Abstract
Background
Meckel syndrome (MKS) is a fatal autosomal recessive condition with prominent renal cystic pathology. Renal protein misexpression was evaluated in the Wpk rat model of human MKS3 gene disease to identify biomarkers for the staging of renal cystic progression.
Methods
Misexpressed proteins were compared between early and late stages of MKS renal cystic disease using proteomic analysis (two-dimensional gel electrophoresis with LC-MS/MS identification) followed by Western blot analysis.
Results
A proteomic analysis identified 76 proteins with statistically different, normalized abundance in at least one group. Subsequently, Western blot was used to confirm differential expression in several of these and polycystic kidney disease (PKD)-associated proteins. Galectin-1 and vimentin were identified as overexpressed proteins, which have been previously found in the jck mouse model of nephronophthisis 9. Ciliopathic PKD proteins, polycystins 1 & 2, and fibrocystin were also differentially expressed in Wpk kidney.
Conclusion
In the Wpk rat, misexpressed proteins were identified that were also implicated in other forms of cystic disease. Numerous proteins were either over- or underexpressed in late-stage disease. Differences in protein expression may serve as biomarkers of cystic disease and its progression.
Key Words: Polycystic kidney, Biomarker, Proteomics
Introduction
Meckel (a.k.a. Meckel-Gruber) syndrome (MKS) is a lethal autosomal recessive condition associated with multiorgan pathology. MKS gene protein products are localized to cilia, basal bodies and/or centrosomes similar to other hepatorenal fibrocystic diseases. Hepatorenal fibrocystic diseases are characterized by cysts and fibrosis of kidney and liver with polycystic kidney disease (PKD) as the most common form while Meckel syndrome (MKS) appears to be the most severe form. MKS usually causes a fetal or rapid postnatal demise with renal cystic pathology, central nervous system and other systemic abnormalities [1]. The Wpk rat model has a single nucleotide change in the orthologous gene to human Meckel syndrome (MKS3). The MKS3 gene encodes for the transmembrane protein meckelin (108 kDa), with widespread organ expression [2]. Rats homozygous for the Wpk mutation exhibit a phenotype similar to human MKS including prominently cystic kidneys, but are viable [3]. Wistar-Wpk rats survive through weaning, so it was possible to evaluate differences in protein expression that might serve as biomarkers of disease severity.
Proteomics of renal cystic disease is a new field of research. Valkova et al. [4] studied the renal proteome of jck mice (a model of human nephronophthisis 9), finding overexpression of galectin-1, vimentin and sorcin in cystic kidneys. We hypothesize that rodent samples may be a source of important proteomic information, identifying potential biomarkers for the disease and its progression. We performed a proteomic analysis of 10-day (early stage) and 21-day (late stage) Wpk cystic kidneys compared to normal littermates to identify misexpressed proteins that were increased (or decreased) later in the disease that may be useful biomarkers for disease progression.
Materials and Methods
Antibodies
Mouse monoclonal antibodies (mAb) to MDGI (mammary-derived growth inhibitor – heart-type fatty acid-binding protein, sc-58275, 1:400) and GAPDH (sc-47724, 1:1,000); polyclonal antibody (pAb) to laminin-R (sc-20979, 1:200), galectin-1 (sc-19277, 1:1,000), HSC-70 (heat-shock cognate 71, sc-1059, 1:200), VCP (ATPase p97, sc-9783, 1:400) and polycystin-2 (sc-10377) were from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif., USA). Mouse mAb to vimentin (ab8069, 1:35) and rabbit pAb to GRP78 (78-kDa glucose-regulated protein, ab21685, 1:2,000) were from Abcam (Cambridge, Mass., USA). Mouse mAb to polycystin-1 (7e12) and fibrocystin (mAb 11, mAb 5a) were provided by Christopher Ward (Mayo Clinic, Rochester, Minn., USA). Horseradish peroxidase (HRP)-conjugated secondary antibodies to mouse (#115036068), goat (#705035147) and rabbit primary antibodies (#711035152) were from Jackson ImmunoResearch Laboratories (West Grove, Pa., USA).
Animals
A breeding colony of Wpk rats was established at Indiana University School of Medicine [3]. All animal procedures were approved by the Indiana University Institutional Animal Care and Use Committee. Male and female Wistar-Wpk/+ rats were bred producing the homozygous cystic (experimental group) and the phenotypically normal littermates (control group). Male and female offspring were evaluated at 10 and 21 days of age. At termination, the rats were anesthetized (sodium pentobarbital, 100 mg/kg, i.p.), systemically perfused with 0.9% saline, the left kidney removed (and snap frozen in liquid nitrogen) while the right kidney was perfusion fixed (4% paraformaldehyde) and paraffin processed for light microscopy. Prior to proteomic analysis, the left kidney samples were stored at −45°C and processed at one time.
