Abstract
Nitric oxide (NO) regulates proliferation, differentiation and survival of neurons. Although NO is reported to involve in NGF-induced differentiation of PC12 cells, the role of NO has not been characterized in primary neuron cells. Therefore, we investigated the role of NO in neuronal differentiation of primary cortical neuron cells. Primary cortical neuron cells were prepared from rat embryos of embryonic day 18 and treated with NMMA (NOS inhibitor) or PTIO (NO scavenger). Neurite outgrowth of neuron cells was counted and the mRNA levels of p21, p27, c-jun and c-myc were measured by RT-PCR. Neurite outgrowth of primary cortical neuron cells was inhibited a little by NOS inhibitor and completely by NO scavenger. The mRNA levels of p21 and p27, differentiation-induced growth arrest genes were increased during differentiation, but they were decreased by NOS inhibitor or NO scavenger. On the other hand, the level of c-jun mRNA was not changed and the level of c-myc mRNA was increased during differentiation differently from previously reported. The levels of these mRNA were reversed in NOS inhibitor- or NO scavenger-treated cells. The level of nNOS protein was not changed but NOS activity was inhibited largely by NOS inhibitor or NO scavenger. These results suggest that NO is an essential mediator for neuronal differentiation of primary cortical neuron cells.
Keywords: primary cortical neuron cells, neurite outgrowth, nitric oxide, nitric oxide synthase, butyrate
INTRODUCTION
Nitric oxide (NO), a diffusible and unstable gas, regulates proliferation, survival and differentiation of neurons. NO is also known to involve in synaptic activity, neural plasticity, memory function, neuroinflammation and neurodegeneration (Calabrese et al., 2009). NO is synthesized from citrulline and L-arginine by nitric oxide synthases (NOS) (Mayer and Hemmens, 1997). Three forms of NOS have been reported. A neuronal type (nNOS) was discovered originally in neurons, an endothelial type (eNOS) was expressed mainly in endothelial cells, and an inducible form (iNOS) was purified from rat and porcine cerebellum (Orlando et al., 2008).
It has been reported that cytostatic effect of NGF is mediated by NO during differentiation of PC12 cells (Peunova and Enikolopov, 1995). In addition, neuronal NO synthase and nitric oxide are required for NGF-induced differentiation of PC12 cells (Phung et al., 1999). But little is known about effect of NO during differentiation of primary neuron cells. Therefore, we examined the effect of NO on differentiation of rat primary cortical neuron cells by using NMMA, a competitive inhibitor of all three isoforms of NOS, and PTIO, a scavenger of NO. Here, we showed that neurite outgrowth of primary cortical neuron cells was inhibited via regulation of differentiation-related genes by depletion of NO.
MATERIALS AND METHODS
Cell culture
Primary cortical neuron cells were established by a modification of previously described procedure (Kim et al., 2007). The cortices from rat embryos of embryonic day 18 were dissected and freed of meninges. The cells were dissociated by trypsin, followed by trituration. The cortical cells were plated onto poly-D-lysine-coated 35 mm culture dishes containing DMEM with 5% fetal bovine serum and 5% horse serum at 5% CO2 and 37℃. After 48 h, cytosine arabinoside (10µM) was added to the media to inhibit the growth of non-neuronal cells. The cortical cells were cultured up to 9 days.
Measurement of neurite outgrowth
Primary cortical neuron cells were seeded in poly-D-lysine-coated 35 mm dishes at a density of 1×106 cells/dish and incubated in complete medium for 12 h. And then neuron cells were treated with or without 6 mM NMMA or 1 mM PTIO for 5 days. Neurite formation was measured by marking at 3 sites of each dish and counting a minimum of 100 cells under the microscope.
