Abstract
Background
Corticotropin-releasing hormone (CRH) is expressed in the brain, immune cells and the gut, where gene expression is upregulated by lipopolysaccharide (LPS) 6 h after injection. Whether these changes are reflected by increased circulating levels of CRH and adrenocorticotropic hormone (ACTH) is unknown.
Methods
LPS (100 μg/kg) was injected intraperitoneally in conscious rats, and blood processed for CRH using the new RAPID (reduced temperatures, acidification, protease inhibition, isotopic exogenous controls and dilution) method compared with EDTA blood with or without plasma methanol extraction. Hormone levels were measured by commercial radioimmunoassay.
Results
The RAPID method improved blood recovery of 125I-CRH in vitro compared to EDTA only added to the blood without or with methanol extraction (90.8 ± 2.0 vs. 66.9 ± 2.6 and 47.5 ± 2.0%, respectively; p < 0.001 vs. RAPID). Basal CRH levels from blood processed by the RAPID method were 28.9 ± 2.8 pg/ml, and by other methods below the radioimmunoassay detection limit (<10 pg/ml). At 6 h after LPS, CRH plasma levels increased significantly by 2.9 times, and in the proximal colon tended to decrease (−27.6 ± 5.7%; p > 0.05), while circulating levels were unchanged at 3 or 4 h. ACTH levels rose compared to control rats (135.3 ± 13.8 vs. 101.4 ± 6.0 pg/ml; p < 0.05) 30 min after the increase in CRH, while at 3 or 6 h after LPS, the levels were not changed.
Conclusion
Intraperitoneal LPS induces a delayed rise in plasma CRH levels associated with an elevation in ACTH plasma levels 30 min later, suggesting that under conditions of immune challenge, CRH of peripheral origin may also contribute to pituitary activation, as detected using the RAPID method of blood processing, which improves CRH recovery.
Key Words: Adrenocorticotropic hormone, Blood, Colon, Gastric emptying, Lipopolysaccharide, RAPID method
Introduction
Corticotropin-releasing hormone (CRH) is mainly localized in the brain [1,2], and can also be detected in peripheral tissues including the gut [3,4,5] and immune cells and organs [6,7,8] in experimental animals and humans [9,10,11]. Although these tissues can be potential sources of circulating CRH, earlier studies have indicated that CRH levels are either nondetectable in rats or low in human plasma [12] as assessed by radioimmunoassay (RIA). This could be due to peptide binding to CRH-binding protein [13] – whose expression has been originally identified in the plasma [14] and also in the brain [15], liver [16] and placenta [17] – or due to difficulties in detection procedures related to peptide processing, degradation or loss. Alternatively, CRH may act as a paracrine hormone near its site of synthesis and therefore never reach meaningful levels in the blood [18].
We recently established a new method for blood processing, termed RAPID, which uses reduced temperatures, acidification, protease inhibition, isotopic exogenous controls and dilution of blood. The RAPID method improves recovery and eliminates breakdown for most of the gut peptides tested [19]. Therefore, we first assessed whether the RAPID method would also improve the recovery of exogenous radiolabeled CRH added to blood in vitro compared to blood collected with EDTA only or followed by methanol extraction of the plasma as commonly performed in previous plasma assessments of CRH [14,20,21,22,23,24]. To extend our findings to circulating CRH, we next assessed basal plasma CRH levels determined by RIA kit when trunk blood from naïve rats was processed according to the RAPID method or collected with EDTA and plasma subjected or not to methanol extraction. Next, we investigated whether peripheral injection of lipopolysaccharide (LPS) would influence circulating levels of CRH. LPS originating from Gram-negative bacterial cell walls is a well-established systemic immunological stressor stimulating the activity of the hypothalamic pituitary axis (HPA), resulting in increased hypothalamic CRH peptide levels and adrenocorticotropic hormone (ACTH) release [25,26]. In addition to the brain, we recently established that LPS injected peripherally at a low dose upregulates CRH mRNA expression and immunoreactivity in the rat colon at 6 h after injection [4]. However, whether the upregulation of CRH tissue expression by LPS translates into changes in circulating CRH levels at this time period is unknown. Therefore, we performed a time course of changes in plasma CRH induced by LPS to give insight into its regulation by an immune challenge and whether this is associated with elevation of ACTH plasma levels. As a potential source of circulating CRH at the time of the maximal response induced by LPS, we also assessed changes in tissue content of CRH in the proximal and distal colon.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (Harlan, San Diego, Calif., USA; body weight: 280–320 g) were group-housed under conditions of controlled illumination (12:12-hour light/dark cycle), humidity and temperature (22 ± 2°C) and maintained on standard rodent diet (Prolab RMH 2500; LabDiet; PMI Nutrition, Brentwood, Mo., USA) and tap water ad libitum. Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the federal authority for animal research conduct and were approved by the animal research committees at Veterans Affairs Greater Los Angeles Healthcare System (No. 11084-03 and 99059-04).
Blood and Tissue Processing
Blood Processing. Blood collected as described in experimental protocols was transferred to EDTA-containing borosilicate glass tubes on ice and in parallel immediately, within 7–10 min, processed according to the 3 methods. The first set of samples was processed according to the RAPID method as detailed previously [19]. Briefly, the blood was diluted 1:10 in ice-cold buffer (pH 3.6) containing 0.1 M ammonium acetate, 0.5 M NaCl and enzyme inhibitors (diprotin A, E-64-d, antipain, leupeptin, chymostatin, 1 μg/ml; Peptides International, Louisville, Ky., USA), then centrifuged at 3,000 rpm for 10 min at 4°C, and supernatants were collected. Sep-Pak C18 cartridges (360 mg, 55–105 μm; product No. WAT051910; Waters Corporation, Milford, Mass., USA) were charged with 100% acetonitrile, equilibrated with 0.1% trifluoroacetate (TFA) and loaded with the supernatant. Thereafter, they were washed with 0.1% TFA and eluted with 70% acetonitrile containing 0.1% TFA. Eluted samples were dried by vacuum centrifugation and stored at −80°C until RIA CRH determination. In parallel, for comparison, a second set of EDTA-containing blood samples was centrifuged at 3,000 g for 10 min at 4°C within 7 min, and the plasma supernatant transferred to other tubes kept at −80°C until CRH RIA. In a third set of EDTA-containing blood samples, plasma was formed and thereafter extracted with methanol, as previously described by Ellis et al. [23] and Linton et al. [24]. Briefly, plasma samples were mixed with 3 volumes of ice-cold methanol and incubated on ice for 10 min, then centrifuged at 3,000 rpm for 15 min at 4°C. Supernatants were transferred to different tubes, and the remaining pellets washed with another volume of ice-cold methanol and centrifuged. The resulting supernatant was combined with the first one and dried by vacuum centrifugation. Dried samples were stored at −80°C until CRH RIA.
