Abstract
The hippocampus develops rapidly during the late fetal and early postnatal periods. Fetal/neonatal iron deficiency anemia (IDA) alters the genomic expression, neurometabolism and electrophysiology of the hippocampus during the period of IDA and, strikingly, in adulthood despite neonatal iron treatment. To determine how early IDA affects the structural development of the apical dendrite arbor in hippocampal area CA1 in the offspring, pregnant rat dams were given an iron-deficient (ID) diet between gestational day 2 and postnatal day (P) 7 followed by rescue with an iron-sufficient (IS) diet. Apical dendrite morphology in hippocampus area CA1 was assessed at P15, P30 and P70 by Scholl analysis of Golgi-Cox-stained neurons. Messenger RNA levels of nine cytoplasmic and transmembrane proteins that are critical for dendrite growth were analyzed at P7, P15, P30 and P65 by quantitative real-time polymerase chain reaction. The ID group had reduced transcript levels of proteins that modify actin and tubulin dynamics [e.g. cofilin-1 (Cfl-1), profilin-1 (Pfn-1), and profilin-2 (Pfn-2)] at P7, followed at P15 by a proximal shift in peak branching, thinner third-generation dendritic branches and smaller-diameter spine heads. At P30, iron treatment since P7 resulted in recovery of all transcripts and structural components except for a continued proximal shift in peak branching. Nevertheless, at P65–P70, the formerly ID group showed a 32% reduction in 9 mRNA transcripts, including Cfl-1 and Pfn-1 and Pfn-2, accompanied by 25% fewer branches, that were also proximally shifted. These alterations may be due to early-life programming of genes important for structural plasticity during adulthood and may contribute to the abnormal long-term electrophysiology and recognition memory behavior that follows early iron deficiency.
Key Words: Development, Dendritogenesis, Spinehead, Anemia, Cognition, Nutrient, Cofilin, Profilin
Introduction
The normal function of neuronal circuitry in adulthood is often dependent on a series of coordinated and well-timed events during development. Normative development, in turn, is dependent on complex interactions between genetically (experience-independent) and environmentally (experience-dependent) events. Disruption of key genetic or environmental factors during development can cause neural systems to deviate from their trajectory with resultant functional alterations that last into adulthood. These deviations are frequently characterized by significant alterations in neuronal morphology, specifically at the level of the cytoskeleton. Genetic examples of altered morphogenesis include fragile X syndrome, where abnormal dendrite spine morphology plays a role in altered cognitive function and Rett syndrome, where mutations of the methyl-CpG binding protein 2 gene results in abnormal axonal guidance during the formation of neural circuits [1]. Environmental examples include the detrimental effect of early-life stress on rodent synaptogenesis [2,3] and the enhancing effect of choline supplementation during pregnancy on hippocampus dendrite structure, electrophysiology and learning behavior in the offspring [4].
Iron availability is critical during late fetal and early postnatal life for the developing hippocampus and the ontogeny of the declarative memory system. Gestational and early postnatal iron deficiency anemia (IDA) in humans results in concurrent [5] and persistent learning and memory deficits in later childhood [6,7,8] and adulthood [9,10] in spite of prompt treatment upon diagnosis. In rats, IDA from gestation through the period of rapid dendritogenesis between postnatal days (P) 15 and P25 impairs hippocampal development and function, including trace fear conditioning, long-term potentiation (LTP) and paired-pulse facilitation (PPF), microtubule associated protein-2 expression, neurometabolism and expression of genes involved in synaptic plasticity, such as postsynaptic density protein 95 and calcium calmodulin-dependent kinase IIα [11,12,13,14,15,16]. Nonetheless, neuronal dendrite and spine morphology and the potential molecular mechanisms underlying alterations in the dendrite arbor during development, that are key aspects in the development of synaptic efficacy, have not been assessed.
The developing arbor requires external inputs to stimulate and support branching morphogenesis. The arbor relies on extracellular guidance cues and growth factors [e.g. brain-derived neurotrophic factor (BDNF), stromal-derived factor 1α (SDF1) and semaphorins] that signal via their respective transmembrane receptors [e.g. neurotrophic tyrosine kinase, receptor for BDNF (TrkB), chemokine (C-X-C motif) receptor 4 (Cxcr4) and collapsing response mediator protein-1 (Crmp1)]. These signals regulate rho GTPase [e.g. Ras-related C3 botulinum toxin substrate 1 (Rac1) and Ras homolog gene family member A (RhoA)] activity which in turn modulate factors (e.g. cypin, cofilin, profilins) that facilitate elongation and branching by promoting or reducing actin polymerization [17,18,19,20,21,22,23]. The process not only builds the specific architecture during development, but also reshapes the structure during experience-dependent learning throughout the lifespan [21].
Here, we build on our previous finding of reduced apical dendrite length during IDA [13] by demonstrating that fetal/neonatal IDA alters dendrite branching and spine morphology in rat hippocampus area CA1 during its period of rapid apical dendrite growth between P15 and P30. Moreover, we demonstrate persistent long-term changes in dendrite morphology at P65 in spite of complete iron repletion by P30. We also demonstrate that IDA alters expression of salient genes that regulate cytoskeletal structure in the hippocampus beginning at P7 prior to the period of rapid growth. Although gene expression recovers with iron treatment during development, we report these genes to be ultimately suppressed in adulthood in formerly iron-deficient (FID) animals, suggesting a programming effect consistent with the developmental origins of adult disease hypothesis.
