Abstract
Background
Most cystic neoplasms of the pancreas (CNPs) are incidentally discovered. Their management continues to be debated and preoperative diagnosis is often inaccurate.
Methods
Retrospective review of 330 patients with incidentally discovered CNPs. Preoperative and final histological diagnoses were correlated.
Results
41% (136/330) of patients were operated on at diagnosis. 50 patients underwent resection for a presumed branch-duct (Bd) intraductal papillary mucinous neoplasm (IPMN), which was confirmed in only 64% (32/50); of the remaining patients, 20% had main-duct involvement. Mucinous cystic neoplasm was the preoperative diagnosis in 30/136 patients, histologic examination was confirmatory in only 60% (18/30). Most lesions presumed to be main-duct or combined IPMNs or serous cystadenomas were confirmed as such after resection (15/16 and 11/12, respectively). Multifocality was not only associated with Bd-IPMN, and 5% of all cysts were non-neoplastic. Overall, in only 68% of cases did the preoperative and histological diagnoses match.
Conclusions
In an experienced, high-volume center, preoperative diagnosis was incorrect in one-third of incidentally discovered CNPs who underwent resection. Of particular concern, 20% of presumed Bd-IPMN had a main-duct component. Conversely, 5% of resected cysts were not even neoplastic. Clearly, better diagnostic methods are needed to aid in formulating appropriate treatment strategies.
Key Words: Branch-duct IPMNs; Cystic neoplasms; Incidental pancreatic cysts; Intraductal papillary mucinous neoplasms; Main-duct IPMNs; Mucinous cystic neoplasms; Pancreas, cystic neoplasms; Pancreatic cysts
Introduction
The diagnostic work-up and management of incidentally discovered cystic lesions of the pancreas (CLPs) continues to evolve. In 2004, the Sendai consensus conference provided guidelines for the management of intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs) [1]. It recommended resection for all main-duct IPMNs and MCNs, and ‘careful observation’ for asymptomatic branch-duct (Bd) IPMNs measuring <30 mm in the absence of solid components or main-duct dilation. Because the majority of incidentally discovered CLPs are felt to be Bd-IPMNs and fit the Sendai criteria, currently many are managed non-operatively with close clinical follow-up and frequent re-imaging. So far, several studies have validated this approach [2,3,4], although none of them has yet followed patients long term. A major caveat of these follow-up studies is the lack of a definitive diagnosis in most patients since only few have thus far been resected.
Little is known about the accuracy of the clinician's ability to ascertain the identity of CLPs in advance of tissue confirmation. In an attempt to measure this accuracy, the present study compares the preoperative and final histological diagnoses in a large cohort of incidentally discovered CLPs, as evaluated by various imaging strategies.
Methods
A retrospective review identified 566 patients who were evaluated for a possible cystic neoplasm of the pancreas between January 2000 and January 2008 in the Department of Surgery of the Massachusetts General Hospital. Among these, 330 patients were classified as having incidentally discovered lesions either because they were asymptomatic or because the cyst was noted during imaging of an unrelated problem.
Using electronic hospital records and office charts, relevant clinicopathological information was logged into a Microsoft Access database. The collected data included patients’ demographics, details of diagnostic evaluation, presumptive diagnosis, and initial disposition (i.e. surgery or observation). The cysts were measured at their largest diameter on cross-sectional imaging. In those patients who underwent resection, the preoperative clinical diagnosis was compared to the final tissue diagnosis. We specifically avoided re-reviewing available images and pathological blocks a posteriori in order to report our actual experience, rather than an unrealistic best-case scenario.
Data is presented as mean and SD or median and range. Statistical analysis was carried out using the Primer of Biostatistics v6.0 software [5]; t test was used for continuous variables and z test for comparison of proportions. A p value <0.05 was considered statistically significant.
