Abstract
The genomic sequence of Pseudomonas aeruginosa PAO1 was searched for the presence of open reading frames (ORFs) encoding enzymes potentially involved in the formation of Gln-tRNA and of Asn-tRNA. We found ORFs similar to known glutamyl-tRNA synthetases (GluRS), glutaminyl-tRNA synthetases (GlnRS), aspartyl-tRNA synthetases (AspRS), and trimeric tRNA-dependent amidotransferases (AdT) but none similar to known asparaginyl-tRNA synthetases (AsnRS). The absence of AsnRS was confirmed by biochemical tests with crude and fractionated extracts of P. aeruginosa PAO1, with the homologous tRNA as the substrate. The characterization of GluRS, AspRS, and AdT overproduced from their cloned genes in P. aeruginosa and purified to homogeneity revealed that GluRS is discriminating in the sense that it does not glutamylate tRNAGln, that AspRS is nondiscriminating, and that its Asp-tRNAAsn product is transamidated by AdT. On the other hand, tRNAGln is directly glutaminylated by GlnRS. These results show that P. aeruginosa PAO1 is the first organism known to synthesize Asn-tRNA via the indirect pathway and to synthesize Gln-tRNA via the direct pathway. The essential role of AdT in the formation of Asn-tRNA in P. aeruginosa and the absence of a similar activity in the cytoplasm of eukaryotic cells identifies AdT as a potential target for antibiotics to be designed against this human pathogen. Such novel antibiotics could be active against other multidrug-resistant gram-negative pathogens such as Burkholderia and Neisseria as well as all pathogenic gram-positive bacteria.
The formation of correctly aminoacylated tRNAs is the central step of the faithful translation of the genetic code. Some organisms use at least a distinct aminoacyl-tRNA synthetase (aaRS) for each amino acid species to be charged on the cognate tRNA(s). This is the case in the cytoplasm of eukaryotic cells and in some eubacteria such as Escherichia coli, which in addition to its basic set of 20 aaRSs, has an additional lysyl-tRNA synthetase (12). On the other hand, many organisms lack one or several aaRSs (35) and correctly aminoacylate the corresponding tRNA(s) via multistep pathways, such as the transamidation pathway for Gln-tRNAGln formation present in all archaea and in most bacteria (37, 38); the first step of this pathway is the misacylation of tRNAGln with glutamate, catalyzed by a nondiscriminating glutamyl-tRNA synthetase (GluRS-ND) (20), followed by the transamidation of Glu-tRNAGln into Gln-tRNAGln, catalyzed by a tRNA-dependent amidotransferase (AdT) (5). The presence of such alternate pathways for the correct aminoacylation of certain tRNAs reflects the formation of the extant 20 aaRSs by the divergent evolution of the ancestors of the two unlinked classes of aaRSs; for instance, glutaminyl-tRNA synthetase (GlnRS) evolved from a GluRS-ND in primitive eukaryotes that used the transamidation pathway for Gln-tRNAGln formation (18, 30). There may be physiological reasons for the conservation of such ancestral pathways by some organisms. In some cases, pathways may be redundant; when AdT and asparaginyl-tRNA synthetase (AsnRS) are present (as in gram-positive bacteria), aspartyl-tRNA synthetases (AspRS) could be either discriminating (AspRS-D) or nondiscriminating (AspRS-ND). Similarly, when AdT and GlnRS are present (as in Pseudomonas aeruginosa), glutamyl-tRNA synthetase (GluRS) could be either discriminating or nondiscriminating.
In certain archaea (7) and in the gram-negative bacterium Chlamydia trachomatis (25), the AsnRS is missing and Asn-tRNAAsn is synthesized by a transamidation pathway involving an AspRS-ND and a heterotrimeric AdT (10). Such AdTs, encoded by the gatA, gatB, and gatC genes, are present in archaea, some bacteria, and most organelles and can transamidate both Glu-tRNAGln and Asp-tRNAAsn (3, 5, 6, 25, 27). Heterodimeric AdTs, encoded by the gatD and gatE genes, are present in some archaea and transamidate only Glu-tRNAGln (35).
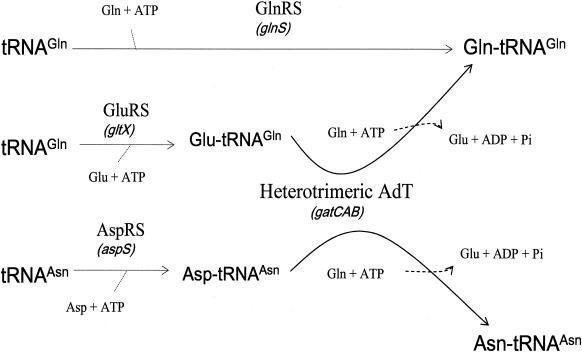
By searching through the complete genomic sequence of the pathogenic bacterium P. aeruginosa PAO1 (33) for genes encoding enzymes potentially involved in the formation of Gln-tRNA and of Asn-tRNA, we found those encoding a GluRS, a GlnRS, an AspRS, and an AdT (Fig. 1), but none were similar to the genes encoding known AsnRSs. We report here that P. aeruginosa contains a discriminating GluRS, an AspRS-ND, a heterotrimeric AdT, and no AsnRS activity (Fig. 2). It is the first organism where this set of enzymes is shown to be used for the formation of Gln-tRNA and Asn-tRNA. More recently, gatCAB has been found to be present and asnS was absent in other beta- and lower gamma-proteobacteria such as Neisseria meningitidis (24) and Pseudomonas putida (23).
FIG. 1.