Tissue Processing. The colon, harvested as detailed in the experimental protocol, was rinsed with ice-cold saline and after opening, separated in proximal (3 cm in length, starting at 1 cm distal to the cecum) and distal (3 cm in length, starting at 2 cm proximal to the anus) segments [4]. Pieces were weighed and whole tissue protein extracted as described previously [27]. Briefly, freshly dissected bowel segments were boiled in a water bath for 1 min, frozen at −20°C and homogenized in a 10-fold volume of 2% TFA. Thereafter, homogenized samples were centrifuged at 3,000 g for 10 min at 4°C, and the supernatant was collected and chromatographed in a step-wise fashion on Sep-Pak C18 cartridges (360 mg, 55–105 μm; product No. WAT051910; Waters Corporation). Eluted samples were dried by vacuum centrifugation, stored at −80°C and reconstituted in a volume of RIA buffer (1 μl/1 mg, according to the original tissue mass) immediately before CRH RIA.
RIA for CRH and ACTH
CRH Radioimmunoassay. Immediately before the RIA, the lyophilized samples obtained by the RAPID method or from plasma that underwent methanol extraction were reconstituted in RIA buffer in the original volume of plasma according to the manufacturer's instructions. Nonextracted plasma samples were thawed. CRH in plasma and colon was determined by a commercially available RIA kit (catalogue No. RK-019-06; Phoenix Pharmaceuticals, Burlingame, Calif., USA), and each sample was assayed in duplicate (sample volume: 100 μl) in 3 batches. Interassay and intraassay variability were 14 and 2%, respectively. The detection range of the assay was 10–1,280 pg/ml, and the CRH antibody used recognizes human, rat, mouse, canine and feline CRH and does not cross-react with other known peptides including urocortin-1 (technical information from Phoenix Pharmaceuticals).
ACTH Radioimmunoassay. Plasma ACTH levels were determined in trunk blood that was processed according to the manufacturer's suggestion. Briefly, EDTA- and aprotinin-containing (0.6 trypsin inhibitory units per milliliter of blood) blood was centrifuged, and the plasma acidified with an equal amount of buffer A (RK-BA-1; Phoenix Pharmaceuticals) and extracted on Sep-Pak C18 cartridges (360 mg, 55–105 μm; product No. WAT051910; Waters Corporation). The eluate was dried by vacuum centrifugation and stored at −80°C until the assay. Immediately prior to the ACTH RIA, samples were reconstituted in RIA buffer in a volume according to the original plasma volume. ACTH was measured by a commercially available RIA kit (catalogue No. RK-001-21; Phoenix Pharmaceuticals) in duplicate (sample volume: 100 μl) in 1 batch. The intraassay variability was <5%. The detection range of the assay was 10–1,280 pg/ml, and the ACTH antibody used recognizes rat and mouse ACTH and does not cross-react with other known peptides (technical information from Phoenix Pharmaceuticals).
CRH Peptide Recovery from RAPID Blood Processing or Plasma with or without Methanol Extraction
To compare the recovery of CRH peptide in blood, exogenous 125I-CRH (4,000–6,000 cpm in 50 μl of 0.1% acetic acid, Phoenix Pharmaceuticals) was added to 1-ml aliquots of blood collected in EDTA-rinsed syringes and obtained by cardiac puncture from naïve rats anesthetized with sodium pentobarbital (70 mg/kg, intraperitoneally; Nembutal; Abbott Laboratories). Thereafter, the blood was processed separately to compare recovery between samples which were either (1) diluted 1:10 in RAPID buffer and centrifuged, 1 ml of the supernatant being counted for radioactivity, or (2) centrifuged, plasma being extracted with ice-cold methanol and the extracts counted for radioactivity, or (3) centrifuged only, the plasma being counted for radioactivity. All samples were centrifuged at 3,000 g for 10 min at 4°C.
Basal Circulating CRH Levels Assessed after RAPID Blood Processing or Plasma Formation with or without Methanol Extraction in Naïve Rats
Naïve rats fed ad libitum (n = 5) were decapitated between 9.00 and 9.30 a.m., and trunk blood was collected in EDTA-containing ice-cold borosilicate glass tubes. Thereafter, 1 ml each was processed in parallel, either according to the RAPID method or to obtain the plasma that was processed or not with methanol extraction, as detailed above and previously [19,23,24]. Plasma CRH levels were determined using the RIA kit described above.
Plasma CRH and ACTH and Colonic CRH Levels in Response to Intraperitoneal Injection of LPS
Rats housed 2 per cage were injected intraperitoneally (300 μl) with either LPS (100 μg/kg body weight; Escherichia coli, serotype 055:B5; Sigma, St. Louis, Mo., USA) or vehicle (pyrogen-free saline) between 9.00 and 10.00 a.m. Animals had access to food and water ad libitum and remained housed 2 per cage throughout the experiment. Trunk blood was obtained from rats euthanized by decapitation at 3, 4 and 6 h after injection in different groups of rats (n = 5–7 per group and each time point) and processed using the RAPID method. Plasma CRH was determined by the RIA kit at 3, 4 and 6 h after LPS injection. As LPS at such dose and route of administration is known to delay gastric emptying in rats [28], the stomachs were also harvested and the contents weighed at 3, 4 and 6 h to evaluate the effectiveness of LPS injection, and the values are expressed as grams per kilogram body weight. Based on the data obtained for CRH levels in blood, in a subsequent study, the whole colon was quickly removed from rats euthanized by decapitation at 6 h after injection of LPS (100 μg/kg, i.p.) or vehicle, and tissue processed as described above to assess tissue CRH concentrations by RIA. Lastly, we assessed whether changes in plasma levels of CRH would translate into stimulation of ACTH release. Trunk blood was obtained from rats euthanized by decapitation at 3, 6 and 6.5 h after intraperitoneal LPS (100 μg/kg body weight) or vehicle injection (n = 5 per group and each time point). Plasma ACTH was measured by RIA according to the manufacturer's instructions. The 6.5-hour time point for ACTH measurement was chosen based on the plasma CRH results and previous studies where ACTH peaked within 5–60 min following a CRH stimulus [29,30].