Results
Incidentally discovered cysts were most frequently identified during the work-up of urologic complaints (21%), other gastrointestinal diseases (21%), non-specific abdominal discomfort (17%), thoracic (9%) or gynecologic complaints (4%). The majority (62%) were found in women, and the mean age was 65 ± 14 years. All patients were evaluated with cross-sectional imaging: 76% had a CT and 41% had an MR (19% had both); 52% also underwent endoscopic ultrasound (EUS). Median cyst size was 20 mm (3–130 mm) and multiple lesions were present in 60/330 patients (18%); the median number of lesions in these patients was 4 (range 2–10).
Early Surgical Treatment
At the time of diagnosis, 41% of patients (136/330) with incidentally discovered CLPs underwent resection generally on the basis of the Sendai criteria suggesting concern for potential malignancy. Their characteristics and preoperative diagnosis are shown in table 1. 44% underwent a distal pancreatectomy, 41% a pancreaticoduodenectomy and 15% a middle pancreatectomy or other atypical resection. Operative mortality was zero. The final pathologic diagnoses are shown in table 2. Invasive cancer was found in 9 patients (7%) all of which met Sendai criteria, having either main-duct dilation, a solid component, or size >3 cm; 16 (12%) had either carcinoma in situ or a neuroendocrine neoplasm.
Table 1.
Characteristics and initial disposition
| Surgery | No surgery | p value | |
|---|---|---|---|
| Total patients | 136 | 194 | |
| Female, % | 60 | 64 | 0.5 |
| Mean age ± SD | 61 ± 15 | 68 ± 13 | <0.01 |
| Median size (range) | 30 (7–130) | 15 (3–65) | <0.01 |
| Multifocal cysts (%) | 6 (4) | 54 (28) | <0.01 |
| Preoperative Dx (%) | |||
| Bd-IPMN | 50 (37) | 152 (78) | <0.01 |
| MCN | 30 (22) | 7 (4) | 0.5 |
| Md-IPMN | 16 (12) | 4 (2) | 0.7 |
| SCA | 12 (9) | 20 (10) | 0.6 |
| CPEN | 8 (6) | ||
| SPPN | 4 (3) | ||
| Other | 3 (3) | 6 (3) | 0.06 |
| Cystic PDAC | 2 (1) | 1 (1) | NS |
| Uncertain diagnosis1 | 11 (7) | 4 (2) | 0.3 |
Characteristics and preoperative diagnosis in 315 patients stratified by initial disposition.
Bd-IPMN = Branch-duct intraductal papillary mucinous neoplasm; MCN = mucinous cystic neoplasm; Md-IPMN = main-duct intraductal papillary cystic neoplasm; SCA = serous cystad-enoma; CPEN = cystic pancreatic endocrine neoplasm; SPPN = solid pseudopapillary neoplasm; PDAC = pancreatic ductal adenocarcinoma.
The preoperative diagnosis was not documented.
Table 2.
Histological diagnoses in 136 cystic lesions of the pancreas resected at presentation
| Degree of dysplasia | Mucinous neoplasms |
Non-mucinous neoplasms |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Bd-IPMN | Md-IPMN | MCN | SCA | SPPN | CPEN | Uncl. | Other | ||
| Benign | Adenoma | 17 | 3 | 20 | 22 | 9 | 6 | 2 | |
| Borderline | 17 | 11 | 4 | ||||||
| Malignant | CIS | – | 8 | – | – | 8 | – | 3 | |
| Invasive | 2 | 3 | 1 | ||||||
| Sum (% of total) | 36 (26) | 25 (18) | 25 (18) | 22 (17) | 9 (7) | 8 (6) | 6 (4) | 5 (4) | |
Bd-IPMN = Branch-duct intraductal papillary mucinous neoplasms; MCN = mucinous cystic neoplasm; Md-IPMN = main-duct intraductal papillary cystic neoplasm; SCA = serous cystadenoma; CPEN = cystic pancreatic endocrine neoplasm; SPPN = solid pseudopapillary neoplasm; Uncl. = unclassified, non-neoplastic cysts; CIS = carcinoma in situ.