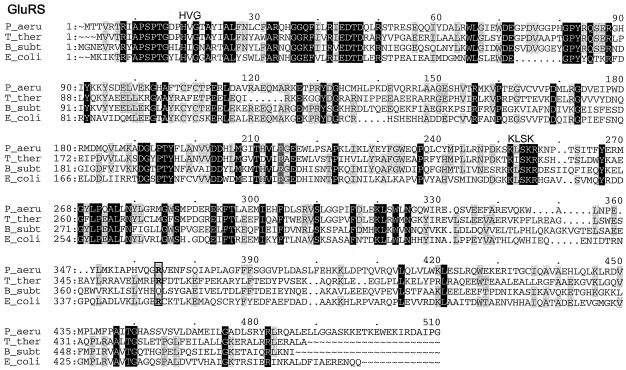
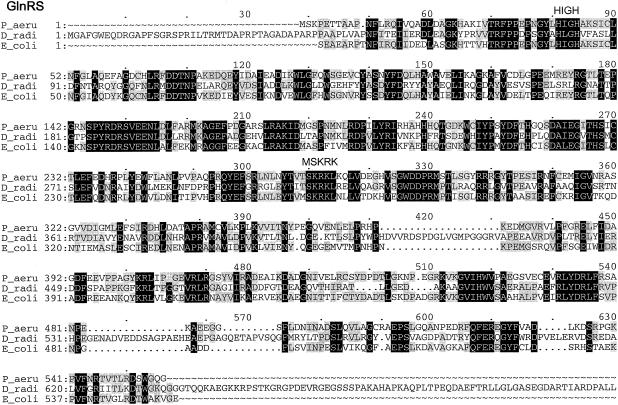
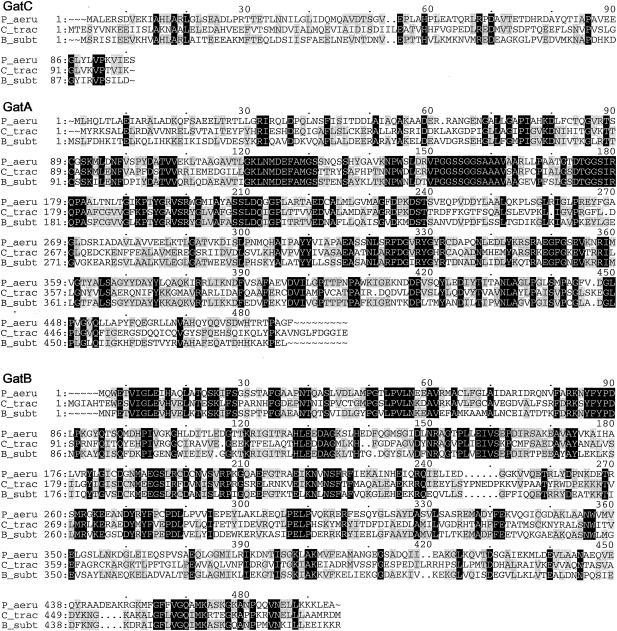
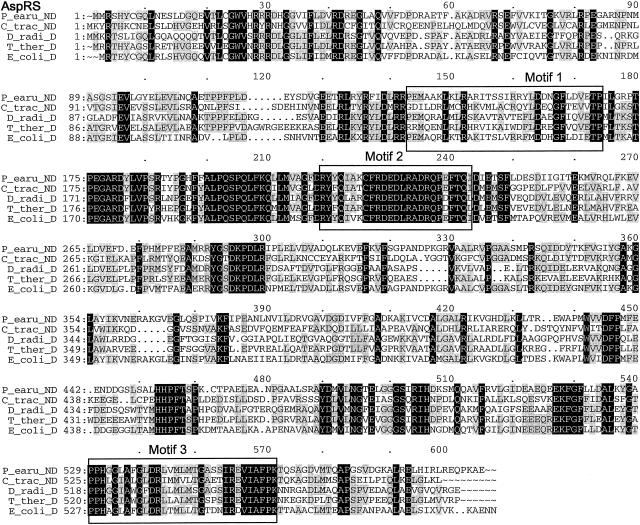
Multiple-sequence alignments of P. aeruginosa PAO1 GluRS, GlnRS, GatC, GatA, GatB, and AspRS with a few respective orthologs from other bacterial species, created by using the Pileup program. The organisms were as follows: P_aeru, P. aeruginosa PAO1; T_ther, T. thermophilus; B_subt, B. subtilis; E_coli, E. coli; D_radi, Deinococcus radiodurans; C_trac, C. trachomatis. The GluRS sequences used were as follows: P_aeru, PA3134; T_ther, P27000; B_subt, P222450; E_coli, P04805. The aligned residues Arg358 of T. thermophilus GluRS-D, Arg358 of P. aeruginosa PAO1, and GluRS and Gln358 of B. subtilis GluRS-ND are boxed. The GlnRS sequences used were as follows: P_aeru, Q9I2U8; D_radi, P56926; E_coli, BAA35328. The GatC, GatA, and GatB sequences used were, respectively, as follows: P_aeru, AAG07870, AAG07871, AAG07872; C_trac, NP_219504, NP_219505, NP_219506; B_subt, O06492, CAB12488, O30509. The AspRS sequences used were as follows: P_aeru, NP_249654; C_trac, O84546; D_radi_D, NP_295070; T_ther_D, P36419; E_coli_D, NP_288303. Residues which were identical in all sequences of each multiple alignment are printed in white on black, and those conserved in at least three of the four GluRS sequences, two of three sequences (GlnRS, GatC, GatA, and GatB), or three or four of the five AspRS sequences are shaded.
FIG. 2.
Putative pathways for Gln-tRNAGln and Asn-tRNAAsn synthesis in P. aeruginosa PAO1 based on the genes identified in its complete genomic sequence and without excluding the possibility that an atypical AsnRS is present.
MATERIALS AND METHODS
Nucleotide and amino acid sequence analyses.
The gapped BLAST algorithm (1) was used for nucleotide sequence analyses of the complete genome of P. aeruginosa PAO1 accessible at ftp://www.pseudomonas.com (33). The multiple alignments of amino acid sequences of homologous proteins were made with ClustalX (16) and the Pileup program of Genetics Computer Group, version 10.3 (Accelrys Inc., San Diego, Calif.), with the blosum 62 matrix, a gap weight of 10, and a gap length weight of 2.
Bacterial strains and plasmids.
P. aeruginosa PAO1 (ATCC 15692), kindly provided by Ann Huletsky (Université Laval, Québec, Canada), was used to purify DNA for gene cloning by PCR. P. aeruginosa ADD1976, carrying the mini-D180 fragment containing the T7 RNA polymerase gene controlled by the isopropyl-β-d-thiogalactopyranoside (IPTG)-inductible UV-5 promoter (4), was kindly provided by John Mattick (University of Queensland, Brisbane, Australia). The E. coli-P. aeruginosa shuttle vectors pUCPSK and pUCPKS were used for protein overproduction. They contain a multiple cloning site, allowing the transcription of the cloned gene from a T7 promoter, and encode a β-lactamase which confers resistance to ampicillin (120 μg/ml) in E. coli and to carbenicillin (500 μg/ml) in P. aeruginosa (36). The preparation and transformation of competent cells were conducted as described by Irani and Rowe (15).
Cloning of P. aeruginosa PAO1 gltX and aspS genes and of the gatCAB operon.
