Abstract
Vibrio vulnificus is a human pathogen that produces lethal septicemia in susceptible persons, and the primary virulence factor for this organism is capsular polysaccharide (CPS). The role of the capsule in V. vulnificus biofilms was examined under a variety of conditions, by using either defined CPS mutants or spontaneous CPS expression phase variants derived from multiple strains. CPS expression was shown to inhibit attachment and biofilm formation, which contrasted with other studies describing polysaccharides as integral to biofilms in related species.
Vibrio vulnificus is indigenous to estuarine environments (9, 18, 24, 36, 43) and causes human infections associated with raw oyster consumption (3). Pathogenesis was recently reviewed, and virulence is primarily attributed to capsular polysaccharide (CPS) expression (34). Opaque (O) colony morphology, indicative of a virulent, encapsulated phenotype, exhibits reversible phase variation to translucent (T) colony types with reduced CPS expression and decreased virulence (30, 47). Defined mutations in the CPS operon confirmed the relationship of CPS and virulence (28, 42, 44, 45). Vibrio spp. attach to algae and zooplankton (5, 15, 16, 17, 19, 22), and V. vulnificus may be more concentrated in oysters and fish which feed on these organisms (9, 29, 36, 43). Microbial communities attached to nutrient-rich surfaces are generally referred to as biofilms and are thought to engage in complex signaling for expression of CPS and other factors (7, 25, 26). For example, V. cholerae biofilms require production of polysaccharide, pili, and flagella (5, 21, 23, 37, 38, 39, 46). Biofilms for V. vulnificus biotype 2 eel pathogens were recently described (20); however, this group differs from human pathogens of biotype 1 in that biotype 2 lipopolysaccharide (LPS) is homogeneous (serovar E) and CPS may not always be required for virulence (2). The role of CPS in biofilms of either biotype has not been addressed; therefore, our studies examined V. vulnificus biofilms in O versus T phase variants and CPS mutants that differed in their abilities to produce capsular polysaccharide.
CPS expression inhibits V. vulnificus biofilm formation.
Surface CPS displays a continuum of expression among V. vulnificus strains (44). Strains for this study are detailed in Table 1 and were stored at −70°C in 50% glycerol to ensure stability of phase variants. O strains are completely encapsulated, while T strains either are acapsular or have reduced, patchy capsules. Mutant strains are acapsular but differ in CPS biosynthesis: CVD752 contains a polar transposon mutation in the CPS operon that eliminates biosynthesis, while MO6-24/31T contains a nonpolar mutation, specifically targeting the CPS transport function of the wza gene, and can synthesize CPS but is unable to transport it to the cell surface (44, 45).
TABLE 1.
V. vulnificus strains and CPS expression
| Strain | Description (reference) |
|---|---|
| MO6-24/O | Opaque clinical isolate; expresses CPS over entire cell surface (44) |
| MO6-24/T | Translucent phase variant of MO6-24/O, reduced patchy capsule (44) |
| MO6-24/31T | Acapsular MO6-24/O mutant; does not transport CPS to the cell surface due to mutation in CPS transport gene, wza (45) |
| CVD752 | Acapsular MO6-24/O mutant; does not produce CPS due to polar transposon insertion (45) |
| LC4/O | Clinical isolate; expresses CPS over the entire cell surface (unpublished) |
| LC4/T | Translucent phase variant of LC4/O; acapsular (unpublished) |
| C7184/O | Clinical isolate; expresses CPS on the entire cell surface (30) |
| C7184/T | Translucent phase variant of C7184/T; acapsular (30) |
Biofilm formation on abiotic surfaces was examined by crystal violet absorption assays, and the relative biofilm content was estimated from the concentration of dye eluted from destained cells and matrix (32). Examination of staining capacity for dilutions of suspended cultures indicated slightly higher absorption (less than twofold) for O or T strains than for mutants, but eluted dye reflected a linear relationship to cell density independent of surface properties for all strains (not shown). Biofilm formation was initially examined with log-phase cultures (1 ml) incubated for 6 h statically in glass tubes with Luria-Bertani broth (LB) at 30°C. Attached cells were washed in phosphate-buffered saline (PBS), stained with crystal violet (1%), rinsed, and destained with acetic acid (33%). The optical density of eluted dye was measured at 570 nm (Molecular Devices). Encapsulated M06-24/O showed a more-than-threefold decrease in attached cells compared to partially encapsulated M06-24/T (Fig. 1), suggesting that CPS expression inhibits biofilm formation. However, the phase variation mutation(s) is not defined and may be pleiotropic; therefore, the inhibitory function of CPS was confirmed by observation of increased biofilms for both defined CPS mutants compared to MO6-24/O. We note that bacteria may produce multiple types of CPS, and a role for other polysaccharides in V. vulnificus biofilms is still a possibility.