Fifty patients underwent resection for a presumed Bd-IPMN, but only 32 of these (64%) were confirmed as such by histopathology. A main-duct extension was identified in 10 patients (20%), and therefore the final pathological diagnosis was combined-duct IPMN. Figure 1 shows an example of this situation. Two cases were serous cystadenomas (SCA), and 1 was a MCN. The remaining 5 were diagnosed histologically as ‘unclassified’ benign pancreatic cysts, which are thought to be non-neoplastic.
Fig. 1.
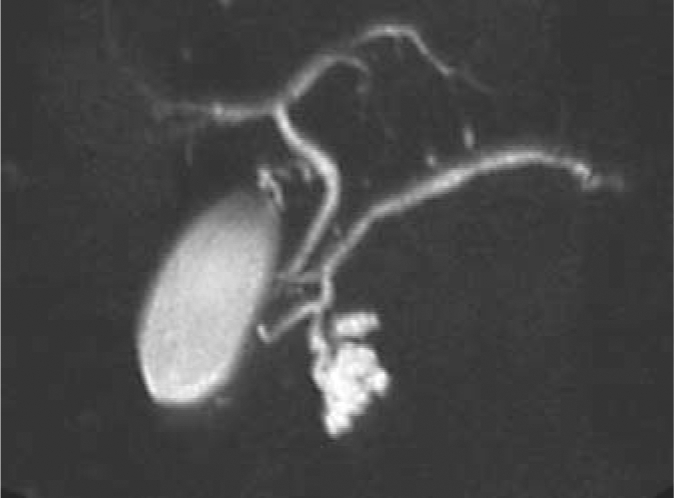
Magnetic resonance cholangiopancreatography of a 64-year-old female with an incidentally discovered cystic lesion at the uncinate process of the pancreas. The lesion measured 2.7 cm and had no associated nodules; the rest of the pancreas was unremarkable. A presumed diagnosis of Bd-IPMN was made. Because of recent onset of diabetes, and a positive family history for pancreatic cancer, the patient underwent a Whipple's resection. Final diagnosis was combined main-duct and Bd-IPMNs.
Thirty patients were operated on with the preoperative diagnosis of MCN, of which only 18 were confirmed by pathologic examination (60%). The other 12 were: Bd-IPMNs (4), cystic pancreatic endocrine neoplasms (3) (fig. 2), solid-pseudopapillary neoplasms (2), SCA (1), cystic acinar-cell carcinoma (1), and ‘unclassified’ benign pancreatic cyst (1).
Fig. 2.
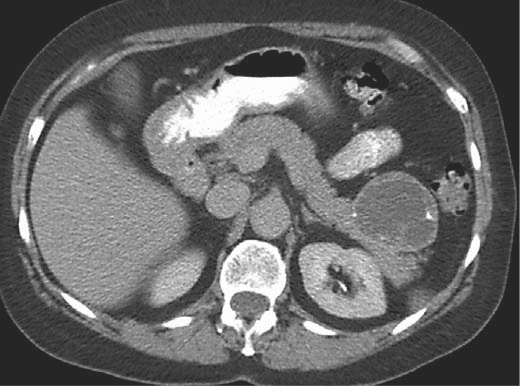
CT scan from a 67-year-old female with an incidentally discovered heterogeneous cystic lesion at the tail of the pancreas measuring 5.2 cm, and with internal calcium deposits. The patient was operated on with a preoperative diagnosis of an MCN, but the final pathology revealed a cystic neuroendocrine neoplasm.
Of the 16 patients presumed to have a main-duct or combined IPMN that were operated on, 15 (94%) were confirmed as such after resection; the other was an MCN.
In 12 patients, the preoperative diagnosis was SCA, and the final histological diagnosis differed in only 1 (MCN).
In 8 patients, the presumed diagnosis was that of a cystic pancreatic endocrine neoplasm (CPEN). This was confirmed by pathology in 4 cases; the remaining were SCAs (2) and solid pseudopapillary neoplasms (2).
Nine patients were thought to have a variety of other diagnoses (including solid pseudopapillary neoplasms, lymphangioma, and pancreatic adenocarcinoma with cystic degeneration), and the diagnosis was accurate only 50% of the time (4/8).
