Abstract
Background
Xanthine oxidase is a major source of superoxide in the vascular endothelium. Previous work in humans demonstrated improved conduit artery function following xanthine oxidase inhibition in patients with obstructive sleep apnea. Objectives: To determine whether impairments in endothelium-dependent vasodilation produced by exposure to chronic intermittent hypoxia are prevented by in vivo treatment with allopurinol, a xanthine oxidase inhibitor.
Methods
Sprague-Dawley rats received allopurinol (65 mg/kg/day) or vehicle via oral gavage. Half of each group was exposed to intermittent hypoxia (FIO2 = 0.10 for 1 min, 15×/h, 12 h/day) and the other half to normoxia. After 14 days, gracilis arteries were isolated, cannulated with micropipettes, and perfused and superfused with physiological salt solution. Diameters were measured before and after exposure to acetylcholine (10−6M) and nitroprusside (10−4M).
Results
In vehicle-treated rats, intermittent hypoxia impaired acetylcholine-induced vasodilation compared to normoxia (+4 ± 4 vs. +21 ± 6 μm, p = 0.01). Allopurinol attenuated this impairment (+26 ± 6 vs. +34 ± 9 μm for intermittent hypoxia and normoxia groups treated with allopurinol, p = 0.55). In contrast, nitroprusside-induced vasodilation was similar in all rats (p = 0.43). Neither allopurinol nor intermittent hypoxia affected vessel morphometry or systemic markers of oxidative stress. Urinary uric acid concentrations were reduced in allopurinol- versus vehicle-treated rats (p = 0.02).
Conclusions
These data confirm previous findings that exposure to intermittent hypoxia impairs endothelium-dependent vasodilation in skeletal muscle resistance arteries and extend them by demonstrating that this impairment can be prevented with allopurinol. Thus, xanthine oxidase appears to play a key role in mediating intermittent hypoxia-induced vascular dysfunction.
Key Words: Hypoxia, Allopurinol, Endothelium, Oxidative stress
Introduction
Obstructive sleep apnea (OSA) is a medical problem increasingly associated with cardiovascular disease and cardiovascular events such as myocardial infarction and stroke [1,2,3,4,5]. OSA is characterized by intermittent obstruction of the upper airway that results in episodes of hypoxemia, hypercapnia and arousal from sleep. Over time, episodes of OSA increase sympathetic nerve activity [6], impair function of the vascular endothelium [7,8,9] and alter arterial structure [10]. Even in the absence of clinical cardiovascular disease, patients with OSA exhibit endothelial dysfunction [7,9] and vascular remodeling [10,11,12,13]. Putative mediators of endothelial dysfunction and vascular remodeling are increased concentrations of vasoconstrictor substances (for example, catecholamines, angiotensin II, and endothelin-1) [14,15,16], vascular inflammation [12,17,18] and oxidative stress [18,19,20,21]. One potential source of OSA-induced oxidative stress in vascular endothelium is the cytosolic and membrane-bound enzyme xanthine oxidase. Superoxide anions generated by this enzyme could limit nitric oxide bioavailability, thereby impairing endothelium-dependent vasodilation [22,23], and contribute to vascular remodeling [24].
During intermittent hypoxia and reoxygenation, xanthine oxidase, NADPH oxidase and ferrylhemoglobin are potent sources of superoxide radicals [25,26,27,28,29]. There are compelling reasons to investigate the role of xanthine oxidase as a source of vascular superoxide in OSA. First, xanthine oxidase activity is increased by hypoxia and by various cytokines such as tumor necrosis factor-α (TNF-α) [30,31,32]. Since patients with OSA experience frequent hypoxic episodes during sleep and TNF-α concentrations are elevated in patients with OSA [33], xanthine oxidase activity may also be increased in patients with OSA. Second, El Solh et al. [34] demonstrated that 2 weeks of xanthine oxidase inhibition improved flow-mediated dilation in the brachial arteries of patients with OSA, suggesting that xanthine oxidase plays a role in the vascular impairments caused by OSA.
We previously demonstrated that a 2-week exposure to intermittent hypoxia in rats, an established animal model for OSA, caused impairments in acetylcholine (ACh)- and hypoxia-induced vasodilation in the skeletal muscle and cerebral circulations [35]. In contrast, the exposure had no effect on vasodilator responses to the nitric oxide donor sodium nitroprusside, suggesting that intermittent hypoxia impairs endothelial function by reducing the bioavailability of nitric oxide. In the present study, we investigated the role of xanthine oxidase-derived superoxide anions in causing those impairments. Accordingly, we tested the hypothesis that xanthine oxidase inhibition with allopurinol would prevent intermittent hypoxia-induced endothelial dysfunction.
Methods
Hypoxic Exposure
Adult male Sprague-Dawley rats, in their home cages, were placed into a Plexiglass chamber (1–3 rats per cage) and exposed to intermittent hypoxia for 12 h/day (from 18:00 to 6:00 h) for 14 days. Rats were housed in accordance with space recommendations set forth in the National Institutes of Health Guide for the Care of Laboratory Animals (NIH publication No. 85-23, revised 1985). Oxygen concentrations in the chamber were monitored using a heated zirconium sensor (Fujikura America, Pittsburgh, Pa., USA) connected to solenoid valves that controlled the flow of oxygen and nitrogen. The valves were operated by a microprocessor-controlled timer. The system was set to provide hypoxic exposures at 4-min intervals. During the first minute of each cycle, nitrogen was flushed into the chamber at a rate sufficient to achieve a fraction of inspired oxygen (FIO2) of 0.10 within 60 s. This level of FIO2 was maintained for an additional 60 s. Oxygen was then introduced at a rate sufficient to achieve a FIO2 of 0.21 within 30 s and to maintain this oxygen level for the duration of the 4-min cycle. The oxygen concentrations were checked daily using a TED60T sensor (Teledyne, City of Industry, Calif., USA). Control rats were housed under normoxic conditions adjacent to the hypoxia chamber. In the chamber and the room, temperature was maintained at 24 ± 1°F and relative humidity was maintained between 20 and 70%. Ad libitum access was provided to standard rat chow and water. The protocol was approved by the University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee and conducted in accordance with all institutional rules and regulations for animal research.