FIG. 1.
Biofilm formation of V. vulnificus on glass. Strains of V. vulnificus, including MO6-24/O (O), MO6-24/T (T), MO6-24/31T (31T), and CVD752, were incubated in LB in glass tubes for 6 h at 30°C and stained with crystal violet (32). The optical density at 570 nm of eluted dye from attached cells is indicative of relative bacterial cell concentration in biofilms.
Influence of growth conditions.
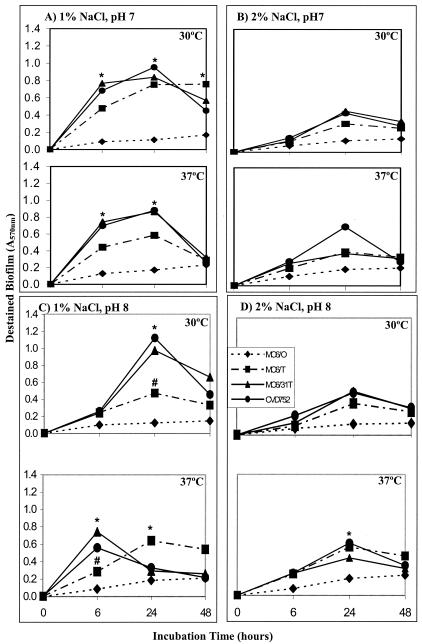
Environmental conditions influence biofilm formation in V. cholerae (1, 15, 16, 19, 21) and CPS expression in V. vulnificus (44); therefore, biofilms were examined for cultures grown statically in LB at different pHs (6, 7, and 8), temperatures (25, 30, and 37°C), and salinities (1 and 2% NaCl). Log-phase cultures were inoculated into fresh media (106 CFU in 100 μl) and monitored over 48 h in a microtiter assay (Immulon 1B; Dynex) by methanol fixation, crystal violet staining, and acetic acid elution (32). Duplicate independent experiments with triplicate samples were performed for each condition. Room temperature and pH 6 produced minimal biofilm for all strains independent of other factors (not shown). Encapsulated MO6-24/O exhibited only minimal biofilm under any condition, and significant differences (P < 0.05) between O and either T or mutant strains were observed at several time points (Fig. 2). Differences could not be attributed to growth kinetics, as strains have similar growth rates (44), and optical densities (A600) of cultures did not correlate with attached biofilms. The greatest biofilm was observed at 1% NaCl for pH 7 or 8 and 30 or 37°C, in contrast with V. cholerae biofilm formation, which was optimum at pH 2 and increased with temperature (15). However, both species generally exhibit greater attachment at 1% than at 2% salinity. CPS inhibition of biofilms was also observed for O versus T variants of other strains (LC4 and C7184), but conditions producing optimum biofilm formation varied among strains (not shown).
FIG. 2.
Biofilm formation for V. vulnificus strains under different growth conditions. Strains of V. vulnificus, including MO6-24/O, MO6-24/T, MO6-24/31T, and CVD752, were examined for biofilm formation in microtiter plates in LB prepared with either 1% NaCl, pH 7 (A), 2% NaCl, pH 7 (B), 1% NaCl, pH 8 (C), or 2% NaCl, pH 8 (D) at incubation temperatures of 30 and 37°C as indicated. The optical density at 540 nm of eluted dye from attached cells is indicative of relative bacterial cell concentration in biofilms. Significant differences (P < 0.05) in destained biofilms between MO6-24/O and other strains (*) and between MO6-24/T and acapsular mutants (#) are noted.
Listeria monocytogenes biofilm formation was greater on hydrophobic polyvinyl chloride than on more-hydrophilic stainless steel surfaces (10), and increased cell surface hydrophobicity may promote biofilms (31). V. vulnificus CPS expression greatly decreases cell surface hydrophobicity (42), presumably because hydrophilic CPS masks more-hydrophobic structures, such as pili. Therefore, the contribution of substrate properties to attachment was examined by using hydrophobic (Immulon 1B) versus more hydrophilic (Immulon 2 or glass) surfaces, but no significant differences were observed (not shown). Thus, although more hydrophobic acapsular strains have increased adherence to surfaces, it would be misleading to conclude that attachment was due solely to hydrophobic interactions.