Preparation of Renal Samples
One kidney from each animal was thawed, cut and blotted onto an absorbent napkin to remove cyst fluid from the grossly cystic specimens. Kidneys were minced, sonicated in a solubilization solution (9 M urea, 1% dithiothreitol and 4% CHAPS), and protein concentration determined (2-D Quant Kit, Amersham Biosciences, Pittsburgh, Pa., USA).
Two-Dimensional Gel Electrophoresis
18 Wpk rats were studied with two-dimensional (2-D) electrophoresis (6 10-day compared with 12 21-day rats, vs. normal kidneys, all gender matched). Kidney samples (750 μg) were loaded onto immobilized pH gradient strips to separate proteins by isoelectric point (pI), then applied to slab gels (Jule Inc., Milford, Conn., USA) and separated in the second dimension based on mass. Gels were stained with colloidal Coomassie Blue, scanned with an ImageScanner III (GE Healthcare, Piscataway, N.J., USA) and analyzed with Progenesis SameSpots software (Nonlinear Dynamics, Ltd, Durham, N.C., USA) to identify significantly different (p < 0.01) protein spots in at least one group using ANOVA. Spots with significant differential expression (>1.5-fold) and with a misexpression pattern of interest were cut from the gels and subjected to LC-MS/MS analysis for identification.
Mass Spectrometry
Gel plugs were destained with acetonitrile in 50 mM NH4HCO3, reduced with 10 mM DTT, alkylated with iodoacetamide, and digested with modified trypsin at 37°C overnight, followed by serial extraction with acetonitrile. Tryptic peptides were injected onto a C18 microbore reversed-phase liquid chromatography (Zorbax 300SB-C18) column at 50 μl/min. Data was collected in the ‘Data dependent MS/MS’ mode with electrospray interface and searched against the International Protein Index rat database (ipi.RAT.v3.51.fasta) using Sequest® (v.28 rev. 12) algorithms in Bioworks (v. 3.3) and validated in the Trans-Proteomic Pipeline (v.3.3.0) (http://tools.proteomecenter.org/software.php).
Western Blot Analysis
10- and 21-day normal and cystic kidneys were homogenized in buffer (1% Triton ×100, 150 mM NaCl, 20 mM Tris, 2 mM EDTA with protease inhibitor at pH 8). Protein concentration was assessed using the micro-BCA protein assay (Pierce Co. part of Thermo Fisher) and proteins separated on a NuPage® Novex® 4–12% Bis-Tris or 3–8% Tris-Acetate Mini gel (Invitrogen, Carlsbad, Calif., USA). Lower-molecular-weight proteins (<20 kDa) were separated using a 20% polyacrylamide gel. All samples were loaded in duplicate at 50 μg protein/lane. Proteins were transferred to a Hybond™-C Extra nitrocellulose membrane (Amersham Biosciences, UK) and nonspecific binding sites blocked by incubating overnight in 3% FBS in TBS containing 0.1% Tween 20 (TBS-T). Blots were washed 3 × 5 min in TBS-T and incubated with the appropriate primary antibody for 1 h at RT. After washing in TBS-T, blots were incubated with species-specific HRP-conjugated secondary antibodies for 1 h. Blots were washed, and then developed with an ECL SuperSignal kit (Thermo Fisher Scientific, Rockford, Ill., USA).
Results
Altered Protein Expression in Wpk Kidneys
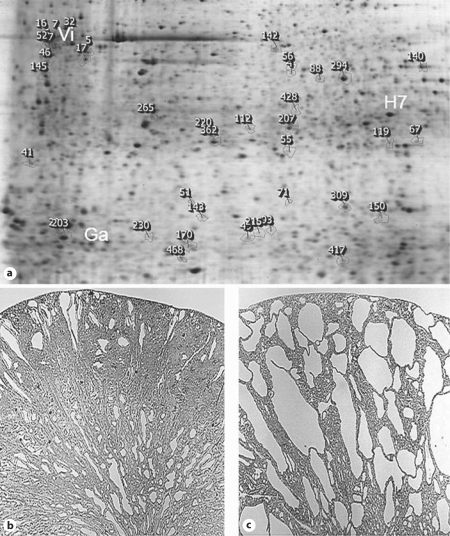
There is a dramatic increase in the cystic pathology between 10 and 21 days Wpk/Wpk homozygous cystic kidney compared with littermate normal kidney (fig. 1b, c). A dataset comparison of 2-D gels identified 76 statistically differentially expressed trypsin protein fragments, i.e. spots (fig. 1a), compared with the noncystic kidney, with 52 spots/proteins selected for identification via LC-MS/MS. Two overexpressed proteins were galectin-1 and vimentin (fig. 2). Other differentially expressed proteins of high interest in the Wpk kidney were: actin, transitional endoplasmic reticulum ATPase (ATPase p97, VCP), laminin receptor 1, fatty acid-binding protein (H-FABP-MDGI), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 78-kDa glucose-regulated protein (GRP78, BiP) (table 1). Galectin-1 and vimentin are overexpressed in cystic kidneys.
