Abstract
Penile growth is under androgenic control. Human chorionic gonadotropin (hCG) has a stimulatory effect on testicular steroidogenesis and penile growth. The purpose of this study was to evaluate the effect of hCG treatment on the gonadal response and penile growth in male idiopathic hypogonadotrophic hypogonadism (IHH) presenting with micropenis. A total of 20 IHH patients who met the criteria for micropenis were included in this study. hCG (1,500-2,000 IU) was administrated intramuscularly, 3 times per week, for 8 weeks. Basic laboratory and hormonal indexes (including serum testosterone and LH levels), penis length (flaccid and stretched), and testicular volume were measured before and 24 weeks after hCG treatment. The patients' mean age was 18.9 years (range, 12 to 24 years). The mean serum testosterone level was significantly increased after hCG treatment (baseline, 2, 4, 12, and 24 weeks: 0.90±1.35 ng/ml, 1.77±1.31 ng/ml, 3.74±2.24 ng/ml, 5.49±1.70 ng/ml, and 5.58±1.75 ng/ml, respectively; p<0.05). Mean penile length also increased significantly 24 weeks after treatment (flaccid length: from 3.39±1.03 cm to 5.14±1.39 cm; stretched length: from 5.41±1.43 cm to 7.45±1.70 cm; p<0.001). Mean testicular volumes increased significantly as well (left: from 5.45 cc to 6.83 cc; right: from 5.53 cc to 7.03 cc). There were no remarkable adverse effects of the hCG treatment. The hCG treatment increased the serum testosterone level, penile length, and testicular volume in IHH patients. Our results suggest that hCG treatment has a beneficial effect on gonadal function and penile growth in patients with IHH presenting with micropenis.
Keywords: Human chorionic gonadotropin, Micropenis, Testosterone
INTRODUCTION
Idiopathic hypogonadotrophic hypogonadism (IHH) is associated with deficient pituitary gonadotropin secretion due to impaired secretion of GnRH from the hypothalamus. The deficiency may be isolated or may occur in conjunction with other disorders.1 Men with IHH present clinically with delayed sexual maturation and may have associated midline defects such as Kallman's syndrome.1 The main goal of treatment in adolescent or young men is to restore serum androgen to normal levels by hormonal application such as testosterone or human chorionic gonadotropin (hCG), thus allowing virilization, penile growth, and puberty to be accomplished, and finally, inducing fertility when appropriate. Normal testosterone levels may be achieved with exogenous testosterone administration. Alternatively, endogenous testosterone secretion can be stimulated by using hCG.2 Penile growth is under androgenic control, but hCG also has a stimulatory effect on testicular steroidogenesis and penile growth. One study reported that the final testicular volume in patients with IHH treated with hCG was substantially greater than that in patients treated with testosterone.3
Micropenis refers to an extremely small penis with a stretched penile length of less than 2.5 SD below the mean for age or stage of sexual development.4 Micropenis is one of the presenting symptoms of IHH; however, few studies have evaluated the response to hCG therapy in adolescent or adult men with IHH in terms of micropenis. The purpose of this study was therefore to determine the effect of hCG therapy on the gonadal response and penile growth in men with IHH who presented with micropenis.
MATERIALS AND METHODS
1. Patients
A total of 20 male patients with IHH who met the criteria for micropenis were included in this study. The results were analyzed retrospectively by chart review and were approved by an institutional review board and ethics committee. Patients with cryptorchidism or its absence according to the imaging studies conducted at the initial presentation were excluded. The hCG stimulation test was performed in all patients to exclude primary testicular insufficiency. Additionally, all men had normal basal thyroid and adrenal function. A pituitary mass lesion or a suprasellar tumor was excluded by skull X-ray and by cranial computed tomography.
2. Diagnosis of IHH
The diagnosis of IHH was made on the basis of low or normal serum LH and FSH concentrations associated with low serum testosterone, otherwise normal anterior pituitary function, and no demonstrable lesion on a high-resolution CT scan or MRI of the hypothalamic-pituitary area.
3. Methods
1) Clinical and laboratory assessment
Testis volume was assessed by using the Prader orchidometer. Gonadotrophin (LH, FSH) levels were measured by immunoradiometric assays. Serum testosterone was measured following organic solvent extraction by radioimmunoassay. Basic laboratory and endocrine assessments were performed (including serum testosterone, LH, and FSH levels) and penis length (flaccid and stretched) and testicular volume were measured before and after hCG treatment.
2) Hormonal therapy
The patients were treated by using a standard protocol of 1,500 IU to 2,000 IU hCG administrated intramuscularly, 3 times per week, for 8 weeks.
3) Penile length measurement
Penile length was measured by one doctor (K. Park). A wooden spatula was pressed against the pubic ramus depressing the suprapubic pad of fat as completely as possible to ensure that the part of the penis that is buried in the subcutaneous fat was measured. Measurement was made along the dorsum of the penis to the tip of the glans penis. The length of foreskin was not included.
4. Statistical analysis
The data are given as the mean±SD unless otherwise indicated. Comparisons of data within a patient were evaluated by Student's t test; comparisons of data from different subsets were evaluated by unpaired t test. Multiple means were compared by ANOVA.
