Abstract
Berberine is an isoquinoline alkaloid isolated from goldenthread, Coptidis Rhizoma and shown to have many biological and pharmacological effects. We previously reported that berberine promotes cell survival and differentiation of neural stem cells. To examine whether berberine has survival promoting effect on damaged neuronal cells, we generated a cellular model under oxidative stress and an neonatal animal model of degenerating brain disease by injecting MK-801. MK801, a noncompetitive antagonist of N-methyl-d-aspartate (NMDA) receptors, acts as a neurotoxin in developing rats by inhibiting NMDA receptors and induce neuronal cell death. We found that the survival rate of the SH-SY5Y cells under oxidative stress was increased by 287% and 344%, when treated with 1.5 and 3.0µg/ml berberine, respectively. In the developing rats injected by MK801, we observed that TUNEL positive apoptotic cells were outspread in entire brain. The cell death was decreased more than 3 fold in the brains of the MK-801-induced neurodegenerative animal model when berberine was treated to the model animals. This suggests that berberine promotes activity dependent cell survival mediated by NMDA receptor because berberine is known to activate neurons by blocking K+ current or lowering the threshold of the action potential. Taken together, berberine has neuroprotective effect on damaged neurons and neurodegenerating brains of neonatal animal model induced by MK-801 administration.
Keywords: Berberine, cell survival, oxidative stress, MK801 animal model
INTRODUCTION
Berberine is an isoquinoline alkaloid and often isolated from goldenthread, Coptidis Rhizoma, and goldenseal, Hydrastis Canadensis (Mirska et al., 1972). Previous reports have shown that berberine has several pharmacological and biological properties including antibiotic (Mirska et al., 1972), anti-inflammatory (Marinova et al., 2000; Zhou and Mineshita, 2000; Yoo et al., 2008) and anti-hypolipidemic (Kong et al., 2004) effects. It has been reported that berberine attenuated neuronal damage in ischemia/reperfusion model (Yoo et al., 2006), and in autoimmune encephalomyelitis model mice (Ma et al., 2010). Berberine showed neuroprotective effects on stroke models (Zhou et al., 2008) and focal cerebral ischemia injury (Xiao et al., 2007). Previously, we reported that berberine enhances neuronal cell survival and differentiation in hippocampal precursor cells and neurons in the rat brains (Lim, 2008). Tan and his colleges (2007) showed that berberine has an antioxidant action on corpus cavenosum smooth muscle cells in which oxidative stress were induced (Tan et al., 2007).
To examine whether berberine has survival promoting effect on damaged neuronal cells, we generated a degenerating brain disease model by injecting neurotoxin to developing rats. MK-801 [(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate, dizocilpine] is a noncompetitive antagonist of N-methyl-d-aspartate (NMDA) receptors (Wong et al., 1986; Javitt and Zukin, 1991). In rodents, MK-801 induces a behavioral syndrome, including hyperlocomotion, head weaving, body rolling, and ataxia (Clineschmidt et al., 1982; Tricklebank et al., 1989; Liljequist et al., 1991), which represents certain aspects of schizophrenia (Carlsson and Carlsson, 1990; Tiedtke et al., 1990). MK-801 is also known to induce neurodegeneration of hippocampal CA1 and entorhinal cortex in adult animals when administrated with high concentrations (10 mg/kg) of MK-801 (Wohrl et al., 2007).
In the developing rat brain, blockade of NMDA receptors with low dose of MK801 during late fetal or early neonatal life triggers widespread apoptotic neurodegeneration (12~26% of cells) (Ikonomidou et al., 1999). This suggests the transient blockade of NMDA receptors can trigger neuronal cell death in the immature mammalian brain during a period of rapid axonal growth and synaptogenesis, and the excitatory neurotransmitter glutamate, acting at NMDA receptors, controls neuronal survival. Thus, Neurodegenerative MK-801 model of the developing rat has relevance to human neurodevelopmental disorders involving postnatal exposure to drugs that block NMDA receptors such as pediatric anesthesia.