Fig. 1.
Proteomic analysis of 10- and 21-day Wpk cystic kidney. a Reference gel from a 21-day Wpk cystic kidney. Galectin-1 (Ga), HSC-70 (H7) and vimentin (Vi) are labeled with white text. HSC-70 and vimentin were identified in multiple gel spots, as indicated in table 1. SameSpots software has generated numerical rank for all differentially expressed spots. b, c Renal histology of 10- and 21-day Wpk cystic kidney. Cystic disease progresses rapidly from minimal disease at 10 days to severe collecting duct cystic pathology by 21 days.
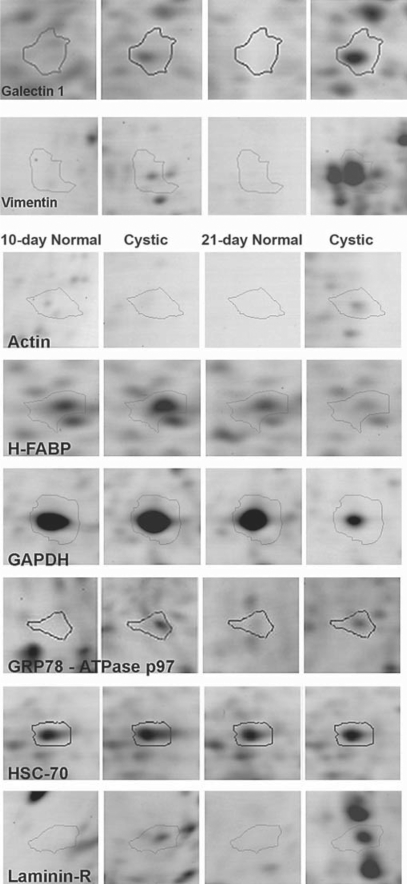
Fig. 2.
Proteomic analysis of differentially expressed proteins. These images represent magnified sections from the 2-D gels. The spots (10-day normal, 10-day cystic, 21-day normal, and 21-day-old cystic kidneys, respectively) were identified via LC-MS/MS as galectin-1 and vimentin. Each set of four images was taken from the same coordinates on four different, representative gels. The differential expression pattern of galectin-1, vimentin, actin, and laminin-R were increased with the extent of cystic pathology while GAPDH and H-FABP were decreased.
Table 1.
Select MS/MS identifications of misexpressed spots from 2-D gel analysis of Wpk kidney
| Spot assignment | Accession No. | Common name of protein | Unique peptides | % coverage | Misexpression patterna |
|---|---|---|---|---|---|
| 5, near 265 | P60711 | actin, cytoplasmic (β-actin) | 9 | 34 | ↑ |
| 41 | P46462 | ATPase p97 (VCP) | 4 | 7.6 | ↓ |
| 32 | P48675 | desmin | 10 | 28.1 | ↑ |
| 71 | P13803 | electron transfer flavoprotein subunit α | 4 | 9.9 | ↓ |
| 56 | Q5U2Q3 | ester hydrolase | 8 | 20.3 | ↓ |
| 170 | P07483 | fatty acid-binding protein, heart (H-FABP) | 3 | 39.1 | ↓ |
| 203 | P11762 | galectin-1 | 3 | 37.8 | ↑ |
| 294 | P04797 | glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | 5 | 20.9 | ↓ |
| 41 | P06761 | 78-kDa glucose-regulated protein (GRP 78, BiP) | 3 | 6.7 | ↑ |
| 17, near 210 | P63018 | heat-shock cognate 71-kDa protein (HSC-70) | 14 | 17.5 | – |
| 145 | P38983 | laminin receptor 1 (40S ribosomal subunit SA) | 8 | 26.8 | ↑ |
| 207 | P48500 | triosephosphate isomerase | 12 | 42.6 | ↑ |
| 6, 7, 16, etc. | P31000 | vimentin | 19 | 37.1 | ↑ |
↑ = Overexpressed; ↓ = underexpressed. SameSpots software spot assignments, Swiss-Prot accession No., number of unique peptides used for identification, protein sequence percent coverage and confirmed misexpression pattern of individual proteins in 21-day cystic compared to 21-day normal kidney.
Misexpression pattern is indicated by a directional arrow.
Galectin-1 and vimentin were overexpressed in cystic kidneys (fig. 2; 1.8- and 9.2-fold at 3 weeks of age, respectively), which was confirmed by Western blot analysis (fig. 3). Galectin-1 and vimentin appeared to be overexpressed at both 10 and 21 days by proteomic analysis but only convincingly overexpressed at 3 weeks by Western blot analysis. Five spots (table 1; fig. 1a) on the original 2-D gels were identified as vimentin, and all spots demonstrated marked overexpression (range 4.0- to 9.2-fold). Interestingly, vimentin was reduced in normal kidney at 21 days compared to the 10-day normal kidney by Western blot analysis (fig. 3) that was not detected in the 2-D gel proteomic analysis (fig. 2).