A specific preoperative diagnosis was not documented in 11 patients. Their final histological diagnoses were: MCN (4), SCA (4), solid pseudopapillary tumor (1), CPEN (1), and lymphoepithelial cyst (1).
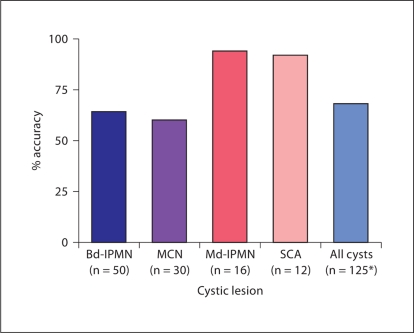
Overall, in patients operated on at presentation, the clinician accurately predicted the final diagnosis only 68% of the time (fig. 3).
Fig. 3.
Accuracy of preoperative diagnosis in 125 incidentally discovered cysts resected at presentation. ∗ Includes 8 cystic endocrine neoplasms, 4 solid pseudopapillary neoplasms, and 5 with various other diagnoses. 11 patients did not have a specific preoperative diagnosis.
Non-Operative Surveillance
194 patients with incidentally discovered pancreatic cysts were initially managed non-operatively, and 179 of them were followed in a surveillance program with annual or semiannual radiological examination. Their clinical characteristics and preoperative diagnoses are listed in table 1. Compared to resected patients, these were older, had smaller cysts, and were seven times more likely to have multifocal cysts. The most common presumed diagnosis was Bd-IPMN 79%, followed by SCA 10%.
Delayed Surgery
Of the 179 patients (13%) in a surveillance program, 23 were eventually operated on after a median follow-up of 23 months (4–90 months). Their characteristics and the reasons for crossover to surgery are detailed in table 3. The most common reason to opt for resection was a significant increase in size. The surgical procedures were: 11 pancreaticoduodenectomies, 11 distal pancreatectomies, and 1 middle pancreatectomy; there was only 1 postoperative death.
Table 3.
Presentation, diagnosis and indications for resection in 23 patients initially managed with observation
| Case | Patient | Initial diagnosis | Dx at the time of surgery | Follow-up months | Reason for resection | Size increase | Tumor markers | Histological diagnosis |
|---|---|---|---|---|---|---|---|---|
| 1 | 62 years M; 21 mm cyst/uncinate (additional 19 mm/neck) | Bd-IPMN (multifocal) | same | 23 | growth | 11 | IPMN main duct borderline | |
| 2 | 62 years M; 37 mm cyst/body | SCA | same | 54 | growth | 21 | serous cystadenoma | |
| 3 | 85 years F, 13 mm cyst/tail | MCN | same | 31 | growth | 7 | IPMN branch duct borderline | |
| 4 | 70 years F; 23 mm cyst/tail | Bd-IPMN | same | 12 | growth | 12 | serous cystadenoma | |
| 5 | 68 years F; 20 mm cyst/head (additional 2–3 mm cyst/body and tail) | Bd-IPMN (multifocal) | same | 39 | growth | 2 | IPMN branch duct adenoma | |
| 6 | 85 years M; 12 mm cyst/head | Bd-IPMN | same | 47 | growth | 21 | IPMN branch duct borderline | |
| 7 | 69 years F; 24 mm cyst/head | pseudocyst | combined IPMN | 14 | growth | 16 | combined IPMN adenoma | |
| 8 | 71 years M; 29 mm cyst/tail | Bd-IPMN | same | 76 | tumor markers | CEA: 143 ng/ml + RedPath® | MCN adenoma | |
| 9 | 77 years M; 20 mm cyst/tail | Md-IPMN | 87 | growth | 10 | IPMN main duct borderline | ||
| 10 | 74 years F; 10 mm cyst/head | Bd-IPMN | same | 61 | growth | 20 | IPMN branch duct borderline | |