Allopurinol Dosing
Rats were randomized to receive either allopurinol (65 mg/kg/day) or vehicle via oral gavage starting 1 week before and continuing through 2 weeks of experimental hypoxia or normoxia exposure (3 weeks total). There were four experimental groups: (1) rats exposed to intermittent hypoxia that received vehicle; (2) rats exposed to intermittent hypoxia that received allopurinol; (3) rats exposed to normoxia that received vehicle; (4) rats exposed to normoxia that received allopurinol. Allopurinol suspension was prepared by mixing 2,000 mg allopurinol into a 1% (w/v) methylcellulose suspension to achieve a 20-mg/ml concentration. Placebo was a 1% (w/v) methylcellulose vehicle. Klein et al. [36] demonstrated that an oral dose of 50 mg/kg/day adequately inhibits xanthine oxidase activity in a rat model. However, previous work indicates that hypoxia upregulates xanthine oxidase activity [31,32]; therefore, we used a higher dose (65 mg/kg/day) in order to inhibit the increased xanthine oxidase activity resulting from intermittent hypoxia. Rats receiving vehicle received equivalent daily oral gavage volumes. Daily gavage volumes ranged between 0.5 and 1.1 ml.
Vessel Harvesting Procedures
The rats were anesthetized (50 mg/kg pentobarbital sodium given intraperitoneally) and the small muscular branch of the femoral artery supplying the gracilis muscle was dissected out. Care was taken to minimize stretching, and the artery was handled only by the surrounding connective tissue. After excision, the arteries were placed in warmed physiological salt solution composed of (in mM): 119.0 NaCl, 4.7 KCl, 1.17 MgSO4, 1.6 CaCl2, 1.18 NaH2PO4, 24.0 NaHCO3, 0.03 EDTA and 5.5 dextrose for transfer to the tissue bath.
Vessel Reactivity Studies
The excised gracilis artery was immersed in warmed physiological salt solution bubbled with oxygen (O2), nitrogen (N2) and carbon dioxide (CO2) blended to achieve a gas composition of 19.3 kPa (145 mm Hg) O2, 5.3 kPa (40 mm Hg) CO2 in a superfusion-perfusion chamber (LSI, Burlington, Vt., USA). The proximal and distal ends of the artery were cannulated with glass micropipettes (120 μm, LSI) and secured to the pipettes using 10-0 nylon sutures. The vessel was stretched to the in situ length, and side branches were singly ligated with small strands teased from a 6-0 silk suture (Ethicon, Somerville, N.J., USA) to ensure optimal pressurization. The inflow pipette was connected to a reservoir perfusion system that allows the intraluminal pressure and luminal gas concentration to be controlled. After the artery was mounted on the pipettes, it was allowed to equilibrate for 1 h before baseline measurements were made.
Vessel diameter was measured using television microscopy and a video micrometer (LSI). The level of baseline tone (%) in the vessel was calculated as follows: [(ΔD × Dmax−1) × 100] where Dmax represents the maximum diameter of the vessel at baseline pressure of 10.6 kPa (80 mm Hg) under calcium-free conditions, and ΔD is the increase in diameter from baseline to maximal relaxation. Arteries exhibiting <20% baseline tone were excluded from analysis. In our previous investigations of the effects of chronic intermittent hypoxia on vascular function, the average amount of baseline tone exhibited by gracilis arteries in vitro was approximately 20% [35]. In the present experiments, we required at least 20% baseline tone to ensure that the arteries were healthy and reactive prior to study.
The responses to ACh (10−6M; Sigma, St. Louis, Mo., USA) and sodium nitroprusside (10−4M; Sigma) were assessed in gracilis arteries from each group of rats. In a previous study, we documented a dose-response relationship for ACh and gracilis artery diameter using concentrations of 10−7, 10−6, 10−5 and 10−4M[35]. Based on that analysis, in the present experiment we chose to study responses to 10−6M ACh, a concentration that consistently produced vigorous vasodilations and clear differences in responses of gracilis arteries from rats exposed to intermittent hypoxia versus normoxia. We chose a single high concentration of sodium nitroprusside (10−4) to ensure a brisk vasodilator response to exogenous nitric oxide. When these drugs were administered, the vessel was pressurized to 10.6 kPa (80 mm Hg) by clamping the outflow pipette, and an appropriate amount of drug was added to the superfusate. Vessel diameter was monitored continuously and was measured at the point of its maximum value after the addition of the dilator agent. Vessel responses were measured by an investigator (N.R.P.) blinded to group assignment.
We also assessed vasodilator responses to acute reductions in PO2. Arteries were pressurized to 10.6 kPa (80 mm Hg) by clamping the outflow pipette, and the PO2 of the perfusate and superfusate was lowered to approximately 5.3 kPa (40 mm Hg) by addition of supplemental N2 [35,37]. Vessel diameters were monitored continuously for 20 min, and were measured at the point of maximum value. After acute hypoxic exposures, normoxic conditions were restored in the perfusate and superfusate, and recovery from hypoxia was verified by measuring vessel diameters.
After the response of the arteries to the vasodilator stimuli had been determined, vessel diameter was determined after the vessels were maximally dilated with calcium-free relaxing solution containing the following constituents (in mM): 92.0 NaCl, 4.7 KCl, 1.17 MgSO4·7H2O, 20.0 MgCl·6H2O, 1.18 NaH2PO4, 24.0 NaHCO3, 0.026 EDTA, 2.0 EGTA and 5.5 dextrose.