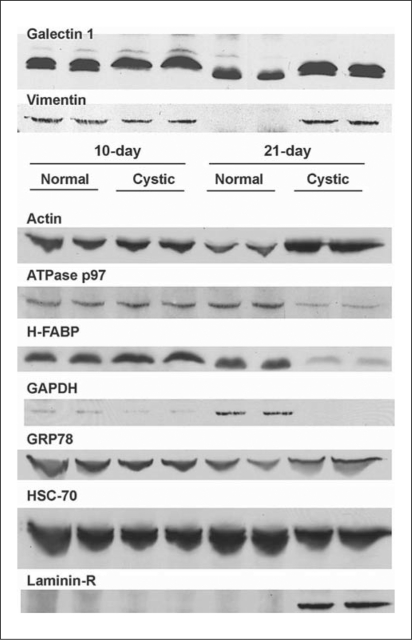
Fig. 3.
Western blots of the differentially expressed proteins. The blots are: 10-day normal (lanes 1 & 2), 10-day cystic (lanes 3 & 4), 21-day normal (lanes 5 & 6), and 21-day-old cystic (lanes 7 & 8) Wpk rat. Primary antibody dilutions were: galectin-1 (14 kDa), vimentin (57 kDa), actin (43 kDa), ATPase p97 (97 kDa) and H-FABP (15 kDa), GAPDH (37 kDa), GRP78 (78 kDa), HSC-70 (70 kDa) and laminin-R (37 kDa). HRP-conjugated secondary antibody dilutions were: anti-mouse, goat and rabbit, respectively.
2-D Gel Analysis Identifies Additional Misexpressed Proteins in Cystic Disease
GRP78 and laminin receptor 1 (laminin-R) were overexpressed at both timepoints on 2-D gel analysis (fig. 2), but Western blot only confirmed the overexpression at 21 days (fig. 3). Cytoplasmic actin was identified with 34% sequence coverage in two separate 2-D gel spots (table 1) with a 9.5-fold overexpression at 21 days in cystic kidneys which Western blot confirmed (fig. 3). Spot 41 (fig. 1a) was overexpressed at both timepoints on the 2-D gels and was co-identified as 78-kDa glucose-regulated protein (GRP78) and ATPase p97 (fig. 2). GRP78 was overexpressed while ATPase p97 was markedly underexpressed at 21 days compared to normal littermates (fig. 3).
Two other proteins were underexpressed (2.8-fold) in 21-day cystic kidneys but normally expressed in 10-day cystic kidney, heart isoform of fatty acid-binding protein (H-FABP, MDGI) (decreased 2.8-fold) and GAPDH (decreased 2.3-fold).
We selected a spot (‘A4’) that was equally expressed on all 2-D gels to serve as an internal control which MS/MS identified as heat-shock cognate 71-kDa protein (HSC-70). Western blot confirmed a 70-kDa band that is equally and highly expressed at both timepoints in normal and cystic kidneys.
Expression of ADPKD and ARPKD Proteins in the Wpk Rat
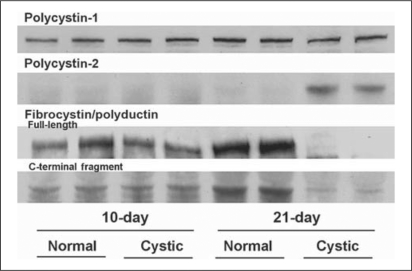
2-D gel analysis is an effective method of proteomic investigation; however, proteins that are hydrophobic, low abundance or with extreme molecular weight and pI, are poorly represented [5]. Cilia-associated proteins are typically hydrophobic, low-abundance proteins, therefore we assessed the expression of the protein products of the human ADPKD genes, PKD1-polycystin-1 (PC-1) and PKD2-polycystin-2 (PC-2), and the ARPKD gene product-fibrocystin/polyductin (FC) with Western blotting (fig. 4). PC-1 was equally expressed in normal and cystic kidney at both ages, while PC-2 was markedly overexpressed in 21-day cystic kidneys. Full-length fibrocystin/polyductin (450 kDa) and its putative 60-kDa fragment (C-terminal portion) were underexpressed in 21-day cystic kidney. This C-terminal fragment was highly expressed in 21-day normal kidney.
Fig. 4.
