Abstract
Aims
To examine awareness of memory abilities by groups (healthy control, suspected dementia/mild cognitive impairment, MCI, and diagnosed dementia/MCI), and to describe group differences in the relationship between awareness and cognitive performance in a community sample.
Methods
In a cross-sectional design, 183 subjects were evaluated in a community setting and categorized into 3 groups based on their cognitive performance and reported medical history. Awareness of memory abilities was quantified using a published anosognosia ratio (AR) comparing the estimated to the objective memory performance by subjects. Each group was further categorized into ‘overestimators’, ‘accurate estimators’, and ‘underestimators’ based on their AR scores.
Results
The suspected and diagnosed dementia/MCI groups had significantly higher AR scores than the controls. The suspected group also had a significantly larger proportion (96.2%) of overestimators than the diagnosed (73.3%) and control groups (26.1%). Impaired awareness in overestimators of the suspected and diagnosed groups was correlated with deficits in executive function, language or global cognition.
Conclusion
Impaired awareness of memory abilities was prevalent in community-dwelling older adults with suspected and diagnosed dementia or MCI. Those with suspected dementia or MCI were more likely to overestimate their memory abilities than their diagnosed counterparts, suggesting that limited awareness of deficits may hinder utilization of dementia diagnostic services.
Key Words: Anosognosia, Dementia, Mild cognitive impairment, Memory, Neuropsychology, Community-dwelling
Introduction
A substantial number of older adults who meet the diagnostic criteria for dementia or mild cognitive impairment (MCI) are not detected because symptoms of a possible neurodegenerative condition are not brought to the attention of caregivers or healthcare providers [1]. While the phenomenon is known to occur in people manifesting relatively mild cognitive and functional declines [1], the contribution of other factors such as self-awareness of deficits remains unclear. Impaired awareness of cognitive deficits [2] is common in patients with neurodegenerative cognitive disorders [3] and predicts progression to dementia among MCI patients [4]. In patients with diagnosed dementia or MCI, as the neuropathological load increases and cognitive and behavioral symptoms progress, awareness of deficits declines [4,5,6,7,8]. However, it is unclear how awareness of deficits manifests in undetected dementia. For example, to what extent is early detection of dementia and MCI hampered by poor insight into deficits?
Characterizing the relationship between impaired awareness and cognitive abilities in undetected dementia is important. If older adults with undetected dementia are unable to recognize their cognitive deficits, they may delay or fail to seek evaluation and treatment in a timely manner. Moreover, older adults with undetected dementia are most likely community dwelling [1]. If they underestimate safety concerns (e.g. driving difficulties, medication errors) due to an impaired ability to accurately appraise their deficits, they may resist assistance from caregivers, making themselves and others more vulnerable to harm. Although impaired awareness is a known component of Alzheimer disease (AD) symptoms, few if any studies have examined impaired awareness in a community-dwelling population demonstrating a broad range of cognitive abilities and diagnostic histories.
Awareness has been suggested to be selectively impaired and domain dissociate [7,9]. For example, impaired awareness in patients with AD or MCI was found to manifest as unawareness of deficits in domains of daily functioning, depression or disinhibition [6,10]. Although the mechanism remains poorly understood, impaired awareness of amnesia has been a primary focus of awareness research [11,12]. Barrett et al. [13] developed an algorithm to examine the ability to accurately appraise one's cognitive abilities, wherein an anosognosia ratio (AR) is calculated by comparing the estimated to the objective performance by subjects on cognitive tests. The current study examined the awareness of memory abilities, using the AR as a method of quantifying accuracy of awareness. We selected a sample of community-dwelling older adults categorized into one of three groups: one group with cognitive scores within expected ranges, one group carrying a diagnosis of dementia or MCI, and a third group demonstrating impairments typical of dementia and MCI on testing but who did not carry a diagnosis of dementia or MCI. We believe the third group represents what others have characterized as undetected cognitive disorders [1]. Because we could not definitively confirm the presence of a cognitive diagnosis, we characterized this group as having ‘suspected’ MCI or dementia. Using this community-dwelling sample, we examined awareness of memory abilities by group and the relationship between awareness and cognitive deficits across the three groups. We hypothesized that (1) a larger proportion of subjects in the suspected dementia/MCI group (i.e. undetected impairments) demonstrated impaired awareness of memory abilities than in the group diagnosed with dementia or MCI, and (2) group differences existed in the relationship of awareness of memory abilities with cognitive deficits between healthy controls and individuals with suspected or diagnosed dementia/MCI. That is, the ability to accurately appraise one's memory abilities is generally independent from their cognitive function in healthy controls; conversely, awareness of memory abilities gradually declines corresponding to the increasing cognitive deficits in individuals with impaired cognition, be it suspected or diagnosed dementia/MCI.