Berberine has been reported to increase action potential by inhibition of voltage dependent potassium current in cat ventricular myocytes (Huang, 1990; Sanchez-Chapula, 1996) and in human myeloma cells (Wu et al., 1998) and hepatocytes (Wang et al., 2003). Berberine suppresses dopamine-induced potassium current and acetylcholine induced potassium current in acutely dissociated CA1 pyramidal neurons (Wu and Jin, 1996; 1997). It is also suggested that berberine contributes to its blockades of potassium currents in damaged ischemic brain (Wang et al., 2004). This leads us a question whether berberine reduces cell death on damaged brain of developing animal model rats induced by MK801. We tested first the cell survival promoting effect of berberine on SH-SY5Y neuronal cells damaged by oxidative stress and then examined whether berberine blocks cell death in vivo developing rat model induced by MK801.
MATERIALS AND METHODS
Cell culture
Human neuroblastoma SH-SY5Y cells were acquired from ATCC. As previously described, SHSY5Y cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS, Thermo, USA), penicillin, and streptomycin at 37℃ (Heo et al., 2009). For the SH-SY5Y cells 0.1 mM MEM non-essential amino acids were also added.
Cell viability assay
To estimate damage of cultured cells which caused by oxidative stress, we performed cell viability assay by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, USA). SH-SY5Y cells were seeded at a density of 7.5×103 cells in 96-well plate. Cells were treated with different concentration (0.25, 1.5, 3 and 3.5µg/ml) of berberine chloride (Sigma-Aldrich, USA). 4 mg/ml of MTT tetrazolium salt was added to each well and incubated for 4 hrs at 37℃. After that, 100µl of solubilization buffer was added and then incubated for 24 hrs. Result were measured by ELISA (Molecular devices, USA).
Neonatal animal model of neuronal cell damaged by MK-801 injection
Sprague Dawley rats were maintained under standard housing conditions and a 12:12 light/dark cycle (lights on at 06:30 AM) with free access to water and standard food. All procedures were performed in accordance with the guidelines of the National Institutes of Health (NIH) for the care and use of laboratory animals. The neonatal rat model of developmental disorder was constructed as described previously (Ikonomidou et al., 1999). In brief, postnatal day 7 animals were randomly divided into a control group (n=3) and a group injected with MK-801 (dizocilpine 0.5 mg/kg of body weight; Sigma, St. Louis, MO, USA), NMDA receptor antagonist (n=3). MK-801 was dissolved in 0.9% saline and injected intraperitoneally (i.p.). Control rats received saline only. Berberine chloride were dissolved in 0.9% saline and injected intraperitoneally every 24 hours for 5 days and control rats received saline again. The degenerating brains were examined at 5th day by TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nickend labeling) assay to detect apoptotic cells.
TUNEL assay
To visualize nuclei with DNA cleavage, brain were cut using a vibratome and brain slices were incubated with 0.1% Triton X-100 in 0.1% sodium citrate (permeable solution) for 2 min on 4℃. Subsequently brain slices were incubated with nucleotide-labeling mixture and terminal deoxynucleotidyl transferase (Roche, Switzerland) for 1 hr at 37℃ to catalytically add peroxidase-labeled digoxigenin nucleotide to DNA fragments. Nuclei displaying DNA cleavage had a dark brown appearance. We counted the number of TUNEL positive cells in the microscophic fields (n=12).
RESULTS
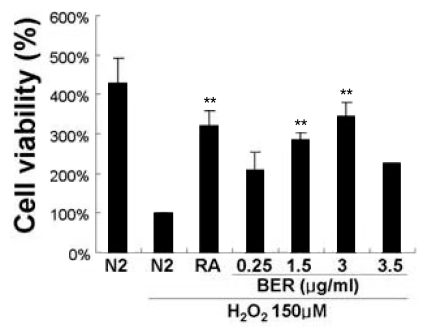
In the previous study, we have demonstrated that berberine promotes cell survival in neuronal stem cells. To investigate the cell survival effect of berberine on damaged neurons, we examined berberine effect in the cultured cellular model under the condition of oxidative stress. SH-SY5Y cells were exposed under oxidative stress by adding 150 µM H2O2 for 30 min after being pretreated with 0.25~3.5µg/ml of berberine for 3 hrs. When serum is removed from media and changed to chemically defined N2 media survival of SH-SY5Y cells are reduced and initiates neurite outgrowth. As shown in Fig. 1, the survival rate of the groups treated by 1.5 and 3.0µg/ml berberine was increased by 287% and 344%, respectively compared to the N2 media control. However, the survival rate of the groups treated by 3.5µg/ml berberine was reduced to about 200%, the similar level of 0.25 µg/ml concentration, suggesting that higher concentration is not more effective.