RNA extraction, Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from cells using RNAzol B as described in manufacturer' instruction (TEL-TEST, INC., Friendswood, Texas, USA) and used directly for the first-strand cDNA synthesis. The primers used for PCR are as follows: 5'-AGT-ATG-CCG-TCG-TCT-GTT-CG-3' (p21 sense), 5'-GAG-TGC-AAG-ACA-GCG-ACA-AG-3' (p21 anti-sense): 5'-GAG-GGC-AGA-TAC-GAA-TGG-CAG-3' (p27 sense), 5'-CTG-GAC-ACT-GCT-CCG-CTA-ACC-3' (p27 anti-sense): 5'-CTG-CAA-CAT-CCC-CAA-TGA-CC-3' (c-fos sense), 5'-AGG-TCC-ACA-TCT-GGC-ACA-GA-3' (c-fos anti-sense): 5'-GCT-TCT-CTA-GTG-CTC-CGT-AA-3' (c-jun sense), 5'-TCT-AGG-AGT-CGT-CAG-AAT-CC-3' (c-jun anti-sense): 5'-AAC-TTA-CAA-TCT-GCG-AGC-CA-3' (c-myc sense), 5'-AGC-AGC-TCG-AAT-TTC-TTC-CAG-ATA-T-3' (c-myc anti-sense). PCR was performed as follows: 95℃ for 8 min, followed by 40 cycles of 95℃ for 1 min, annealing temperature [GAPDH (61℃), p21 (52℃), p27 (56℃), c-fos (54℃), c-jun (52℃), c-myc (60℃)] for 1 min, 72℃ for 2 min. After the last cycle, the final elongation was performed at 72℃ for 10 min. PCR product was analyzed by gel electrophoresis. The sizes of PCR products for the various genes are 495 bp (GAPDH), 311 bp (p21), 238 bp (p27), 272 bp (c-fos), 775 bp (c-jun), 342 bp (c-myc), respectively.
Immunohistochemical staining
Cells were fixed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and incubated at room temperature for 25 min. Cells were incubated with anti-nNOS antibody at 4℃ overnight. Secondary antibody (biotin-conjugated anti-mouse IgG rabbit serum) was added and incubated for 2 h. And then horseradish peroxidase-conjugated Steptavidin was added and incubated for 2 h. After washing with 0.1 M phosphate buffer (pH 7.4), 0.05% diaminobenzidine solution was added and incubated for 1 min. Brown color-stained cells were observed and photographed at 400 times magnification under phase contrast and bright-field microscope.
Determination of NOS activity
Cells were homogenized in 20 mM HEPES buffer (pH 7.2) containing 0.32 M sucrose, 0.5 mM EDTA, 1 mM DTT, 1 mM PMSF, 10 mg/l leupeptin, 10 mg/l aprotinin. For the [3H-arginine-citrulline] conversion assay, 1µCi of [3H-arginine] was incubated with 20µg of cell extracts in 50 mM HEPES buffer (pH 7.5) containing 0.45 mM CaCl2, 2 mM NADPH, 500µM arginine, 10µg/ml calmodulin, at 37℃ for 30 min as described previously (Bredt and Snyder, 1990; Peunova and Enikolopov, 1995). Assays were terminated by addition of 2 ml of ice cold 20 mM HEPES buffer (pH 5.5) containing 2 mM EDTA and reaction mixtures were applied to 1 ml columns of Dowex AG 50WX-8 (Na+ form), which were pre-eluted with 2 ml of distilled water. Citrulline was separated from arginine on Dowex AG 50 columns and the radioactivity in the flow-through (citrulline-cotaining fraction) was determined. Control determinations of the enzyme activity were done in the presence of 20µl of heat-treated extract, or in the absence of NADPH. [3H]-citrulline in 4 ml flow-through was quantified by using the scintillation counter.