Statistical Analysis
Data are expressed as means ± SEM, and they were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey post hoc test, or by two-way ANOVA followed by the Holm-Šidák method. Differences between groups were considered significant at p < 0.05.
Results
Recovery of Exogenous Radiolabeled CRH Added to Rat Blood
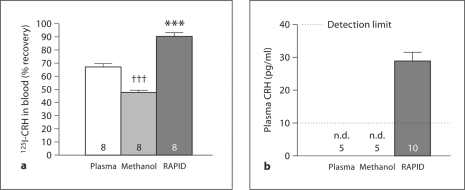
The RAPID method for blood processing significantly improved the in vitro recovery of 125I-CRH (4,000–5,000 cpm in 50 μl of 0.1% acetic acid) added to 1 ml rat blood compared to plasma formation without or with methanol extraction (90.8 ± 2.0, 66.9 ± 2.6 and 47.5 ± 2.0%, respectively; p < 0.001, RAPID vs. standard and methanol extraction; p < 0.001 standard vs. methanol extraction; n = 8) (fig. 1a).
Fig. 1.
Bars: means ± SEM of the number of rats indicated at the bottom. a The RAPID method (dark grey bar) significantly improves recovery of iodinated CRH added to rat blood compared to plasma formation (white bar) or methanol extraction (light grey bar). ∗∗∗ p < 0.001 vs. plasma formation or methanol extraction; ††† p < 0.001 vs. methanol extraction. b Plasma CRH levels after different blood processing methods: plasma formation, plasma methanol extraction or the RAPID method. Blood was withdrawn from nontreated rats fed ad libitum, and processed according to the 3 methods. Plasma CRH levels were measured by RIA. The RAPID method (dark grey bar) allows detection of plasma CRH levels, whereas CRH is not detectable (n.d.) after plasma formation or plasma methanol extraction.
CRH Levels in Blood Processed by RAPID Method versus Plasma Formation Only, Subjected or Not to Methanol Extraction
Trunk blood collected from naïve rats and processed by the RAPID method yields basal plasma CRH levels of 28.9 ± 2.8 pg/ml (n = 10) (fig. 1b). However, when the same EDTA-containing blood samples were only centrifuged to obtain plasma that was subjected or not to methanol extraction, CRH plasma levels were below the detectable threshold (not detectable, n.d., <10 pg/ml) (fig. 1b).
Gastric Contents after LPS Injection
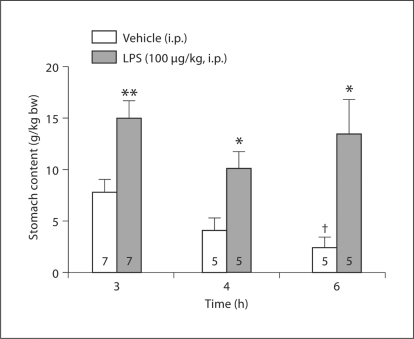
We assessed stomach content as an internal control for the effectiveness of LPS injected intraperitoneally at 100 μg/kg. The vehicle-injected groups had a steady emptying rate of their gastric content throughout the 6-hour experimental period, resulting in a significantly lower gastric content at 6 h compared to 3 h after vehicle injection (p < 0.05) (fig. 2). By contrast, in the LPS-injected groups, gastric content remained significantly increased, with similar values at 3, 4 and 6 h after injection, resulting in 1.9-, 2.4- and 5.2-fold higher values at 3, 4 and 6 h after LPS injection compared to the respective vehicle groups.
Fig. 2.
LPS injected intraperitoneally delays gastric emptying as reflected by increased stomach content in rats. The animals were left undisturbed in their cages and had access to food and water ad libitum until measurement points. After the rats had been decapitated for blood collection, their stomachs were carefully harvested and the contents weighed. Rats injected with vehicle had significantly lower gastric contents at 6 h compared to 3 h. bw = Body weight. Bars: means ± SEM of the number of rats indicated at the bottom. ∗ p < 0.05, ∗∗ p < 0.01 compared with vehicle at the respective time point; † p < 0.05 compared with vehicle at 3 h.
Plasma and Colonic CRH Levels after LPS Injection
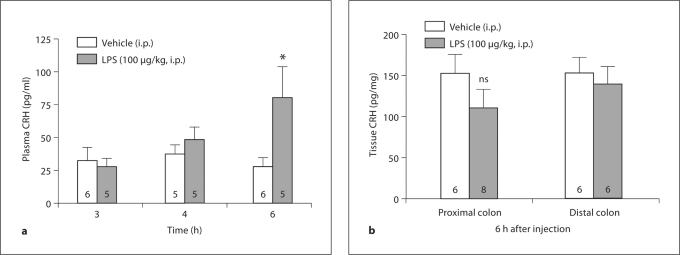
In intraperitoneally vehicle-injected rats, CRH plasma levels were not significantly different throughout the 3- to 6-hour postinjection blood sampling period (3 h: 32.2 ± 10.3 pg/ml; 4 h: 37.0 ± 7.2 pg/ml; 6 h: 27.3 ± 6.8 pg/ml). Intraperitoneal injection of LPS (100 μg/kg body weight) increased circulating CRH at 6 h after injection by 2.9 times compared to vehicle (80.0 ± 24.0 vs. 27.3 ± 6.9 pg/ml; p < 0.05), whereas at 3 and 4 h, no significant differences could be detected (3 h: 27.5 ± 6.1 pg/ml; 4 h: 47.8 ± 9.4 pg/ml; p > 0.05) (fig. 3a) compared to the respective vehicle groups. Two-way ANOVA showed a significant effect of treatment (F1, 24 = 4.6; p < 0.05) and treatment × time (F2, 24 = 3.4; p < 0.05). In the colon, the mean CRH tissue levels in the proximal segment were 27.6 ± 5.7% lower at 6 h after LPS injection compared to vehicle-treated rats (110.6 ± 22.7 vs. 152.8 ± 22.9 pg/mg; p > 0.05) (fig. 3b), although the difference did not reach statistical significance, and in the distal segment, values were not changed (139.2 ± 22.0 vs. 152.5 ± 19.5 pg/mg; p > 0.05) (fig. 3b).