Vessel Morphometry
After the gracilis artery was harvested for vessel reactivity studies, the animal was euthanized and the contralateral gracilis artery was perfused in situ with 4% paraformaldehyde at 10.6 kPa (80 mm Hg) for 45 min and then excised. The vessel was embedded in paraffin, cross-sectioned and stained with hemotoxylin and eosin for vessel morphometry measurements (Histo-Scientific Research Laboratories, Mount Jackson, Va., USA). The sections were visualized on an inverted microscope (TE-2000; Nikon, Melville, N.Y., USA) and were captured using a Spot camera and software for image analysis (MetaVue; Optical Analysis Systems, Nashua, N.H., USA) by a single observer blinded to experimental condition (C.E.B.). In the hematoxylin- and eosin-stained tissue sections, intima-media thickness (IMT) was assessed with line measurement tools (after appropriate calibration) by averaging 12 equally spaced positions around the entire vessel circumference. To calculate the lumen diameter, circumference was determined by averaging the sizes of two circles – one drawn at the ‘peaks’ of the endothelial folds and the other drawn at the ‘valleys’. Wall to lumen ratio was calculated by dividing IMT by lumen diameter.
Assays
Blood for hematocrit determination and measurements of plasma lipid peroxidation was withdrawn from the femoral vein in anesthetized rats at the end of the experimental period. Three samples of blood were placed in glass capillary tubes and centrifuged at 11,500 rpm in an IEC MB microhematocrit centrifuge (International Equipment Co., Needham Heights, Mass., USA) for 8 min. All samples were measured using a Spiracrit microhematocrit reading device (Lourdes Instrument Corp., Brooklyn, N.Y., USA) and the results were averaged to yield a single value for each rat. To obtain plasma, blood was anticoagulated with citrate, centrifuged, and plasma was stored at −80°C until analysis. Plasma lipid peroxidation was measured by quantitating 4-hydroxynonenal (4-HNE)-His protein adducts using an enzyme immunoassay kit (Cell Biolabs, San Diego, Calif., USA).
Urine was obtained from the bladder of anesthetized rats with a tuberculin syringe and stored at −80°C until analysis. Urinary uric acid was measured using enzymatic colorimetry (BioVision Inc., Mountain View, Calif., USA). Urinary concentrations of malondialdehyde were measured using a spectrophotometric thiobarbituric acid reactive substances (TBARS) kit to assess lipid peroxidation (a marker of oxidative stress) (Cell Biolabs). Urinary creatinine concentrations were measured using a colorimetric kit (Cayman Chemical, Ann Arbor, Mich., USA). Since urinary creatinine excretion is relatively constant, it can be used as an index to standardize other urinary measurements. Subsequently, malondialdehyde and uric acid concentrations were divided by urinary creatinine concentrations to normalize their rates of excretion. Urinary creatinine was converted from mg/dl to mmol/l by multiplying by 88.4 g/mol so that units were the same as urinary malondialdehyde. Urinary malondialdehyde has been shown to be a marker of lipid peroxidation in animal tissues [38].
Data Analysis
All data are presented as means ± SEM. Vessel reactivity to ACh, sodium nitroprusside and acute hypoxia are expressed as change in diameter (μm). Between-group differences in baseline characteristics, biomarkers of oxidative stress and vascular responses were assessed using an analysis of variance (ANOVA). Post hoc pair-wise comparisons were made using Fisher's protected least significance difference tests. All data were rank transformed prior to analysis in order to better meet the assumptions of ANOVA. Pooled analysis of intermittent hypoxia versus normoxic control rats (hematocrit) and allopurinol versus vehicle (urinary uric acid) was conducted using a Wilcoxon rank sum test. p values less than 0.05 were considered as significant. The relationship between uric acid excretion and ACh-induced vasodilation was evaluated using Pearson correlation coefficient. All analyses were performed using SAS statistical software version 9.1 (SAS Institute Inc., Cary, N.C., USA).
Results
Characteristics of Study Animals
Rats in the 4 groups were of similar age and weight when studied (table 1). Baseline gracilis artery diameters, maximal diameters and levels of spontaneous baseline tone were similar in the 4 groups, as were hematocrits (table 1). Urinary uric acid divided by urinary creatinine concentrations were lower in allopurinol- versus vehicle-treated rats, providing evidence for xanthine oxidase inhibition (0.18 ± 0.01 vs. 0.23 ± 0.01, p = 0.02).
Table 1.
Characteristics of rats exposed to normoxia or intermittent hypoxia (means ± SEM)
| Normoxia-vehicle (n = 12) | Normoxia-allopurinol (n = 9) | Hypoxia-vehicle (n = 11) | Hypoxia-allopurinol (n = 9) | |
|---|---|---|---|---|
| Age, weeks | 12.4 ± 0.3 | 12.8 ± 0.4 | 12.7 ± 0.3 | 12.2 ± 0.2 |
| Body weight, g | 326 ± 10 | 311 ± 6 | 313 ± 6 | 304 ± 9 |
| Hematocrit, % | 53 ± 1 | 51 ± 1 | 56 ± 1 | 54 ± 2 |
| Baseline tone, % | 30 ± 3 | 25 ± 3 | 28 ± 5 | 28 ± 3 |
| Baseline gracilis artery diameter, μm | 135 ± 6 | 147 ± 7 | 141 ± 9 | 148 ± 7 |
| Maximum gracilis artery diameter, μm | 195 ± 7 | 196 ± 7 | 196 ± 7 | 207 ± 5 |
Response of Resistance Arteries to ACh and Sodium Nitroprusside
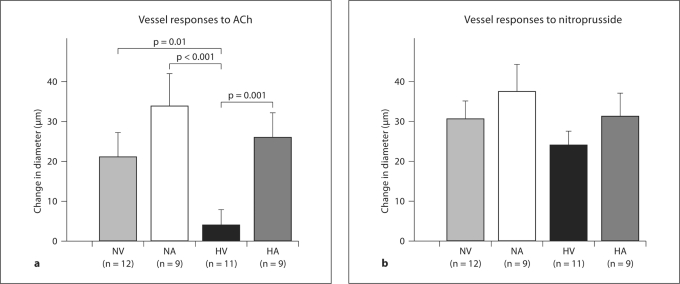
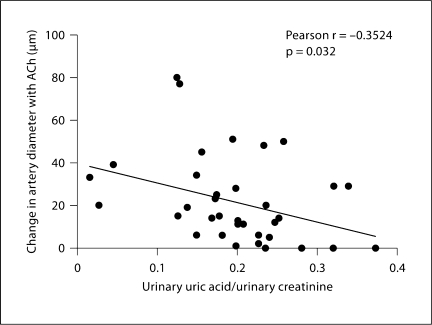
Gracilis artery responses to ACh differed in the 4 groups (p = 0.001; fig. 1a). Arteries from hypoxia-vehicle rats showed impaired ACh-induced vasodilation compared to those from normoxia-vehicle rats (p = 0.01). ACh-induced vasodilation of arteries from the hypoxia-vehicle rats were also significantly smaller than both the hypoxia-allopurinol (p = 0.001) and normoxia-allopurinol rats (p <0.001). Vessel responses to ACh were not different between the normoxia-vehicle, normoxia-allopurinol and hypoxia-allopurinol groups. Arterial responses to sodium nitroprusside were not significantly different in the four experimental groups (p = 0.43; fig. 1b). A modest negative correlation was found between urinary uric acid and ACh-induced vasodilation (Pearson r = −0.35, p = 0.032; fig. 2).