RESULTS
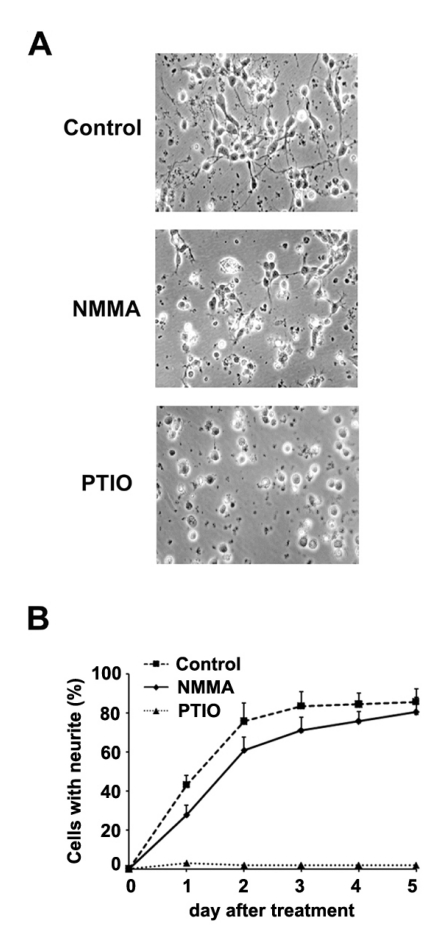
Neurite outgrowth of rat primary cortical neuron cells was inhibited slightly by NMMA (NOS inhibitor) and completely by PTIO (NO scavenger)
To investigate the effect of NO on neurite outgrowth of rat primary cortical neuron cells, NMMA, a NOS inhibitor or PTIO, a NO scavenger was treated and the neurite outgrowth was measured. Maximum concentration of NMMA or PTIO that does not result in cell death of rat primary cortical neuron cells was determined and used for treatment. In untreated neuron cells, neurite outgrowth was formed in about 80% of neuron cells within 2 days and in all neuron cells in 5 days. There is no change in neurite outgrowth of neuron cells treated with 1 mM or 3 mM NMMA (data not shown). Slight reduction was observed in 6 mM NMMA treatment. However, the neurite outgrowth of 6 mM NMMA-treated cells was also completed in 5 days as in untreated cells. On the other hand, neurite outgrowth of primary cortical neuron cells was inhibited completely by treatment of 1 mM PTIO (Fig. 1). These results suggest that NO is involved in differentiation of primary cortical neuron cells.
Fig. 1.
The effect of NO scavenger and NOS inhibitor on neurite outgrowth in rat primary cortical neuron cells. (A) Phase-contrast photographs of untreated or 6 mM NMMA (inhibitor of three NOS isoforms) or 1 mM PTIO (NOS scavenger) treated primary cortical neuron cells for 1 day. (B) The number of neurite of primary cortical neuron cells were counted under the microscope for 5 days.
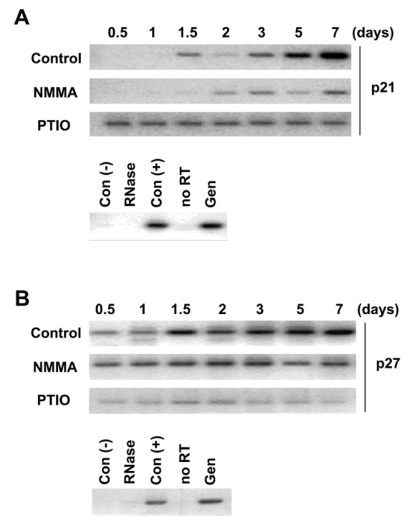
The levels of p21 and p27 mRNAs were increased during neurite outgrowth but their increases were inhibited by treatment of NMMA or PTIO
Because p21 and p27 were known to be involved in various differentiation-related growth arrests, the levels of p21 and p27 mRNA were measured by RT-PCR during neurite outgrowth of rat primary cortical neuron cells. The level of p21 mRNA was gradually increased from 1.5 to 7 days during neuronal differentiation (Fig. 2A). The level of p21 mRNA was gradually increased from 2 to 7 days in 6 mM NMMA-treated cells but the level was reduced considerably when compared to untreated cells. The level of p21 mRNA maintained without change from 0.5 to 7 days in 1 mM PTIO-treated cells. On the other hand, the level of p27 mRNA was gradually increased from 0.5 to 7 days as seen in p21 (Fig. 2B) during neuronal differentiation. But the level of p27 mRNA was not changed in 6 mM NMMA or 1 mM PTIO-treated cells. The level of p27 mRNA in 1 mM PTIO-treated cells was interestingly much lower than untreated cells. These results show that the levels of p27 and p21 mRNAs were increased during neurite outgrowth in primary cortical neuron cells. Differentition-induced change in their mRNA levels was inhibited by NMMA and PTIO.