Fig. 3.
Bars: means ± SEM of the number of rats indicated at the bottom. a LPS injected intraperitoneally increases plasma CRH levels after 6 h. Rats were injected intraperitoneally with LPS (100 μg/kg body weight) or vehicle (saline) and decapitated at the time points indicated at the x-axis. Trunk blood was collected and processed according to the RAPID method. ∗ p < 0.05 compared to vehicle. b Mean CRH tissue levels in the proximal colon show a trend towards a decrease at 6 h after LPS injection. Tissue of the proximal and distal colon was harvested at 6 h after LPS or vehicle injection, and total protein was extracted for RIA. ns = Not significant.
Plasma ACTH Levels after LPS Injection
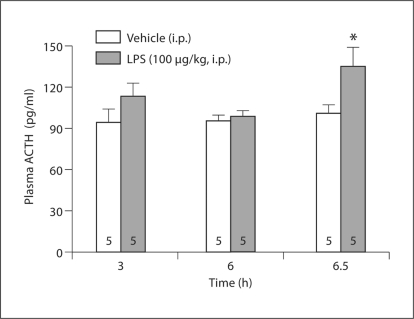
At 3, 6 and 6.5 h after intraperitoneal injection of vehicle, ACTH plasma levels remained unchanged (3 h: 94.8 ± 9.9 pg/ml; 6 h: 95.2 ± 5.0 pg/ml; 6.5 h: 101.4 ± 6.0 pg/ml). Plasma ACTH levels were significantly increased by 33% compared to vehicle (135.3 ± 13.8 pg/ml vs. 101.4 ± 6.0 pg/ml; p < 0.05) (fig. 4) at 6.5 h after LPS, while they were unchanged at 3 and 6 h after injection (113.0 ± 10.3 pg/ml and 99.0 ± 3.9 pg/ml) compared to the respective vehicle groups. Two-way ANOVA showed a significant effect of treatment (F1, 23 = 7.3; p < 0.05).
Fig. 4.
LPS injected intraperitoneally increases plasma ACTH levels after 6.5 h. Rats were injected intraperitoneally with LPS (100 μg/kg body weight) or vehicle (saline) and decapitated at the time points indicated at the x-axis. Trunk blood was collected and processed for ACTH RIA. ∗ p < 0.05 compared to vehicle.
Discussion
The present study showed that in trunk blood processed by the RAPID method, basal plasma CRH levels were 28.9 ± 2.8 pg/ml and remained in this range (27–37 pg/ml) at 3, 4 or 6 h after an intraperitoneal injection of saline in naïve rats thereafter maintained in their home cages. In addition, we showed that LPS at a low dose induced a rise in plasma CRH levels occurring at 6 h after injection that resulted in an elevation of plasma ACTH, while there were no changes at earlier time points.
Nunez et al. [31] obtained similar basal CRH plasma levels in 40-day-old Wistar rat pups (29.1 ± 4.2 pg/ml) in plasma after acidification and C18 column extraction using the same RIA kit. In earlier studies, low basal plasma levels of CRH ranging from 5 to 13 pg/ml were detected in rats [20,32,33] as monitored using CRH antibodies, tracers and RIA conditions developed within investigators’ laboratories, leading to detection limits of approximately 5 pg/ml [20,23,33]. We previously reported that the use of the RAPID method for blood processing enables accurate measurement of circulating gut peptide concentrations, as assessed for acylated ghrelin, cholecystokinin-58, gastrin-releasing peptide and somatostatin-28, for instance [19], and extended here to endogenous CRH. Indeed, when the trunk blood of naïve rats was processed directly to obtain plasma that was subjected or not to methanol extraction, CRH values were below the detection limit (10 pg/ml) of the commercial CRH RIA kit. Therefore, we determined the recovery of radiolabeled CRH from blood, and added 125I-CRH directly to whole blood prior to processing, as previously performed for a variety of gastrointestinal peptides [19]. We recovered 91, 67 and 47% of 125I-CRH added to blood processed either by the RAPID method or plasma formation alone followed or not by methanol extraction, respectively. Previously, when synthetic peptide was added to already formed plasma, either 55% [34] or 90% [32] CRH recovery was reported, while synthetic peptide added to methanol-extracted plasma from different species was recovered at 94–100% [24]. Thus, 125I-CRH added directly to blood, and synthetic CRH added to plasma, can give different recoveries, leading to the assumption that the native form of CRH may not be identical to the 125I-CRH in its recovery properties or that the CRH-binding protein may not bind 125I-CRH as avidly as endogenous CRH in rats, as has been suggested before [14]. Thus, if the binding protein is pelleted during centrifugation, less loss of labeled peptide versus endogenous peptide could occur. It is to note that the reduced recovery of 125I-CRH in the direct plasma formation or plasma methanol extraction method compared to the RAPID method does not fully account for the lack of endogenous plasma CRH detection, and there is no clear explanation for this discrepancy. However, irrespective of whether such a difference may be related to the reduction in peptide degradation by diluting samples and using enzyme inhibitors along with the elimination of the binding of CRH to its binding protein, achieved by acid treatment, the present data indicate that the RAPID blood processing allows the detection of basal circulating levels of CRH in rats with a commercial RIA kit presently predominantly used to detect CRH release in cell cultures or hypothalamic tissue extracts [35,36,37]. It is to note that the CRH antibody was raised against the full-length synthetic 41-aa CRH peptide that is conserved in humans and rats and recognizes full-length 41-aa CRH (technical note of the company). Nonetheless, it cannot be excluded that the CRH immunoreactivity measured in the plasma by us and others [31,38] with this CRH antibody originates from CRH precursor or degraded and/or processed pro-CRH to form endogenously circulating different CRH molecular forms, as it has been characterized for other circulating peptides such as cholecystokinin and peptide YY [39,40]. Existing evidence indicates that the major form of CRH detected in human placenta under conditions of adequate protease inhibition is the unprocessed pro-CRH, unlike the mature CRH [41]. However, in the blood, the mature 41-aa CRH was the major molecular form detected in pregnant women [41]. In a follow-up study, HPLC characterization of circulating CRH will give new insight whether different molecular forms of CRH exist in rat blood.