Fig. 1.
a Response to 10−6M ACh in isolated gracilis arteries of rats exposed to 14 days of intermittent hypoxia or normoxia. Vasodilatory responses were measured under no flow conditions with intraluminal pressures equal to 10.6 kPa (80 mm Hg). Data are presented as means ± SEM changes in vessel diameter from control measured before the application of ACh. Normoxia-vehicle (NV, light gray bar), normoxia-allopurinol (NA, white bar) and hypoxia-allopurinol (HA, dark gray bar) rats had a significantly greater increase in diameter to ACh compared to hypoxia-vehicle (HV, black bar) (p = 0.001 for model). b Response to 10−4M sodium nitroprusside in isolated gracilis arteries of rats exposed to 14 days of intermittent hypoxia or normoxia. Data are presented as means ± SEM changes in vessel diameter from control measured before the application of nitroprusside. Nitroprusside responses were not significantly different in vessels from the four groups of rats (p = 0.43).
Fig. 2.
Correlation of gracilis artery vasodilatory responses to ACh and urinary uric acid in all rats. Change in diameter to ACh was negatively correlated with corrected urinary uric acid concentrations (r = −0.35, p = 0.032).
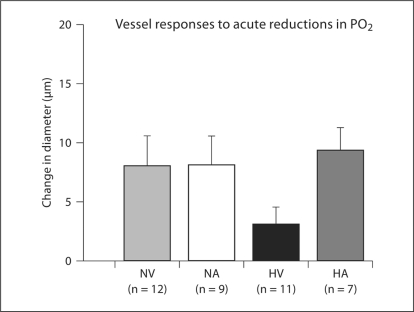
Responses of Resistance Arteries to Acute Reductions in PO2
During acute reductions in perfusate and superfusate PO2, vessels from hypoxia-vehicle rats had somewhat attenuated dilation compared to vessels from rats in the other groups, but the differences were not statistically significant (p = 0.08; fig. 3). Superfusate and perfusate oxygen tensions were similar in the 4 groups during these in vitro tests of hypoxic vasodilation. There were no between-group differences in superfusate O2 tensions for the normoxia (p = 0.13) or acute hypoxia conditions (p = 0.81; table 2).
Fig. 3.
Responses to acute reductions in perfusate and superfusate PO2 in gracilis arteries of animals exposed to 14 days of intermittent hypoxia or normoxia. Vasodilatory responses were measured under no flow conditions with intraluminal pressures equal to 10.6 kPa (80 mm Hg). Data are presented as means ± SEM changes in diameter from the baseline level. ANOVA revealed no significant differences among groups (p = 0.08).
Table 2.
O2 tensions in the superfusate during in vitro tests of hypoxic vasodilation in gracilis arteries from rats exposed to normoxia or intermittent hypoxia (means ± SEM)
| Normoxia-vehicle (n = 12) | Normoxia-allopurinol (n = 9) | Hypoxia-vehicle (n = 11) | Hypoxia-allopurinol (n = 9) | |
|---|---|---|---|---|
| Normoxia PO2 | ||||
| kPa | 19.8 ± 0.1 | 20 ± 0.03 | 19.7 ± 0.2 | 20.1 ± 0.2 |
| mm Hg | 149 ± 1 | 150 ± 0.2 | 148 ± 1 | 151 ± 1 |
| Hypoxia PO2 | ||||
| kPa | 5.9 ± 0.3 | 5.5 ± 0.3 | 5.7 ± 0.2 | 5.9 ± 0.3 |
| mm Hg | 44 ± 2 | 41 ± 2 | 43 ± 1 | 44 ± 2 |
Vessel Morphometry Analysis
Neither intermittent hypoxia nor allopurinol influenced gracilis artery morphometry measures. Wall:lumen ratios were 0.40 ± 0.09, 0.49 ± 0.17, 0.46 ± 0.12 and 0.48 ± 0.13 (p = 0.43), while intima-media thickness measurements were 23.9 ± 0.7, 25.7 ± 1.1, 26.1 ± 1 and 24.3 ± 1.1 μm (p = 0.52) in the normoxia-vehicle, normoxia-allopurinol, hypoxia-vehicle and hypoxia-allopurinol groups, respectively.
Biomarkers of Oxidative Stress
Urinary concentrations of malondialdehyde corrected for urinary creatinine concentration were similar in all four groups of rats. Urinary malondialdehyde/urinary creatinine concentrations were 1.0 ± 0.1, 1.0 ± 0.1, 0.9 ± 0.1 and 1.0 ± 0.1 in the normoxia-vehicle, normoxia-allopurinol, hypoxia-vehicle and hypoxia-allopurinol groups, respectively (p = 0.95). Plasma HNE-His protein concentrations were also similar in all 4 groups of rats. Mean plasma HNE-adduct concentrations were 9.1 ± 0.5, 9.2 ± 0.3, 9.2 ± 0.4 and 8.4 ± 0.6 μg/ml in the normoxia-vehicle, normoxia-allopurinol, hypoxia-vehicle and hypoxia-allopurinol groups, respectively (p = 0.55).