Fig. 2.
The effect of NO scavenger and NOS inhibitor on expression levels of p21 and p27 mRNAs in rat primary cortical neuron cells. The primary cortical neuron cells were treated with 6 mM NMMA or 1 mM PTIO as indicated. Cells were harvested at 0.5, 1, 1.5, 2, 3, 5 and 7 days and total RNA was extracted. (A) mRNA expression of p21 was measured by RT-PCR. The PCR product (311 bp) was resolved in 1.5% agarose gel. (B) mRNA expression of p27 was measured by RT-PCR. The PCR product (238 bp) was resolved in 1.5% agarose gel. Con (-): negative control, RNase: RNase treatment, Con (+): positive control, no RT: PCR reaction without reverse transcriptase, Gen: genomic DNA 500 ng.
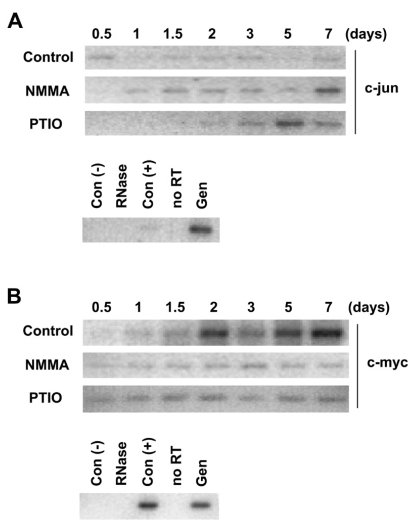
The effects of NO on levels of c-jun and c-myc mRNAs of rat primary cortical neuron cells
c-myc and c-jun are well known as immediate early genes that are activated in early developmental period. To investigate the expression of c-jun and c-myc genes during neurite outgrowth of rat primary cortical neuron cells, the levels of c-jun and c-myc mRNAs were measured by RT-PCR. The level of c-jun mRNA was not changed during differentiation in neuron cells (Fig. 3A). But it was gradually increased in 6 mM NMMA treated-cells or in the 1 mM PTIO treated-cells. On the other hand, the level of c-myc mRNA was gradually increased during differentiation in cortical neuron cells, but was not changed in 6 mM NMMA or 1 mM PTIO treated-cells (Fig. 3B).
Fig. 3.
The effect of NO scavenger and NOS inhibitor on expression levels of c-jun and c-myc mRNAs in rat primary cortical neuron cells. The primary cortical neuron cells were treated with 6 mM NMMA or 1 mM PTIO as indicated. Cells were harvested at 0.5, 1, 1.5, 2, 3, 5 and 7 days and total RNA was extracted. (A) mRNA expression of c-jun was measured by RT-PCR. The PCR product (775 bp) was resolved in 1.5% agarose gel. (B) mRNA expression of c-myc was measured by RT-PCR and PCR product (342 bp) was resolved in 1.5% agarose gel. Con (-): negative control, RNase: RNase treatment, Con (+): positive control, no RT: PCR reaction without reverse transcriptase, Gen: genomic DNA 500 ng.