We next used the RAPID blood processing method to assess whether stress in form of an acute immune challenge alters circulating levels of CRH. LPS at a low dose of 100 μg/kg injected intraperitoneally is well established to delay gastric transit of nutrients and reduce food intake as part of the functional responses to this immune stressor [28]. We previously reported that LPS injected intraperitoneally at a similar dose inhibited gastric emptying of a nonnutrient viscous solution by 87%, as monitored during the 20-min period at 5 h after injection in overnight-fasted rats [28]. We extended these findings by showing that gastric contents of freely fed rats remained significantly increased by 2 and 5 times at 3 and 6 h after LPS injection, respectively, compared to the vehicle group, which had a linear time-related decrease in gastric contents over the 6-hour period. The significant difference between values of stomach content at 6 versus 3 h after vehicle injection reflects the physiological time course of gastric emptying in control rats fed ad libitum in response to nocturnal feeding followed by a low drive to eat during the light phase [42].
Under these conditions of stress-related functional changes in upper gut transit induced by intraperitoneal LPS [43], plasma CRH levels were increased by 2.9 times compared to vehicle at 6 h after injection, whereas at earlier time points, no significant differences could be detected. Although the origin of circulating CRH occurring at 6 h after intraperitoneal LPS injection cannot be ascertained from the present study, previous and present evidence points towards a possible more prominent contribution of the gut and/or immune cells rather than of the hypothalamus. First, the time course of plasma CRH response to LPS and the reported activation of the HPA are not synchronized. The activation of ACTH and corticosterone release following LPS at a similar dose as used in the present study displayed a rapid onset that peaked within 1–2 h after injection [44,45,46], at a time when we observed no change in plasma levels of CRH. Secondly, the rise in plasma CRH levels observed at 6 h after LPS injection is temporarily correlated with a 2.5-fold upregulation of CRH mRNA levels in the proximal colon 6 h after injection of LPS under the same conditions in rats [4]. At the cellular level, CRH immunoreactivity was localized in epithelial enterochromaffin and lamina propria cells, and CRH immunoreactivity increased in myenteric neurons 6 h after LPS injection [4]. In the present study, the CRH levels in the proximal and distal colon were not significantly different in the LPS and vehicle groups, although the values were 28% lower in the proximal colon 6 h after LPS. Other studies showed that neonatal maternal separation stress increased prepro-CRH in distal colonic mucosal lamina propria and crypts in adult rats, as assessed by immunohistochemical labeling, while distal colonic CRH measured by ELISA was unchanged [47]. In addition, intestinal immune cells including eosinophils, macrophages and T cells expressed CRH and could respond to stress by releasing the peptide [8]. The coupling of increased CRH synthesis and release after LPS may explain the nonsignificant decrease observed in the colon and rise in plasma levels at the same time point. Although these data suggest that the activation of the intestinal CRH signaling system following immune challenge stress may represent a peripheral source of circulating CRH under these conditions, it cannot be ruled out that CRH could also be derived from nonintestinal immune cells where CRH was found, including the thymus, spleen and circulating immune cells [6,10,48,49]. Furthermore, CRH gene expression was reported to be higher in blood cells of infected cattle compared with noninfected [50], and LPS increased the secretion of CRH from B and T lymphocytes [10], and other T-cell-activating agents had similar enhancing effects on CRH mRNA levels in T lymphocytes in humans [51]. These data support the hypothesis that upon activation by LPS, immune cells could release CRH into the circulation and thus may represent another source of CRH.
The mechanisms underlying the delayed-onset rise of plasma CRH may involve time required for upregulation of CRH gene expression by cytokines acting downstream of LPS action consistent with an immune origin of circulating CRH [52]. The Toll-like receptor 4 is the principal mediator of the macrophage response to LPS [53], and an interaction between CRH and LPS has recently been suggested as CRH augments LPS-induced proinflammatory cytokine production mediated via the corticotropin-releasing factor 2 receptor in mouse macrophages, further increasing their sensitivity to LPS [54]. Thus, is can be speculated that the delayed LPS-induced rise in circulating CRH is linked with the late macrophage response to an immune challenge via the Toll-like receptor 4.
The present study also showed that the rise in circulating levels of CRH at 6 h after LPS injection was associated with a significant elevation in plasma ACTH levels measured 30 min later. Such a response is consistent with the temporal relation between ACTH release and exogenous elevation of circulating levels of CRH [55]. By contrast, at 3 and 6 h after LPS injection, plasma ACTH plasma levels were similar to those in the vehicle groups. These findings indicate that circulating CRH may act on the pituitary to induce a second rise in plasma ACTH in addition to the initial ACTH elevation. Indeed, activation of the HPA induced by intraperitoneal injection of LPS has been well established to occur within the first hour and to decay thereafter [29,44,45]. However, it cannot be ruled out that CRH acts directly on leukocytes, which in turn release ACTH into the circulation [56]. Taken together, these data lend support to earlier observations that several hours after injection, LPS was able to activate the pituitary-adrenal system in the absence of hypophysiotropic neuropeptides of paraventricular hypothalamic origin [57].
In summary, we demonstrated that the RAPID method of blood processing improved the recovery of 125I-CRH and allowed the detection of CRH plasma levels under basal conditions in naïve rats, using CRH RIA kits. This contrasts with CRH levels below detection limits when plasma was directly formed and subjected or not to methanol extraction. In addition, we showed that peripheral injection of LPS at a dose of 100 μg/kg, altering gastric propulsive motor function, induced a 2.9-fold elevation of CRH plasma levels occurring at 6 h after injection, while levels were unchanged at earlier time points. The delayed rise in circulating CRH results in an elevation of plasma ACTH levels occurring 30 min later. The colon, which in addition to the brain is a target site for LPS-induced upregulation of CRH gene expression, at 6 h after injection of LPS at a similar dose [4] showed a trend towards reduced tissue levels (present study), and may therefore contribute to the pool of circulating CRH, although other peripheral sources of immune origin may also contribute.