| 11 | 46 years F; 16 mm cyst/head (additional 5–10 mm cyst/body and tail) | Bd-IPMN (multifocal) | same | 10 | growth | 14 | unclassified ‘benign’ cysts | |
| 12 | 86 years M; ‘growing’ lesion/head | MCN | 15 | growth | initial: unknown final: 60 mm | IPMN branch duct invasive | ||
| 13 | 54 years F; ‘growing’ lesion/body and tail | uncertain | uncertain | 7 | growth | initial: unknown final: 25 mm | serous cystadenoma | |
| 14 | 54 years F; 40 mm cyst/tail | MCN | 15 | nodule | MCN CIS | |||
| 15 | 49 years F; 20 mm cyst/head | Bd-IPMN | same | 10 | nodule | lymphangioma | ||
| 16 | 53 years F; 18 mm cyst/head | MCN | IPMN | 8 | growth | 7 | serous cystadenoma | |
| 17 | 24 years M; 20 mm cyst/tail | lympho-epithelial cyst | same | 9 | growth | 10 | lymphoepithelial cyst | |
| 18 | 36 years F; 30 mm cyst/tail | SCA | MCN | 40 | growth | 13 | MCN adenoma | |
| 19 | 62 years F; 10 mm cyst/body | Bd-IPMN | same | 21 | tumor markers | CA 19.9: 68 U/ml | IPMN branch duct borderline | |
| 20 | 83 years F; 15 mm cyst/tail | uncertain | uncertain | 42 | nodule | MCN adenoma | ||
| 21 | 63 years F; 20 mm cyst/head | Bd-IPMN | same | 4 | anxiety | unclassified ‘benign’ cysts | ||
| 22 | 67 years F; 18 mm cyst/tail | Bd-IPMN | MCN invasive | 90 | nodule | IPMN branch duct invasive | ||
| 23 | 64 years F; 27 mm cyst/head | Bd-IPMN | same | 23 | nodule | combined IPMN – borderline | ||
CEA = Carcinoembryonic antigen; CA 19.9 = cancer antigen 19.9.
During follow-up, the clinician reconsidered the initial diagnosis in 4 patients (17%). In patients who underwent delayed surgery, preoperative and final histological diagnosis matched 52% of the time; the final histological diagnoses are shown in table 1. Two of these patients were found to have invasive carcinoma after 1.2 and 7.5 years of follow-up, respectively (cases 12 and 22; table 3).
Multifocal Disease
15% (9/60) of patients with multiple cysts underwent resection. Two were presumed to have combined-duct IPMN, and this was confirmed by histological examination. Multifocal Bd-IPMN was the preoperative diagnosis in the remaining 7 cases, however, histological diagnoses confirmed this only in 2 cases. The other 5 were combined-duct-IPMNs (2) and ‘unclassified’ multifocal benign cysts (3) (fig. 4). The preoperative and final diagnosis matched only 44% of the time.
Fig. 4.
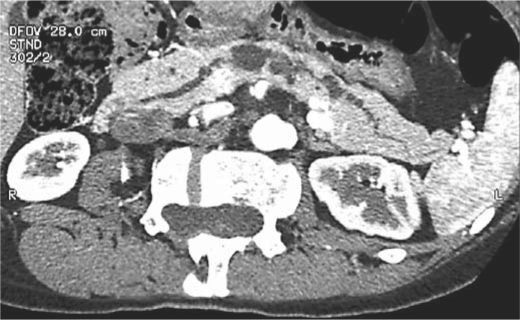
Reformatted CT images from a 46-year-old female with the incidental discovery of multiple pancreatic cysts during the evaluation of non-specific lower abdominal pain. This was presumed to be multifocal Bd-IPMN or combined IPMN. The suspicion of an associated nodule, and a mildly dilated pancreatic duct to 6 mm, prompted an operation. The final pathological diagnosis was multifocal non-neoplastic cysts.
‘Unclassified’ Benign Cysts
Eight of 159 (5%) patients operated on for a presumed cystic neoplasm (7 Bd-IPMNs, 1 MCN) were found to have ‘unclassified’ non-neoplastic cysts. These cysts did not show communication with the ductal system or a mucinous epithelium and are believed to be non-neoplastic.