Materials and Methods
Animals and Study Design
All protocols were approved by the Institution Animal Care Utilization Committee at the University of Minnesota. Pregnant Harlan Sprague Dawley rat dams were fed an iron-deficient (ID) diet (3–6 mg/kg iron) from gestational day 2 to P7, the approximate equivalent of term human birth with respect to hippocampal development. Control dams were fed an iron-sufficient (IS) diet (198 mg/kg iron) as were the ID dams beginning at P7. This dietary model induces a 40–50% reduction in brain iron concentration in P15 pups with complete brain iron sufficiency by P56 [13,14,15,16]. Litters were culled to 8 pups and weaned on P21. A total of 128 pups were used in the experiments; 36 each for hematocrit/iron content, morphometric analysis, and quantitative real-time quantitative polymerase chain reaction (qPCR), and 8 for Western blot analysis. Pups were sacrificed with an overdose of pentobarbital, blood was obtained for hematocrit measurement, whole brains were obtained for Golgi, and hippocampi for qPCR and Western blot analysis were isolated in ice-cold phosphate-buffered saline at pH 7.4, flash frozen and stored at −80°C. Samples for morphometric analysis were obtained at P15, P30 and P70. The P15 and P30 time points represent the beginning and end of rapid dendritogenesis in the rat hippocampus [24], while P70 represents the young adult animal. Golgi analysis was not performed at P7 because arborization in area CA1 is rudimentary at this age [24]. Samples for qPCR were obtained at P7, P15, P30 and P65. Brains for histochemistry were obtained at P65 to assess protein localization.
Hematocrit and Tissue Iron Concentration
Hematocrits were determined as previously described [13,14]. Tissue iron concentration was measured by atomic absorption spectroscopy as previously described and expressed as micrograms Fe per gram wet weight [14].
Golgi-Cox Staining
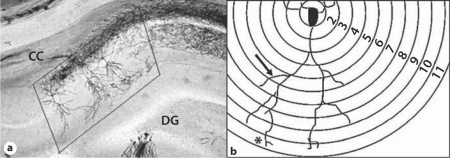
Approximately 18 rats per dietary group were utilized for Golgi-Cox analysis at P15, P30 and P70. The brains were placed directly into Golgi solution (1 g potassium chromate, 1 g mercuric chloride, 0.8 g potassium chloride and 100 ml double-distilled water) where they remained in a foil-wrapped jar for 6 weeks. Thereafter, the solution was exchanged sequentially in 10, 20 and 30% sucrose solutions in light-protected jars to aid in maintaining histological structure. The brains were sectioned at 200 μm thickness with a vibratome (OTS3000–03; FHC, Brunswick, Me., USA) and placed onto gelatin-coated glass slides [25]. Slides were processed in an exhaust hood and covered with aluminum foil to prevent light contamination of their development. After rinsing with double-distilled water, slides were incubated in ammonium hydroxide for 30 min. Following a water wash, the slides were incubated for 30 min in a black and white film developer diluted 1:9 with water (Sprint Systems of Photography, Scituate, R.I., USA) and then rinsed with final double-distilled water. Slides were mounted with glycerol and cover-slipped. Staining was visualized using a Nikon Eclipse E600 light microscope (Nikon Instruments Inc., Melville, N.Y., USA). Neurons were chosen from a proscribed area of CA1 (fig. 1a). Labeled neurons with little or no overlap from neighboring cells and no visibly truncated dendrite branches were selected for analysis. Digital images (Nikon DXM 1200) were taken at 1,000× to measure apical dendrite length, third-generation branch width, third-generation branch spine density and spine head diameter. The neurons were traced manually at 200× on the live computer image (Nikon Act I version 2.20© 2000) with a permanent pen on transparent acetate. The entire apical tree was traced by focusing through several planes to follow each branch. Any break in the continuity of a branch was assessed by determining whether the branch appeared to continue after the break with a similar caliber, directionality and plane of focus as the initial segment. Branches that met these criteria were considered to be continuous and were traced. All subsequent quantitative analyses were conducted in an unbiased manner to group designation with repeated measurements.
Fig. 1.
a Proscribed area of CA1 from which Golgi-Cox-stained neurons were selected for analysis. CC = Corpus callosum; DG = dentate gyrus. b Schematic of neuron tracing over Scholl rings with the soma abutting ring 1. The asterisk marks the point of maximal length. The arrow marks the proximal aspect of a third-generation branch immediately after bifurcation from the second-generation branch.
Morphometric Analysis
Dendritic length and branching were assessed using Scholl analysis [26]. A concentric ring template was constructed with a scale corresponding to the live image, wherein rings were 31 μm apart. The transparent neuron tracings were then laid over the ring diagram with the neuron positioned so that the end of the soma and the beginning of the apical shaft lay on the first ring (fig. 1b). The maximal apical dendrite length for each neuron was defined as the furthest distal extent of the dendrite to cross a Scholl ring. This was measured to the nearest half ring. Dendrite width, spine density and spine head diameter were measured in a third-generation branch. The third-generation branch was chosen because it falls within mid-stratum radiatum, a place of high-density synaptogenesis and spinogenesis [24]. The measurement was made as proximal as possible to the take-off from the second-generation branch. For each branch image (a single image or a stack), Image J [27] was used to calibrate the scale, enlarge the segment and measure the dendrite shaft width. The segment width was measured across the most clearly visible section between the spines within the 20 μm length.