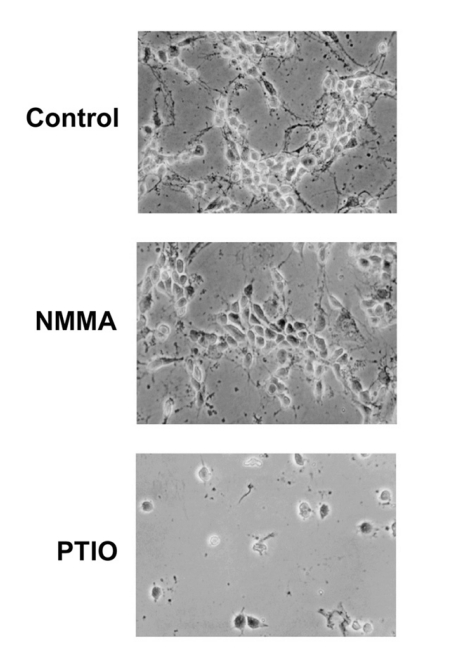
The expression level of neuronal NOS (nNOS) protein is not apparently related with neuronal differentiation of rat primary cortical neuron cells
Because nNOS is expressed in neurons and is possibly related with neurite outgrowth, we analyzed the level of nNOS protein by immunostaining using anti-nNOS antibody. The level of nNOS protein was not changed considerably in NMMA- or PTIO-treated cells (Fig. 4). These results show that the expression level of nNOS protein is not apparently related with neuronal differentiation of the primary cortical neuron cells.
Fig. 4.
Immunochemical staining analysis of neuronal NOS (nNOS). The primary cortical neuron cells were treated with 6 mM NMMA or 1 mM PTIO as indicated. Cells were fixed by 4% paraformaldehyde and incubated with anit-nNOS monoclonal antibody as describes in materials and methods.
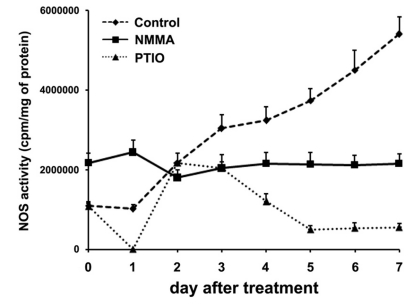
NOS activity was increased during differentiation of primary cortical neuron cells and inhibited by NMMA or PTIO
Because NO is involved in differentiation of primary cortical neuron cells (Fig. 1), we measured total NOS activity during differentiation. NOS activity was increased during differentiation of untreated primary cortical neuron cells. However, NOS activity was inhibited in NMMA-treated cells and largely inhibited in PTIO-treated cells (Fig. 5). These results show that total NOS activity was increased during differentiation of primary cortical neuron cells but was inhibited by NMMA or PTIO as expected.
Fig. 5.
Measurement of NOS activity in rat primary cortical neuron cells. The primary cortical neuron cells were harvested and prepared cell extracts. NOS activity was measured as described in materials and methods.
DISCUSSION
Several reports suggested that NGF-induced neurite outgrowth of PC12 cells were inhibited by NO scavengers and NOS inhibitors. The chronic inhibition of NOS increases cell proliferation in dentate gyrus (Park et al., 2003) and nNOS-derived NO suppresses neurogenesis in adult dentate gyrus (Zhu et al., 2006). However, since PC12 cells were not neuronal origin, the role of NO in neuronal differentiation should be investigated in primary neuronal cells. We demonstrated that NO scavenger and NOS inhibitor inhibit neurite outgrowth in rat primary cortical neuron cells (Fig. 1). NO turned also out to be an important mediator of neuronal differentiation in primary cortical neuron cells as well as PC12 cells.