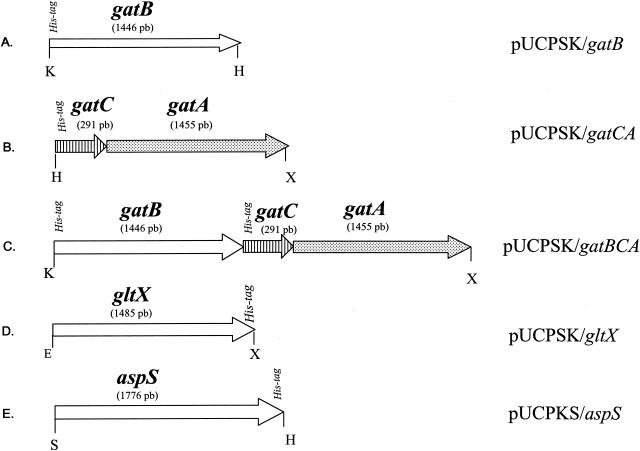
The gltX gene (1.48 kb) was amplified by PCR from P. aeruginosa genomic DNA with the following upstream and downstream oligonucleotides, respectively: 5′-CGCCCCTGAATTCCCGTTTTAACTTCC-3′ and 5′-CCCGGATCCTTATTAGTGGTGGTGGTGGTGGTGGCTGCTGCCGCGGCCCTCGGCGCCGGGAATGGCGTCGCG-3′. It was then inserted into pUCPSK (Fig. 3D). The aspS gene (1.77 kb) was similarly amplified by PCR with the following oligonucleotides: 5′-GGCAGCCGGGAGCTCCACAGAA-3′ and 5′-GCTCTTTGCGAAGCTTGATGTTGG-3′. It was then inserted into pUCPKS (Fig. 3E). The gatCAB operon was cloned in three steps. First, gatB (1.44 kb) was PCR amplified with the oligonucleotides 5′-CCCGGTACCAGGAGGTCTCCATGGGCAGCAGCCACCACCACCACCACCACCACCACAGCAGCGCCGAGGGCCGCATGCAATGGGAAACCG-3′ and 5′-CGAACCCGGC GTCAAGCTTTGACTCACGCTTC-3′ and cloned into pUCPSK (Fig. 3A). Second, gatCA (1.72 kb) was PCR amplified with the oligonucleotides 5′-CGCCCAAGCTTAGGAGGTCTCCATGGGCAGCAGCCACCACCACCACCACCACCACCACAGCAGCGCCGAGGGCCGCATGGCGCTTGAACGCTCCGAC-3′ and 5′-GCTCTAGAGCTTATTAGAAGCCGGCCGGGG-3′ and cloned into pUCPSK (Fig. 3B). Finally, the KpnI-HindIII fragment of pUCPSK/gatB was inserted upstream of gatC in pUCPSK/gatCA, generating the gatBCA operon (3.2 kb) (Fig. 3C). The integrity of the cloned genes was checked by sequencing.
FIG. 3.
P. aeruginosa PAO1 genes amplified by PCR and inserted into the multiple cloning site of the E. coli-P. aeruginosa shuttle vector pUCPSK or pUCPKS, which differ only by the orientation of their multiple cloning sites (36). The resulting vectors, identified on the right, express the inserted gene(s) from a proximal T7 promoter. The letters K, H, X, E, and S represent the KpnI, HindIII, XbaI, EcoRI, and SacI restriction sites, respectively. Upstream of gatB and gatC, we inserted by PCR the Shine-Dalgarno sequence AGGAGG frequently found in P. aeruginosa, 8 His codons (CAC), and a sequence encoding the factor Xa digestion site Arg-Glu-Gly-Arg (with codons preferentially used in P. aeruginosa). Downstream of gltX and aspS, we inserted a sequence encoding the factor Xa digestion site, 6 His codons, and two stop codons.
Enzyme purification.
P. aeruginosa was grown with strong agitation in modified Luria-Bertani medium (2.5 g of NaCl per liter) (C. Hancock, personal communication) to reduce the synthesis of alginate, which interferes with enzyme purification. The overproduced P. aeruginosa GluRS and AspRS, carrying C-terminal His tags, were purified to homogeneity by affinity chromatography on nickel nitrilotriacetate (Ni-NTA) superflow agarose (Qiagen). P. aeruginosa AdT was overproduced in P. aeruginosa ADD1976 carrying the plasmid pUCPSK/gatBCA, with His tags on the C and B subunits but none on the A subunit. The AdT purified by Ni-NTA affinity chromatography contains equimolar amounts of each of the three subunits. These purified enzymes were concentrated by filtration on Centricon membranes (YM-5, -10, or -30). P. aeruginosa GlnRS was partially purified from a clarified (S-10) crude extract of P. aeruginosa PAO1 by partition in a polyethylene glycol-dextran two-phase system, as previously used for E. coli GlnRS (17), followed by chromatography on a Q-Sepharose column (Amersham Pharmacia Biotech).
Purification of unfractionated tRNA from P. aeruginosa PAO1.
Cells were grown as described above to reduce the synthesis of alginate, which strongly interferes with phenol extraction of tRNA. All the operations were conducted at 4°C. The cell pellet was suspended in 20 mM Tris-HCl (pH 7.9) and shaken with an equal volume of phenol previously equilibrated with Tris-HCl (pH 7.9). After separation of the phases by centrifugation, the aqueous phase was submitted to a second phenol extraction. Nucleic acids were precipitated from the resulting aqueous phase by the addition of 0.1 volume of 20% potassium acetate (pH 5.0) and 3 volumes of 95% ethanol. The precipitate was resuspended in 1 M NaCl, which allowed the small RNAs to be solubilized but kept the large ribosomal RNAs as the precipitate. Unfractionated tRNA was then precipitated from the supernatant in the presence of 3 volumes of 95% ethanol at −20°C for at least 30 min, solubilized in sterilized distilled and deionized water, and purified from remaining traces of rRNA by filtration through Centriplus 100,000-molecular-weight membranes (Millipore).
Purification of P. aeruginosa tRNAAsp, tRNAAsn, and tRNAGln.
The P. aeruginosa genome contains a single species of each of tRNAAsp, tRNAAsn, and tRNAGln, encoded by four, two, and one genes, respectively (http://rna.wustl.edu/GtRDB/Paer/Paer-summary.html). Each of these tRNA species was purified to homogeneity, as a complex with a complementary oligonucleotide (24-mer), by polyacrylamide gel electrophoresis under nondenaturing conditions and recovered by electroelution (the choice of the complementary oligonucleotides and the detailed experimental conditions will be described elsewhere).