Acknowledgements
This study was supported by German Research Foundation fellowship grants GO 1718/1-1 (M.G.) and STE 1765/1-1 (A.S.), the VA Research Career Scientist Award, a Department of Veterans Affairs Merit Award (Y.T.), NIHDK 57238 (Y.T.) and Center grant DK-41301 (Animal Core, Y.T.; Peptidomic RIA Proteomic Core, J.R. Jr.). We are grateful to Mrs. Honghui Liang for her excellent technical support and Ms. Eugenia Hu for reviewing the manuscript.
References
- 1.Bloom FE, Battenberg EL, Rivier J, Vale W. Corticotropin releasing factor (CRF): immunoreactive neurones and fibers in rat hypothalamus. Regul Pept. 1982;4:43–48. doi: 10.1016/0167-0115(82)90107-0. [DOI] [PubMed] [Google Scholar]
- 2.Suda T, Tozawa F, Mouri T, Demura H, Shizume K. Presence of immunoreactive corticotropin-releasing factor in human cerebrospinal fluid. J Clin Endocrinol Metab. 1983;57:225–226. doi: 10.1210/jcem-57-1-225. [DOI] [PubMed] [Google Scholar]
- 3.van Tol EA, Petrusz P, Lund PK, Yamauchi M, Sartor RB. Local production of corticotropin releasing hormone is increased in experimental intestinal inflammation in rats. Gut. 1996;39:385–392. doi: 10.1136/gut.39.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan PQ, Wu SV, Wang L, Taché Y. Corticotropin releasing factor in the rat colon: expression, localization and upregulation by endotoxin. Peptides. 2010;31:322–331. doi: 10.1016/j.peptides.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Gao N, Hu HZ, Wang X, Wang GD, Fang X, Gao X, Xia Y, Wood JD. Distribution and chemical coding of corticotropin-releasing factor-immunoreactive neurons in the guinea pig enteric nervous system. J Comp Neurol. 2006;494:63–74. doi: 10.1002/cne.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aird F, Clevenger CV, Prystowsky MB, Redei E. Corticotropin-releasing factor mRNA in rat thymus and spleen. Proc Natl Acad Sci USA. 1993;90:7104–7108. doi: 10.1073/pnas.90.15.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baigent SM, Lowry PJ. mRNA expression profiles for corticotrophin-releasing factor (CRF), urocortin, CRF receptors and CRF-binding protein in peripheral rat tissues. J Mol Endocrinol. 2000;25:43–52. doi: 10.1677/jme.0.0250043. [DOI] [PubMed] [Google Scholar]
- 8.Zheng PY, Feng BS, Oluwole C, Struiksma S, Chen X, Li P, Tang SG, Yang PC. Psychological stress induces eosinophils to produce corticotrophin releasing hormone in the intestine. Gut. 2009;58:1473–1479. doi: 10.1136/gut.2009.181701. [DOI] [PubMed] [Google Scholar]
- 9.Baker C, Richards LJ, Dayan CM, Jessop DS. Corticotropin-releasing hormone immunoreactivity in human T and B cells and macrophages: colocalization with arginine vasopressin. J Neuroendocrinol. 2003;15:1070–1074. doi: 10.1046/j.1365-2826.2003.01099.x. [DOI] [PubMed] [Google Scholar]
- 10.Kravchenco IV, Furalev VA. Secretion of immunoreactive corticotropin releasing factor and adrenocorticotropic hormone by T- and B-lymphocytes in response to cellular stress factors. Biochem Biophys Res Commun. 1994;204:828–834. doi: 10.1006/bbrc.1994.2534. [DOI] [PubMed] [Google Scholar]
- 11.Kawahito Y, Sano H, Kawata M, Yuri K, Mukai S, Yamamura Y, Kato H, Chrousos GP, Wilder RL, Kondo M. Local secretion of corticotropin-releasing hormone by enterochromaffin cells in human colon. Gastroenterology. 1994;106:859–865. doi: 10.1016/0016-5085(94)90743-9. [DOI] [PubMed] [Google Scholar]
- 12.Linton EA, McLean C, Nieuwenhuyzen Kruseman AC, Tilders FJ, van der Veen EA, Lowry PJ. Direct measurement of human plasma corticotropin-releasing hormone by ‘two-site’ immunoradiometric assay. J Clin Endocrinol Metab. 1987;64:1047–1053. doi: 10.1210/jcem-64-5-1047. [DOI] [PubMed] [Google Scholar]
- 13.Suda T, Iwashita M, Ushiyama T, Tozawa F, Sumitomo T, Nakagami Y, Demura H, Shizume K. Responses to corticotropin-releasing hormone and its bound and free forms in pregnant and nonpregnant women. J Clin Endocrinol Metab. 1989;69:38–42. doi: 10.1210/jcem-69-1-38. [DOI] [PubMed] [Google Scholar]
- 14.Orth DN, Mount CD. Specific high-affinity binding protein for human corticotropin-releasing hormone in normal human plasma. Biochem Biophys Res Commun. 1987;143:411–417. doi: 10.1016/0006-291x(87)91369-6. [DOI] [PubMed] [Google Scholar]
- 15.Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW. The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci USA. 1992;89:4192–4196. doi: 10.1073/pnas.89.9.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behan DP, de Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995;16:362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- 17.Linton EA, Wolfe CD, Behan DP, Lowry PJ. A specific carrier substance for human corticotrophin releasing factor in late gestational maternal plasma which could mask the ACTH-releasing activity. Clin Endocrinol (Oxf) 1988;28:315–324. doi: 10.1111/j.1365-2265.1988.tb01218.x. [DOI] [PubMed] [Google Scholar]
- 18.Gravanis A, Makrigiannakis A, Chatzaki E, Zoumakis E, Tsatsanis C, Margioris AN. Stress neuropeptides in the human endometrium: paracrine effects on cell differentiation and apoptosis. Hormones (Athens) 2002;1:139–148. doi: 10.14310/horm.2002.1161. [DOI] [PubMed] [Google Scholar]