Since growth arrest is a prerequisite of neuronal differentiation, expression levels of growth-related genes were tested during differentiation of primary cortical neuron cells. Several reports demonstrated that p21 expression was involved in neurite outgrowth. NGF increases p21 and cyclin D1 for growth arrest during NGF-induced neurite outgrowth of PC12 cells (Yan and Ziff, 1995; Yan and Ziff, 1997). It was also reported that NGF induces NOS and the resulting synthesized-NO acts as a second messenger. NGF-induced NO increases p21 during NGF-induced neuronal differentiation of PC12 cells. By using p21 inducible system, induction of p21 restored neurite extension (Poluha et al., 1997). On the other hand, cell cycle arrest and differentiation were induced in neuroblastoma cells by retinoic acid (RA). Two sublines of SK-N-SH, SK-N and SH-F displayed strikingly different responses to RA. SK-N was induced to neuronal differentiation by RA but SH-F was transformed from the small neuroblastic cells into large, flattened, epithelium-like cells by RA (Wainwright et al., 2001). RA induces high expression of cyclin D1 and accumulation of p21 in SH-N cells. But in SH-F, RA induces elevation of p18, down-regulation of cyclin D1, and swift inhibition of cyclin D-dependent kinases (cdks). Overexpression of p21 also induces neural differentiation in RA untreated neuroblastoma cells (Wainwright et al., 2001). During differentiation of rat primary cortical neuron cells in our work, p21 mRNA was also increased (Fig. 2A). The level of p21 mRNA was decreased in NMMA-treated cells but unchanged in PTIO-treated cells as expected.
p27 is involved in cell cycle arrest and differentiation in neuroblastoma cells (Perez-Juste and Aranda, 1999) and embryonal carcinoma cells (Baldassarre et al., 2000). p27 is accumulated in proliferating neuroblastoma N2A cells, and is degraded for triggering differentiation and re-synthesis of active p27 occurs between 24 and 48 h after treatment of cAMP (Munoz et al., 2003). During differentiation of primary cortical neuron cells, the level of p27mRNA was increased in our work (Fig. 2B). The level of p27 mRNA was not changed during differentiation and was much lower in NMMA- or PTIO-treated cells when compared to untreated cells.
Early inducible genes such as c-fos, jun-B, zif/ 268, and c-jun, were induced transiently by stimulation of glutamate receptors in primary cultures of rat cerebellar neurons (Szekely et al., 1990). Immediately early genes were also expressed in differentiated neurons by cyclic AMP (Vaccarino et al., 1993). c-jun expression and phosphorylation were induced in various conditions (Raivich and Behrens, 2006) including neuronal differentiation and survival in PC12 cells (Dragunow et al., 2000) and neuronal and non-neuronal cell death (Dragunow and Preston, 1995). However, the level of c-jun mRNA was not changed during differentiation of primary cortical neurons in our work (Fig. 3A), indicating that c-jun may not be associated with neuronal differentiation of primary cortical neuron cells. On the other hand, the level of c-myc mRNA was gradually increased in untreated cells but was not changed in NMMA- or PTIO-treated cells (Fig. 3B). Since high expression of c-myc was known to be related with prevention of differentiation (Henriksson and Luscher, 1996), c-myc may have a specific role during neuronal differentiation of rat primary cortical neurons, although we do not have further evidence.
NO produced by nNOS reduced brain neurogenesis as antiproliferative molecule (Matarredona et al., 2004; Moreno-Lopez et al., 2004). However, NO produced by iNOS and eNOS stimulate brain neurogenesis (Zhang et al., 2001; Zhu et al., 2003; Reif et al., 2004). The level of nNOS protein was not changed in NMMA- or PTIO-treated cells in our work (Fig. 4). iNOS and eNOS but not nNOS seem to be involved in stimulation of neuronal differentiation. Total NOS activity was increased during differentiation of primary cortical neuron cells and was inhibited in NMMA- or PTIO-treated cells (Fig. 5), indicating that NOS activity is required for neuronal differentiation in primary cortical neuron cells. The level of NO and NOS activity appear to play key roles in mediating neuronal differentiation of primary cortical neuron cells. Our result also suggests that NO and NOS activity appear to play key roles in mediating neuronal differentiation of brain cortical neuron.
ACKNOWLEDGEMENTS
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0094074).