Apical dendrite branching was assessed by counting the number of crossings at each of the Scholl rings in order to estimate the amount of total dendrite area available for synaptic contact [28]. A dendrite that branched directly on a ring was counted as two branches at that distance. Total crossings at each ring across the entire apical dendrite arbor in each neuron were recorded. The location of peak dendrite branching and the number of branches at that location may affect synaptic efficacy [28]. The distance from the soma to the Scholl ring with the largest number of crossings was termed the peak branching distance. The mean number of crossings at that ring was recorded for each neuron. Since branch crossings could be broadly distributed across an area rather than concentrated at one peak distance, peak branching regions were defined as contiguous Scholl rings that had 4 or more dendrite crossings. The total number of crossings and an area under the curve (AUC) of crossings for the peak branching region were calculated for each neuron.
Spine density and spine head diameter were counted within the same segment of the proximal third-generation apical dendrite branch in which dendrite branch width was assessed. The number of spines per 20 μm of dendrite length was recorded for each neuron. Spine heads were measured across the widest part of the protrusion and averaged for each neuron. No assessment of spine head morphology type was made and all spine head types were counted.
For all Golgi analyses, individual neuron values were averaged for a given animal to generate a single mean per animal. Values for each animal within each dietary and age group were then averaged to generate a group mean for statistical comparison at each age by the unpaired t test with Welch's correction for unequal variances.
Quantitative Real-Time PCR
Hippocampal tissues were collected at P7, P15, P30 and P65 with 6 animals per dietary group at each age. The P7 time point was used to determine whether changes at that time were associated with structural changes at P15. qPCR was carried out as described previously [29]. Briefly, messenger RNA levels of nine proteins relevant to cytoskeletal dynamics were measured by real-time qPCR using Taqman® gene expression probes (Applied Biosystems Inc., Foster City, Calif., USA). These selected genes included modulators of neurite extension/growth (Crmp1 and Cxcr4), intracellular signaling rho GTPases [RhoA, Rac1 and cell division control protein 42 (Cdc42)] that translate guidance cues, and downstream effectors of rho GTPase activity that regulate actin and tubulin dynamics [profiling-1 (Pfn-1), profiling-2 (Pfn-2), cofilin-1 (Cfl-1) and cypin] [30,31]. Reverse transcription was carried out using high-capacity RNA to a cDNA kit (Applied Biosystems) and random hexamers per manufacturer recommendation. Approximately 4 μg of total RNA was used to generate each cDNA sample. The resulting cDNA was diluted tenfold to give a final volume of 200 μl. All qPCR experiments were performed with half the manufacturer-recommended volume consisting of 4 μl of diluted cDNA, 5 μl 2× Taqman qPCR universal mix, and 0.5 μl 20× Taqman Gene Expression Assay primers/probes mix (Applied Biosystems). Exon-exon spanning Taqman probes were selected to minimize potential amplification of genomic DNA. Ribosomal protein 18S was used as an internal control. The expression of this housekeeping gene is not altered by iron deficiency [29]. Thermocycling was carried out according to the manufacturer's protocol (Applied Biosystems) using an MX3000P instrument (Statagene, La Jolla, Calif., USA). Two-way ANOVA was used to assess changes in gene expression over time by dietary group with Bonferroni corrected t tests for post-hoc analysis at each age. Significance was set at a p value <0.05.
Immunohistochemistry
Immunohistochemistry was used to examine the hippocampal subarea localization of six proteins (Rac1, Cdc42, RhoA, Cxcr4, Cfl-1 and Crmp-1) whose mRNA levels were assessed by qPCR and where antibodies suitable for immunohistochemistry were commercially available (Abcam). Deeply anesthetized rats were perfused transcardially and fixed according as previously described [13]. Briefly, the staining procedure used Tris-buffered saline with 0.1% Tween for all washes, which occurred between all steps prior to blocking. Antigen unmasking was facilitated with 5 mM sodium citrate followed by 15% hydrogen peroxide. Antigen blocking was accomplished using purified goat serum and bovine serum albumin before the overnight incubation with the primary polyclonal antibody at 4°C. Staining was detected with an ABC Elite (Vector) antibody kit. Diaminobenzidine with nickel was used as the substrate and was developed for a minimum of 5 min. An average of 4 animals per group was used for analysis. All sets of the same primary antibody were incubated simultaneously. The light microscope and camera were the same as those used for Golgi imaging.
Staining was quantified by measuring the optical density on a gray scale ranging from zero (black) to 254 (white). Adobe Photoshop CS4 Extended (Adobe Systems Inc.) was used to draw a loop around the entire cell body layer of interest (e.g. CA1) and the background staining of nonneuronal tissue to compare the total level of stain for each section. The protein levels in the cell body layers of CA1 hippocampus were assessed by optical density with background subtraction at 100× magnification as previously described [12]. Optical-density values were averaged across animals within each group, and expressed in ID as a percentage of IS control.