Biofilms and nutrient status.
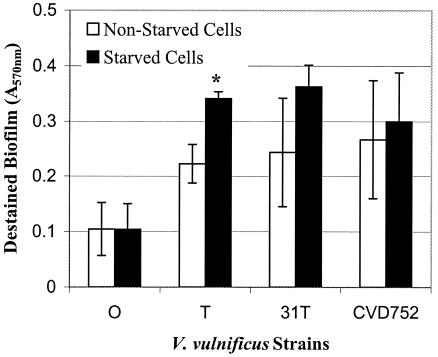
Biofilm formation may be a response to nutrient limitation, with biofilms initially forming at nutrient-rich surfaces and then detaching as nutrient availability declines following extended incubation (26). For example, starvation of a marine vibrio increased adhesion to glass surfaces (8). As shown in Fig. 3, nutrient-depleted (48 h of preincubation in PBS) V. vulnificus MO6-24/T (P < 0.05) and mutants (not significant) showed increased biofilm formation in comparison to nonstarved cells. Also, the V. vulnificus biofilm generally accumulated over 24 h and then leveled off or declined by 48 h, suggesting that nutrient-limited cells were detaching (Fig. 2).
FIG. 3.
Biofilm formation of starved versus nonstarved V. vulnificus strains. Strains of V. vulnificus (MO6-24/O, MO6-24/T, MO6-24/31T, and CVD752) were assayed for biofilms with or without prior starvation (48 h in PBS) in LB for 6 h at 30°C. The optical density at 540 nm of eluted dye from attached cells is indicative of relative bacterial cell concentration in biofilms. Significant differences (P < 0.05) in biofilm formation between starved and nonstarved cells of the same strain are noted (*).
Starvation may also increase phase variation. Extracellular polysaccharide (EPS) is required for V. cholerae biofilm and shows phase variation whereby rugose (wrinkled) colonies express EPS and smooth colonies do not. Starved V. cholerae demonstrates increased phase shift to rugose, biofilm-forming variants (21). However, comparable increases in V. vulnificus phase shift as a function of starvation or extended incubation were not observed, and strains maintained the original phenotype. Thus, the V. vulnificus starvation response was independent of phase variation. Increased rugose-colony phase shift in response to specific growth medium, through induction of high-frequency phase variation, was also reported (1). Perhaps more extended incubation or as yet unidentified nutrient parameters may influence V. vulnificus phase variation and biofilm formation.
Biofilm structure.
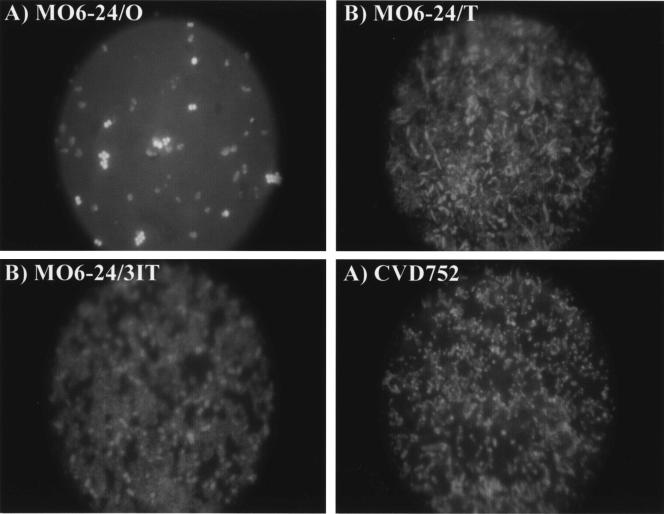
Fluorescence microscopy (HB-10101A; Nikon; DC290 camera; Eastman Kodak), using BacLight viability staining (Molecular Probes), confirmed strain differences in biofilm formation. After 24 h at 30°C in polystyrene plates (Corning), cells were rinsed twice with PBS and stained. Attached MO6-24/O cells appeared mostly as singles or doublets, while dense biofilms through multiple focal planes were observed for acapsular strains (Fig. 4). Interestingly, all attached O cells were viable (as indicated by yellow-green fluorescence), while biofilms of other strains consisted of both live and dead (red) cells. Encapsulated cells did not form the monolayers seen with EPS-negative, biofilm-defective V. cholerae (38, 46) or Escherichia coli (6); thus, V. vulnificus CPS probably relates more to initial attachment rather than the later stages of biofilm development postulated for the role of other polysaccharides.