Fig. 1.
Neuronal cell viability was improved by berberine treatment in cultured SH-SY5Y cells under oxidative stress induced by hydrogen peroxide (H2O2). SH-SY5Y cells were pretreated with berberine (BER, 0.25, 1.5, 3 and 3.5µg/ml) 3 hours before H2O2 treatment (150µM, 30 min) in chemically defined N2 media. SH-SY5Y cells treated with retinoic acid (RA, 5µM) represents positive control. Cell survival was measured by MTT assay and cell viability was normalized to H2O2 added control. Data represents meansαstandard error measures. **p<0.001 compared with the control, one-way ANOVA.
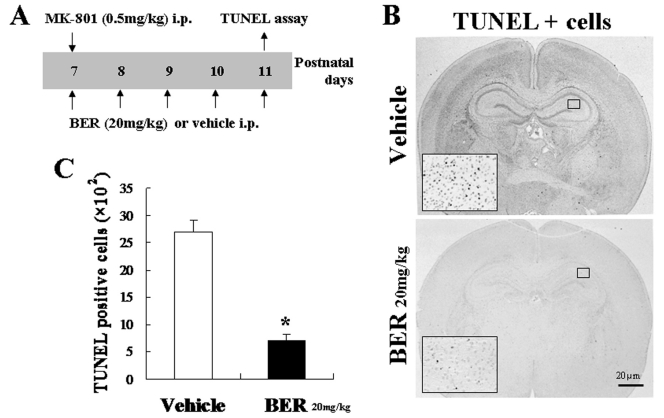
To determine the protective effect of berberine in the damaged neuronal cells of developing animal model, we generated neonatal animal model of degenerating brain by injecting intraperitoneally to postnatal day 7 aged rats 0.5 mg/kg of MK-801, which is a non-competitive antagonist of the Nmethyl-d-aspartate (NMDA) receptor, as described previously (Ikonomidou et al., 1999). As described previously, we also observed changes of cell shape including intracytoplasmatic vacuoles in the cortex of rats treated with a low dose of MK-801 (0.5 mg/kg body weight) when stained with haematoxylin and eosin (Olney et al., 1989; Fix et al., 1994). We intraperitoneally injected 20 mg/kg of berberine, or vehicle, to the model animals for 5 days. After sacrificing the animals, the brain slices were prepared to perform TUNEL assay (Fig. 2). TUNEL positive apoptotic cells were outspread in entire brain of the developing rats injected by MK801.
Fig. 2.
Berberine reduced cell death in the developing neonatal rats damaged by MK-801. (A) A schematic diagram represents experimental schedule. MK-801 (0.5 mg/kg; i.p.) injected at postnatal day 7. (B) TUNEL positive apoptotic cells were reduced by BER injection (20 mg/kg, 5 days). (C) Quantification graph indicates number of TUNEL positive cells in the microscopic field (×200; Vehicle n=3, BER n=3). Data indicates means ±standard error measures. *p<0.01 compared with the vehicle injected group, one-way ANOVA.
However, the cell death was decreased more than 3 fold in the brains of berberine administrated animals. The number of TUNEL positive cells of the control group were 27×102 cells per microscophic field (×200; Vehicle n=3, BER n=3), on the other hand, the number of TUNEL positive cells of the berberine group were 7×102 cells per microscophic field (×200; Vehicle n=3, BER n=3).
DISCUSSION
Recently, berberine is shown to enhance cell survival by reducing reactive oxygen species (ROS), the release of cytochrome c and apoptosis-inducing factors (AIFs) in PC12 cells damaged by oxygenglucose deprivation (Zhou et al., 2008). In this report we have demonstrated that survival promoting effect of berberine in both damaged neuronal cell model and degenerating brain of neonatal animal model. SH-SY5Y cell culture model induced by adding H2O2 are well established as a damaged neuronal cell model for ischemic studies. Ischemic conditions induce apoptosis by generating excessive ROS (Zhang et al., 2009). We found berberine increases cell viability of SH-SY5Y under oxidative stress about 3 fold. This result implies the antiapoptotic effect of berberine under oxidative stress condition.