P. aeruginosa tRNA aminoacylation.
Unfractionated tRNA, isolated as described above, was aminoacylated with [14C]glutamate, [14C]aspartate, or [14C]glutamine with pure GluRS or AspRS or partially purified GlnRS, respectively. [14C]asparagine was used to search for the presence of an AsnRS. The aminoacylation reactions were conducted at 37°C in 50 mM Na HEPES (pH 7.2), 25 mM MgCl2, 15 mM KCl, 5 mM dithiothreitol, 1 mM ATP, 50 μM unfractionated tRNA, 125 μΜ amino acid substrate ([14C]glutamate, 238 mCi/mmol; [14C]glutamine, 244 mCi/mmol; [14C]aspartate, 216 mCi/mmol; or [14C]asparagine, 210 mCi/mmol), and the enzyme (4.2 μg of pure GluRS or AspRS/ml or 12.5 μg of partially purified GlnRS/ml). The 14C-labeled amino acids were purchased from Amersham Pharmacia Biotech.
Asp-tRNAAsn and Glu-tRNAGln transamidation reactions.
The transamidation reactions were conducted at 37°C in 50 mM Na HEPES (pH 7.2), 25 mM MgCl2, 15 mM KCl, 5 mM dithiothreitol, 1 mM ATP, 50 μM unfractionated tRNA, and 125 μM [14C]glutamate or [14C]aspartate, as described by Curnow et al. (5) and Raczniak et al. (25). In a preliminary step, the aminoacyl-tRNA substrates for AdT were prepared by adding either AspRS or GluRS to this mixture and incubating it for 15 min at 37°C, which led to plateaux of Asp-tRNA and Glu-tRNA. The transamidation reaction was then started by the addition of 320 μg of pure AdT/ml and 5 mM unlabeled glutamine. The reaction was carried out for 30 min at 37°C and was stopped by the addition of 2.5 M Na acetate (pH 5.2) to a final concentration of 0.3 M. This reaction mixture was shaken with 1 volume of phenol at pH 5.2 and 1 volume of chloroform. After centrifugation, tRNA was ethanol precipitated from the aqueous phase, washed with 70% ethanol, dried and solubilized in 50 μl of 25 mM KOH, and incubated for 1 h at 65°C to deacylate aminoacyl-tRNAs. This solution was then neutralized by the addition of 1.3 μl of 100 mM HCl and dried under vacuum. The residue was solubilized in 10 ml of distilled and deionized water, and 2 μl of this solution was analyzed for its amino acid content by ascending chromatography on microcrystalline cellulose thin-layer plates (Whatman) with ammonia-water-chloroform-methanol (2:1:6:6) as described by Curnow et al. (5). The thin-layer chromatography (TLC) plate was then dried at room temperature, and the position and radioactivity of the spots were measured and analyzed with a Fuji BAS 1000 phosphorimager, with the Image gauge, version 4.0 software.
RESULTS
P. aeruginosa genes encoding enzymes potentially involved in the formation of Gln-tRNA and Asn-tRNA.
Analysis of the complete genomic sequence of P. aeruginosa PAO1 (33) (ftp://www.pseudomonas.com) by gapped BLAST (1), ClustalX (16), and version 10.3 of the Genetics Computer Group (Accelrys Inc.) revealed the presence of open reading frames (ORFs) similar to known GluRSs, GlnRSs, AspRSs, and trimeric AdT (Fig. 1 ) but none similar to known AsnRSs. As trimeric AdT characterized in other bacteria can transamidate both Glu-tRNAGln and Asp-tRNAAsn (3, 5, 25), this set of genes suggests the existence of two pathways of Gln-tRNAGln formation (direct, via GlnRS; indirect, via GluRS-ND and AdT) and of only the indirect pathway for Asn-tRNAAsn formation. However, firm conclusions about the pathways used in P. aeruginosa for the formation of Glu-tRNAGln and Asp-tRNAAsn cannot rely only on the presence of these genes, first because it is not possible at this point to determine from its amino acid sequence whether a GluRS is discriminating or nondiscriminating and second because the absence of a gene similar to the known asnS genes does not imply that P. aeruginosa has no AsnRS activity. Indeed, some aaRSs such as class I Lys-tRNA synthetase of Methanococcus maripaludis (13) and Cys-tRNA synthetase of Methanococcus jannaschii and Methanobacterium thermoautotrophicum (9, 32) are too different from their cognate aaRSs in other organisms to be identified by sequence comparisons. The same set of genes encoding enzymes potentially involved in the formation of Gln-tRNA and Asn-tRNA (Fig. 2) is also present in all beta- and some gamma-proteobacteria whose complete genomes have been reported: P. aeruginosa (gamma), P. putida (gamma), Pseudomonas syringae (gamma), Neisseria gonorrhoeae (beta), N. meningitidis (beta), Nitrosomonas europaea (beta), Bordetella pertussis (beta), Ralstonia solanacearum (beta), and Burkholderia pseudomallei (beta). No biochemical characterization of these enzymes has been reported. Therefore, we undertook the search for AsnRS activity in P. aeruginosa and the biochemical characterization of its AspRS, GluRS, GlnRS, and AdT.
Absence of an AsnRS in P. aeruginosa.
To detect AsnRS activity in crude extracts of P. aeruginosa, we measured the incorporation of [14C]asparagine into homologous unfractionated tRNA and observed the incorporation of the 14C label into this tRNA fraction, even in the presence of unlabeled aspartate; however, identification of the charged amino acid by TLC, following the deacylation of the charged tRNAs, revealed that it was [14C]aspartate. This result does not rule out the presence of AsnRS activity, since very active asparaginases in the crude extract could rapidly transform [14C]asparagine into [14C]aspartate (31). We thus fractionated P. aeruginosa PAO1 extracts by several chromatographic steps, selecting the fractions endowed with the above-mentioned activity. We found that the purest active fraction contained AspRS and the GatA and GatB subunits of the heterotrimeric AdT. In the presence of this fraction and of [14C]asparagine, only [14C]aspartate was found acylated to tRNA, indicating that aspartate was formed by an asparaginase activity, and charged on tRNA by the copurified AspRS. No AsnRS activity was detected in pure AspRS nor in pure AdT. Therefore, we conclude that P. aeruginosa does not contain an AsnRS.
Cloning and (over)expression of the P. aeruginosa genes encoding GluRS, AspRS, and AdT in E. coli and P. aeruginosa.