Preoperative Test, Size and Accuracy of Diagnosis
The diagnosis was correctly predicted in 63% of cases when cross-sectional (CT or MRI) imaging was used alone; when both studies were done, the accuracy was also 63%. It was not improved in patients who had EUS in addition to either CT or MRI (69%, p = NS). Only 4 patients were evaluated by the three modalities (CT + MRI + EUS), and only in 2 was the preoperative diagnosis accurate.
Stratifying cysts by size at diagnosis (<30, 31–40, 41–50, >51 mm) did not impact the accuracy of preoperative diagnosis, which was 61, 64, 61, and 60%, respectively (p = NS).
Discussion
The widespread use of high-resolution imaging studies has resulted in detection of unsuspected findings in many organs, and the pancreas is no exception. As a consequence, incidental CLPs are being reported with increasing frequency. A recent study looking at multidetector CT in 2,832 outpatients reported the prevalence of pancreatic cysts as 2.6% [6]. The average size of these cysts was 8.9 mm, and their incidence directly correlated with age. There is concern that a number of these incidentally discovered cysts may represent precursors of pancreatic cancer, such as IPMNs and MCNs, but it is unclear how many of them carry a risk of malignant degeneration, how to identify those accurately, and, once recognized, how to establish which ones are likely to harbor incipient cancer.
The Sendai consensus proposed guidelines for management of IPMNs and MCNs that have been widely adopted in the surgical decision-making process [1.] However, they are based on the premise that we can accurately classify these lesions on the basis of imaging characteristics, an assumption yet to be proven. Through pattern recognition (i.e. demographics, presentation, radiologic appearance, fluid analysis, etc.), a diagnosis is usually reached, but our experience suggests that the histological diagnosis will commonly differ. Although the discrepancy between the preoperative and final diagnosis is unimportant in some cases (e.g. 6-cm cyst in the tail of the pancreas harboring nodules merits resection whether it is a Bd-IPMN, an MCN, or any other cystic neoplasm), in other situations alternative diagnoses might influence the clinician towards either more aggressive or more conservative management (e.g. the management of an asymptomatic 2-cm cystic lesion in the tail of the pancreas without a visible solid component differs if it is believed to be an MCN or a Bd-IPMN).
The present study attempts to provide insight into the presumed accuracy of preoperative diagnosis. In a referral center that currently manages over 100 new patients with CLPs per year, and which has clinicians, endoscopists and radiologists who believe themselves to be very familiar with the differential diagnosis of these lesions, we found that the accuracy of preoperative diagnosis is only 68%, and that it is not the same for all cystic lesions. On the one hand, when the preoperative diagnosis is that of a main-duct IPMN or a SCA, this was almost always correct. On the other hand, when the diagnosis is Bd-IPMN or MCN, the postoperative diagnosis was incorrect about 40% of the time. While it is not surprising that diagnostic overlap exists between these two entities since they have morphologic similarities, misdiagnosis in either direction (i.e. Bd-IPMN that turned out to be MCN, or MCN that was a Bd-IPMN) only accounts for 16% of misdiagnosed cases (5/30). The error in diagnosis does matter, nonetheless, because current recommendations are to resect all MCNs whereas observation is considered appropriate for Bd-IPMN <3 cm. We also found that as many as 20% of presumed Bd-IPMNs have main-duct extension. This is of particular concern because combined Bd- and main-duct IPMNs, like pure main-duct IPMNs, are felt to have an increased likelihood of malignancy [7,8,9] (although it is unclear if a microscopic involvement of the main pancreatic duct has the same implications).
On the opposite end of the spectrum, 5% of all resected cysts were histologically diagnosed as non-neoplastic, benign pancreatic cysts. Little is known about these lesions, which have neither mucinous epithelium nor communication with the ductal system. Their radiological characteristics closely mimic and are indistinguishable from Bd-IPMN. They ranged in size between 2 and 3.5 cm, and 50% were multifocal. Had we known that these cysts were non-neoplastic, we would have avoided resection in these asymptomatic patients.