We also found the anti-apoptotic effect of berberine in the MK801 induced animal model. To make the model animals, we injected low dose of MK-801 into the developing postnatal day 7 rats, and MK801 induced TUNEL positive apoptotic cells were found in entire brain of the developing rats as Ikonomidou and his colleges previously described (1999). Although half life of MK-801, NMDA receptor anatagoist is only for a few hours (Vezzani et al., 1989), neuronal apoptosis in the immature mammalian brain can be triggered by the transient blockade of glutamate NMDA receptors. This is probably caused by mechanism of activity dependant cell survival which is induced by release of neurotransmitter, glutamate and activation of NMDA receptor on target cells. Activation of the NMDA receptor by glutamate on post-synaptic neurons releases retrograde signal which is required for cell survival of presynaptic neurons. During a period of rapid brain growth or synaptogenesis period, such target derived survival signals from post-synaptic neurons regulate strengthening of the synapses as well as cell survival of pre and post-synaptic neurons, thereby forming neuronal networks. Blockage of NMDA receptors inhibits to produce target derived survival factors and cause apoptosis of pre and post-synaptic neurons.
When berberine was injected to the MK801 model rats, apoptotic cells were decreased more than 3 fold. Other researcher group also reported neuroprotective action of berberine in organotypic hippocampal slice culture induced by oxygen and glucose deprivation (Cui et al., 2009). Berberine has also been reported to block transient outward potassium current (IA) and delayed rectifier potassium current (IK) in acutely isolated CA1 pyramidal neurons of rat hippocampus by using the whole-cell patch-clamp techniques. These biological functions were suggested as a protective mechanism against ischemic brain damage (Wang et al., 2004). In our animal model of neonatal brain damaged by NMDA receptor antagonist, MK801, berberine probably stimulates cell survival of neuronal cells expressing NMDA receptors by blocking potassium current or lowering the threshold of the action potential. This may elevate synaptic depolarization and activate channel opening of NMDA receptors and calcium influx.
Thus, we suggest that these anti-apoptotic effect of berberine on the neurodegenerating brain of neonatal animal model caused by promoting activity dependent cell survival.
In the immature mammalian brain during a period of rapid synaptogenesis, the transient blockade of glutamate NMDA receptors, or the excessive activation of gamma-aminobutyric acid (GABA(A)) receptors trigger neuronal apoptosis. Apoptogenic agents include anesthetics and drugs of abuse including phencyclidine, ketamine, and ethanol. In humans, the brain growth period are between the sixth month of pregnancy and the third year after birth. Agents used in pediatric and obstetrical medicine for purposes of sedation, anesthesia, and seizure management may cause apoptotic neuronal degeneration in the developing human brain such as dysmorphogenic changes in the fetal brain and consequent neurobehavioral disturbances.
In conclusion, berberine has cell survival promoting effect on damaged neuronal cells under oxidative stress and degenerating brains of developing animal model induced by MK801.
ACKNOWLEDGEMENTS
This research was supported by a grant (2010 K000803) from the Brain Research Center of the 21st Century Frontier Research Program, funded by the Ministry of Science and Technology.