Western blots of the PKD-associated proteins. Immunoblot analysis of Wpk cystic kidney to assess the expression pattern of the ciliopathic proteins polycystin-1 (>250 kDa), polycystin-2 (110 kDa) and fibrocystin (full-length 450 kDa, C-terminal fragment ∼60 kDa). Full-length fibrocystin is labeled with an N-terminal antibody (mAb 11) and the C-terminal fragment is labeled with mAb 5a. The four samples on each blot are, respectively, 10-day normal, 10-day cystic, 21-day normal and 21-day-old cystic Wpk rat. Samples were run in duplicate lanes.
Discussion
Several differential mRNA expression and mRNA microarray studies were published using kidney tissues from polycystic humans [6,7], animals [8] or cultured cells from ADPKD kidneys [9]. These studies largely focused on late-stage cystic disease and/or cells from latter-stage disease. While RNA is necessary for cellular translation, proteins can undergo posttranscriptional modifications which can modify their activity. Therefore, mRNA expression is frequently poorly correlated to the abundance of a fully functional protein. 2-D gel analysis of proteins, such as those in tissue homogenates, enables an analysis of differential expression as well as changes in protein mass and/or pI. Protein expression patterns on 2-D gel may reflect biologically significant processing and charge alterations in addition to posttranslational modifications [5] that would not be predicted by mRNA assessment. Disease stage proteomic studies can identify misexpression specific to cystic disease and its progression.
We sought a ‘housekeeping’ protein to serve as an internal control for renal cystic disease studies. Two were selected from the 2-D gel and identified by MS/MS as HSC-70 and β-actin. HSC-70 was equally and highly expressed among all samples on 2-D gel (fig. 2) and in Western blots that were loaded with equal amounts of protein which confirmed its utility as an equally expressed protein in both the 2-D gel and in the Western blot studies (fig. 3). Cytoplasmic β-actin was overexpressed in 21-day cystic kidneys in 2-D gel (fig. 2b) and by immunoblot (fig. 3). Cui et al. [10] recently validated internal RT-PCR controls for use in renal cystic disease (cpk mouse), suggesting Gapdhand Actb genes represent suitable controls. Our results suggest the corresponding protein products, GAPDH and β-actin, are misexpressed in Wpk cystic kidneys and would make poor proteomic controls. We concluded that HSC-70 appears to be the best control for proteomics.
Galectin-1 and vimentin were overexpressed in Wpk cystic kidney relative to the normal kidney. Valkova et al. [4], also using 2-D gel electrophoresis and mass spectrometry, similarly demonstrated galectin-1 and vimentin overexpression in jck cystic kidneys, a model of human nephronophthisis 9. These data suggest that there are commonly misexpressed proteins in multiple forms of cystic disease that could serve as biomarkers of the disease condition and/or progression. Galectin-1 was overexpressed in both nephronophthisis and MKS, but no studies have characterized a role for galectin-1 in cystic disease.
However, galectins may be important in renal cystic diseases. Galectin-3, a related cilia protein, is involved in cyst-forming MDCK cells [11] and cpk mouse PKD [12]. Galectin-3 colocalizes with laminin in the cultured epithelium and augmenting media with galectin-3 inhibited MDCK cyst enlargement [11]. However, when cpk mice were crossed with galectin-3 null mice, the renal cystic pathology was increased [12]. Therefore, galectins (1 and/or 3) contribution to renal cystic disease may be complex in pathological epithelia. For example, endometrial carcinoma cells overexpress galectin-1 and laminin-R and underexpress galectin-3 [13]. The overexpression of galectin 1 and laminin-R in the Wpk kidney may inhibit galectin 3 expression, a protein necessary for renal epithelial morphogenesis [14].
Vimentin is an intermediate filament protein found in fibroblasts, endothelial cells, and mesenchymal cells. Confluent, cultured ADPKD epithelial cells express 5 times more vimentin than normal epithelial cells [15] and we found a 4.0- to 9.2-fold overexpression of vimentin in the 21-day cystic kidney. However, vimentin is overexpressed in a number of renal pathologies including radiation nephropathy [16], the initial reparation phase of ischemic acute renal failure [17] and tubulocystic renal carcinoma [18]. While it is currently unclear what role vimentin overexpression plays in MKS renal pathology, polycystin-1 can interact with vimentin [19]. Overexpression of vimentin is also associated with epithelial to mesenchymal transition [20]. In the PCK rat model of ARPKD, mesenchymal expression of vimentin in cholangiocytes is discussed as mesenchymal transition that could contribute to the development of hepatic fibrosis [21]. Vimentin expression by renal and biliary cystic tissues appears to be a feature of the cystic cell phenotype. In our study, vimentin overexpression was associated with a severe cystic phenotype.