Subjects and Methods
The secondary data analysis was performed using baseline data from the Midwest Initiative for Dementia Screening (MINDS) project. The MINDS project is a prospective study evaluating the efficacy of regional, community-based dementia screening and educational outreach in Southern Wisconsin launched in 2006 by the University of Wisconsin's Alzheimer Disease Research Center (ADRC). A clinical neuropsychologist (C.E.G.) and a geriatrician (S.A.) provided the free-of-charge cognitive assessment service for each participant, and they were responsible for contacting the primary care physicians of the participants to inform them when findings suggested cognitive impairment such as a suspected MCI or dementia. The project was approved by the University of Wisconsin-Madison health science institutional review board.
Subjects
English-speaking residents aged 50 years and over were invited to the free educational lectures provided by the ADRC in residential assisted living facilities, senior centers and other community organizations (e.g. churches) in 8 communities. A total of 260 residents agreed to undergo cognitive screening following the lectures, of which 218 subjects (84%) provided data sufficient to calculate their AR. Data from subjects who reported, in either their medical history form or clinical interview, major medical, non-AD neurological conditions (e.g. brain tumor, Parkinson's disease, stroke) or suspected alcohol-, medication- or depression-related reversible cognitive impairment (n = 35) were excluded from the analyses. All subjects provided informed consent in accordance with the health science institutional review board of the University of Wisconsin-Madison.
Diagnostic Assessment
In MINDS screening, the participants were first administered a questionnaire addressing demographic information and health history including the personal and family history of any diagnosed cognitive disorder (e.g. MCI, dementia). This was followed by a structured clinical interview about the daily memory performance of the participants and their level of independence in day-to-day activities by interviewing participants and their caregivers (if available). The final portion was a comprehensive neuropsychological test battery. In addition, depressive symptoms were assessed using the 15-item Geriatric Depression Scale (GDS) [14]. Testing was conducted by a trained technician.
The evaluations were intended to serve as a memory screening service, wherein experienced diagnosticians (a neuropsychologist and a geriatrician) went into community settings to conduct portions of a dementia evaluation, i.e. cognitive testing and an interview. The results of the screening evaluation were sent to primary care providers with specific recommendations such as to evaluate for possible MCI. The screening evaluations did not entail typical dementia diagnostic laboratory tests or neurological examinations; thus, definitive diagnoses were not provided to the subjects. For the purpose of these analyses, subjects whose cognitive scores were within expected ranges were labeled as ‘controls’ (n = 115). Their intact cognition was based on their age- or age-and-education-corrected standard scores on cognitive tests (score no more than 1.5 SD below standardized mean from normative data), and interview data on functional abilities. The ‘suspected’ group (n = 53) was characterized according to the Diagnostic and Statistical Manual of Mental Disorders criteria by the American Psychiatric Association [15] (n = 26), or based on the International Psychogeriatric Association Expert Conference on Mild Cognitive Impairment [16] (n = 27). For the ‘suspected’ group, permission to send evaluation results to the subjects’ primary care providers was sought, and a release-of-information form completed and signed by the participant prior to any communication with a volunteer's provider. If the participant agreed, a letter summarizing the test results and recommendations was faxed or delivered to the provider. This procedure further confirmed the lack of a diagnostic history in the ‘suspected’ group. The ‘diagnosed’ group (n = 15) was based on a self- or caregiver-reported diagnostic history of any type of dementia or MCI.
Cognitive Tests
Cognitive tests included the Trail Making Test, part B (Trail B time) [17]; number of words on the Stroop color-word interference subtask [18]; number of words generated on the Animal Fluency task [19]; Neurobehavioral Cognitive Status Examination [20] (Cognistat) domains including construction, memory, language, reasoning, calculation, attention and orientation; Mini-Mental State Exam (MMSE) [21]; Clock Draw Test [22], and Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Word List Learning Test [23].
Estimated Memory Performance
We used a 20-item structured interview derived from our memory clinic interview protocol, labeled here as the ‘everyday memory interview’ (EMI) to collect data on subjects’ estimation of their everyday retrospective memory performance (for online suppl. table 1, see www.karger.com/doi/10.1159/000318752). Retrospective memory, such as recall of past events, or memory for word lists is typically impaired in patients with AD and amnestic MCI [24]. Content validity was evaluated by a group of clinicians in the ADRC. Subjects responded to items using a 0–5 scale. In the event of missing items, an algorithm was applied to calculate the percentile score. The rating of each item was transformed into score Xi as Xi = rating/5, and the percentile score of estimated memory performance was computed as
with a possible range of 0–100%. Higher percentile scores indicate fewer complaints of memory difficulties. The internal consistency (Cronbach's α) of the transformed 20-item EMI questionnaire was 0.87.
Objective Memory Performance
The CERAD Word List Learning Test is a 10-word list memory task. The age-adjusted percentile score for delayed free recall, based on normative data [25], was used to measure the objective memory performance with a possible range of 1–100%. Higher percentile scores indicate better memory performance. The CERAD Word List Learning Test had been widely applied in assessing functioning of retrospective memory in nondemented older adults and differential diagnosis of dementia across ethnic/racial groups [26].