Discussion
The major findings of this study are that (1) 2 weeks of intermittent hypoxia exposure impaired ACh-induced vasodilation in skeletal muscle resistance arteries and (2) this impairment was greatly attenuated by in vivo treatment with the xanthine oxidase inhibitor allopurinol. In contrast, neither intermittent hypoxia nor xanthine oxidase inhibition influenced responsiveness to sodium nitroprusside or maximal dilation under calcium-free conditions. Thus, differences in ACh responses between groups cannot be explained by differences in smooth muscle responsiveness to nitric oxide and were not due to structural impairments that limited vessels’ ability to dilate. This study confirms our previous finding that chronic exposure to intermittent hypoxia impairs endothelial function in the skeletal muscle circulation and it extends it by demonstrating that xanthine oxidase plays a role in mediating this vascular dysfunction. The negative correlation we observed between urinary uric acid concentration and ACh-induced vasodilation provides additional support for this notion.
Mechanisms of Intermittent Hypoxia-Induced Impairment in Endothelial Function
Taken together, our findings of impaired ACh-induced and preserved nitroprusside-induced vasodilation provide indirect evidence that exposure to chronic intermittent hypoxia causes a decrease in the bioavailability of nitric oxide. The precise mechanism by which chronic exposure to intermittent hypoxia reduces nitric oxide is not known; however, previous research from our laboratory indicates that intermittent hypoxia does not diminish endothelial or inducible nitric oxide synthase content in gracilis artery [39]. One often proposed culprit is ‘oxidative stress’ (that is, an upset of the balance between reactive oxygen species and antioxidant capacity) in vascular tissue. Previous investigators have demonstrated that exposure to intermittent hypoxia in rats triggers production of superoxide anion in mesenteric arteries [40]. Because this oxygen radical is known to react quickly with nitric oxide to form peroxynitrite [41], it may contribute importantly to reduced availability of nitric oxide in the setting of intermittent hypoxia. A potential intracellular source of superoxide during episodes of hypoxia and reoxygenation is NADPH oxidase [25,26,27,28,29,42].
We propose that vascular xanthine oxidase is also an important source of superoxide ion during intermittent hypoxia cycles. During the hypoxia phase, xanthine dehydrogenase is converted to xanthine oxidase and concentrations of xanthine oxidase substrates (that is, hypoxanthine) are increased [32,43]. Once reoxygenation occurs, activated xanthine oxidase uses available oxygen and hypoxanthine to produce superoxide [26]. Inhibition of xanthine oxidase with allopurinol would be expected to reduce vascular superoxide production and hence attenuate the impact of intermittent hypoxia exposure on bioavailability of nitric oxide. In vitro studies indicate that allopurinol in high doses can act as a direct antioxidant, in addition to its effect on xanthine oxidase inhibition [44,45,46]. We consider it unlikely that allopurinol had such an effect in our study, however, because previous investigators determined that direct antioxidant activity is not present in doses up to 100 mg/kg (approximately 1.5 times the dose we used) [36].
The involvement of xanthine oxidase in mediating impairments in conduit vessel function in patients with clinical OSA has been reported by previous investigators [34]. El Solh et al. [34] demonstrated that xanthine oxidase inhibition with allopurinol reduced markers of oxidative stress and improved flow-mediated dilation in a conduit artery of the forearm in patients with OSA. Our data expand upon this finding by demonstrating that allopurinol prevents impaired endothelium-dependent vasodilation in skeletal muscle resistance arteries in an experimental model of OSA. We would like to emphasize that these two studies provide complementary, not overlapping, information because concordant responses are not always observed in conduit and resistance arteries [7,47]. The previous and present findings are consistent in that they point to an important role for xanthine oxidase in causing the vascular dysfunction produced by OSA in humans and intermittent hypoxia in rats. We consider it unlikely that xanthine oxidase acts alone in mediating these impairments; nevertheless, nearly full reversal of endothelial dysfunction has been observed after administration of xanthine oxidase inhibitors in smokers and hypertensive subjects [48,49].
Effects of Chronic Intermittent Hypoxia and Xanthine Oxidase Inhibition on Vascular Structure
In the present study, neither chronic intermittent hypoxia nor xanthine oxidase inhibition influenced measures of arterial thickening in the gracilis artery. In clinical studies, subjects with OSA have increased IMT compared to control subjects [10,11,12]; however, it is likely that these changes develop relatively slowly during years of exposure and, if so, would not be expected to materialize after only 2 weeks of intermittent hypoxia in our animal model. In a separate study from our laboratory that was run concurrently to this one, we found that IMT was unchanged, even after 8 weeks of intermittent hypoxia exposure [39], although the abundance of both type I and III collagen fibers in gracilis arteries was increased [39]. Our paradigm of chronic exposure to intermittent hypoxia in rats elicits vascular changes consistent with the cardiovascular phenotype of OSA; however, it is possible that characteristics of OSA other than intermittent hypoxia may also play a pathogenetic role. In addition to intermittent hypoxia, patients with OSA experience intermittent hypercapnia and frequent arousals from sleep. Previous evaluations of IMT in patients with OSA have found that high end-tidal CO2, daytime sleepiness and frequency of arousals are all well correlated with increased IMT [50,51]. Our model of chronic intermittent hypoxia produces hypocapnia, rather than hypercapnia, and it does not cause significant sleep disruption. Thus, we suspect that our negative findings regarding vascular structure may be due, at least in part, to the relatively brief exposure period we employed and the failure of our model to mimic all of the insults inherent in clinical OSA, including the presence of comorbid conditions such as increased insulin resistance and obesity.