While laminin-R initially appeared to be overexpressed at both 10 and 21 days, it was only markedly overexpressed at 21 days as determined by Western blotting. Laminin-R is a 67-kDa, membrane-associated protein that forms from the dimerization of a 37-kDa precursor [22]. The mature receptor interacts with extracellular laminin while the precursor has a dual function as the 40S ribosomal subunit. Laminin-R is well described in oncology literature to play a role in tumor aggressiveness by modifying laminin-1, resulting in the activation of proteolytic enzymes that promote cellular invasion through extracellular matrix degradation [23]. As previously discussed, endometrial carcinoma cells overexpress both laminin-R and galectin-1 [13] similar to our findings in the Wpk kidney suggesting that these proteins may act in concert. The Han:SPRD rat model of ADPKD develops laminin deposits in cyst wall basement membrane, especially in areas of cellular immaturity [24], suggesting an interrelationship between basement membrane thickening, laminin and cellular dedifferentiation. Joly et al. [25] have shown that purified laminin-5 augments cyst growth in culture. Laminin-R is believed to be involved in regulating or stabilizing the interaction of laminin with the α6β4 integrin [26]. Laminin α5 chain is critical for normal tubule formation/maintenance since mice null for this laminin chain develop cystic kidneys [27]. These findings suggest that extracellular interactions between laminin, integrins, and laminin-R regulate cystic cell proliferation and possibly cystogenesis.
ATPase p97 and H-FABP were underexpressed in 21-day Wpk cystic kidneys. ATPase p97 (VCP) is a component of the endoplasmic reticulum (ER)-associated degradation (ERAD) pathway and participates in the retrotranslocation of ubiquinated PC-2 from the ER to the cytosol [28]. In the present study, underexpression of ATPase p97 coincides temporally (21 days) with a marked overexpression of PC-2. Likewise, Liu et al. [29] found a 3-fold underexpression of ATPase p97 in advanced human ADPKD tissue. H-FABP is expressed in distal tubule and has been validated as a biomarker of distal tubule injury [30]. H-FABP was markedly underexpressed at 21 days in the cystic kidney, possibly due to a reduction in distal tubule mass as a percent of the kidney secondary to cystic expansion and renal fibrosis.
Three proteins involved in human PKD were also found in the Wpk kidney by Western blot (fig. 4). While polycystin-1 was not differentially expressed, polycystin-2 was overexpressed in 21-day cystic kidney. We hypothesize that PC-2 overexpression may be secondary to underexpression of ERAD pathway components (i.e. ATPase p97) responsible for degrading PC-2. Meckelin has been identified as being required for ERAD [31]. Full-length fibrocystin/polyductin, and its putative 60-kDa fragment were underexpressed at 21 days. A 60-kDa C-terminal fragment, identified in cultured cells (HEK 293, mIMCD-3 cell lines) [32,33], appears to correspond to the FCD fragment []33[]. This FCD fragment represents the transmembrane/cytosolic domain remaining after proteolytic shedding of the fibrocystin extracellular domain. Interestingly, a global gene profile of human ADPKD tissue demonstrated down-regulation of mRNA for PKHD1 and upregulated PKD2 expression [7], a pattern similar to our findings.
In summary, renal misexpression of galectin-1, vimentin, H-FABP, laminin-R, PC-2 and fibrocystin were identified in late-stage renal cystic disease in the Wpk rat model of MKS. Several of the identified proteins coincide with data from other animal models (cpk and jck mouse) and/or human forms of the renal cystic disease. Such differences in protein expression need to be further evaluated in order to identify proteins that may serve as biomarkers of disease and/or progression for renal cystic pathology.
Acknowledgements
Supported by grant NIH R01 DK68581. The polycystin-2 and fibrocystin antibodies were a generous gift from Christopher Ward. The authors appreciate the technical help of Tracy Eggleston, Traci Miller, and Weimin Xu.