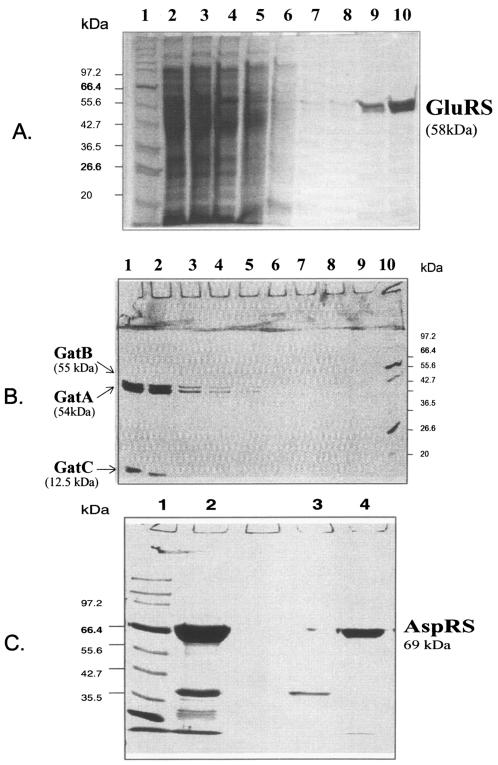
The P. aeruginosa PAO1 gltX and aspS genes, flanked at their 3′ ends by His tag-encoding extensions, have been amplified by PCR from genomic DNA and cloned into the P. aeruginosa-E. coli multicopy (about 14 copies per cell) shuttle vector pUCPSK (36) under the control of a T7 promoter (Fig. 3A and B). The E. coli thermosensitive strain JP1449 altered in gltX (19, 26), which does not contain the T7 RNA polymerase gene, grows at restrictive temperature (42°C) when transformed with pUCPSK/gltX but does not grow under these conditions when transformed with pUCPKS alone. This result shows that even in the absence of T7 RNA polymerase, the level of expression of P. aeruginosa gltX provides enough GluRS to complement the temperature-sensitive character of this E. coli mutant and suggests that P. aeruginosa GluRS is discriminating. On the other hand, no transformants of E. coli BL21(DE3) (which carries an IPTG-inducible T7 RNA polymerase gene) with pUCPSK/gltX were obtained, indicating the toxicity for E. coli of high levels of P. aeruginosa GluRS. The mechanism responsible for this toxicity is yet unknown. On the other hand, no transformants by the pUCPSK/aspS of E. coli DH5α or CS89 (29), which carries a thermosensitive AspRS, were obtained, suggesting that P. aeruginosa AspRS is nondiscriminating. Therefore, we overproduced P. aeruginosa GluRS and AspRS in P. aeruginosa ADD1976 and purified these His-tagged enzymes to near homogeneity by affinity chromatography (Fig. 4A and C). As removing His tags from the purified GluRS or AspRS did not significantly affect the activity, we used the His-tagged forms in the work described below.
FIG. 4.
SDS-PAGE characterization of P. aeruginosa PAO1 GluRS (A), the three subunits of the heterotrimeric AdT (B), and AspRS (C) overproduced in P. aeruginosa ADD1976 and purified by affinity chromatography on Ni-NTA. (A) Lanes: 1, protein standard; 2 to 8, wash with 30 mM imidazole; 9 to 10, GluRS elution with 90 mM imidazole. (B) Lanes: 1 to 9, amidotransferase elution with 100 mM imidazole, after an initial wash with 30 mM imidazole; 10, protein standard. (C) Lanes: 1, protein standard; 2, AspRS elution with 85 mM imidazole, after an initial wash with 30 mM imidazole; 3 and 4, contaminant and pure AspRS, respectively, removed after Superdex 200 chromatography. Numbers indicate the molecular mass (in kilodaltons) of protein standards. The gels (8% polyacrylamide) are stained with Coomassie blue.
We did not succeed in cloning gatCAB in the same vector after its amplification by PCR, probably because of the large size of this operon (3.5 kbp). Independent clonings of gatCA and gatB were successful (Fig. 3A and B) and allowed the overproduction in P. aeruginosa ADD1976 of the His-tagged B subunit of AdT and its purification by affinity chromatography. However, the untagged A subunit, overproduced in the same cells as the His-tagged C subunit, was not retained during the affinity chromatography. This indicates that the interaction between A and C is weak, at least in the absence of B. Therefore, we inserted gatB upstream of gatC in the pUCPSK/gatCA (see Materials and Methods) to overproduce the three subunits in the same cell (Fig. 3B). Although the expression of this artificial gatBCA operon in E. coli(DE3) did not affect the growth of this host, we conducted gatBCA overexpression in P. aeruginosa ADD1976; purification of the overproduced P. aeruginosa AdT by affinity chromatography yielded equimolar ratios of the three subunits (Fig. 4B).
P. aeruginosa has GlnRS activity, and its GluRS is discriminating.
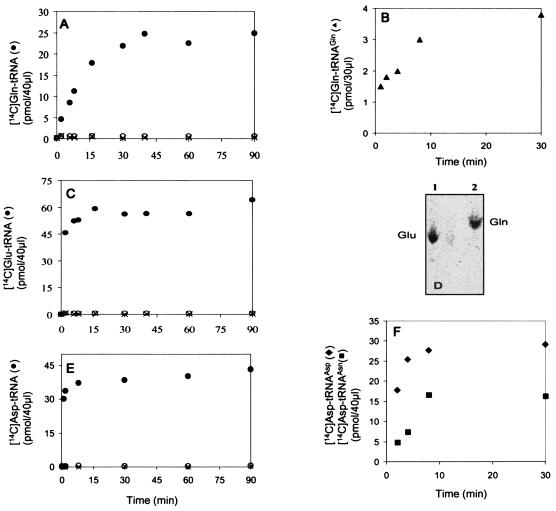
The presence of GlnRS activity was detected in the supernatant of a crude extract (centrifugation at 10,000 × g). This GlnRS was partially purified by Q-Sepharose chromatography and used to charge unfractionated tRNA from P. aeruginosa with [14C]glutamine (Fig. 5A). From the value of the plateau obtained, we calculated that 2.4% of these tRNA molecules accept glutamine. Moreover, we verified by chromatography that glutamine, and not glutamate, was acylated to tRNA (Fig. 5D). This result excludes the possibility that tRNAGln was glutaminylated via the transamidation pathway involving a GluRS-ND and AdT, because in that case both glutamate and glutamine would have been found to be acylated to unfractionated tRNA. This control was made to exclude the possibility that the 14C-labeled amino acid measured in this experiment is glutamic acid. This could have happened by the conversion of [14C]glutamine into [14C]glutamate by a glutaminase in the partially purified GlnRS fraction followed by the charging of [14C]glutamate on tRNA. The presence in the P. aeruginosa genome of an ORF similar to known glnS genes is not sufficient to conclude that this ORF encodes a GlnRS because GluRSs and GlnRSs are closely related (11, 39), and some bacteria have no GlnRS and two GluRSs (one is discriminating and the other is nondiscriminating) (34). The presence of only glutamine acylated to tRNA (Fig. 5D) in that experiment demonstrates the existence of a GlnRS activity in P. aeruginosa. Finally, tRNAGln purified to homogeneity by gel electrophoresis (see Materials and Methods) is glutaminylated by this GlnRS fraction (Fig. 5B).