Results
Iron Status
The ID animals were anemic with lower hematocrits at P15 (IS: 39.7 ± 0.6% vs. ID: 24.2 ± 0.9%, p < 0.001). The groups had similar hematocrits on P30 (IS: 38.8 ± 0.4% vs. ID: 45.0 ± 1.0%). Hippocampal iron concentrations were lower at P15 in the ID group (IS: 4.3± 0.6 μg Fe/g vs. ID: 1.2± 0.5 μg Fe/g, p = 0.003) but were no longer different from the IS group at P30 (IS: 5.8 ±0.4 μg Fe/g vs. ID: 5.3 ± 1.1μg Fe/g).
CA1 Dendrite Morphometry (table 1)
Table 1.
Dendrite morphometry at P15, P30 and P70
| Age, group: | P15 |
P30 |
P70 |
|||
|---|---|---|---|---|---|---|
| IS (n = 5) | ID (n = 7) | IS (n = 5) | ID (n = 7) | IS (n = 6) | ID (n = 5) | |
| Maximal apical length, μm | 329±18 | 309±25 | 362±11 | 384±21 | 370±22 | 427±26 |
| Branch width, μm | 0.52±0.03 | 0.29±0.02∗∗ | 0.42±0.03 | 0.39±0.04 | 0.48±0.02 | 0.51±0.07 |
| Total Scholl ring crossings, n | 39.4±1.2 | 35.4±4.1 | 53.1±3.0 | 55.4±4.6 | 64.3±4.5 | 61.4±3.3 |
| Peak branching distance, μm | 136±16 | 88±5∗ | 180±18 | 120±18∗ | 176±15 | 136±8∗ |
| AUC of peak branching region (fig. 3a) | 324±20 | 287±37∗ | 627±65 | 585±51 | 645 ± 44 | 491±49∗ |
| Crossings at peak branching distance, n | 6.7±0.3 | 5.9±0.4 | 8.3±0.6 | 8.9±0.6 | 10.9±0.6 | 8.2±0.6∗ |
| Crossings at IS peak Sholl ring, n | 6.3±0.4 | 4.9±0.2∗ | 8.2±0.7 | 8.0±0.6 | 9.6±0.7 | 7.9±0.6∗ |
p < 0.05
p < 0.001 comparing ID to IS at same age. Values are expressed as mean ± SEM.
The maximal length of the apical dendrite from the soma was not different between the groups at any age. Third-generation dendrite width was 44% lower in the ID group at P15 but was similar to IS at P30 or 70. The total branch number across the entire apical dendrite tree was similar between dietary groups at all ages. However, the location of peak branching was more proximal to the soma in the ID group at all three time points (fig. 2). The ID group had a smaller AUC in the peak branching region and less branching at the control (IS) peak distance at P15 (fig. 2a). The FID group had fewer branches at the peak branch distance and a smaller AUC in the peak branching region at P70 (fig. 2c).
Fig. 2.
Dendrite branching distributions at P15 (a), P30 (b) and P70 (c). Average crossings are shown at each ring in intervals 31 μm apart, starting from the soma. The IS group is represented by black squares and ID and FID groups by open triangles. Peak branching distance for the IS group is labeled with a black arrow and with a dashed arrow for the ID group.
Spine head diameters were 43% smaller in the ID group at P15 (IS: 0.96 ± 0.03 μm vs. ID: 0.55 ± 0.04 μm, p < 0.0001) although spine densities were not different between groups at any age. The dendrite branches and spine heads of the ID group at P15 were thinner and more poorly differentiated (fig. 3b, d). Differences in diameters resolved with iron treatment by P30 (IS: 0.64 ± 0.08 μm vs. ID: 0.59 ± 0.05 μm) and remained so at P70 (IS: 0.60 ± 0.04 μm vs. ID: 0.55 ± 0.03 μm).
Fig. 3.
Representative images of third-generation dendrite branches in P15 IS (a) and higher magnification of the highlighted area (rectangular box, b) and P15 ID (c, d). Arrows denote the bifurcation of the thirdgeneration branch from the second generation. Arrowheads illustrate the smaller third-generation branch widths and spine head diameters in the ID compared to the IS animal. Scale bar = 20 μm.
Regulation of Cytoskeletal Structures (table 2)
Table 2.