Effects of Chronic Intermittent Hypoxia and Xanthine Oxidase Inhibition on Systemic Markers of Oxidative Stress
Although our vascular reactivity data indicate that exposure to intermittent hypoxia limits nitric oxide bioavailability, and previous studies indicate that it causes oxidative stress by stimulating vascular superoxide production [40,52,53,54], we did not detect effects of intermittent hypoxia or xanthine oxidase inhibition on measures of lipid peroxidation and malondialdehyde. One possible explanation is that our stimulus for production of oxidative stress was more subtle than in previous investigations where oxidative stress was documented. We used an oxygen nadir of 10% with a frequency of 15/h for 2 weeks, whereas other studies used oxygen nadirs of 5% [40,52,53,54] and frequencies of 20 or 30/h for as long as 6 weeks [40,52,54,55]. A previous study from our laboratory that employed the present intermittent hypoxia paradigm for durations up to 8 weeks did reveal a significant effect of intermittent hypoxia on urinary excretion of 8-isoprostane PGF2α, another systemic marker of oxidative stress [39]. Many studies that have demonstrated increased oxidative stress in animal models of intermittent hypoxia [40,52,53,54,56] have measured tissue concentrations rather than systemic levels of reactive oxygen species and antioxidant substances. It is possible that our intervention caused oxidative stress in vascular tissue that was not reflected in our systemic measures (that is, urinary malondialdehyde and plasma lipid peroxidation). We would like to emphasize that previous human studies have used systemic markers to demonstrate increased oxidative stress in patients with OSA, diabetes and heart failure, and that these studies report beneficial effects of xanthine oxidase inhibition on systemic markers [34,49,57,58]. It is possible, however, that comorbid conditions such as obesity and increased insulin resistance [59,60] may have contributed to an oxidative stress ‘burden’ in those human subjects that was heightened relative to that experienced by our rats.
Methodological Considerations
Our rat model fails to mimic OSA in humans in a number of important respects. It does not produce upper airway occlusions, negative intrathoracic pressure swings or sleep disruption, and it causes hypocapnia rather than hypercapnia. These features, along with intermittent hypoxia, may be important determinants of OSA-induced vascular dysfunction; nevertheless, our model does recapitulate the OSA phenotype in several ways (that is, endothelial dysfunction [31], blood pressure elevation [61,62], increased sympathetic nervous system activity [62] and increased vascular stiffness [63]). Our model is advantageous in that it allowed us to evaluate the effects of allopurinol on vessel function in the absence of confounding effects of comorbid conditions and other medications. Also, the relatively short (2-week) intermittent hypoxia exposure allowed us to evaluate vascular function in the absence of structural changes that might occur with more prolonged exposure.
We acknowledge that our study has several limitations. First, although our measures of urinary uric acid suggest that xanthine oxidase was inhibited in rats that received allopurinol, we did not make direct measurements of xanthine oxidase activity. We consider it unlikely that decreased urinary uric acid concentrations were due to differences in dietary purine content or decreased renal elimination of uric acid because diet was identical for all animals and excretion of uric acid was accounted for by dividing by urinary creatinine. Therefore, we consider it likely that allopurinol successfully inhibited xanthine oxidase activity. We did not find significantly elevated uric acid levels in intermittent hypoxia-exposed rats treated with vehicle; however, cell culture studies indicate that a much more severe hypoxic stimulus (continuous exposure to 10% oxygen) is required to increase endothelial xanthine oxidase activity [64]. Also, we relied on systemic (plasma and urinary) markers of oxidative stress, not gracilis artery tissue measurements. We did this because we chose to use one gracilis artery for functional studies and the contralateral gracilis artery for analysis of structural changes. Systemic measures of oxidative stress did not differ in the 4 groups of rats. Thus, although our findings indicate xanthine oxidase plays a role in chronic intermittent hypoxia-induced vascular impairments, they do not allow the definitive conclusion that xanthine oxidase inhibition attenuated these impairments via reduction of oxidative stress.
Conclusion
Our study showed that in vivo treatment with allopurinol prevented the development of intermittent hypoxia-induced endothelial dysfunction in skeletal muscle resistance arteries in rats. Our findings are consistent with a previous study that demonstrated improvements in conduit vessel function in patients with established OSA after 2 weeks of allopurinol treatment [34]. Future research is needed to evaluate the potential cardiovascular benefits of long-term xanthine oxidase inhibition as a preventive cardiovascular therapy in patients with OSA.
Financial Disclosure and Conflicts of Interest
None to declare.
Acknowledgements
The authors wish to thank Drs. Glen Leverson and Victoria Rajamanickam in the University of Wisconsin School of Medicine and Public Health Department of Surgery for their statistical assistance. This project was supported by an American College of Clinical Pharmacy Investigator Development Grant (J.M.D.) and by UW Institutional Clinical and Translational Science Award (University of Wisconsin Madison) (KL2) RR025012-01 (J.M.D.) and by NIH grant HL-074072 (B.J.M.).