FIG. 5.
Aminoacylation of unfractionated tRNA, pure tRNAGln, tRNAAsp, and tRNAAsn from P. aeruginosa PAO1. The reactions shown in panels A, C, and E were conducted in the presence of unfractionated tRNA and catalyzed by partially purified GlnRS, pure GluRS, and pure AspRS from P. aeruginosa PAO1, respectively. Panel B shows the glutaminylation of pure tRNAGln by partially purified GlnRS, and panel F shows the aspartylation of pure tRNAAsp and tRNAAsn by pure AspRS. (D) Identification by TLC (see Methods) of the amino acid charged on tRNA by GluRS in the reaction shown in panel C (lane 1) and by GlnRS in the reaction shown in panel A (lane 2). Shown are complete reactions (filled circles) and controls without either tRNA (X) or enzyme (empty circles).
The pure GluRS charges 6.4% of unfractionated tRNA from P. aeruginosa with [14C]glutamate (Fig. 5C). When this [14C]glutamyl-tRNA was incubated in the presence of pure AdT from P. aeruginosa, no [14C]glutaminyl-tRNA was formed (Fig. 6A). Moreover, P. aeruginosa GluRS does not aminoacylate pure tRNAGln from P. aeruginosa (results not shown). These results demonstrate that P. aeruginosa GluRS is discriminating.
FIG. 6.
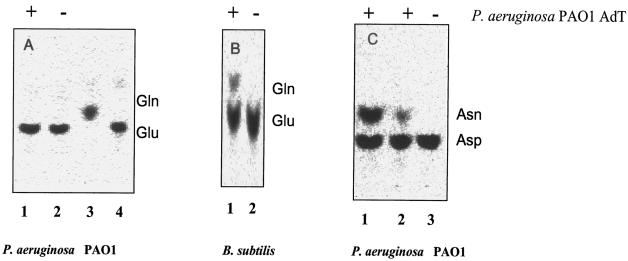
Activity of P. aeruginosa PAO1 heterotrimeric AdT. (A) P. aeruginosa Glu-tRNA. Lanes: 1, with (+) AdT; 2, without (−) AdT; 3 and 4, glutamine and glutamate, respectively, as standards. (B) B. subtilis Glu-tRNA. Lanes: 1, with AdT; 2, without AdT. (C) P. aeruginosa Asp-tRNA. Lanes: 1, with AdT for 30 min; 2, with AdT for 15 min; 3, without AdT. These panels show phosphorimages of TLC of the 14C-labeled amino acids after their removal from these tRNAs (see Materials and Methods) or free glutamine and glutamate as standards (panel A, lanes 3 and 4).
P. aeruginosa AspRS is nondiscriminating.
The pure AspRS charges 6.9% of unfractionated tRNA from P. aeruginosa with [14C]aspartate (Fig. 5E). When this [14C]aspartyl-tRNA was incubated in the presence of pure AdT from P. aeruginosa, about 34% was transformed into [14C]asparaginyl-tRNA (Fig. 6C). This result demonstrates that P. aeruginosa AspRS is nondiscriminating and indicates that the 6.9% of the tRNA molecules charged with aspartate correspond to about 4.6% tRNAAsp and 2.3% tRNAAsn. These proportions are consistent with the presence of 4 tRNAAsp genes and 2 tRNAAsn genes in the genome of this bacterium (http://rna.wustl.edu/GtRDB/Paer/Paer-summary.html) and with the observation that the levels of individual tRNA genes in bacteria are generally controlled by gene dosage (8, 14). Finally, the nondiscriminating character of P. aeruginosa AspRS is demonstrated directly by its capacity to aspartylate efficiently both pure tRNAAsp and tRNAAsn from P. aeruginosa (Fig. 5F).
The heterotrimeric AdT of P. aeruginosa has no Glu-tRNAGlnsubstrate in its host, but it can transamidate heterologous Glu-tRNAGln.
The pure heterotrimeric AdT of P. aeruginosa transamidates its homologous Asp-tRNAAsn into Asn-tRNAAsn (Fig. 6C). The characterized heterotrimeric AdTs can use both Asp-tRNAAsn and Glu-tRNAGln, even if the latter substrate is lacking in P. aeruginosa because of the discriminating character of its GluRS (Fig. 6A), its AdT has kept its capacity to transamidate Glu-tRNAGln, as shown with the corresponding tRNA of Bacillus subtilis (Fig. 6B).
DISCUSSION
By analyzing the genome of P. aeruginosa and characterizing several enzymatic activities potentially involved in the formation of Gln-tRNA and of Asn-tRNA, we have found that it does not have an AsnRS and that it is the first known organism to synthesize Gln-tRNA, via the direct pathway (GlnRS), and Asn-tRNA, via the transamidation pathway. Up to now, the absence of AsnRS was correlated to the absence of GlnRS in archaea (7) and in Chlamydia (25). Genomic data indicate that all beta- and some gamma-proteobacteria have a GlnRS and lack an AsnRS.
The presence of a GlnRS in P. aeruginosa is consistent with the biochemical evidence for the discriminating character of its GluRS (Fig. 6A). Moreover, structural evidence for this character comes from the presence of an Arg residue at the position corresponding to Arg 358 of domain 4 of Thermus thermophilus discriminating GluRS (GluRS-D) (Fig. 1), which interacts with the third nucleotide anticodon (C-36) of tRNAGlu,and whose replacement by a Gln residue makes this a GluRS-ND (28). Finally, physiological evidence for the discriminating character of P. aeruginosa GluRS is that its production at a low level in E. coli JP 1449 allows this strain carrying a thermosensitive GluRS to grow at a restrictive temperature. On the other hand, stronger expression is toxic for this heterologous host (see above). The mechanism for the toxicity for E. coli of higher levels of this GluRS-D is unknown.
