-528. [DOI] [PubMed] [Google Scholar]
- 17.Hofreuter, D., S. Odenbreit, and R. Haas. 2001. Natural transformation competence in Helicobacter pylori is mediated by the basic components of a type IV secretion system. Mol. Microbiol. 41:379-391. [DOI] [PubMed] [Google Scholar]
- 18.Hofreuter, D., S. Odenbreit, G. Henke, and R. Haas. 1998. Natural competence for DNA transformation in Helicobacter pylori: identification and genetic characterization of the comB locus. Mol. Microbiol. 28:1027-1038. [DOI] [PubMed] [Google Scholar]
- 19.Israel, D. A., A. S. Lou, and M. J. Blaser. 2000. Characteristics of Helicobacter pylori natural transformation. FEMS Microbiol. Lett. 186:275-280. [DOI] [PubMed] [Google Scholar]
- 20.Josenhans, C., R. L. Ferrero, A. Labigne, and S. Suerbaum. 1999. Cloning and allelic exchange mutagenesis of two flagellin genes of Helicobacter felis. Mol. Microbiol. 33:350-362. [DOI] [PubMed] [Google Scholar]
- 21.Josenhans, C., L. Vossebein, S. Friedrich, and S. Suerbaum. 2002. The neuA/flmD gene cluster of Helicobacter pylori is involved in flagellar biosynthesis and flagellin glycosylation. FEMS Microbiol. Lett. 210:165-172. [DOI] [PubMed] [Google Scholar]
- 22.Kahrs, A. F., S. Odenbreit, W. Schmitt, D. Heuermann, T. F. Meyer, and R. Haas. 1995. An improved TnMax mini-transposon system suitable for sequencing, shuttle mutagenesis and gene fusions. Gene 167:53-57. [DOI] [PubMed] [Google Scholar]
- 23.Keener, S. L., K. P. McNamee, and K. McEntee. 1984. Cloning and characterization of recA genes from Proteus vulgaris, Erwinia carotovora, Shigella flexneri, and Escherichia coli B/r. J. Bacteriol. 160:153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon, D. H., J. S. Woo, C. L. Perng, M. F. Go, D. Y. Graham, and F. A. El-Zaatari. 1998. The effect of galE gene inactivation on lipopolysaccharide profile of Helicobacter pylori. Curr. Microbiol. 37:144-148. [DOI] [PubMed] [Google Scholar]
- 25.Linton, D., E. Allan, A. V. Karlyshev, A. D. Cronshaw, and B. W. Wren. 2002. Identification of N-acetylgalactosamine-containing glycoproteins PEB3 and CgpA in Campylobacter jejuni. Mol. Microbiol. 43:497-508. [DOI] [PubMed] [Google Scholar]
- 26.Marsh, J. L., M. Erfle, and E. J. Wykes. 1984. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene 32:481-485. [DOI] [PubMed] [Google Scholar]
- 27.Masure, H. R., B. J. Pearce, H. Shio, and B. Spellerberg. 1998. Membrane targeting of RecA during genetic transformation. Mol. Microbiol. 27:845-852. [DOI] [PubMed] [Google Scholar]
- 28.Matic, I., C. Rayssiguier, and M. Radman. 1995. Interspecies gene exchange in bacteria: the role of SOS and mismatch repair systems in evolution of species. Cell 80:507-515. [DOI] [PubMed] [Google Scholar]
- 29.Peek, R. M. J., and M. J. Blaser. 2002. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2:28-37. [DOI] [PubMed] [Google Scholar]
- 30.Pham, P., S. Rangarajan, R. Woodgate, and M. F. Goodman. 2001. Roles of DNA polymerases V and II in SOS-induced error-prone and error-free repair in Escherichia coli. Proc. Natl. Acad. Sci. USA 98:8350-8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pohlner, J., J. Krämer, and T. F. Meyer. 1993. A plasmid system for high-level expression and in-vitro processing of recombinant proteins. Gene 130:121-126. [DOI] [PubMed] [Google Scholar]
- 32.Power, P. M., and M. P. Jennings. 2003. The genetics of glycosylation in Gram-negative bacteria. FEMS Microbiol. Lett. 218:211-222. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Sandler, S. J., and K. J. Marians. 2000. Role of PriA in replication fork reactivation in Escherichia coli. J. Bacteriol. 182:9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schirm, M., E. C. Soo, A. J. Aubry, J. Austin, P. Thibault, and S. M. Logan. 2003. Structural, genetic and functional characterization of the flagellin glycosylation process in Helicobacter pylori. Mol. Microbiol. 48:1579-1592. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt, W., S. Odenbreit, D. Heuermann, and R. Haas. 1995. Cloning of the Helicobacter pylori recA gene and functional characterization of its product. Mol. Gen. Genet. 248:563-572. [DOI] [PubMed] [Google Scholar]
- 37.Sciochetti, S. A., G. W. Blakely, and P. J. Piggot. 2001. Growth phase variation in cell and nucleoid morphology in a Bacillus subtilis recA mutant. J. Bacteriol. 183:2963-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steffen, S. E., and F. R. Bryant. 2000. Purification and characterization of the RecA protein from Streptococcus pneumoniae. Arch. Biochem. Biophys. 382:303-309. [DOI] [PubMed] [Google Scholar]
- 39.Strebel, K., E. Beck, K. Strohmaier, and H. Schaller. 1986. Characterization of foot-and-mouth disease virus gene products with antisera against bacterially synthesized fusion proteins. J. Virol. 57:983-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suerbaum, S., and P. Michetti. 2002. Helicobacter pylori infection. N. Engl. J. Med. 347:1175-1186. [DOI] [PubMed] [Google Scholar]
- 41.Suerbaum, S, J. M. Smith, K. Bapumia, G. Morelli, N. H. Smith, E. Kunstmann, I. Dyrek, and M. Achtman. 1998. Free recombination within Helicobacter pylori. Proc. Natl. Acad. Sci. USA 95:12619-12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szymanski, C. M., R. Yao, C. P. Ewing, T. J. Trust, and P. Guerry. 1999. Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol. Microbiol. 32:1022-1030. [DOI] [PubMed] [Google Scholar]
- 43.Taha, M. K., M. So, H. S. Seifert, E. Billyard, and C. Marchal. 1988. Pilin expression in Neisseria gonorrhoeae is under both positive and negative transcriptional control. EMBO J. 7:4367-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson, S., and M. J. Blaser. 1995. Isolation of the Helicobacter pylori recA gene and involvement of the recA region in resistance to low pH. Infect. Immun. 63:2185-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Virji, M., J. R. Saunders, G. Sims, K. Makepeace, D. Maskell, and D. J. P. Ferguson. 1993. Pilus-facilitated adherence of Neisseria meningitidis to human epithelial and endothelial cells: modulation of adherence phenotype occurs concurrently with changes in primary amino acid sequence and the glycosylation status of pilin. Mol. Microbiol. 10:1013-1028. [DOI] [PubMed] [Google Scholar]
- 46.Wessel, D., and U. I. Flügge. 1984. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138:141-143. [DOI] [PubMed] [Google Scholar]
- 47.Young, N. M., J. R. Brisson, J. Kelly, D. C. Watson, L. Tessier, P. H. Lanthier, H. C. Jarrell, N. Cadotte, F. St. Michael, E. Aberg, and C. M. Szymanski. 2002. Structure of the N-linked glycan present on multiple glycoproteins in the Gram-negative bacterium, Campylobacter jejuni. J. Biol. Chem. 277:42530-42539. [DOI] [PubMed] [Google Scholar]