Awareness Quantification
The awareness of one's memory abilities was estimated using the AR [13], calculated as
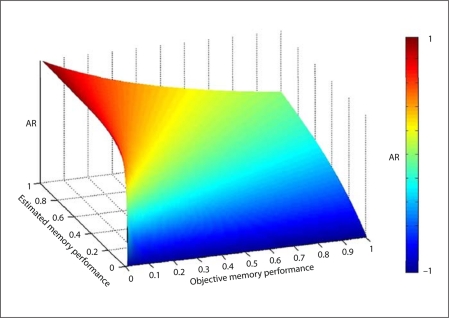
Figure 1 displays the conceptual relationship between AR, estimated memory performance and objective memory performance. Compared to traditional discrepancy scores between estimated and objective memory performance [27], the equation by Barrett et al. [13] adjusts for the increased likelihood of overestimation occurring in those with poor objective memory performance. The estimated memory performance was quantified as a percentile score for the EMI. The objective memory performance was obtained using the CERAD percentile score. The AR score ranged from −1 to 1. In the study by Barrett et al. [13], positive scores suggested an overestimation of memory abilities, negative scores an underestimation, while accurate estimation was equated with an AR of 0. In our study, we used the controls as the normative sample and defined accurate estimators as having an AR score within ±1.5 SD of the AR obtained from the controls, i.e. ±0.375. In this manner, subjects were characterized as under-, over- or accurate estimators of their memory abilities.
Fig. 1.
Conceptual relationship between estimated and objective memory performance, described by AR equation.
Data Analysis
Analyses were conducted using SPSS 16.0 [28]. Data analysis specifically addressed the nature of the variable (i.e. continuous vs. categorical), unequal sample size across groups, multiple comparisons [29] and lack of homoskedasticity in variables. All statistical significance tests were evaluated using an overall α of 0.05. Levene's test was used to test equality of variances across groups. Group differences in demographic information, health history and cognitive tests were assessed by χ2 tests for categorical variables, one-way analyses of variance (ANOVA) for equal variances across groups with Bonferroni's correction for α to 0.01, and Welch's ANOVA for unequal variances followed, when appropriate, by Games-Howell post hoc analysis for continuous variables. To examine group differences in estimated memory abilities and accuracy of appraisal (EMI and AR scores), we used ordinary least squares regression models that produce heteroskedasticity-consistent standard error estimators [30]. Heteroskedastic residuals were assumed and standard errors were adjusted accordingly. All regressions were estimated using an SPSS macro [31], taking percentile score on the EMI or AR as dependent variables and group membership as an independent variable, controlling for corresponding noncognitive and cognitive covariates. A Mann-Whitney test was used to examine whether EMI and AR scores differed between individuals suspected to have dementia versus MCI. The correlations between AR score with cognitive and noncognitive factors for subjects who overestimated their memory abilities (overestimators) in the 3 groups was computed by Spearman's ρ.
Results
Demographic Information, Health History and Cognitive Tests by Group
Table 1 displays the group differences (control, suspected and diagnosed) in demographic information, health history and cognitive tests. There were significant group differences in age and gender. For cognitive tests, there were significant group differences in all tests except for Cognistat calculation and the Clock Draw Test. Thus, 2 noncognitive factors and 10 cognitive factors were added as covariates in the regression analyses described below. In addition, the CERAD percentile scores were significantly lower in the suspected and diagnosed groups than in the controls. Significant differences between the suspected and diagnosed groups were noted in 2 tests of executive function, such that subjects carrying a diagnosis performed more poorly on the Stroop color-word interference task and the Animal Fluency test than those merely suspected of demonstrating an MCI or dementia. There were no significant differences between the suspected and diagnosed groups in either memory measure (CERAD Word List and Cognistat memory).
Table 1.