- 19.Stengel A, Keire D, Goebel M, Evilevitch L, Wiggins B, Taché Y, Reeve JR., Jr The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology. 2009;150:5113–5118. doi: 10.1210/en.2009-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokoe T, Audhya T, Brown C, Hutchinson B, Passarelli J, Hollander CS. Corticotropin-releasing factor levels in the peripheral plasma and hypothalamus of the rat vary in parallel with changes in the pituitary-adrenal axis. Endocrinology. 1988;123:1348–1354. doi: 10.1210/endo-123-3-1348. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe CD, Patel SP, Linton EA, Campbell EA, Anderson J, Dornhorst A, Lowry PJ, Jones MT. Plasma corticotrophin-releasing factor (CRF) in abnormal pregnancy. Br J Obstet Gynaecol. 1988;95:1003–1006. doi: 10.1111/j.1471-0528.1988.tb06504.x. [DOI] [PubMed] [Google Scholar]
- 22.Alexander SL, Irvine CH, Ellis MJ, Donald RA. The effect of acute exercise on the secretion of corticotropin-releasing factor, arginine vasopressin, and adrenocorticotropin as measured in pituitary venous blood from the horse. Endocrinology. 1991;128:65–72. doi: 10.1210/endo-128-1-65. [DOI] [PubMed] [Google Scholar]
- 23.Ellis MJ, Livesey JH, Donald RA. Circulating plasma corticotrophin-releasing factor-like immunoreactivity. J Endocrinol. 1988;117:299–307. doi: 10.1677/joe.0.1170299. [DOI] [PubMed] [Google Scholar]
- 24.Linton EA, Perkins AV, Hagan P, Poole S, Bristow AF, Tilders F, Corder R, Wolfe CD. Corticotrophin-releasing hormone (CRH)-binding protein interference with CRH antibody binding: implications for direct CRH immunoassay. J Endocrinol. 1995;146:45–53. doi: 10.1677/joe.0.1460045. [DOI] [PubMed] [Google Scholar]
- 25.Turnbull AV, Lee S, Rivier C. Mechanisms of hypothalamic-pituitary-adrenal axis stimulation by immune signals in the adult rat. Ann NY Acad Sci. 1998;840:434–443. doi: 10.1111/j.1749-6632.1998.tb09582.x. [DOI] [PubMed] [Google Scholar]
- 26.Beishuizen A, Thijs LG. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J Endotoxin Res. 2003;9:3–24. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- 27.Reeve JR, Jr, Liddle RA, McVey DC, Vigna SR, Solomon TE, Keire DA, Rosenquist G, Shively JE, Lee TD, Chew P, Green GM, Coskun T. Identification of nonsulfated cholecystokinin-58 in canine intestinal extracts and its biological properties. Am J Physiol Gastrointest Liver Physiol. 2004;287:G326–G333. doi: 10.1152/ajpgi.00520.2003. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St-Pierre DH, Taché Y. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am J Physiol Gastrointest Liver Physiol. 2006;291:G611–G620. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- 29.Rivier C, Brownstein M, Spiess J, Rivier J, Vale W. In vivo corticotropin-releasing factor-induced secretion of adrenocorticotropin, β-endorphin, and corticosterone. Endocrinology. 1982;110:272–278. doi: 10.1210/endo-110-1-272. [DOI] [PubMed] [Google Scholar]
- 30.Rivier CL, Grigoriadis DE, Rivier JE. Role of corticotropin-releasing factor receptors type 1 and 2 in modulating the rat adrenocorticotropin response to stressors. Endocrinology. 2003;144:2396–2403. doi: 10.1210/en.2002-0117. [DOI] [PubMed] [Google Scholar]
- 31.Nunez H, Ruiz S, Soto-Moyano R, Navarrete M, Valladares L, White A, Perez H. Fetal undernutrition induces overexpression of CRH mRNA and CRH protein in hypothalamus and increases CRH and corticosterone in plasma during postnatal life in the rat. Neurosci Lett. 2008;448:115–119. doi: 10.1016/j.neulet.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Suda T, Tomori N, Yajima F, Sumitomo T, Nakagami Y, Ushiyama T, Demura H, Shizume K. Immunoreactive corticotropin-releasing factor in human plasma. J Clin Invest. 1985;76:2026–2029. doi: 10.1172/JCI112204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sumitomo T, Suda T, Tomori N, Yajima F, Nakagami Y, Ushiyama T, Demura H, Shizume K. Immunoreactive corticotropin-releasing factor in rat plasma. Endocrinology. 1987;120:1391–1396. doi: 10.1210/endo-120-4-1391. [DOI] [PubMed] [Google Scholar]
- 34.Nishioka T, Iyota K, Nakayama T, Suemaru S, Numata Y, Hashimoto K. Effects of ether-laparotomy and water immersion-restraint stress on CRH concentration in the hypothalamus, extrahypothalamic tissues and peripheral blood. Endocr J. 1993;40:213–220. doi: 10.1507/endocrj.40.213. [DOI] [PubMed] [Google Scholar]
- 35.Dijkstra I, Tilders FJ, Aguilera G, Kiss A, Rabadan-Diehl C, Barden N, Karanth S, Holsboer F, Reul JM. Reduced activity of hypothalamic corticotropin-releasing hormone neurons in transgenic mice with impaired glucocorticoid receptor function. J Neurosci. 1998;18:3909–3918. doi: 10.1523/JNEUROSCI.18-10-03909.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kappeler L, Zizzari P, Grouselle D, Epelbaum J, Bluet-Pajot MT. Plasma and hypothalamic peptide-hormone levels regulating somatotroph function and energy balance in fed and fasted states: a comparative study in four strains of rats. J Neuroendocrinol. 2004;16:980–988. doi: 10.1111/j.1365-2826.2004.01259.x. [DOI] [PubMed] [Google Scholar]
- 37.Smagin GN, Howell LA, Redmann S, Jr, Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. Am J Physiol. 1999;276:R1461–R1468. doi: 10.1152/ajpregu.1999.276.5.R1461. [DOI] [PubMed] [Google Scholar]