References
- 1.Baldassarre G, Boccia A, Bruni P, Sandomenico C, Barone MV, Pepe S, Angrisano T, Belletti B, Motti ML, Fusco A, Viglietto G. Retinoic acid induces neuronal differentiation of embryonal carcinoma cells by reducing proteasome-dependent proteolysis of the cyclin-dependent inhibitor p27. Cell Growth Differ. 2000;11:517–526. [PubMed] [Google Scholar]
- 2.Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calabrese V, Cornelius C, Rizzarelli E, Owen JB, Dinkova-Kostova AT, Butterfield DA. Nitric oxide in cell survival: a janus molecule. Antioxid Redox Signal. 2009;11:2717–2739. doi: 10.1089/ars.2009.2721. [DOI] [PubMed] [Google Scholar]
- 4.Dragunow M, Preston K. The role of inducible transcription factors in apoptotic nerve cell death. Brain Res Brain Res Rev. 1995;21:1–28. doi: 10.1016/0165-0173(95)00003-l. [DOI] [PubMed] [Google Scholar]
- 5.Dragunow M, Xu R, Walton M, Woodgate A, Lawlor P, MacGibbon GA, Young D, Gibbons H, Lipski J, Muravlev A, Pearson A, During M. c-Jun promotes neurite outgrowth and survival in PC12 cells. Brain Res Mol Brain Res. 2000;83:20–33. doi: 10.1016/s0169-328x(00)00191-1. [DOI] [PubMed] [Google Scholar]
- 6.Henriksson M, Luscher B. Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv Cancer Res. 1996;68:109–182. doi: 10.1016/s0065-230x(08)60353-x. [DOI] [PubMed] [Google Scholar]
- 7.Kim MJ, Oh SJ, Park SH, Kang HJ, Won MH, Kang TC, Park JB, Kim JI, Kim J, Lee JY. Neuronal loss in primary long-term cortical culture involves neurodegeneration-like cell death via calpain and p35 processing, but not developmental apoptosis or aging. Exp Mol Med. 2007;39:14–26. doi: 10.1038/emm.2007.3. [DOI] [PubMed] [Google Scholar]
- 8.Matarredona ER, Murillo-Carretero M, Moreno-Lopez B, Estrada C. Nitric oxide synthesis inhibition increases proliferation of neural precursors isolated from the postnatal mouse subventricular zone. Brain Res. 2004;995:274–284. doi: 10.1016/j.brainres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Mayer B, Hemmens B. Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem Sci. 1997;22:477–481. doi: 10.1016/s0968-0004(97)01147-x. [DOI] [PubMed] [Google Scholar]
- 10.Moreno-Lopez B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J Neurosci. 2004;24:85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munoz JP, Sanchez JR, Maccioni RB. Regulation of p27 in the process of neuroblastoma N2A differentiation. J Cell Biochem. 2003;89:539–549. doi: 10.1002/jcb.10525. [DOI] [PubMed] [Google Scholar]
- 12.Orlando GF, Wolf G, Engelmann M. Role of neuronal nitric oxide synthase in the regulation of the neuroendocrine stress response in rodents: insights from mutant mice. Amino Acids. 2008;35:17–27. doi: 10.1007/s00726-007-0630-0. [DOI] [PubMed] [Google Scholar]
- 13.Park C, Sohn Y, Shin KS, Kim J, Ahn H, Huh Y. The chronic inhibition of nitric oxide synthase enhances cell proliferation in the adult rat hippocampus. Neurosci Lett. 2003;339:9–12. doi: 10.1016/s0304-3940(02)01422-2. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Juste G, Aranda A. The cyclin-dependent kinase inhibitor p27(Kip1) is involved in thyroid hormone-mediated neuronal differentiation. J Biol Chem. 1999;274:5026–5031. doi: 10.1074/jbc.274.8.5026. [DOI] [PubMed] [Google Scholar]
- 15.Peunova N, Enikolopov G. Nitric oxide triggers a switch to growth arrest during differentiation of neuronal cells. Nature. 1995;375:68–73. doi: 10.1038/375068a0. [DOI] [PubMed] [Google Scholar]