Group differences in demographic information, health history and cognitive tests
| Control (n = 115) | Suspected (n = 53) | Diagnosed (n = 15) | Welch's F | pk | |
|---|---|---|---|---|---|
| Age, years | 76.06 (8.10) | 81.47 (7.66)∗∗∗ | 79.07 (6.01) | 8.83 (2,41) | 0.001 |
| Education, years | 15.04 (2.44) | 14.90 (3.99) | 14.34 (3.42) | 0.30 (2, 34) | NS |
| Health history, number of 12 chronic conditions/behaviorsa | 2.69 (1.31) | 2.45 (1.80) | 2.64 (1.15) | 0.45 (2, 37) | NS |
| Depression, GDS scoreb | 1.66 (1.69) | 2.72 (2.43) | 2.53 (2.17) | 4.71 (2, 35) | NS |
| Gender – male, n | 96 (83.5%) | 35 (66.0%)∗∗∗ | 8 (46.7%)∗∗∗ | 13.23c | 0.001 |
| Family history of cognitive disorders – yes, n | 49 (42.6%) | 17 (32.1%) | 3 (20%) | 2.01c | NS |
| Trail B time, s | 105.17 (136.87) | 195.45 (176.73)∗∗ | 356.67 (347.86)∗ | 32.69 (2, 33) | 0.001 |
| Stroop color-word, number of words | 34.66 (10.06) | 23.04 (9.29)∗∗∗ | 16.40 (8.93)∗∗∗† | 43.28 (2, 39) | <0.001 |
| Animal Fluency, number of words | 20.14 (5.16) | 15.45 (4.55)∗∗∗ | 11.27 (3.52)∗∗∗†† | 43.19 (2,43) | <0.001 |
| Cognistat – construction, pointsd | 4.99 (0.97) | 4.19 (1.44)∗∗∗ | 3.20 (2.04)∗∗ | 11.36 (2,33) | <0.001 |
| Cognistat – memory, pointse | 10.58 (1.63) | 7.68 (2.87)∗∗∗ | 6.20 (2.71)∗∗∗ | 35.24 (2, 33) | <0.001 |
| Cognistat – language, pointsf | 27.16 (1.13) | 25.46 (2.54)∗∗∗ | 24.40 (2.80)∗∗ | 16.73 (2, 31) | <0.001 |
| Cognistat – reasoning, pointsg | 13.27 (1.02) | 12.32 (1.95) | 12.33 (1.57) | 6.21 (2,32) | NS |
| Cognistat – calculation, pointsh | 3.92 (0.44) | 3.85 (0.66) | 3.47 (0.99) | 1.70 (2, 32) | NS |
| Cognistat – attention, pointsi | 7.83 (0.64) | 7.30 (1.27) | 7.07 (1.67) | 5.28 (2, 32) | NS |
| Cognistat – orientation, pointsj | 11.97 (0.18) | 11.68 (0.85) | 10.80 (1.78) | 5.98 (2, 30) | NS |
| MMSE, points out of 30 | 28.98 (1.24) | 27.55 (1.81)∗∗∗ | 25.53 (3-34)∗∗ | 20.04 (2, 32) | <0.001 |
| Clock Draw Test – fail, n | 22 (19%) | 12 (23%) | 4 (27%) | 0.62c | NS |
| Objective memory performance (CERAD), percentile score | 52.53% (24.09%) | 5.19% (9.66%)∗∗∗ | 15.93% (28.57%)∗∗∗ | 161.60 (2,36) | <0.001 |
Values denote numbers with percentages or means with SD in parentheses, except for Welch's F, where d.f. are displayed in parentheses. Post hoc testing revealed significant differences between control and suspected or diagnosed groups:
p < 0.05
p < 0.01
p < 0.001. Post hoc testing revealed significant differences between suspected and diagnosed groups:
p< 0.05
p< 0.01.
Twelve health conditions/behaviors: stroke, hypertension, high cholesterol, colon disease, liver problems, phlebitis/circulation problems, cancer, vision problems, diabetes, swallowing problems, and tobacco and alcohol use; possible range: 0-12.
15-item GDS scale.
χ2 test.
Possible range: 0-6.
Possible range: 0-12.
Possible range: 0-28.
Possible range: 0-14.
Possible range: 0-4.
Possible range: 0-8.
Possible range: 0-12.
a adjusting to 0.003.
Group Differences in EMI Scores and AR
The mean EMI scores were 0.71 (SD = 0.12) for the control, 0.68 (SD = 0.12) for the suspected, and 0.56 (SD = 0.15) for the diagnosed participants. After controlling for noncognitive covariates, group was not a predictor of EMI scores (β = −0.02; p > 0.05), indicating the 3 groups did not differ in their report of everyday memory abilities (table 2).
Table 2.
Ordinary least squares regression results of EMI scores and AR
| Characteristics | Dependent variable |
|||||
|---|---|---|---|---|---|---|
| EMI score |
AR |
|||||
| β | SEa | t | β | SEa | t | |
| Constant | 0.57 | 0.10 | 5.95∗∗∗ | 1.19 | 0.39 | 3.06∗∗ |
| Covariates | ||||||
| Age | 0.01 | 0.01 | 0.94 | 0.01 | 0.01 | 0.04 |
| Gender – male | 0.04 | 0.03 | 1.70 | −0.03 | 0.05 | −0.68 |
| Trail B time | <0.001 | 0.01 | 0.12 | |||
| Stroop color-word | −0.01 | 0.01 | −0.96 | |||
| Animal Fluency | −0.01 | 0.01 | −0.73 | |||
| Cognistat – construction | 0.01 | 0.02 | 0.52 | |||
| Cognistat – memory | −0.02 | 0.01 | −2.32∗ | |||
| Cognistat – language | 0.01 | 0.01 | 0.45 | |||
| MMSE | −0.03 | 0.01 | −2.73∗∗ | |||
| Independent variable | ||||||
| Group membership | −0.02 | 0.01 | −1.71 | 0.28 | 0.02 | 12.01∗∗∗ |
p < 0.05
p < 0.01
p < 0.001.
Heteroskedasticity-consistent SE.
The mean AR were 0.18 (SD = 0.25) for the control, 0.87 (SD = 0.19) for the suspected, and 0.71 (SD = 0.38) for the diagnosed group. After controlling for cognitive and noncognitive covariates, group was a significant predictor of AR (β = 0.28; p < 0.001) (table 2). Post hoc testing revealed that the suspected and diagnosed groups had significantly higher AR than the control group (both p < 0.001), but no differences were found between the suspected and diagnosed groups.