References
- 1.Harris PC. 2008 Homer W. Smith Award: insights into the pathogenesis of polycystic kidney disease from gene discovery. J Am Soc Nephrol. 2009;20:1188–1198. doi: 10.1681/ASN.2009010014. [DOI] [PubMed] [Google Scholar]
- 2.Smith UM, Consugar M, Tee LJ, McKee BM, Maina EN, Whelan S, Morgan NV, Goranson E, Gissen P, Lilliquist S, Aligianis IA, Ward CJ, Pasha S, Punyashthiti R, Malik Sharif S, Batman PA, Bennett CP, Woods CG, McKeown C, Bucourt M, Miller CA, Cox P, Algazali L, Trembath RC, Torres VE, Attie-Bitach T, Kelly DA, Maher ER, Gattone VH, Harris PC, Johnson CA. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the Wpk rat. Nat Genet. 2006;38:191–196. doi: 10.1038/ng1713. [DOI] [PubMed] [Google Scholar]
- 3.Gattone VH, Tourkow BA, Trambaugh CM, Yu AC, Whelan S, Phillips CL, Harris PC, Peterson RG. Development of multiorgan pathology in the Wpk rat model of polycystic kidney disease. Anat Rec A Discov Mol Cell Evol Biol. 2004;277:384–395. doi: 10.1002/ar.a.20022. [DOI] [PubMed] [Google Scholar]
- 4.Valkova N, Yunis R, Mak SK, Kang K, Kültz D. Nek8 mutation causes overexpression of galectin-1, sorcin, and vimentin and accumulation of the major urinary protein in renal cysts of jck mice. Mol Cell Proteomics. 2005;4:1009–1018. doi: 10.1074/mcp.M500091-MCP200. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed FE. Mining the oncoproteome and studying molecular interactions for biomarker development by 2DE, ChIP and SPR technologies: Expert Rev Proteomics. 2008;5:469–496. doi: 10.1586/14789450.5.3.469. [DOI] [PubMed] [Google Scholar]
- 6.Schieren G, Rumberger B, Klein M, Kreutz C, Wilpert J, Geyer M, Faller D, Timmer J, Quack I, Rump LC, Walz G, Donauer J. Gene profiling of polycystic kidneys. Nephrol Dial Transplant. 2006;21:1816–1824. doi: 10.1093/ndt/gfl071. [DOI] [PubMed] [Google Scholar]
- 7.Song X, Di Giovanni V, He N, Wang K, Ingram A, Rosenblum ND, Pei Y. Systems biology of autosomal dominant polycystic kidney disease: computational identification of gene expression pathways and integrated regulatory networks. Hum Mol Genet. 2009;18:2328–2343. doi: 10.1093/hmg/ddp165. [DOI] [PubMed] [Google Scholar]
- 8.Gattone VH, Ricker JL, Trambaugh CM, Klein RM. Multiorgan mRNA misexpression in murine autosomal recessive polycystic kidney disease. Kidney Int. 2002;62:1560–1569. doi: 10.1046/j.1523-1755.2002.00632.x. [DOI] [PubMed] [Google Scholar]
- 9.Husson H, Manavalan P, Akmaev VR, Russo RJ, Cook B, Richards B, Barberio D, Liu D, Cao X, Landes GM, Wang CJ, Roberts BL, Klinger KW, Grubman SA, Jefferson DM, Ibraghimov-Beskrovnaya O. New insights into ADPKD molecular pathways using combination of SAGE and microarray technologies. Genomics. 2004;84:497–510. doi: 10.1016/j.ygeno.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Cui X, Zhou J, Qiu J, Johnson MR, Mrug M. Validation of endogenous internal real-time PCR controls in renal tissues. Am J Nephrol. 2009;30:413–417. doi: 10.1159/000235993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao Q, Hughes RC. Galectin-3 expression and effects on cyst enlargement and tubulogenesis in kidney epithelial MDCK cells cultured in three-dimensional matrices in vitro. J Cell Sci. 1995;108:2791–2800. doi: 10.1242/jcs.108.8.2791. [DOI] [PubMed] [Google Scholar]
- 12.Chiu MG, Johnson TM, Woolf AS, Dahm-Vicker EM, Long DA, Guay-Woodford L, Hillman KA, Bawumia S, Venner K, Hughes RC, Poirier F, Winyard PJ. Galectin-3 associates with the primary cilium and modulates cyst growth in congenital polycystic kidney disease. Am J Pathol. 2006;169:1925–1938. doi: 10.2353/ajpath.2006.060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van den Brule FA, Buicu C, Berchuck A, Bast RC, Deprez M, Liu FT, Cooper DN, Pieters C, Sobel ME, Castronovo V. Expression of the 67-kDa laminin receptor, galectin-1, and galectin-3 in advanced human uterine adenocarcinoma. Hum Pathol. 1996;27:1185–1191. doi: 10.1016/s0046-8177(96)90313-5. [DOI] [PubMed] [Google Scholar]
- 14.Koch A, Poirier F, Jacob R, Delacour D. Galectin-3, a novel centrosome-associated protein, required for epithelial morphogenesis. Mol Biol Cell. 2010;21:219–231. doi: 10.1091/mbc.E09-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silberberg M, Charron AJ, Bacallao R, Wandinger-Ness A. Mispolarization of desmosomal proteins and altered intercellular adhesion in autosomal dominant polycystic kidney disease. Am J Physiol Renal Physiol. 2005;288:F1153–F1163. doi: 10.1152/ajprenal.00008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu DG, Wang TM. Role of connective tissue growth factor in experimental radiation nephropathy in rats. Chin Med J. 2008;121:1925–1931. [PubMed] [Google Scholar]