By extrapolating from the preoperative and final histopathological diagnosis in patients with incidentally discovered pancreatic cysts who underwent resection, we attempted to gain insight into the accuracy of diagnosis in the patients managed expectantly. It could be argued that this cannot be done, since the population is different (older patients with smaller cysts). However, the lack of correlation between preoperative and operative diagnosis was also seen in the 23 patients within the observation group who eventually came to surgery (only 52% accuracy), and we found that neither cyst size nor multifocality (which was more commonly seen in patients managed with observation) influenced this. Inasmuch as the majority of patients with asymptomatic cysts that are being followed have the presumptive diagnosis of Bd-IPMN, it is quite probable that this diagnosis is incorrect in about a third.
This study does not address the issue of which imaging modality is optimal for the differential diagnosis of CLPs. Its retrospective nature, as well as the fact that most patients had only one imaging test and that many of the studies were done in community hospitals with varying techniques and technologies, precludes such a conclusion. We did find that those patients who had both CT and MRI did not have a more accurate preoperative diagnosis. Other than finding a minimal increment in the accuracy of preoperative diagnosis with EUS, our study does not allow us to evaluate its role in refining the diagnosis, or to consider the possible contribution of guided aspiration of cysts for evaluation of the fluid contents. We have previously reported our experience with CEA measurement and cytological fluid analysis [4,10], and we believe that prospective studies are needed to re-define their role.
In summary, analysis of this large cohort of incidental pancreatic cysts shows that diagnosis based on clinical and radiological information, with or without EUS, is inaccurate in over a third of patients. Some of these have more concerning features than expected, and others, comprising 5% of incidental cysts, are not even neoplastic.
These results underscore the difficulty in preoperative classification of cystic neoplasms of the pancreas with current methods. We continue to face the dilemma of too much versus too little treatment. We do not want to miss the opportunity for cure, but also seek to avoid unnecessary pancreatic resections. In the present state of the art, we await reliable tools, perhaps using new biochemical or genetic markers in the cyst fluid or refinement in radiological imaging [11], that allow for a more precise preoperative diagnosis of incidentally discovered, asymptomatic pancreatic cysts.
References
- 1.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 2.Allen PJ, D'Angelica M, Gonen M, Jaques DP, Coit DG, Jarnagin WR, DeMatteo R, Fong Y, Blumgart LH, Brennan MF. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006;244:572–582. doi: 10.1097/01.sla.0000237652.84466.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das A, Wells CD, Nguyen CC. Incidental cystic neoplasms of pancreas: what is the optimal interval of imaging surveillance? Am J Gastroenterol. 2008;103:1657–1662. doi: 10.1111/j.1572-0241.2008.01893.x. [DOI] [PubMed] [Google Scholar]
- 4.Ferrone CR, Correa-Gallego C, Warshaw AL, Brugge WR, Forcione DG, Thayer SP, Fernandez-del Castillo C. Current trends in pancreatic cystic neoplasms. Arch Surg. 2009;144:448–454. doi: 10.1001/archsurg.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glantz SA. Primer of Biostatistics. ed 6. New York: McGraw-Hill Medical; 2005. [Google Scholar]
- 6.Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnelldorfer T, Sarr MG, Nagorney DM, Zhang L, Smyrk TC, Qin R, Chari ST, Farnell MB. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639–646. doi: 10.1001/archsurg.143.7.639. [DOI] [PubMed] [Google Scholar]
- 8.Salvia R, Fernandez-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, Pederzoli P, Warshaw AL. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685. doi: 10.1097/01.sla.0000124386.54496.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–434. doi: 10.1001/archsurg.138.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correa-Gallego C, Warshaw AL, Fernandez-del Castillo C. Fluid CEA in IPMNS: a useful test or the flip of a coin? Am J Gastroenterol. 2009;104:796–797. doi: 10.1038/ajg.2008.158. [DOI] [PubMed] [Google Scholar]
- 11.Kinney TP, Freeman ML. Pancreatic imaging: current state of the art. Gastroenterology. 2009;136:776–779. doi: 10.1053/j.gastro.2009.01.023. [DOI] [PubMed] [Google Scholar]