In addition, Mann-Whitney tests showed that within the suspected group, neither EMI scores nor AR significantly differed between individuals labeled as MCI or dementia.
Awareness of Memory Abilities
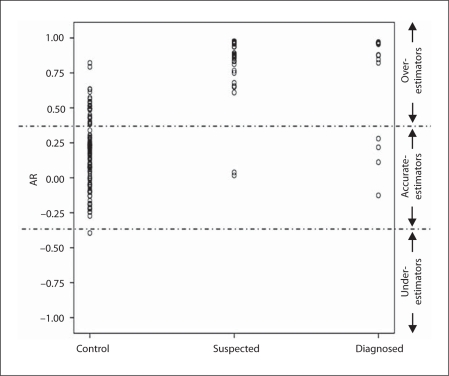
Our first hypothesis was that a larger proportion of individuals with a suspected disorder (MCI or dementia) would demonstrate impaired awareness of their memory problems than individuals already diagnosed with dementia or MCI. As described earlier, subjects with AR deviating from 0 by more than 1.5 control group SD (AR >0.375) were labeled as overestimators; those with AR scores under −0.375 (less than −1.5 SD from 0) were considered underestimators, and those with AR scores between −0.375 and 0.375 were labeled as accurate estimators. The proportion of overestimators was significantly higher in the suspected (n = 51; 96.2%) and diagnosed (n = 11; 73.3%) groups (indicating limited awareness of memory deficits) than in the control group (n = 30; 26.1%; χ2 = 71.48 and 13.72; both p < 0.001). Additionally, the proportion of overestimators in the suspected group was higher than that in the diagnosed group (χ2 = 7.62; p = 0.006). Eighty-four control subjects (73.0%), 2 suspected subjects (3.9%) and 4 diagnosed subjects (26.7%) were accurate estimators (fig. 2).
Fig. 2.
Distribution of AR scores in the 3 groups.
Relationship of AR to Cognitive and Noncognitive Factors in Overestimators
Table 3 displays the AR correlations with cognitive and noncognitive factors in overestimators by group. We hypothesized that there were group differences in the relationship between awareness of memory abilities and cognitive function or, specifically, differences between cognitively healthy individuals and those with suspected or previously diagnosed dementia or MCI. In the controls, AR was positively correlated to Cognistat construction (ρ = 0.42; p = 0.020), indicating overestimation was associated with a strong performance on a simple block design task. In contrast, subjects who overestimated their memory performance in the suspected and diagnosed groups were more likely to show impairments in selected domains. In the suspected participants, AR was negatively correlated to Stroop color-word (ρ = −0.30; p = 0.031) and MMSE scores (ρ = −0.31; p = 0.027). In the diagnosed participants, AR was negatively correlated to Animal Fluency (ρ = −0.76; p = 0.007) and Cognistat language scores (ρ = −0.62; p = 0.041).
Table 3.
Correlations of AR with noncognitive and cognitive factors in subsample of overestimators
| AR |
|||
|---|---|---|---|
| control (n = 30) | suspected (n = 51) | diagnosed (n=ll) | |
| Age | 0.12 | 0.12 | 0.58 |
| Education | −0.08 | −0.14 | −0.33 |
| Health history | 0.07 | −0.11 | 0.10 |
| CDS | −0.29 | −0.12 | 0.21 |
| Trail B time | −0.30 | 0.15 | 0.21 |
| Stroop color-word | 0.27 | −0.30∗ | 0.02 |
| Animal Fluency | 0.23 | −0.14 | −0.76∗∗ |
| Cognistat – construction | 0.42∗ | −0.01 | −0.01 |
| Cognistat – memory | −0.03 | −0.05 | −0.38 |
| Cognistat – language | 0.19 | −0.16 | −0.62∗ |
| Cognistat – reasoning | −0.23 | −0.25 | −0.47 |
| Cognistat – calculation | −a | −0.01 | −0.18 |
| Cognistat – attention | −0.15 | −0.17 | −0.29 |
| Cognistat – orientation | 0.10 | −0.06 | −0.35 |
| MMSE | −0.09 | −0.31∗ | −0.22 |
p < 0.05
p< 0.01.
No variety in score.
Discussion
To our knowledge, this is the first study attempting to extend the understanding of awareness of memory abilities to community-dwelling older adults, as opposed to clinical patients. Although the history and test performance were suggestive of dementia or MCI, a substantial number of participants were not diagnosed with dementia/MCI. These individuals ‘suspected’ to have a diagnosable disorder showed important differences when compared to cognitively healthy older adults (controls) and participants already diagnosed with dementia or MCI (diagnosed). While the groups reported a similar number of memory complaints, AR were significantly different across the three groups, with the suspected group reporting the highest scores; moreover, 96.2% of participants suspected to have MCI or dementia overestimated their memory abilities, compared to 73.3% in the diagnosed group and 26.1% in the control group. The findings indicate subjects with a possible undetected dementia/MCI are more likely to demonstrate impaired awareness of memory abilities than both those carrying a diagnosis of dementia/MCI and healthy controls. The suspected subjects were also older than the diagnosed and control groups. Similar to previous studies of diagnosed dementia and MCI [32,33,34], no differences in memory complaints or awareness were detected between individuals with suspected MCI and suspected dementia. While conjecture, our finding that those with suspected dementia were disproportionately likely to overestimate their memory abilities might explain why these individuals have not sought formal clinical evaluation of their deficits.