Comparison of structural determining gene expression in the IS and ID rat hippocampus
| Gene | Ratio of P7 IS |
2-WayANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P7 |
P15 |
P30 |
P65 |
p values |
|||||||
| IS | ID | IS | ID | IS | ID | IS | FID | iron status | age | interaction | |
| Crmpl | 1.00d | 0.77∗ | 0.54c | 0.58 | 0.25b | 0.23 | 0.07a | 0.06 | 0.07 | <0.01 | 0.03 |
| Cxcr4 | 1.00d | 0.84 | 0.69c | 0.55 | 0.24b | 0.27 | 0.12a | 0.06 | 0.06 | <0.01 | 0.35 |
| Cdc42 | 1.00a | 0.53∗ | 1.72b | 1.80 | 2.45c | 2.52 | 3.48d | 2.08∗ | <0.01 | <0.01 | <0.01 |
| RhoA | 1.00a | 1.45∗ | 2.26b | 1.10∗ | 2.67b | 2.39 | 1.14a | 0.58∗ | 0.02 | <0.01 | <0.01 |
| Racl | 1.00a | 1.30∗ | 1.99b | 2.37 | 2.26b | 2.38 | 0.93a | 0.47∗ | 0.57 | 0.18 | <0.01 |
| Cypin | 1.00b | 0.70∗ | 1.08b | 0.67∗ | 0.63a | 0.68 | 0.58a | 0.55 | <0.01 | <0.01 | <0.01 |
| Cfll | 1.00b | 0.53∗ | 0.68a | 0.68 | 0.54a | 0.47 | 0.67a | 0.39∗ | <0.01 | <0.01 | <0.01 |
| Pfnl | 1.00b | 0.48∗ | 0.59a | 0.60 | 0.49a | 0.57 | 0.90b | 0.54∗ | <0.01 | <0.01 | <0.01 |
| Pfn2 | 1.00b | 0.58∗ | 0.93b | 1.26∗ | 0.56a | 0.58 | 1.12b | 0.52∗ | <0.01 | <0.01 | <0.01 |
Values are means ± SEM; n = 4-6. Means in a row without a common letter differ, p < 0.05, a<b<c<d.
Different from IS at given time point at p < 0.05.
Transcript levels were normalized to an endogenous control ribosomal protein 18S and standardized relative to P7 IS for each gene. The relative mRNA levels of the neurite guidance proteins Crmp-1 and Cxcr4 were highest at P7 and declined with postnatal age in both groups (table 2). At P7, the ID group had lower Crmp-1, Cypin, Cfl-1, Cdc42, Pfn-1 and Pfn-2 accompanied by higher RhoA and Rac1 compared to the IS group. At P15, after 1 week of treatment, the ID group showed normalization of most genes with the exception for cypin, which subsequently completely recovered to IS expression levels by P30. In spite of this recovery, the mRNA levels for Cdc42, RhoA, Rac1, Cfl-1, Pfn-1, and Pfn-2 were again reduced by an average of 32% in the FID group at P70. Immunohistochemical assessment demonstrated lower CA1 expression of Crmp-1 (−48%), Cxcr4 (−42%), RhoA (−21%), Rac1 (−30%), Cfl-1 (21%) and Cdc42 (−38%) in the FID group compared to the always-IS group (fig. 4).
Fig. 4.
Representative images of coronal sections of hippocampus with immunohistochemical staining for Crmp-1, Cxcr4, RhoA, Rac1, Cfl-1 and Cdc42 at P65 IS (af) and FID (a′f′). Arrows denote cell body layer of CA1 pyramidal cells. Scale bar = 100 μm.
Discussion
The developmental ontogeny of the hippocampus and specifically CA1 relies on access to adequate metabolic substrates such as glucose, amino acids, oxygen and iron to support the highly energy dependent process of neurite extension and differentiation. From an iron biology standpoint, rapid dendritogenesis begins at P15 in the rat hippocampus and is preceded from P5 to P15 by high rates of iron import [32,33]. The shafts and spines of apical dendrites of CA1 pyramidal neurons are highly metabolic areas that demonstrate rapid local iron transmembrane cycling via the iron transporter, transferrin receptor-1 [34]. The specific requirement for iron in these processes was underscored recently in a mouse model with a late-gestation, hippocampus pyramidal cell neuron-specific knockout of the gene for the iron transporter, Slc11a2. This mouse remains ID throughout life and has abnormal neuronal hippocampal CA1 apical dendrite branching patterns characterized by shortened shaft lengths, excessive proximal branches and abnormal learning on the Morris Water Maze in adulthood [35]. The current study demonstrates that iron is critical for normal activity of the signaling cascade regulating cytoskeletal dynamics and subsequent apical dendrite formation in CA1.
We have previously demonstrated that iron deficiency during the period of hippocampal dendritic differentiation in the rat shortens the main apical dendrite length of CA1 pyramidal neurons [13]. That analysis was limited because the staining methodology that was employed could not provide information about the branching characteristics of the more distal arbor and the integrity of spines. In the current study, we utilized the more informative Golgi-Cox staining to confirm the previous finding while documenting additional structural defects in the CA1 apical dendrite arbor including reduced dendrite width, spine head diameter and branching during the period of iron deficiency. Moreover, the branching abnormalities persisted at P65 after complete iron repletion. While this study specifically assessed hippocampal area CA1 because of its role in learning and memory, dietary iron deficiency affects the entire developing brain and thus we speculate that morphologic changes may be present in other areas as well. Morphologic effects in the hippocampus are mediated through the alteration of genes involved in a signaling cascade that regulates actin and tubulin dynamics [17,18,19,20,21,22,23,29,31]. In the current study, we demonstrated that these genes are most highly expressed in the IS hippocampus in early neonatal life just prior to rapid dendrite differentiation, but continue to be expressed at low levels in adult life, presumably to mediate experience-dependent changes in the arbor. Gestational IDA altered their expression during the period of iron deficiency and was accompanied by significant morphologic changes. The temporal lag between abnormal gene expression and dendritic structure between P7 and P15 was interesting to note. Apical dendritic structures at P7 are likely too rudimentary for the detection of significant structural abnormalities by the Golgi-staining approach [24]. We speculate that under IS circumstances, developing CA1 pyramidal neurons increase the expression of these genes at P7 in order to support the significant dendritic growth and arborization that occurs between P7 and P15 [24]. The altered gene expression noted at the time of peak IDA at P7 is thus likely to be responsible for the dendritic growth impairment subsequently seen at P15 in the IDA pups. The more remarkable and concerning finding was the persistent suppression of these genes in adulthood accompanied by persistent morphologic abnormalities in spite of an initial and complete recovery following early iron therapy. This long-term suppression suggests early-life programming of these genes (or their regulators) and is consistent with the concept of the developmental origins of adult neurologic dysfunction. Collectively, the findings begin to reveal molecular and structural underpinnings for the long-term electrophysiologic and cognitive impairment following early iron deficiency observed in multiple rodent and human studies [6,7,8,9,10,36,37].