- 38.Vesely DL, San Miguel GI, Hassan I, Schocken DD. Atrial natriuretic hormone, vessel dilator, long-acting natriuretic hormone, and kaliuretic hormone decrease the circulating concentrations of CRH, corticotropin, and cortisol. J Clin Endocrinol Metab. 2001;86:4244–4249. doi: 10.1210/jcem.86.9.7829. [DOI] [PubMed] [Google Scholar]
- 39.Grandt D, Schimiczek M, Beglinger C, Layer P, Goebell H, Eysselein VE, Reeve JR., Jr Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY1–36 and PYY3–36. Regul Pept. 1994;51:151–159. doi: 10.1016/0167-0115(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 40.Reeve JR, Jr, Green GM, Chew P, Eysselein VE, Keire DA. CCK-58 is the only detectable endocrine form of cholecystokinin in rat. Am J Physiol Gastrointest Liver Physiol. 2003;285:G255–G265. doi: 10.1152/ajpgi.00523.2002. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed I, Glynn BP, Perkins AV, Castro MG, Rowe J, Morrison E, Linton EA. Processing of procorticotropin-releasing hormone (pro-CRH): molecular forms of CRH in normal and preeclamptic pregnancy. J Clin Endocrinol Metab. 2000;85:755–764. doi: 10.1210/jcem.85.2.6351. [DOI] [PubMed] [Google Scholar]
- 42.Rosenwasser AM, Boulos Z, Terman M. Circadian organization of food intake and meal patterns in the rat. Physiol Behav. 1981;27:33–39. doi: 10.1016/0031-9384(81)90296-1. [DOI] [PubMed] [Google Scholar]
- 43.Stengel A, Taché Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol. 2009;71:219–239. doi: 10.1146/annurev.physiol.010908.163221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takemura T, Makino S, Takao T, Asaba K, Suemaru S, Hashimoto K. Hypothalamic-pituitary-adrenocortical responses to single vs repeated endotoxin lipopolysaccharide administration in the rat. Brain Res. 1997;767:181–191. doi: 10.1016/s0006-8993(97)00460-5. [DOI] [PubMed] [Google Scholar]
- 45.Lenczowski MJ, van Dam AM, Poole S, Larrick JW, Tilders FJ. Role of circulating endotoxin and interleukin-6 in the ACTH and corticosterone response to intraperitoneal LPS. Am J Physiol. 1997;273:R1870–R1877. doi: 10.1152/ajpregu.1997.273.6.R1870. [DOI] [PubMed] [Google Scholar]
- 46.Watanobe H, Yoneda M. A mechanism underlying the sexually dimorphic ACTH response to lipopolysaccharide in rats: sex steroid modulation of cytokine binding sites in the hypothalamus. J Physiol. 2003;547:221–232. doi: 10.1113/jphysiol.2002.032169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barreau F, Cartier C, Leveque M, Ferrier L, Moriez R, Laroute V, Rosztoczy A, Fioramonti J, Bueno L. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J Physiol. 2007;580:347–356. doi: 10.1113/jphysiol.2006.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redei E. Immuno-reactive and bioactive corticotropin-releasing factor in rat thymus. Neuroendocrinology. 1992;55:115–118. doi: 10.1159/000126104. [DOI] [PubMed] [Google Scholar]
- 49.Chowdrey HS, Lightman SL, Harbuz MS, Larsen PJ, Jessop DS. Contents of corticotropin-releasing hormone and arginine vasopressin immunoreactivity in the spleen and thymus during a chronic inflammatory stress. J Neuroimmunol. 1994;53:17–21. doi: 10.1016/0165-5728(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Aodon-geril H, Shu Y, Momotani Y, Wang X, Mori Y, Momotani E. Corticotropin-releasing hormone and urocortin expression in peripheral blood cells from experimentally infected cattle with Mycobacterium avium subsp. paratuberculosis. Microbes Infect. 2007;9:1061–1069. doi: 10.1016/j.micinf.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 51.Ekman R, Servenius B, Castro MG, Lowry PJ, Cederlund AS, Bergman O, Sjögren HO. Biosynthesis of corticotropin-releasing hormone in human T-lymphocytes. J Neuroimmunol. 1993;44:7–13. doi: 10.1016/0165-5728(93)90262-w. [DOI] [PubMed] [Google Scholar]
- 52.Givalois L, Dornand J, Mekaouche M, Solier MD, Bristow AF, Ixart G, Siaud P, Assenmacher I, Barbanel G. Temporal cascade of plasma level surges in ACTH, corticosterone, and cytokines in endotoxin-challenged rats. Am J Physiol. 1994;267:R164–R170. doi: 10.1152/ajpregu.1994.267.1.R164. [DOI] [PubMed] [Google Scholar]
- 53.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 54.Tsatsanis C, Androulidaki A, Alissafi T, Charalampopoulos I, Dermitzaki E, Roger T, Gravanis A, Margioris AN. Corticotropin-releasing factor and the urocortins induce the expression of TLR4 in macrophages via activation of the transcription factors PU.1 and AP-1. J Immunol. 2006;176:1869–1877. doi: 10.4049/jimmunol.176.3.1869. [DOI] [PubMed] [Google Scholar]
- 55.Rivier CL, Plotsky PM. Mediation by corticotropin releasing factor (CRF) of adenohypophysial hormone secretion. Annu Rev Physiol. 1986;48:475–494. doi: 10.1146/annurev.ph.48.030186.002355. [DOI] [PubMed] [Google Scholar]
- 56.Smith EM, Morrill AC, Meyer WJ, 3rd, Blalock JE. Corticotropin releasing factor induction of leukocyte-derived immunoreactive ACTH and endorphins. Nature. 1986;321:881–882. doi: 10.1038/321881a0. [DOI] [PubMed] [Google Scholar]
- 57.Elenkov IJ, Kovacs K, Kiss J, Bertok L, Vizi ES. Lipopolysaccharide is able to bypass corticotrophin-releasing factor in affecting plasma ACTH and corticosterone levels: evidence from rats with lesions of the paraventricular nucleus. J Endocrinol. 1992;133:231–236. doi: 10.1677/joe.0.1330231. [DOI] [PubMed] [Google Scholar]