Not surprisingly, our findings show that individuals with either a possible undetected or diagnosed dementia/MCI are more likely to overestimate their memory abilities than healthy controls. Interestingly, a larger proportion of participants in the suspected group overestimated their memory abilities than in the diagnosed group, in spite of generally similar cognitive impairment in the two groups. The impaired awareness in individuals with suspected dementia or MCI may in part account for the lack of a formal clinical evaluation/diagnosis of deficits. On the other hand, this study expands previous work by demonstrating that deterioration of awareness appears to correspond with cognitive decline. Specifically, the degree to which participants with suspected or diagnosed dementia/MCI overestimated their memory abilities correlated with cognitive deficits in executive function, language or global cognition. In contrast, level of awareness was generally unrelated to cognitive performance in healthy controls, confirming other reports [13,35]. These data support an emerging notion that awareness, like traditional cognitive domains and functional abilities, follows a trajectory of decline in progressive cognitive illnesses that corresponds to disease burden. Ries et al. [36] studied amnestic MCI subjects and found that those with less awareness of cognitive deficits showed a reduced neuronal response in the anterior medial frontal cortex and posterior cingulate during a self-appraisal task. Similar results were also found among asymptomatic subjects at risk for AD [37].
Another interesting finding was that the three groups did not differ in their estimated memory performance after adjusting for age and gender differences. As posited in previous reports [38,39,40], the current study raises further concern about overreliance on subjective memory complaints in prompting evaluation of early cognitive decline among community-dwelling older adults. That is, if the impaired awareness of an individual limits the number of subjective memory complaints, the patient will fail to report signs or symptoms of a cognitive disorder to either their family or their healthcare providers. Importantly, the MINDS project organized free memory screenings in community settings. In contrast, the patient or a family member who is concerned about a patient's memory function usually initiates a clinical evaluation. Thus, while studies showed that subjective memory complaints could be an important prompt in a subset of patients with mild decline in clinical settings [41], our findings suggest the need to identify other appropriate triggers, such as assessing their accuracy of self-appraisal, to prompt evaluation of early cognitive decline.
Secondary data analysis of the MINDS project presents informative and novel results regarding the potential manifestation of impaired awareness of deficits in a community-dwelling sample. Still, the limitations of the study need be acknowledged. First, consistent with previous studies [1], we characterized a group of individuals suspected of manifesting a dementia or MCI using comprehensive neuropsychological tests, a medical history questionnaire and a brief clinical interview which did not always involve the family. In support of the accuracy of our categorization as suspected MCI or dementia, we observed a similar pattern of cognitive deficits in the suspected and diagnosed groups. However, our assessment did not include standard dementia-screening laboratory tests (thyroid function, vitamin B12, etc.), neurological examination or neuroimaging. We can only state that individuals were suspected of having a dementia or MCI diagnosis. On the other hand, the category of suspected dementia/MCI was reserved for those cases where a possible cause of a memory problem could not be discerned from health or medication history, such that individuals with depressive symptoms and those using sedating and/or psychoactive medications were excluded from this group. Second, although adopted from our clinical work, the EMI was first used to quantify participants’ self-estimate of memory abilities in this research project. We recognize the importance of validating the instrument by other developed measurements in future projects. Next, because the purpose of the MINDS project was to offer a free-of-charge service to detect cognitive impairment among community-dwelling people without diagnosed dementia, few with a previously diagnosed dementia/MCI were included. This limitation is common in observational studies, and we statistically controlled for unequal numbers across groups. Nonetheless, our findings need replication with a larger sample. Given that a community-based sampling approach was not used, our study may have limited generalizability. For example, individuals self-selected to undergo memory screening. Furthermore, we were unable to differentiate the reasons why residents participated in the screening, i.e. as a preventative measure or due to concerns about a memory problem. Finally, like many cross-sectional studies, we were unable to identify the amount of time that a participant's awareness of deficits had been compromised, or the temporal relationship between limited awareness and cognitive decline in suspected dementia/MCI.
In consideration of these findings, we must acknowledge the ongoing methodological discussion regarding measuring awareness. Despite intense interest in the cognitive phenomenon of insight, there is no ‘gold standard’ for testing awareness. Three common approaches include measuring (1) the discrepancy between the predicted and actual performance by patients (the method used in this study), (2) the discrepancy between patient and caregiver ratings of patient performance, and (3) the clinician's judgment of the level of awareness of the patients. The pros and cons of the three approaches were discussed elsewhere [35]. For this project, we attempted to expand the methodology used by Barrett et al. [13]. Specifically, we utilized a subject interview to estimate retrospective memory performance in daily living, rather than a single-item self-evaluation, and we considered accurate awareness as a range of AR around 0 in contrast to the single-point value of 0, as has previously been done [13]. In taking these steps, we sought to improve the comparability between everyday retrospective memory performance and laboratory-based measurement of retrospective memory performance (CERAD Word List Learning Test), and to permit a distinction between mildly optimistic and grossly inaccurate appraisal of one's memory performance.