- 17.Villanueva S, Cespedes C, Vio CP. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am J Physol. 2006;290:R861–R870. doi: 10.1152/ajpregu.00384.2005. [DOI] [PubMed] [Google Scholar]
- 18.Osunkoya AO, Young AN, Wang W, Netto GJ, Epstein JI. Comparison of gene expression profiles in tubulocystic carcinoma and collecting duct carcinoma of the kidney. Am J Surg Pathol. 2009;33:1103–1106. doi: 10.1097/PAS.0b013e3181a13e7b. [DOI] [PubMed] [Google Scholar]
- 19.Xu GM, Sikaneta T, Sullivan BM, Zhang Q, Andreucci M, Stehle T, Drummond I, Arnaout MA. Polycystin-1 interacts with intermediate filaments. J Biol Chem. 2001;276:46544–46552. doi: 10.1074/jbc.M107828200. [DOI] [PubMed] [Google Scholar]
- 20.Chea SW, Lee KB. TGF-β-mediated epithelial-mesenchymal transition in autosomal dominant polycystic kidney disease. Yonsei Med J. 2009;50:105–111. doi: 10.3349/ymj.2009.50.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato Y, Harada K, Ozaki S, Furubo S, Kizawa K, Sanzen T, Yasoshima M, Ikeda H, Sasaki M, Nakanuma Y. Cholangiocytes with mesenchymal features contribute to progressive hepatic fibrosis of the polycystic kidney rat. Am J Pathol. 2007;171:1859–1871. doi: 10.2353/ajpath.2007.070337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fatehullah A, Doherty C, Pivato G, Allen G, Devine L, Nelson J, Timson DJ. Interactions of the 67 kDa laminin receptor and its precursor with laminin. Biosci Rep. 2009;30:73–79. doi: 10.1042/BSR20090023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berno V, Porrini D, Castiglioni F, Campiglio M, Casalini P, Pupa SM, Balsari A, Ménard S, Tagliabue E. The 67 kDa laminin receptor increases tumor aggressiveness by remodeling laminin-1. Endocr Relat Cancer. 2005;12:393–406. doi: 10.1677/erc.1.00870. [DOI] [PubMed] [Google Scholar]
- 24.Cowley BD, Jr, Gudapaty S, Kraybill AL, Barash BD, Harding MA, Calvet JP, Gattone VH. Autosomal-dominant polycystic kidney disease in the rat. Kidney Int. 1993;43:522–534. doi: 10.1038/ki.1993.79. [DOI] [PubMed] [Google Scholar]
- 25.Joly D, Berissi S, Bertrand A, Strehl L, Patey N, Knebelmann B. Laminin-5 regulates polycystic kidney cell proliferation and cyst formation. J Biol Chem. 2006;281:29181–29189. doi: 10.1074/jbc.M606151200. [DOI] [PubMed] [Google Scholar]
- 26.Ardini E, Tagliabue E, Magnifico A, Butò S, Castronovo V, Colnaghi MI, Ménard S. Co-regulation and physical association of the 67-kDa monomeric laminin receptor and the α6β4 integrin. J Biol Chem. 1997;272:2342–2345. doi: 10.1074/jbc.272.4.2342. [DOI] [PubMed] [Google Scholar]
- 27.Shannon MB, Patton BL, Harvey SJ, Miner JH. A hypomorphic mutation in the mouse laminin α5 gene causes polycystic kidney disease. J Am Soc Nephrol. 2006;17:1913–1922. doi: 10.1681/ASN.2005121298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang G, Li Q, Tang Y, Kokame K, Kikuchi T, Wu G, Chen XZ. Polycystin-2 is regulated by endoplasmic reticulum-associated degradation. Hum Mol Genet. 2008;17:1109–1119. doi: 10.1093/hmg/ddm383. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Dai B, Mei C, Zhang Y, Xiong X, Sandford R. Identification of phosphoproteins in kidney tissues from patients with autosomal dominant polycystic kidney disease. Proteomics Clin Appl. 2008;2:1153–1166. doi: 10.1002/prca.200780172. [DOI] [PubMed] [Google Scholar]
- 30.Pelsers MM. Fatty acid-binding protein as marker for renal injury. Scand J Clin Lab Invest Suppl. 2008;241:73–77. doi: 10.1080/00365510802150133. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Bridges J, Cheng-Lun N, Xu Y, Weaver TE. The Meckel-Gruber syndrome protein MKS3 is required for ER-associated degradation of surfactant protein. J Biol Chem. 2010;284:33377–33383. doi: 10.1074/jbc.M109.034371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaimori JY, Nagasawa Y, Menezes LF, Garcia-Gonzalez MA, Deng J, Imai E, Onuchic LF, Guay-Woodford LM, Germino GG. Polyductin undergoes notch-like processing and regulated release from primary cilia. Hum Mol Genet. 2007;16:942–956. doi: 10.1093/hmg/ddm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiesberger T, Gourley E, Erickson A, Koulen P, Ward CJ, Masyuk TV, Larusso NF, Harris PC, Igarashi P. Proteolytic cleavage and nuclear translocation of fibrocystin is regulated by intracellular Ca2+ and activation of protein kinase C. J Biol Chem. 2006;281:34357–34364. doi: 10.1074/jbc.M606740200. [DOI] [PubMed] [Google Scholar]