The development of the hippocampus-based declarative memory system and its maintenance during adulthood relies on the integrity of three interacting signaling cascades. These include proteins involved in LTP which support the cellular basis of memory [38], the BDNF system which regulates dendrite growth and branching during development and neuronal plasticity across the lifespan [39], and the cascade regulating actin and microtubule dynamics [17,18,19]. The latter bears an important relationship to both the LTP and BDNF signaling cascades. For example, SDF1 binding to Cxcr4 stimulates ERK/CREB/Egr1 activity, which is a critical aspect of LTP [40,41,42,43]. We have previously demonstrated that IDA during gestation dysregulates SDF1/Cxcr4 signaling and delays the ontogeny of LTP in CA1 between P15 and P30 [14,15,16]. Similarly, BDNF interacts with Cdc42 to inhibit RhoA, a negative regulator of Cfl-1, thus stimulating dendrite branching and spine stability [21,22,31]. Early IDA induces acute and long-term suppression of BDNF, its receptor (TrkB) and its downstream effectors [29,38] and thus likely contribute to the dysregulation of genes involved in regulating actin and tubulin dynamics.
The short- and long-term gene expression changes in these pathways induced by IDA were generally consistent with the morphologic alterations noted at P15 and P70. Figure 5 summarizes the proposed interactions of the three pathways and illustrates the acute and long-term changes induced by iron deficiency. Ultimately, Cfl-1, Pfn-1 and Pfn-2 are responsible for interacting with actin elements to promote dendrite arbor extension and branching during development and to maintain plasticity during adulthood [17,18,19,23]. IDA suppressed the expression of each at both time points, accompanied by an arbor characterized by fewer and more proximal branches. Similarly, the expression of Crmp1 and Cdc42 was suppressed, suggesting that IDA impaired cell surface-signaling mechanisms that influence downstream Cfl1, Pfn-1 and Pfn-2 expression. The contrast between the elevation of RhoA and Rac1 levels at P7 and their suppression at P65 was interesting to note. One possible explanation resides in the finding that the activities of rho GTPases may be a function of the GTP/GDP-associated state rather than the overall gene or protein levels. Although such data remain to be characterized in IDA rats, the changes in expression level might reflect the state of functional demands. Thus, it is possible that IDA-induced upregulation of RhoA and Rac1 at P7 reflects a compensation for a deficit in GTPases activity due to lower energy (GTP/ATP) availability in IDA rats [15], while the changes at P65 may reflect reduced activity-dependent gene regulation [11,14,36].
Fig. 5.
The proposed effects of early-life IDA on expression of factors regulating actin and tubulin dynamics in rat hippocampus (derived from ref. [17, 18, 19, 20, 21, 22, 23, 29, 31]. The dotted arrows show the direction of regulatory change at P7 while ID and solid arrows show directional changes at P65 following resolution of iron deficiency.
The signaling and structural anomalies may account for the impaired electrophysiology (e.g. persistence of an immature LTP pattern and impaired PPF) and poorer learning documented during the period of iron deficiency in young rats [11,12,14]. The physiologic consequences likely stem from the reduced branching area as well as the thinner third-generation dendrite branches and the smaller spine head diameters observed in the ID animals at P15. The latter may reduce conduction velocity, resulting in less coordinated input to the soma [44]. The smaller spine head diameters likely represent a smaller postsynaptic density, lower concentrations of AMPA and NMDA receptors and less smooth endoplasmic reticulum for local calcium storage and release [45,46,47].
It is not surprising that the hippocampus functions abnormally while it is ID. It has been less clear why prompt, early and complete iron repletion does not reverse the genomic, biochemical, structural, electrophysiologic and behavioral effects that appear to underlie the long-lasting sequelae of early IDA in the hippocampus [11,13,15,16,36]. For example, the CA1 field isolated from P65 FID rats shows a 48% lower ratio of presynaptic input to postsynaptic output (p < 0.05), a 15% delay in synaptic vesicle reloading rate (p < 0.02), and an 11% lower LTP output (p < 0.05) compared to never-ID controls [14]. From a behavioral standpoint, FID rats require 35% more trials to achieve criteria on a Win-Shift learning task [36]. In the current study, P65 FID adult rats had, on average, 32% lower levels of factors regulating cytoskeletal dynamics compared to always-IS controls accompanied by a 25% reduction in branching density. The findings were surprising given the apparent recovery of their levels at P30 following iron treatment. The degree of structural abnormalities at P65 suggests that the ID group actually lost ground relative to the IS group since P30. While the more proximal maximal branching point in the FID group may represent the long-term consequence of growth failure from previous growth stages, the persistent suppression of the genes that regulate actin dynamics are consistent with the continuing compromise of hippocampal plasticity we have documented in this model in adulthood [13,36].