References
- 1.Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia--implications for schizophrenia and Parkinson's disease. Trends Neurosci. 1990;13:272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- 2.Clineschmidt BV. Effect of the benzodiazepine receptor antagonist Ro 15-1788 on the anticonvulsant and anticonflict actions of MK-801. Eur J Pharmacol. 1982;84:119–121. doi: 10.1016/0014-2999(82)90167-4. [DOI] [PubMed] [Google Scholar]
- 3.Cui HS, Matsumoto K, Murakami Y, Hori H, Zhao Q, Obi R. Berberine exerts neuroprotective actions against in vitro ischemia-induced neuronal cell damage in organotypic hippocampal slice cultures: involvement of B-cell lymphoma 2 phosphorylation suppression. Biol Pharm Bull. 2009;32:79–85. doi: 10.1248/bpb.32.79. [DOI] [PubMed] [Google Scholar]
- 4.Eyjolfsson EM, Brenner E, Kondziella D, Sonnewald U. Repeated injection of MK801: an animal model of schizophrenia? Neurochem Int. 2006;48:541–546. doi: 10.1016/j.neuint.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Fix AS, Horn JW, Truex LL, Smith RA, Gomez E. Neuronal vacuole formation in the rat posterior cingulate/retrosplenial cortex after treatment with the N-methyl-D-aspartate (NMDA) antagonist MK-801 (dizocilpine maleate) Acta Neuropathol. 1994;88:511–519. doi: 10.1007/BF00296487. [DOI] [PubMed] [Google Scholar]
- 6.Heo SR, Han AM, Kwon YK, Joung I. p62 protects SH-SY5Y neuroblastoma cells against H2O2-induced injury through the PDK1/Akt pathway. Neurosci Lett. 2009;450:45–50. doi: 10.1016/j.neulet.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Huang W. Ventricular tachyarrhythmias treated with berberine. Zhonghua Xin Xue Guan Bing Za Zhi. 1990;18:155–156. [PubMed] [Google Scholar]
- 8.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 9.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, Wang S, Wu J, Li Z, Liu J, Jiang JD. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 10.Liljequist S, Ossowska K, Grabowska-Anden M, Anden NE. Effect of the NMDA receptor antagonist, MK-801, on locomotor activity and on the metabolism of dopamine in various brain areas of mice. Eur J Pharmacol. 1991;195:55–61. doi: 10.1016/0014-2999(91)90381-y. [DOI] [PubMed] [Google Scholar]
- 11.Lim J, Kim H, Choi YS, Kwon H, Shin KS, Joung I, Kwon YK. Neuroprotective effects of berberine in neurodegeneration model rats induced by ibotenic acid. Animal Cells and Systems. 2008;12:203–209. [Google Scholar]
- 12.Ma X, Jiang Y, Wu A, Chen X, Pi R, Liu M, Liu Y. Berberine attenuates experimental autoimmune encephalomyelitis in C57 BL/6 mice. PLoS One. 2010;5:e13489. doi: 10.1371/journal.pone.0013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marinova EK, Nikolova DB, Popova DN, Gallacher GB, Ivanovska ND. Suppression of experimental autoimmune tubulointerstitial nephritis in BALB/c mice by berberine. Immunopharmacology. 2000;48:9–16. doi: 10.1016/s0162-3109(99)00189-7. [DOI] [PubMed] [Google Scholar]
- 14.Mirska I, Kedzia H, Kowalewski Z, Kedzia W. The effect of berberine sulfate on healthy mice infected with Candida albicans. Arch Immunol Ther Exp (Warsz) 1972;20:921–929. [PubMed] [Google Scholar]
- 15.Nussenzveig IZ, Sircar R, Wong ML, Frusciante MJ, Javitt DC, Zukin SR. Polyamine effects upon N-methyl-D-aspartate receptor functioning: differential alteration by glutamate and glycine site antagonists. Brain Res. 1991;561:285–291. doi: 10.1016/0006-8993(91)91606-2. [DOI] [PubMed] [Google Scholar]
- 16.Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Chapula J. Increase in action potential duration and inhibition of the delayed rectifier outward current IK by berberine in cat ventricular myocytes. Br J Pharmacol. 1996;117:1427–1434. doi: 10.1111/j.1476-5381.1996.tb15302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan Y, Tang Q, Hu BR, Xiang JZ. Antioxidant properties of berberine on cultured rabbit corpus cavernosum smooth muscle cells injured by hydrogen peroxide. Acta Pharmacol Sin. 2007;28:1914–1918. doi: 10.1111/j.1745-7254.2007.00705.x. [DOI] [PubMed] [Google Scholar]
- 19.Tiedtke PI, Bischoff C, Schmidt WJ. MK-801-induced stereotypy and its antagonism by neuroleptic drugs. J Neural Transm Gen Sect. 1990;81:173–182. doi: 10.1007/BF01245040. [DOI] [PubMed] [Google Scholar]