All of the characterized trimeric AdTs from microorganisms or organelles catalyze in vitro the transamidation of Glu-tRNAGln and Asp-tRNAAsn (10). Even in the organisms where only one of these substrates is present in vivo, the trimeric AdT has not lost the property of transamidating the other substrate provided by another organism, in vitro or in vivo (3, 5, 25). This is also the case for P. aeruginosa AdT, which finds in its host only Asp-tRNAAsn but which can transamidate B. subtilis Glu-tRNAGln (Fig. 6B).
The C subunit of T. thermophilus AdT interacts weakly with the A and B subunits, and the A-B interaction is strong, as evidenced by the fact that only the A-B heterodimer was obtained at the end of several steps of purification from a crude extract of normal cells (2). In the P. aeruginosa AdT, there is also a weak interaction between the C and A subunits, since following the overproduction of the His-tagged C subunit together with the untagged A subunit (Fig. 3B), the only peptide retained on the affinity column was the C subunit (see above). To purify this enzyme by affinity chromatography, we overproduced the His-tagged C and B subunits together with the untagged A subunit (Fig. 3C), counting on the strong A-B interaction to retain A on the column. This strategy led to a pure and active AdT, with stoichiometric amounts of the three subunits (Fig. 4B).
In some organisms lacking an asnA or asnB gene encoding asparagine synthetase, AdT participates in asparagine biosynthesis via Asp-tRNAAsn transamidation (21, 30). As P. aeruginosa has an asnB gene, the only function of AdT in its host appears to be its participation in the formation of Asn-tRNAAsn involved in ribosomal protein biosynthesis.
AdT is present in all archaea, most bacteria, and all known organelles, with the exception of Leishmania tarentolae mitochondria (22). On the other hand, it is absent from the cytoplasm of eukaryotes, so inhibitors of bacterial AdT are expected to have a low toxicity. Therefore, the essential role of AdT reported here for the formation of Asn-tRNA in P. aeruginosa identifies this enzyme as a potential target for antibiotics to be designed against this and other pathogenic gram-negative bacteria that lack asnS, such as Moraxella, Neisseria, and Burkholderia, and all gram-positive bacteria that lack glnS.
Acknowledgments
This work was supported by grant OGP0009597 from the Natural Sciences and Engineering Research Council of Canada (NSERC) to J.L. and grant 2003-ER-2481 from the “Fonds pour la Formation de Chercheurs et l'Aide à la Recherche du Québec” (FCAR) to P.H.R. and J.L. P.M.A. was a doctoral fellow from the “Ministère de l'Enseignement Supérieur et de la Recherche Scientifique de Côte d'Ivoire.” D.B. was an FCAR-FRSQ doctoral fellow.
We thank Ann Huletsky and John Mattick for the gift of P. aeruginosa strains and plasmids.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker, H. D., and D. Kern. 1998. Thermus thermophilus: a link in evolution of the tRNA-dependent amino acid amidation pathways. Proc. Natl. Acad. Sci. USA 95:12832-12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, H. D., B. Min, C. Jacobi, G. Raczniak, J. Pelaschier, H. Roy, S. Klein, D. Kern, and D. Soll. 2000. The heterotrimeric Thermus thermophilus Asp-tRNAAsn amidotransferase can also generate Gln-tRNAGln. FEBS Lett. 476:140-144. [DOI] [PubMed] [Google Scholar]
- 4.Brunschwig, E., and A. Darzins. 1992. A two-component T7 system for overexpression of gene in Pseudomonas aeruginosa. Gene 111:35-41. [DOI] [PubMed] [Google Scholar]
- 5.Curnow, A. W., K. Hong, R. Yuan, S. Kim, O. Martins, W. Winkler, T. M. Henkin, and D. Soll. 1997. Glu-tRNAGln amidotransferase: a novel heterotrimeric enzyme required for correct decoding of glutamine codons during translation. Proc. Natl. Acad. Sci. USA 94:11819-11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curnow, A. W., D. L. Tumbula, J. T. Pelaschier, B. Min, and D. Soll. 1998. Glutamyl-tRNA(Gln) amidotransferase in Deinococcus radiodurans may be confined to asparagine biosynthesis. Proc. Natl. Acad. Sci. USA 95:12838-12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curnow, A. W., M. Ibba, and D. Soll. 1996. tRNA dependent asparagine formation. Nature 382:589-590. [DOI] [PubMed] [Google Scholar]
- 8.Dong, H., L. Nilsson, and C. G. Kurland. 1996. Co-variation of tRNA abundance and codon usage in Escherichia coli at different growth rates. J. Mol. Biol. 260:649-663. [DOI] [PubMed] [Google Scholar]
- 9.Fabrega, C., M. A. Farrow, B. Mukhopadhyay, V. de Crécy-Lagard, A. R. Ortiz, and P. Schimmel. 2001. An aminoacyl-tRNA synthetase whose sequence fits into neither of the two known classes. Nature 411:110-114. [DOI] [PubMed] [Google Scholar]
- 10.Feng, L., H. D. Tumbula, B. Min, S. Namgoog, J. Salazar, O. Orrellena, and D. Soll. Transfer RNA-dependant amidotransferase. In M. Ibba, C. Francklyn, and S. Cusack (ed.), Aminocyl-tRNA synthetases, in press. Landes Biosience, Georgetown, Tex.