The long-term abnormalities suggest two not necessarily mutually exclusive mechanisms. The first invokes the concept of critical periods of development [48]. The fetal/neonatal period is characterized by greater neural plasticity in the hippocampus than will be evident later in life. This plasticity allows the developing hippocampus to recover from negative environmental processes, including presumably the absence of iron. However, periods of heightened plasticity are temporally finite [48]. We speculate that the lack of complete iron repletion during the critical developmental period for CA1 pyramidal cell arborization alters its physical developmental trajectory permanently. The structural changes induced by early IDA may result in an adult animal that is generally less responsive to its environment. Lower responsiveness may subsequently lower activity of genes responsible for mediating adult plasticity. The second mechanism involves epigenetic modification of genes involved in experience-dependent plasticity. We have shown that fetal-neonatal IDA downregulates long-term hippocampal BDNF expression in adulthood in spite of complete iron repletion of the structure [38]. BDNF is an epigenetically modifiable gene [49,50,51], whose expression can be regulated environmentally through modification of CpG sites [51]. While it remains unknown whether the genes involved in regulation of cytoskeletal dynamics are similarly modifiable, the fact that BDNF expression modulates the activity of these genes suggests they are at least potentially susceptible to indirect epigenetic influences. Future work will determine whether stimulating growth factors, specifically BDNF, can positively influence dendritogenesis in this model. Such stimulation may increase the ability to promote normal dendritogenesis during early IDA and allow the system to remain more plastic after repletion.
References
- 1.Degano AL, Pasterkamp RJ, Ronnett GV. MeCP2 deficiency disrupts axonal guidance, fasciculation, and targeting by altering semaphorin 3F function. Mol Cell Neurosci. 2009;42:243–254. doi: 10.1016/j.mcn.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joels M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learn Mem. 2009;92:292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joels M, Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams CL. Food for thought: brain, genes, and nutrition. Brain Res. 2008;1237:1–4. doi: 10.1016/j.brainres.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 5.Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier RA. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res. 2004;55:1034–1041. doi: 10.1203/01.pdr.0000127021.38207.62. [DOI] [PubMed] [Google Scholar]
- 6.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 7.Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140:165–170. doi: 10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- 8.Riggins T, Miller NC, Bauer PJ, Georgieff MK, Nelson CA. Electrophysiological indices of memory for temporal order in early childhood: implications for the development of recollection. Dev Sci. 2009;12:209–219. doi: 10.1111/j.1467-7687.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med. 2006;160:1108–1113. doi: 10.1001/archpedi.160.11.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–43. doi: 10.1301/nr.2006.may.S34-S43. discussion S72–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEchron MD, Cheng AY, Liu H, Connor JR, Gilmartin MR. Perinatal nutritional iron deficiency permanently impairs hippocampus-dependent trace fear conditioning in rats. Nutr Neurosci. 2005;8:195–206. doi: 10.1080/10284150500162952. [DOI] [PubMed] [Google Scholar]
- 12.Gewirtz JC, Hamilton KL, Babu MA, Wobken JD, Georgieff MK. Effects of gestational iron deficiency on fear conditioning in juvenile and adult rats. Brain Res. 2008;1237:195–203. doi: 10.1016/j.brainres.2008.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;25:412–420. doi: 10.1159/000075667. [DOI] [PubMed] [Google Scholar]
- 14.Jorgenson LA, Sun M, O'Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–1102. doi: 10.1002/hipo.20128. [DOI] [PubMed] [Google Scholar]
- 15.Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–3221. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- 16.Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus. 2007;17:679–691. doi: 10.1002/hipo.20307. [DOI] [PubMed] [Google Scholar]
- 17.Ackermann M, Matus A. Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat Neurosci. 2003;6:1194–1200. doi: 10.1038/nn1135. [DOI] [PubMed] [Google Scholar]
- 18.Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol. 2005;75:161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int. 2007;51:92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Pujol F, Kitabgi P, Boudin H. The chemokine SDF-1 differentially regulates axonal elongation and branching in hippocampal neurons. J Cell Sci. 2005;118:1071–1080. doi: 10.1242/jcs.01694. [DOI] [PubMed] [Google Scholar]
- 21.Chen TJ, Gehler S, Shaw AE, Bamburg JR, Letourneau PC. Cdc42 participates in the regulation of ADF/cofilin and retinal growth cone filopodia by brain derived neurotrophic factor. J Neurobiol. 2006;66:103–114. doi: 10.1002/neu.20204. [DOI] [PubMed] [Google Scholar]