- 20.Tricklebank MD, Singh L, Oles RJ, Preston C, Iversen SD. The behavioural effects of MK-801: a comparison with antagonists acting non-competitively and competitively at the NMDA receptor. Eur J Pharmacol. 1989;167:127–135. doi: 10.1016/0014-2999(89)90754-1. [DOI] [PubMed] [Google Scholar]
- 21.Vezzani A, Serafini R, Stasi MA, Caccia S, Conti I, Tridico RV, Samanin R. Kinetics of MK-801 and its effect on quinolinic acid-induced seizures and neurotoxicity in rats. J Pharmacol Exp Ther. 1989;249:278–283. [PubMed] [Google Scholar]
- 22.Wang F, Zhao G, Cheng L, Zhou HY, Fu LY, Yao WX. Effects of berberine on potassium currents in acutely isolated CA1 pyramidal neurons of rat hippocampus. Brain Res. 2004;999:91–97. doi: 10.1016/j.brainres.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Zhou HY, Cheng L, Zhao G, Zhou J, Fu LY, Yao WX. Effects of palmatine on potassium and calcium currents in isolated rat hepatocytes. World J Gastroenterol. 2003;9:329–333. doi: 10.3748/wjg.v9.i2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wohrl R, Eisenach S, Manahan-Vaughan D, Heinemann U, von Haebler D. Acute and long-term effects of MK-801 on direct cortical input evoked homosynaptic and heterosynaptic plasticity in the CA1 region of the female rat. Eur J Neurosci. 2007;26:2873–2883. doi: 10.1111/j.1460-9568.2007.05899.x. [DOI] [PubMed] [Google Scholar]
- 25.Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Jin GZ. Tetrahydroberberine blocks membrane K+ channels underlying its inhibition of intracellular message-mediated outward currents in acutely dissociated CA1 neurons from rat hippocampus. Brain Res. 1997;775:214–218. doi: 10.1016/s0006-8993(97)00960-8. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Jin GZ. Tetrahydroberberine suppresses dopamine-induced potassium current in acutely dissociated CA1 pyramidal neurons from rat hippocampus. Neurosci Lett. 1996;207:155–158. doi: 10.1016/0304-3940(96)12522-2. [DOI] [PubMed] [Google Scholar]
- 28.Wu SN, Yu HS, Jan CR, Li HF, Yu CL. Inhibitory effects of berberine on voltage- and calcium-activated potassium currents in human myeloma cells. Life Sci. 1998;62:2283–2294. doi: 10.1016/s0024-3205(98)00209-4. [DOI] [PubMed] [Google Scholar]
- 29.Xiao B, Bi FF, Hu YQ, Tian FF, Wu ZG, Mujlli HM, Ding L, Zhou XF. Edaravone neuroprotection effected by suppressing the gene expression of the Fas signal pathway following transient focal ischemia in rats. Neurotox Res. 2007;12:155–162. doi: 10.1007/BF03033912. [DOI] [PubMed] [Google Scholar]
- 30.Yoo KY, Hwang IK, Kim JD, Kang IJ, Park J, Yi JS, Kim JK, Bae YS, Won MH. Antiinflammatory effect of the ethanol extract of Berberis koreana in a gerbil model of cerebral ischemia/reperfusion. Phytother Res. 2008;22:1527–1532. doi: 10.1002/ptr.2527. [DOI] [PubMed] [Google Scholar]
- 31.Yoo KY, Hwang IK, Lim BO, Kang TC, Kim DW, Kim SM, Lee HY, Kim JD, Won MH. Berberry extract reduces neuronal damage and N-Methyl-D-aspartate receptor 1 immunoreactivity in the gerbil hippocampus after transient forebrain ischemia. Biol Pharm Bull. 2006;29:623–628. doi: 10.1248/bpb.29.623. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Su X, Gao Y, Sun B, Yu Y, Wang X, Zhang F. Berberine protects mesenchymal stem cells against hypoxia-induced apoptosis in vitro. Biol Pharm Bull. 2009;32:1335–1342. doi: 10.1248/bpb.32.1335. [DOI] [PubMed] [Google Scholar]
- 33.Zuo DY, Zhang YH, Cao Y, Wu CF, Tanaka M, Wu YL. Effect of acute and chronic MK-801 administration on extracellular glutamate and ascorbic acid release in the prefrontal cortex of freely moving mice on line with open-field behavior. Life Sci. 2006;78:2172–2178. doi: 10.1016/j.lfs.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 34.Zhou H, Mineshita S. The effect of berberine chloride on experimental colitis in rats in vivo and in vitro. J Pharmacol Exp Ther. 2000;294:822–829. [PubMed] [Google Scholar]
- 35.Zhou XQ, Zeng XN, Kong H, Sun XL. Neuroprotective effects of berberine on stroke models in vitro and in vivo. Neurosci Lett. 2008;447:31–36. doi: 10.1016/j.neulet.2008.09.064. [DOI] [PubMed] [Google Scholar]