FIG. 4.
Biofilm formation of V. vulnificus strains as observed with fluorescence microscopy. Strains of V. vulnificus (MO6-24/O [A], MO6-24/T [B], MO6-24/31T [C], and CVD752 [D]) were observed for biofilm formation after 6 h of incubation in LB at 30°C by fluorescence microscopy as described in the text. BacLight staining provides discrimination between live and dead cells (green versus red; color not shown). MO6-24/O cells were observed as viable, while the other strains were a mixture of live and dead cells.
Conclusions.
Polysaccharides are not always critical to initial adhesion but are considered major constituents of the complex architecture of later stages of biofilm formation (6, 12, 38). Surface polysaccharides include CPS, EPS (slime), and LPS, but distinctions are not clear. For example, bacteria may produce multiple types of CPS or have LPS capsules (E. coli KLPS) comprised of CPS sugars attached to lipid A (40). Polysaccharides, derived from the same genetic locus, are referred to as EPSs in mucoid strains and CPS in nonmucoid isolates; however, EPS-producing V. cholerae strains are not mucoid but instead exhibit rugose morphology. Further, EPS has also been referred to as an extracellular polymeric substance (41), and polysaccharide may or may not be a component of this matrix.
Our results indicated that V. vulnificus CPS expression actually inhibited attachment and biofilm formation, and similar observations were reported elsewhere (D. Ramos, K. Piechaczek, and P. Watnick, Abstr. 103rd Gen. Meet. Am. Soc. Microbiol, abstr. J-022, p. 358, 2003). Thus, contrasting roles for V. vulnificus and V. cholerae polysaccharides are proposed and may be related to their divergent biochemical properties. Uronic acid sugars, common to V. vulnificus CPS (4, 14), contribute to increased negative charge and hydrophilicity (42), while V. cholerae EPS is composed primarily of neutral sugars glucose and galactose (46). Unlike V. vulnificus, EPS-producing cells are strongly adherent to each other as well as surfaces. Further, oral biofilms are also composed primarily of neutral sugars (35). Uronic acid sugars from mucoid E. coli (6) and Pseudomonas spp. (11) were previously implicated in biofilm formation; however, recent analysis indicated that glucose, not alginate, predominates in the Pseudomonas aeruginosa EPS (41). This study demonstrated that an alginate-negative algD mutant formed a biofilm equivalent to those formed by encapsulated, nonmucoid wild-type strains and questioned the role of uronic acid in biofilm formation in nonmucoid strains. These data underscore the importance of CPS composition and indicate that polysaccharide function may relate to both structure and relative quantity of capsule expressed.
Our studies suggest that environmental conditions can decrease biofilm. In light of the purported contributions of biofilms to survival (7, 8, 21, 23, 26, 27, 31, 33, 46), manipulating V. vulnificus biofilms could be used to reduce seafood contamination. Increased biofilm capacity of T variants compared to that of O variants might predict the prevalence of T variants in estuarine environments; however, environmental isolates are almost always opaque and presumably encapsulated (43). Additional factors, such as avoidance of phagocytic cells, may provide increased selection for encapsulated variants in molluscan hosts (13). Alternatively, attachment to surfaces may vary with the biological context. For example, encapsulated Klebsiella pneumoniae was less adherent than acapsular mutants to most tissue culture cell lines but attached well to mucus-producing cells (12). Eel mucous also increased the adhesion of V. vulnificus biotype 2 (2). Sutherland (35) emphasized that assumptions about biofilm are frequently based on structures derived from monocultures and polysaccharides extracted from planktonic cells; therefore, further examination of the relationship of polysaccharide structure, biochemistry, and genetics to natural biofilms is needed to delineate the complex parameters influencing these microbial communities.
Acknowledgments
This research was funded in part by the Florida First Program and by an NRI from the USDA.