- 11.Freist, W., D. H. Gauss, D. Soll, and J. Lapointe. 1997. Glutamyl-tRNA sythetase. Biol. Chem. 378:1313-1329. [PubMed] [Google Scholar]
- 12.Ibba, M., and D. Soll. 2000. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 69:617-650. [DOI] [PubMed] [Google Scholar]
- 13.Ibba, M., I. Celic, A. Curnow, H. Kim, J. Pelaschier, D. Tumbula, U. Vothknecht, C. Woese, and D. Soll. 1997. Aminoacyl-tRNA synthesis in Archaea. Nucleic Acids Symp. Ser. 37:305-306. [PubMed] [Google Scholar]
- 14.Ikemura, T. 1981. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J. Mol. Biol. 151:389-409. [DOI] [PubMed] [Google Scholar]
- 15.Irani, V. R., and J. J. Rowe. 1997. Enhancement of transformation in P. aeruginosa PAO1 by Mg2+ and heat. BioTechniques 22:54-56. [DOI] [PubMed] [Google Scholar]
- 16.Jeanmougin, F., J. D. Thompson, M. Gouy, D. G. Higgins, and T. J. Gibson. 1998. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 23:403-405. [DOI] [PubMed] [Google Scholar]
- 17.Kern, D., S. Potier, J. Lapointe, and Y. Boulanger. 1980. The glutamyl-tRNA synthetase of Escherichia coli. Purification, structure and function relationship. Biochim. Biophys. Acta 607:65-80. [DOI] [PubMed] [Google Scholar]
- 18.Lamour, V., S. Quevillon, S. Diriong, V. C. N′Guyen, M. Lipinski, and M. Mirande. 1994. Evolution of the Glx-tRNA synthetase family: the glutaminyl enzyme as a case of horizontal gene transfer. Proc. Natl. Acad. Sci. USA 91:8670-8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lapointe, J., and G. Delcuve. 1975. Thermosensitive mutants of Escherichia coli K-12 altered in the catalytic subunit and in a regulatory factor of the glutamy-transfer ribonucleic acid synthetase. J. Bacteriol. 122:352-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lapointe, J., L. Duplain, and M. Proulx. 1986. A single glutamyl-tRNA synthetase aminoacylates tRNAGlu and tRNAGln in Bacillus subtilis and efficiently misacylates Escherichia coli tRNAGln in vitro. J. Bacteriol. 165:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Min, B., J. T. Pelaschier, D. E. Graham, D. Tumbula-Hansen, and D. Soll. 2002. Transfer RNA-dependent amino acid biosynthesis: an essential route to asparagine formation. Proc. Natl. Acad. Sci. USA 99:2678-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nabholz, C. E., R. Hauser, and A. Schneider. 1997. Leishmania tarentolae contains distinct cytosolic and mitochondrial glutaminyl-tRNA synthetase activities. Proc. Natl. Acad. Sci. USA 94:7903-7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson, K. E., C. Weinel, I. T. Paulsen, R. J. Dodson, H. Hilbert, V. A. Martins dos Santos, D. E. Fouts, S. R. Gill, M. Pop, M. Holmes, L. Brinkac, M. Beanan, R. T. DeBoy, S. Daugherty, J. Kolonay, R. Madupu, W. Nelson, O. White, J. Peterson, H. Khouri, I. Hance, P. C. Lee, E. Holtzapple, D. Scanlan, K. Tran, A. Moazzez, T. Utterback, M. Rizzo, K. Lee, D. Kosack, D. Moestl, H. Wedler, J. Lauber, D. Stjepandic, J. Hoheisel, M. Straetz, S. Heim, C. Kiewitz, J. Eisen, K. N. Timmis, A. Dusterhoft, B. Tummler, and C. M. Fraser. 2002. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 4:799-808. [DOI] [PubMed] [Google Scholar]
- 24.Parkhill, J., M Achtman, K. D. James, S. D. Bentley, C. Churcher, S. R. Klee, G. Morelli, D. Basham, D. Brown, T. Chillingworth, R. M. Davies, P. Davis, K. Devlin, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, S. Leather, S. Moule, K. Mungall, M. A. Quail, M. A. Rajandream, K. M. Rutherford, M. Simmonds, J. Skelton, S. Whitehead, B. G. Spratt, and B. G. Barrell. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502-506. [DOI] [PubMed] [Google Scholar]
- 25.Raczniak, G., H. D. Becker, B. Min, and D. Soll. 2001. A single amidotransferase forms asparaginyl-tRNA and glutaminyl-tRNA in Chlamydia trachomatis. J. Biol. Chem. 276:45862-45867. [DOI] [PubMed] [Google Scholar]
- 26.Russell, R. R. B., and A. J. Pittard. 1971. Mutants of Escherichia coli unable to make protein at 42°C. J. Bacteriol. 108:790-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salazar, J. C., Z. Roberto, G. Raczniak, H. Becker, D. Soll, and O. Orellena. 2001. A dual-specific Glu-tRNAGln and Asp-tRNAAsn amidotransferase is involved in decoding glutamine and asparagine codons in Acidithiobacillus ferroxidans. FEBS Lett. 500:129-131. [DOI] [PubMed] [Google Scholar]
- 28.Sekine, S., O. Nureki, A. Shimada, D. G. Vassylyev, and S. Yokoyama. 2001. Structural basis for anticodon recognition by discriminating glutamyl-tRNA synthetase. Nat. Struct. Biol. 8:203-206. [DOI] [PubMed] [Google Scholar]
- 29.Sharples, G. J., and R. G. Lloyd. 1991. Location of a mutation in the aspartyl-tRNA synthetase gene of Escherichia coli K12. Mutat. Res. 264:93-96. [DOI] [PubMed] [Google Scholar]
- 30.Siatecka, M., M. Rozek, J. Barciszewski, and M. Mirande. 1998. Modular evolution of the glx-tRNA synthetase family: rooting of the evolutionary tree between the Bacteria and Archaea/Eucarya branches. Eur. J. Biochem. 256:80-87. [DOI] [PubMed] [Google Scholar]
- 31.Sonawane, A., U. Kloppner, C. Derst, and K. H. Rohm. 2003. Utilization of acidic amino acids and their amides by pseudomonads: role of periplasmic glutaminase-asparaginase. Arch. Microbiol. 179:151-159. [DOI] [PubMed] [Google Scholar]
- 32.Stathopoulos, C., T. Li, R. Longman, U. C. Vothknecht, H. D. Becker, M. Ibba, and D. Soll. 2000. One polypeptide with two aminoacyl-tRNA synthetase activities. Science 287:479-482. [DOI] [PubMed] [Google Scholar]
- 33.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 34.Tomb, J.-F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, G. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 35.Tumbula, D. L., H. D. Becker, W. Z. Chang, and D. Soll. 2000. Domain-specific recruitment of amide amino acids for protein synthesis. Nature 407:106-110. [DOI] [PubMed] [Google Scholar]
- 36.Watson, A. A., R. A. Alam, and J. S. Mattick. 1996. Construction of improved vectors for protein production in Pseudomonas aeruginosa. Gene 172:163-164. [DOI] [PubMed] [Google Scholar]
- 37.White, B. N., and S. T. Bayley. 1972. Further codon assignments in an extremely halophilic bacterium using a cell-free protein-synthesizing system and a ribosomal binding assay. Can. J. Biochem. 50:600-609. [DOI] [PubMed] [Google Scholar]
- 38.Wilcox, M., and M. Nirenberg. 1968. Transfer RNA as a cofactor coupling synthesis with that of protein. Proc. Natl. Acad. Sci. USA 61:229-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woese, C. R., G. J. Olsen, M. Ibba, and D. Soll. 2000. Aminoacyl-tRNA synthetases, the genetic code, and the evolutionary process. Microbiol. Mol. Biol. Rev. 64:202-326. [DOI] [PMC free article] [PubMed] [Google Scholar]