- 22.Charych EI, Akum BF, Goldberg JS, Jornsten RJ, Rongo C, Zheng JQ, Firestein BL. Activity-independent regulation of dendrite patterning by postsynaptic density protein PSD 95. J Neurosci. 2006;26:10164–10176. doi: 10.1523/JNEUROSCI.2379-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramakers GJ. Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci. 2002;25:191–199. doi: 10.1016/s0166-2236(00)02118-4. [DOI] [PubMed] [Google Scholar]
- 24.Pokorny J, Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. 2. Development of ultrastructure in stratum lacunosum and moleculare. Brain Res Bull. 1981;7:121–130. doi: 10.1016/0361-9230(81)90076-9. [DOI] [PubMed] [Google Scholar]
- 25.Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- 26.Scholl D. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- 27.Rasband W. Image J, US National Institutes of Health, Bethesda, Maryland, USAhttp://rsbinfonihgov/ij/1997-2008
- 28.Spruceton N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008:9. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- 29.Tran PV, Carlson ES, Fretham SJ, Georgieff MK. Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J Nutr. 2008;138:2495–2501. doi: 10.3945/jn.108.091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang BL. Inhibitors of neuronal regeneration: Mediators and signaling mechanisms. Neurochem Int. 2003;42:189–203. doi: 10.1016/s0197-0186(02)00094-3. [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Firestein BL. RhoA regulates dendrite branching in hippocampal neurons by decreasing cypin protein levels. J Neurosci. 2007;27:8378–8386. doi: 10.1523/JNEUROSCI.0872-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rihn LL, Claiborne BJ. Dendritic growth and regression in rat dentate granule cells during late postnatal development. Brain Res Dev Brain Res. 1990;54:115–124. doi: 10.1016/0165-3806(90)90071-6. [DOI] [PubMed] [Google Scholar]
- 33.Taylor EM, Morgan EH. Developmental changes in transferrin and iron uptake by the brain in the rat. Brain Res Dev Brain Res. 1990;55:35–42. doi: 10.1016/0165-3806(90)90103-6. [DOI] [PubMed] [Google Scholar]
- 34.Blanpied TA, Scott DB, Ehlers MD. Age-related regulation of dendritic endocytosis associated with altered clathrin dynamics. Neurobiol Aging. 2003;24:1095–1104. doi: 10.1016/j.neurobiolaging.2003.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Carlson ES, Tkac I, Magid R, O'Connor MB, Andrews NC, Schallert T, Gunshin H, Georgieff MK, Petryk A. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr. 2009;139:672–679. doi: 10.3945/jn.108.096354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt AT, Waldow KJ, Grove WM, Salinas JA, Georgieff MK. Dissociating the long-term effects of fetal/neonatal iron deficiency on three types of learning in the rat. Behav Neurosci. 2007;121:475–482. doi: 10.1037/0735-7044.121.3.475. [DOI] [PubMed] [Google Scholar]
- 37.Burden MJ, Westerlund AJ, Armony-Sivan R, Nelson CA, Jacobson SW, Lozoff B, Angelilli ML, Jacobson JL. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics. 2007;120:e336–e345. doi: 10.1542/peds.2006-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran PV, Fretham SJ, Carlson ES, Georgieff MK. Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr Res. 2009;65:493–498. doi: 10.1203/PDR.0b013e31819d90a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malenka RC, Nicoll RA. Long-term potentiation – a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 40.Tapia-Arancibia L, Rage F, Givalois L, Arancibia S. Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol. 2004;25:77–107. doi: 10.1016/j.yfrne.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Baudouin SJ, Pujol F, Nicot A, Kitabgi P, Boudin H. Dendrite-selective redistribution of the chemokine receptor CXCR4 following agonist stimulation. Mol Cell Neurosci. 2006;33:160–169. doi: 10.1016/j.mcn.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Luo Y, Lathia J, Mughal M, Mattson MP. SDF1α/CXCR4 signaling, via ERKs and the transcription factor Egr1, induces expression of a 67-kDa form of glutamic acid decarboxylase in embryonic hippocampal neurons. J Biol Chem. 2008;283:24789–24800. doi: 10.1074/jbc.M800649200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Z, Alzenman C, Holis C. Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron. 2002;33:741–750. doi: 10.1016/s0896-6273(02)00621-9. [DOI] [PubMed] [Google Scholar]
- 44.Hodgkin AL. A note on conduction velocity. J Physiol. 1954;125:221–224. doi: 10.1113/jphysiol.1954.sp005152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris KM, Kater SB. Dendritic spines: Cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 46.Wiens KM, Lin H, Liao D. Rac1 induces the clustering of AMPA receptors during spinogenesis. J Neurosci. 2005;25:10627–10636. doi: 10.1523/JNEUROSCI.1947-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Y, Wang XB, Frerking M, Zhou Q. Spine expansion and stabilization associated with long-term potentiation. J Neurosci. 2008;28:5740–5751. doi: 10.1523/JNEUROSCI.3998-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 49.Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylationrelated chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 50.Jiang Y, Langley B, Lubin FD, Renthal W, Wood MA, Yasui DH, Kumar A, Nestler EJ, Akbarian S, Beckel-Mitchener AC. Epigenetics in the nervous system. J Neurosci. 2008;28:11753–11759. doi: 10.1523/JNEUROSCI.3797-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]