REFERENCES
- 1.Ali, A., M. H. Rashid, and D. K. Karaolis. 2002. High-frequency rugose exopolysaccharide production by Vibrio cholerae. Appl. Environ. Microbiol. 68:5773-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaro, C., E. G. Biosca, B. Fouz, E. Alcaide, and C. Esteve. 1995. Evidence that water transmits Vibrio vulnificus biotype 2 infections to eels. Appl. Environ. Microbiol. 61:1133-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blake, P. A., M. H. Merson, R. E. Weaver, D. G. Hollis, and P. C. Heublein. 1979. Disease caused by a marine Vibrio: clinical characteristics and epidemiology. N. Engl. J. Med. 300:1-5. [DOI] [PubMed] [Google Scholar]
- 4.Bush, C. A., P. Patel, S. Gunawardenal, J. Powell, A. Joseph, J. A. Johnson, and J. G. Morris, Jr. 1997. Classification of Vibrio vulnificus strains by the carbohydrate composition of their capsular polysaccharides. Anal. Biochem. 250:186-195. [DOI] [PubMed] [Google Scholar]
- 5.Chiavelli, D. A., J. W. Marsh, and R. K. Taylor. 2001. The mannose-sensitive hemagglutinin of Vibrio cholerae promotes adherence to zooplankton. Appl. Environ. Microbiol. 67:3220-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danese, P. N., L. A. Pratt, and R. Kolter. 2000. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 182:3593-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davey, M. E., and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson, M. P., B. A. Humphrey, and K. C. Marshall. 1981. Adhesion, a tactic in the survival strategy of a marine Vibrio during starvation. Curr. Opin. Microbiol. 6:195-198. [Google Scholar]
- 9.DePaola, A., G. M. Capers, and D. Alexander. 1994. Densities of Vibrio vulnificus in the intestines of fish from the U.S. gulf coast. Appl. Environ. Microbiol. 60:984-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djordjevic, D., M. Wiedmann, and L. A. McLandsborough. 2002. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 68:2950-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans, L. R., and A. Linker. 1973. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J. Bacteriol. 116:915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favre-Bonte, S., B. Joly, and C. Forestier. 1999. Consequences of reduction of Klebsiella pneumoniae capsule expression on interactions of this bacterium with epithelial cells. Infect. Immun. 67:554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris-Young, L., M. L. Tamplin, W. S. Fisher, and J. W. Mason. 1993. Effects of physicochemical factors and bacterial colony morphotype on association of Vibrio vulnificus with hemocytes of Crassostrea virginica. Appl. Environ. Microbiol. 59:1012-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayat, U., G. P. Reddy, C. A. Bush, J. A. Johnson, A. C. Wright, and J. G. Morris, Jr. 1993. Capsular types of Vibrio vulnificus: an analysis from clinical and environmental sources. J. Infect. Dis. 168:758-762. [DOI] [PubMed] [Google Scholar]
- 15.Hood, M. A., and P. A. Winter. 1997. Attachment of Vibrio cholerae under various environmental conditions and to selected substrates. FEMS Microbiol. Ecol. 22:215-223. [Google Scholar]
- 16.Huq, A., P. A. West, E. B. Small, M. I. Huq, and R. R. Colwell. 1984. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar O1 associated with live copepods in laboratory microcosms. Appl. Environ. Microbiol. 48:420-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Islam, M. S., B. S. Drasar, and D. J. Bradley. 1989. Attachment of toxigenic Vibrio cholerae O1 to various freshwater plants and survival with filamentous green alga, Rhizoclonium fontanum. J. Trop. Med. Hyg. 92:396-401. [PubMed] [Google Scholar]
- 18.Kaysner, C. A., M. L. Tamplin, M. W. Wekell, R. F. Scott, and K. G. Colburn. 1987. Virulent strains of Vibrio vulnificus isolated from estuaries of the United States west coast. Appl. Environ. Microbiol. 53:1349-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumazawa, N. H., N. Fukuma, and Y. Komoda. 1991. Attachment of Vibrio parahaemolyticus strains to estuarine algae. J. Vet. Med. Sci. 53:201-205. [DOI] [PubMed] [Google Scholar]
- 20.Marco-Noales, E., M. Milan, B. Fouz, E. Sanjuan, and C. Amaro. 2001. Transmission to eels, portals of entry, and putative reservoirs of Vibrio vulnificus serovar E (biotype 2). Appl. Environ. Microbiol. 67:4717-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizunoe, Y., S. N. Wai, A. Takade, and S. Yoshida. 1999. Isolation and characterization of rugose form of Vibrio cholerae O139 strain MO10. Infect. Immun. 67:958-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montanari, M. P., C. Pruzzo, L. Oane, and R. R. Colwell. 1999. Vibrios associated with plankton in a coastal zone of the Adriatic Sea (Italy). FEMS Microbiol. Ecol. 29:241-247. [Google Scholar]