‘Memory decline in everyday life’ is a familiar concept to lay people and healthcare providers who commonly question patients about these changes when assessing cognitive ability. Our findings show that the ability to accurately self-report memory ability declines concurrently with cognitive abilities in individuals with impairments. Hence, they will likely fail to report memory problems to healthcare providers, forgoing the potential to intervene early. More importantly, the current study found a large proportion of community-dwelling older adults with suspected dementia/MCI demonstrated just such an impaired awareness of memory abilities. These findings emphasize the importance of initiatives directed toward primary care providers and caregivers as they are more likely than patients to provide the impetus for an evaluation of early cognitive and functional changes associated with MCI and dementia.
Supplementary Material
Everyday Memory Interview (published online only)
Acknowledgements
This research was supported by NIH-National Institute on Aging grants K23 AG024302 (PI CEG) and P50 AG033514, and by the Extendicare Foundation Inc. The Biostatistics and Data Management Core of the Wisconsin ADRC provided statistical consultation and assistance with analyses.
References
- 1.Sternberg SA, Wolfson C, Baumgarten M. Undetected dementia in community-dwelling older people: the Canadian Study of Health and Aging. J Am Geriatr Soc. 2000;48:1430–1434. doi: 10.1111/j.1532-5415.2000.tb02633.x. [DOI] [PubMed] [Google Scholar]
- 2.Babinski J. Contribution to the study of mental disturbance in organic cerebral hemiplegia (anosognosia) (in French) Rev Neurol (Paris) 1914;12:845–848. [Google Scholar]
- 3.Twamley EW, Ropacki SA, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease. J Int Neuropsychol Soc. 2006;12:707–735. doi: 10.1017/S1355617706060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabert MH, Albert SM, Borukhova-Milov L, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58:758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 5.Kashiwa Y, Kitabayashi Y, Narumoto J, Nakamura K, Ueda H, Fukui K. Anosognosia in Alzheimer's disease: association with patient characteristics, psychiatric symptoms and cognitive deficits. Psychiatry Clin Neurosci. 2005;59:697–704. doi: 10.1111/j.1440-1819.2005.01439.x. [DOI] [PubMed] [Google Scholar]
- 6.Starkstein SE, Jorge R, Mizrahi R, Robinson RG. A diagnostic formulation for anosognosia in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006;77:719–725. doi: 10.1136/jnnp.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannesdóttir K, Morris RG. Primary and secondary anosognosia for memory impairment in patients with Alzheimer's disease. Cortex. 2007;43:1020–1030. doi: 10.1016/s0010-9452(08)70698-1. [DOI] [PubMed] [Google Scholar]
- 8.Gallo DA, Chen JM, Wiseman AL, Schacter DL, Budson AE. Retrieval monitoring and anosognosia in Alzheimer's disease. Neuropsychology. 2007;21:559–568. doi: 10.1037/0894-4105.21.5.559. [DOI] [PubMed] [Google Scholar]
- 9.Agnew SK, Morris RG. The heterogeneity of anosognosia for memory impairment in Alzheimer's disease: a review of the literature and a proposed model. Aging Ment Health. 1998;2:7–19. [Google Scholar]
- 10.Okonkwo OC, Griffith HR, Vance DE, Marson DC, Ball KK, Wadley VG. Awareness of functional difficulties in mild cognitive impairment: a multidomain assessment approach. J Am Geriatr Soc. 2009;57:978–984. doi: 10.1111/j.1532-5415.2009.02261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel A, Stokholm J, Gade A, Andersen BB, Hejl AM, Waldemar G. Awareness of deficits in mild cognitive impairment and Alzheimer's disease: do MCI patients have impaired insight? Dement Geriatr Cogn Disord. 2004;17:181–187. doi: 10.1159/000076354. [DOI] [PubMed] [Google Scholar]
- 12.Souchay C. Metamemory in Alzheimer's disease. Cortex. 2007;43:987–1003. doi: 10.1016/s0010-9452(08)70696-8. [DOI] [PubMed] [Google Scholar]
- 13.Barrett AM, Eslinger PJ, Ballentine NH, Heilman KM. Unawareness of cognitive deficit (cognitive anosognosia) in probable AD and control subjects. Neurology. 2005;64:693–699. doi: 10.1212/01.WNL.0000151959.64379.1B. [DOI] [PubMed] [Google Scholar]
- 14.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 15.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, ed 4 (DSM-IV) Washington: American Psychiatric Association; 1994. [Google Scholar]
- 16.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 17.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 18.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol Gen. 1935;18:643–662. [Google Scholar]
- 19.Spreen O, Strauss E. A Compendium of Neuropsychological Tests. New York: Oxford University Press; 1998. [Google Scholar]