References
- 1.Dyken ME, Somers VK, Yamada T, Ren ZY, Zimmerman MB. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27:401–407. doi: 10.1161/01.str.27.3.401. [DOI] [PubMed] [Google Scholar]
- 2.Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest. 1996;109:659–663. doi: 10.1378/chest.109.3.659. [DOI] [PubMed] [Google Scholar]
- 3.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 5.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher EC. Sympathetic activity and blood pressure in the sleep apnea syndrome. Respiration. 1997;64(suppl 1):22–28. doi: 10.1159/000196732. [DOI] [PubMed] [Google Scholar]
- 7.Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 8.Büchner NJ, Quack I, Woznowski M, Stähle C, Wenzel U, Rump LC. Microvascular endothelial dysfunction in obstructive sleep apnea is caused by oxidative stress and improved by continuous positive airway pressure therapy. Respiration. 2011;82:409–417. doi: 10.1159/000323266. [DOI] [PubMed] [Google Scholar]
- 9.Oflaz H, Cuhadaroglu C, Pamukcu B, Meric M, Ece T, Kasikcioglu E, Koylan N. Endothelial function in patients with obstructive sleep apnea syndrome but without hypertension. Respiration. 2006;73:751–756. doi: 10.1159/000094183. [DOI] [PubMed] [Google Scholar]
- 10.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:613–618. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 11.Baguet JP, Hammer L, Levy P, Pierre H, Launois S, Mallion JM, Pepin JL. The severity of oxygen desaturation is predictive of carotid wall thickening and plaque occurrence. Chest. 2005;128:3407–3412. doi: 10.1378/chest.128.5.3407. [DOI] [PubMed] [Google Scholar]
- 12.Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Tanaka A, Oda N, Okada S, Ohta S, Naito H, Adachi M. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:625–630. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 13.Tanriverdi H, Evrengul H, Kara CO, Kuru O, Tanriverdi S, Ozkurt S, Kaftan A, Kilic M. Aortic stiffness, flow-mediated dilatation and carotid intima-media thickness in obstructive sleep apnea: non-invasive indicators of atherosclerosis. Respiration. 2006;73:741–750. doi: 10.1159/000093531. [DOI] [PubMed] [Google Scholar]
- 14.Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med. 2007;3:409–415. [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler MG, Nelesen R, Mills P, Ancoli-Israel S, Kennedy B, Dimsdale JE. Sleep apnea, norepinephrine-release rate, and daytime hypertension. Sleep. 1997;20:224–231. doi: 10.1093/sleep/20.3.224. [DOI] [PubMed] [Google Scholar]
- 16.Erami C, Zhang H, Tanoue A, Tsujimoto G, Thomas SA, Faber JE. Adrenergic catecholamine trophic activity contributes to flow-mediated arterial remodeling. Am J Physiol Heart Circ Physiol. 2005;289:H744–H753. doi: 10.1152/ajpheart.00129.2005. [DOI] [PubMed] [Google Scholar]
- 17.Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–2278. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavie L, Polotsky V. Cardiovascular aspects in obstructive sleep apnea syndrome – molecular issues, hypoxia and cytokine profiles. Respiration. 2009;78:361–370. doi: 10.1159/000243552. [DOI] [PubMed] [Google Scholar]
- 19.Lattimore JL, Wilcox I, Skilton M, Langenfeld M, Celermajer DS. Treatment of obstructive sleep apnoea leads to improved microvascular endothelial function in the systemic circulation. Thorax. 2006;61:491–495. doi: 10.1136/thx.2004.039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monneret D, Pepin JL, Godin-Ribout D, Ducros V, Baguet JP, Levy P, Faure P. Association of urinary 15-F2t-isoprostane level with oxygen desaturation and carotid intima-media thickness in nonobese sleep apnea patients. Free Rad Biol Med. 2010;48:619–625. doi: 10.1016/j.freeradbiomed.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Polidori MC, Pratico D, Parente B, Mariani E, Cecchetti R, Yao Y, Sies H, Cao P, Mecocci P, Stahl W. Elevated lipid peroxidation biomarkers and low antioxidant status in atherosclerotic patients with increased carotid or iliofemoral intima media thickness. J Invest Med. 2007;55:163–167. doi: 10.2310/6650.2007.06043. [DOI] [PubMed] [Google Scholar]
- 22.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol. 1986;250:H222–H227. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- 23.Price DT, Vita JA, Kearney JF., Jr Redox control of vascular nitric oxide bioavailability. Antiox Redox Signal. 2000;2:919–935. doi: 10.1089/ars.2000.2.4-919. [DOI] [PubMed] [Google Scholar]
- 24.Zalba G, Beloqui O, San Jose G, Moreno MU, Fortuno A, Diez J. NADPH-oxidase dependent superoxide production is associated with carotid intima-media thickness in subjects free of atherosclerotic disease. Arterioscler Thromb Vasc Biol. 2005;25:1452–1457. doi: 10.1161/01.ATV.0000168411.72483.08. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227–C241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- 26.McCord JM. Oxygen-derived free radicals in post-ischaemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 27.McLeod L, Alayash AI. Detection of a ferrylhemoglobin intermediate in an endothelial cell model after hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 1999;277:H92–H99. doi: 10.1152/ajpheart.1999.277.1.H92. [DOI] [PubMed] [Google Scholar]
- 28.Terada LS, Guidot DM, Leff JA, Willingham IR, Hanley ME, Piermattei D, Repine JE. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proc Natl Acad Sci USA. 1992;89:3362–3366. doi: 10.1073/pnas.89.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei Z, Costa K, Al-Mehdi AB, Dodia C, Muzyantov V, Fisher AB. Simulated ischemia in flow-adapted endothelial cells leads to generation of reactive oxygen species in cell signaling. Circ Res. 1999;85:682–689. doi: 10.1161/01.res.85.8.682. [DOI] [PubMed] [Google Scholar]
- 30.Friedl HP, Till GO, Ryan US, Ward PA. Mediator-induced activation of xanthine oxidase in endothelial cells. FASEB J. 1989;3:2512–2518. doi: 10.1096/fasebj.3.13.2806779. [DOI] [PubMed] [Google Scholar]
- 31.Hassoun PM, Feng-Sheng Y, Cote CG, Zulueta JJ, Sawhney R, Skinner KA, Skinner HB, Parks DA, Lanzillo JJ. Upregulation of xanthine oxidase by lipopolysaccharide, interleukin-1 and hypoxia. Role in acute lung injury. Am J Respir Crit Care Med. 1998;158:299–305. doi: 10.1164/ajrccm.158.1.9709116. [DOI] [PubMed] [Google Scholar]