- 23.Morris, J. G., Jr., M. B. Sztein, E. W. Rice, J. P. Nataro, G. A. Losonsky, P. Panigrahi, C. O. Tacket, and J. A. Johnson. 1996. Vibrio cholerae O1 can assume a chlorine-resistant rugose survival form that is virulent for humans. J. Infect. Dis. 174:1364-1368. [DOI] [PubMed] [Google Scholar]
- 24.Oliver, J. D., R. A. Warner, and D. R. Cleland. 1982. Distribution and ecology of Vibrio vulnificus and other lactose-fermenting marine vibrios in coastal waters of the southeastern United States. Appl. Environ. Microbiol. 44:1404-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 26.O'Toole, G. A., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 27.Pedros, A. C., and T. D. Brock. 1983. The importance of attachment to particles for planktonic bacteria. Arch. Hydrobiol. 98:354-379. [Google Scholar]
- 28.Powell, J. L., A. C. Wright, S. S. Wasserman, D. M. Hone, and J. G. Morris, Jr. 1997. Release of tumor necrosis factor alpha in response to Vibrio vulnificus capsular polysaccharide in in vivo and in vitro models. Infect. Immun. 65:3713-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prieur, D., G. Mevel, J. L. Nicolas, A. Plusquellec, and M. Vigneulle. 1990. Interactions between bivalve mollusks and bacteria in the marine environment. Oceanogr. Mar. Biol. Annu. Rev. 28:277-352. [Google Scholar]
- 30.Simpson, L. M., V. K. White, S. F. Zane, and J. D. Oliver. 1987. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect. Immun. 55:269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sommer, P., C. Martin-Rouas, and E. Mettler. 1999. Influence of the adherent population level on biofilm population, structure, and resistance to chlorination. Food Microbiol. 16:503-515. [Google Scholar]
- 32.Stepanovic, S., D. Vukovic, I. Dakie, B. Savie, and M. Svabic-Vlahovic. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 40:175-179. [DOI] [PubMed] [Google Scholar]
- 33.Stewart, P. S., L. Grab, and J. A. Diemar. 1998. Analysis of biocide transport limitation in an artificial biofilm system. J. Appl. Microbiol. 85:495-500. [DOI] [PubMed] [Google Scholar]
- 34.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microb. Infect. 2:177-188. [DOI] [PubMed] [Google Scholar]
- 35.Sutherland, I. W. 2001. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3-9. [DOI] [PubMed] [Google Scholar]
- 36.Tamplin, M. L., G. E. Rodrick, N. J. Blake, and T. Cuba. 1982. The isolation and characterization of Vibrio vulnificus from two Florida estuaries. Appl. Environ. Microbiol. 44:1466-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thelin, K. H., and R. K. Taylor. 1996. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect. Immun. 64:2853-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watnick, P. I., and R. Kolter. 1999. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 34:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watnick, P. I., K. J. Fullner, and R. Kolter. 1999. A role for the mannose-sensitive hemagglutination in biofilm formation by Vibrio cholerae El Tor. J. Bacteriol. 181:3606-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitfield, C., and I. W. Roberts. 1999. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 31:1307-1319. [DOI] [PubMed] [Google Scholar]
- 41.Wozniak, D. J., T. J. O. Wyckoff, M. Starkey, R. Keyser, P. Azadi, G. A. O'Toole, and M. R. Parsek. 2003. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PA01 Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA 100:7907-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright, A. C., L. M. Simpson, J. D. Oliver, and J. G. Morris, Jr. 1990. Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect. Immun. 58:1769-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright, A. C., R. T. Hill, J. A. Johnson, M. Roghman, R. R. Colwell, and J. G. Morris, Jr. 1996. Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl. Environ. Microbiol. 62:717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright, A. C., J. L. Powell, M. K. Tanner, L. A. Ensor, A. B. Karpas, J. G. Morris, Jr., and M. B. Sztein. 1999. Differential expression of Vibrio vulnificus capsular polysaccharide. Infect. Immun. 67:2250-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright, A. C., J. L. Powell, J. B. Kaper, and J. G. Morris, Jr. 2001. Identification of a group 1-like capsular polysaccharide operon for Vibrio vulnificus. Infect. Immun. 69:6893-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yildiz, F. H., and G. K. Schoolnik. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm production. Proc. Natl. Acad. Sci. USA 96:4028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida, S., M. Ogawa, and Y. Mizuguchi. 1985. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect. Immun. 47:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]