- 20.Northern California Neurobehavioral Group . Manual for the Neurobehavioral Cognitive Status Examination. Fairfax: Northern California Neurobehavioral Group; 2001. [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Sunderland T, Hill JL, Mellow AM, et al. Clock drawing in Alzheimer's disease: a novel measure of dementia severity. J Am Geriatr Soc. 1989;37:725–729. doi: 10.1111/j.1532-5415.1989.tb02233.x. [DOI] [PubMed] [Google Scholar]
- 23.Morris JC, Edland S, Clark C, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). 4. Rates of cognitive change in the longitudinal assessment of probable Alzheimer's disease. Neurology. 1993;43:2457–2465. doi: 10.1212/wnl.43.12.2457. [DOI] [PubMed] [Google Scholar]
- 24.Smith G, della Sala S, Logie RH, Maylor EA. Prospective and retrospective memory in normal ageing and dementia: a questionnaire study. Memory. 2000;8:311–321. doi: 10.1080/09658210050117735. [DOI] [PubMed] [Google Scholar]
- 25.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). 5. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 26.Mitrushina MN, Boone KB. Handbook of Normative Data for Neuropsychological Assessment. New York: Oxford University Press; 2005. [Google Scholar]
- 27.Wagner MT, Spangenberg KB, Bachman DL, O'Connell P. Unawareness of cognitive deficit in Alzheimer disease and related dementias. Alzheimer Dis Assoc Disord. 1997;11:125–131. doi: 10.1097/00002093-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 28.SPSS 16.0 for Windows. Chicago: SPSS; 2007. [Google Scholar]
- 29.Games PA, Howell JF. Pairwise multiple comparison procedures with unequal N's and/or variances: a Monte Carlo study. J Educ Behav Stat. 1976;1:113. [Google Scholar]
- 30.MacKinnon JG, Davidson R. Estimation and Inference in Econometrics. New York: Oxford University Press; 1993. [Google Scholar]
- 31.Hayes AF, Cai L. Using heteroskedasticity-consistent standard error estimators in OLS regression: an introduction and software implementation. Behav Res Methods. 2007;39:709. doi: 10.3758/bf03192961. [DOI] [PubMed] [Google Scholar]
- 32.Clement F, Belleville S, Gauthier S. Cognitive complaint in mild cognitive impairment and Alzheimer's disease. J Int Neuropsychol Soc. 2008;14:222–232. doi: 10.1017/S1355617708080260. [DOI] [PubMed] [Google Scholar]
- 33.Vogel A, Hasselbalch SG, Gade A, Ziebell M, Waldemar G. Cognitive and functional neuroimaging correlate for anosognosia in mild cognitive impairment and Alzheimer's disease. Int J Geriatr Psychiatry. 2005;20:238–246. doi: 10.1002/gps.1272. [DOI] [PubMed] [Google Scholar]
- 34.Kalbe E, Salmon E, Perani D, et al. Anosognosia in very mild Alzheimer's disease but not in mild cognitive impairment. Dement Geriatr Cogn Disord. 2005;19:349–356. doi: 10.1159/000084704. [DOI] [PubMed] [Google Scholar]
- 35.Clare L. Awareness in early-stage Alzheimer's disease: a review of methods and evidence. Br J Clin Psychol. 2004;43:177–196. doi: 10.1348/014466504323088042. [DOI] [PubMed] [Google Scholar]
- 36.Ries ML, Jabbar BM, Schmitz TW, et al. Anosognosia in mild cognitive impairment: relationship to activation of cortical midline structures involved in self-appraisal. J Int Neuropsychol Soc. 2007;13:450–461. doi: 10.1017/S1355617707070488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson SC, Ries ML, Hess TM, et al. Effect of Alzheimer disease risk on brain function during self-appraisal in healthy middle-aged adults. Arch Gen Psychiatry. 2007;64:1163–1171. doi: 10.1001/archpsyc.64.10.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer K, Backman L, Winblad B, Fratiglioni L. Detection of Alzheimer's disease and dementia in the preclinical phase: population-based cohort study. BMJ. 2003;326:245. doi: 10.1136/bmj.326.7383.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts JL, Clare L, Woods RT. Subjective memory complaints and awareness of memory functioning in mild cognitive impairment: a systematic review. Dement Geriatr Cogn Disord. 2009;28:95–109. doi: 10.1159/000234911. [DOI] [PubMed] [Google Scholar]
- 40.Purser JL, Fillenbaum GG, Wallace RB. Memory complaint is not necessary for diagnosis of mild cognitive impairment and does not predict 10-year trajectories of functional disability, word recall, or short portable mental status questionnaire limitations. J Am Geriatr Soc. 2006;54:335–338. doi: 10.1111/j.1532-5415.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- 41.Crowe M, Andel R, Wadley V, et al. Subjective cognitive function and decline among older adults with psychometrically defined amnestic MCI. Int J Geriatr Psychiatry. 2006;21:1187–1192. doi: 10.1002/gps.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Everyday Memory Interview (published online only)