- 32.Kayyali US, Donaldson C, Huang H, Abdelnour R, Hassoun PM. Phosphorylation of xanthine dehydrogenase/oxidase in hypoxia. J Biol Chem. 2001;276:14359–14365. doi: 10.1074/jbc.M010100200. [DOI] [PubMed] [Google Scholar]
- 33.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 34.El Solh AA, Saliba R, Bosinski T, Grant BJ, Berbary E, Miller N. Allopurinol improves endothelial function in sleep apnoea: a randomised controlled study. Eur Respir J. 2006;27:997–1002. doi: 10.1183/09031936.06.00101005. [DOI] [PubMed] [Google Scholar]
- 35.Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol. 2004;286:H388–H393. doi: 10.1152/ajpheart.00683.2003. [DOI] [PubMed] [Google Scholar]
- 36.Klein AS, Joh JW, Rangan U, Wang D, Bulkley GB. Allopurinol: discrimination of antioxidant from enzyme inhibitory activities. Free Rad Biol Med. 1996;21:713–717. doi: 10.1016/0891-5849(96)00158-x. [DOI] [PubMed] [Google Scholar]
- 37.Fredricks KT, Liu Y, Lombard JH. Response of extraparenchymal resistance arteries of rat skeletal muscle to reduced PO2. Am J Physiol Heart Circ Physiol. 1994;267:H706–H715. doi: 10.1152/ajpheart.1994.267.2.H706. [DOI] [PubMed] [Google Scholar]
- 38.Draper HH, Polensek L, Hadley M, McGirr LG. Urinary malondialdehyde as an indicator of lipid peroxidation in the diet and in the tissues. Lipids. 1984;19:836–843. doi: 10.1007/BF02534512. [DOI] [PubMed] [Google Scholar]
- 39.Philippi NR, Bird CE, Marcus NJ, Olson EB, Chesler NC, Morgan BJ. Time course of intermittent hypoxia-induced impairments in resistance artery structure and function. Respir Physiol Neurobiol. 2010;170:157–163. doi: 10.1016/j.resp.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Troncoso Brindeiro CM, da Silva AQ, Allahdadi KJ, Youngblood V, Kanagy NL. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol. 2007;293:H2971–H2976. doi: 10.1152/ajpheart.00219.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad and the ugly. Am J Physiol. 1996;271:C1421–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi T, Yamashita C, Matsumoto C, Kwak CJ, Fujii K, Hirata T, Miyamura M, Mori T, Ukimura A, Okada Y, Matsumura Y, Kitaura Y. Role of gp91-phox-containing NADPH oxidase in left ventricular remodeling induced by intermittent hypoxic stress. Am J Physiol Heart Circ Physiol. 2008;294:H2197–H2203. doi: 10.1152/ajpheart.91496.2007. [DOI] [PubMed] [Google Scholar]
- 43.Lavie L. Obstructive sleep apnoea syndrome – an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 44.Das DK, Engelman RM, Clement R, Otani H, Prasad MR, Rao PS. Role of xanthine oxidase inhibitor as free radical scavenger: a novel mechanism of action of allopurinol and oxypurinol in myocardial salvage. Biochem Biophys Res Commun. 1987;148:314–319. doi: 10.1016/0006-291x(87)91112-0. [DOI] [PubMed] [Google Scholar]
- 45.Hoey BM, Butler J, Halliwell B. On the specificity of allopurinol and oxypurinol as inhibitors of xanthine oxidase. A pulse radiolysis determination of rate constants for reaction of allopurinol and oxypurinol with hydroxyl radicals. Free Radic Res Commun. 1988;4:259–263. doi: 10.3109/10715768809055151. [DOI] [PubMed] [Google Scholar]
- 46.Moorhouse PC, Grootveld M, Halliwell B, Quinlan JG, Gutteridge JMC. Allopurinol and oxypurinol are hydroxyl radical scavengers. FEBS Lett. 1987;213:23–28. doi: 10.1016/0014-5793(87)81458-8. [DOI] [PubMed] [Google Scholar]
- 47.Green DJ, Walsh JH, Maiorana A, Burke V, Taylor RR, O'Driscoll JG. Comparison of resistance and conduit vessel nitric oxide-mediated vascular function in vivo: effects of exercise training. J Appl Physiol. 2004;97:749–755. doi: 10.1152/japplphysiol.00109.2004. [DOI] [PubMed] [Google Scholar]
- 48.Guthikonda S, Sinkey C, Barenz T, Haynes WG. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107:416–421. doi: 10.1161/01.cir.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- 49.Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 50.Saletu M, Sauter C, Lalouschek W, Saletu B, Kapfhammer G, Benesch T, Zeithofer J. Is excessive sleepiness a predictor of carotid atherosclerosis in sleep apnea. Atherosclerosis. 2008;196:810–816. doi: 10.1016/j.atherosclerosis.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 51.Anderson DE, Scuteri A, Metter EJ, Chesney MA. Association of high resting end tidal CO2 with carotid artery thickness in women, but not men. J Hypertens. 2001;19:459–463. doi: 10.1097/00004872-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med. 2005;172:915–920. doi: 10.1164/rccm.200504-560OC. [DOI] [PubMed] [Google Scholar]
- 53.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–323. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 55.Sweazea KL, Kanagy NL, Walker BR. Increased adiposity does not exacerbate impaired vasodilation in rats exposed to eucapnic intermittent hypoxia. Respiration. 2011;81:47–56. doi: 10.1159/000320322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med. 2003;167:1548–1553. doi: 10.1164/rccm.200209-1050OC. [DOI] [PubMed] [Google Scholar]
- 57.Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, Hambrecht R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 58.Farquharson CAJ, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 59.Bugianesi E, Moscatiello S, Ciaravella MF, Marchesini G. Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des. 2010;16:1941–1951. doi: 10.2174/138161210791208875. [DOI] [PubMed] [Google Scholar]
- 60.De Lima AM, Franco CM, de Castro CM, Bezerra Ade A, Ataide L, Jr, Halpern A. Effects of nasal continuous positive airway pressure treatment on oxidative stress and adiponectin levels in obese patients with obstructive sleep apnea. Respiration. 2010;79:370–376. doi: 10.1159/000227800. [DOI] [PubMed] [Google Scholar]
- 61.Marcus NJ, Olson EB, Jr, Bird CE, Philippi NR, Morgan BJ. Time-dependent adaptation in the hemodynamic response to hypoxia. Respir Physiol Neurobiol. 2009;165:90–96. doi: 10.1016/j.resp.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol. 2010;171:36–45. doi: 10.1016/j.resp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol. 2004;286:H388–H393. doi: 10.1152/ajpheart.00683.2003. [DOI] [PubMed] [Google Scholar]
- 64.Kelley EE, Hock T, Khoo NK, Richardson GR, Johnson KK, Powell PC, Giles GI, Agarwal A, Lancaster JR, Jr, Tarpey MM. Moderate hypoxia induces xanthine oxidoreductase activity in arterial endothelial cells. Free Rad Biol Med. 2006;40:952–959. doi: 10.1016/j.freeradbiomed.2005.11.008. [DOI] [PubMed] [Google Scholar]