- 16.Phung YT, Bekker JM, Hallmark OG, Black SM. Both neuronal NO synthase and nitric oxide are required for PC12 cell differentiation: a cGMP independent pathway. Brain Res Mol Brain Res. 1999;64:165–178. doi: 10.1016/s0169-328x(98)00315-5. [DOI] [PubMed] [Google Scholar]
- 17.Poluha W, Schonhoff CM, Harrington KS, Lachyankar MB, Crosbie NE, Bulseco DA, Ross AH. A novel, nerve growth factor-activated pathway involving nitric oxide, p53, and p21WAF1 regulates neuronal differentiation of PC12 cells. J Biol Chem. 1997;272:24002–24007. doi: 10.1074/jbc.272.38.24002. [DOI] [PubMed] [Google Scholar]
- 18.Raivich G, Behrens A. Role of the AP-1 transcription factor c-Jun in developing, adult and injured brain. Prog Neurobiol. 2006;78:347–363. doi: 10.1016/j.pneurobio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Reif A, Schmitt A, Fritzen S, Chourbaji S, Bartsch C, Urani A, Wycislo M, Mossner R, Sommer C, Gass P, Lesch KP. Differential effect of endothelial nitric oxide synthase (NOS-III) on the regulation of adult neurogenesis and behaviour. Eur J Neurosci. 2004;20:885–895. doi: 10.1111/j.1460-9568.2004.03559.x. [DOI] [PubMed] [Google Scholar]
- 20.Sulz L, Bacigalupo J. Role of nitric oxide during neurogenesis in the olfactory epithelium. Biol Res. 2006;39:589–599. doi: 10.4067/s0716-97602006000500002. [DOI] [PubMed] [Google Scholar]
- 21.Szekely AM, Costa E, Grayson DR. Transcriptional program coordination by N-methyl-D-aspartate-sensitive glutamate receptor stimulation in primary cultures of cerebellar neurons. Mol Pharmacol. 1990;38:624–633. [PubMed] [Google Scholar]
- 22.Vaccarino FM, Hayward MD, Le HN, Hartigan DJ, Duman RS, Nestler EJ. Induction of immediate early genes by cyclic AMP in primary cultures of neurons from rat cerebral cortex. Brain Res Mol Brain Res. 1993;19:76–82. doi: 10.1016/0169-328x(93)90151-e. [DOI] [PubMed] [Google Scholar]
- 23.Wainwright LJ, Lasorella A, Iavarone A. Distinct mechanisms of cell cycle arrest control the decision between differentiation and senescence in human neuroblastoma cells. Proc Natl Acad Sci U S A. 2001;98:9396–9400. doi: 10.1073/pnas.161288698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan GZ, Ziff EB. Nerve growth factor induces transcription of the p21 WAF1/CIP1 and cyclin D1 genes in PC12 cells by activating the Sp1 transcription factor. J Neurosci. 1997;17:6122–6132. doi: 10.1523/JNEUROSCI.17-16-06122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan GZ, Ziff EB. NGF regulates the PC12 cell cycle machinery through specific inhibition of the Cdk kinases and induction of cyclin D1. J Neurosci. 1995;15:6200–6212. doi: 10.1523/JNEUROSCI.15-09-06200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang R, Zhang L, Zhang Z, Wang Y, Lu M, Lapointe M, Chopp M. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol. 2001;50:602–611. doi: 10.1002/ana.1249. [DOI] [PubMed] [Google Scholar]
- 27.Zhu DY, Liu SH, Sun HS, Lu YM. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci. 2003;23:223–229. doi: 10.1523/JNEUROSCI.23-01-00223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu XJ, Hua Y, Jiang J, Zhou QG, Luo CX, Han X, Lu YM, Zhu DY. Neuronal nitric oxide synthase-derived nitric oxide inhibits neurogenesis in the adult dentate gyrus by down-regulating cyclic AMP response element binding protein phosphorylation. Neuroscience. 2006;141:827–836. doi: 10.1016/j.neuroscience.2006.04.032. [DOI] [PubMed] [Google Scholar]