RESULTS
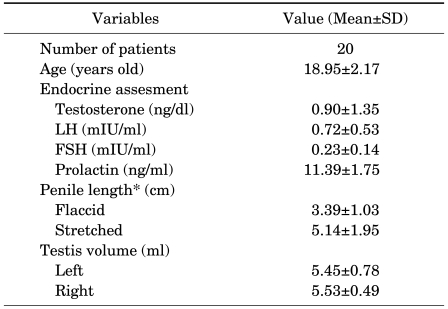
The clinical features of the patients are shown in Table 1. The patients' mean age was 18.9 years (range, 12 to 24 years). The mean testicular volume of the patients was less than 6 ml (measured by Prader orchidometer) at the time of assessment. The basal serum LH (mIU/ml), FSH (mIU/ml), and prolactin (ng/ml) levels were 0.72±0.53 (reference range, 1.3-13.0), 0.23±0.14 (reference range, 0.9-15.0), and 11.39±1.75 (reference range, 2.0-15.0), respectively (Table 1). There were no remarkable adverse events related to the hCG treatment.
TABLE 1.
The basal characteristics of the patients before hCG treatment
SD: standard deviation, *Mean penile length: circumcised length (From Hwang IS, Study on penile length of Korean young adults. Korean J Urol 2005;46:621-5).
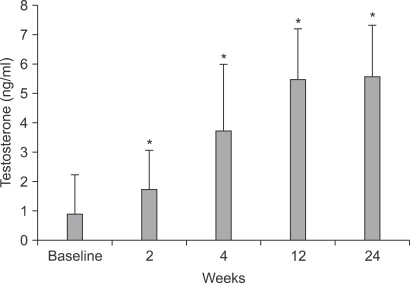
1. Increase in serum testosterone after hCG treatment
The mean serum testosterone level was significantly increased after hCG treatment. The serum testosterone levels at baseline and after 2, 4, 12, and 24 weeks of hCG treatment were 0.90±1.35 ng/ml, 1.77±1.31 ng/ml, 3.74±2.24 ng/ml, 5.49±1.70 ng/ml, and 5.58±1.75 ng/ml, respectively (p<0.05) (Fig. 1).
FIG. 1.
Change in the mean serum testosterone level after hCG treatment. *: p<0.05 vs baseline.
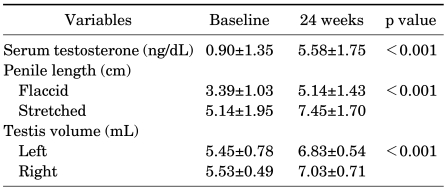
2. Increase in penile length after hCG treatment
Penile length was measured with the penis flaccid and fully stretched. The mean penile length also increased significantly after hCG treatment. The flaccid and stretched length after hCG treatment increased from 3.39±1.03 cm to 5.14±1.39 cm and from 5.41±1.43 cm to 7.45±1.70 cm, respectively (p<0.001) (Table 2).
TABLE 2.
Testosterone levels, penile length, and testis volume before and after hCG treatment
3. Increase in testicular volume after hCG treatment
The mean testicular volume measured by orchidometer increased significantly as well after hCG treatment. Testis volume increased from 5.45 cc to 6.83 cc on the left side and from 5.53 cc to 7.03 cc on the right side (p<0.005) (Table 2).
DISCUSSION
In this study, patients with isolated IHH had a good response to hCG therapy in terms of penile growth, testicular growth, and elevation of serum testosterone. Although the present study included relatively severe cases whose initial mean testicular volume was less than 6 mL, the majority of patients achieved a good response. The hCG treatment increased serum concentrations of testosterone, testicular volume, and penile length. Therefore, our results suggest that patients with IHH will have a good response to hCG therapy in terms of testicular growth, improvement in serum testosterone, and increased penile growth, even patients with severe forms of hypogonadotropic hypogonadism.
IHH is a congenital disorder with deficient pituitary gonadal secretion that may occur in conjunction with other disorders.1 The central defect in most men with IHH is the loss of pulsatile secretion of GnRH from the hypothalamus.5-8 The objectives of therapy in adolescent and young adult males are to restore normal serum androgen levels to induce penile growth and puberty, and finally, to induce fertility. Although penile growth is dependent on androgenic control, hCG is also known to have a stimulatory effect on testicular steroidogenesis and penile growth.
Micropenis, a presenting symptom of IHH, refers to an extremely small penis with a stretched penile length of less than 2.5 SD below the mean for age or stage of sexual development.4 The most important concern in a patient with micropenis is whether the patient will have sufficient penile growth to allow sexual function as an adult. It is well known that micropenis results from a lack of adequate androgen action during early fetal life that hinders full masculinization of the external genitalia and induces inadequate androgen stimulation for normal penile growth. Among the suggested etiologic factors of micropenis, the most common cause of micropenis is failure of the hypothalamus to secrete gonadotropins or hypophysial dysfunction.9 When treating patients with anterior pituitary or hypothalamic dysfunction, hCG can be used to induce puberty instead of testosterone, which has the additional advantage of producing testicular enlargement and initiating spermatogenesis.10 Few studies, however, have evaluated penile growth in response to hCG therapy in adolescent patients with IHH. In cases of idiopathic IHH combined with micropenis, hCG alone has been reported to increase penile length.2 In the current study, we studied the effect of hCG therapy on the gonadal response and penile growth in male patients with IHH presenting with micropenis. Our results show that hCG treatment can successfully improve penile length in IHH patients.
Normal testosterone levels may be achieved with exogenous testosterone administration. Alternatively, endogenous testosterone secretion can be stimulated by using hCG. Additionally, the final testicular volumes in patients with IHH treated with hCG are substantially greater than in patients treated with testosterone.3 Previously, many other studies reported a good response to gonadotropin therapy in males with IHH. Schopohl et al11 reported that gonadotropin therapy with 3×2,500 IU hCG as a weekly intramuscular injection restored endocrine and exocrine testicular function to the normal range in male patients with IHH. They showed that the serum level of testosterone, positive sperm count, and testicular volume were increased significantly in the gonadotropin-injected group. Burris et al12 investigated the effect of exogenous hCG alone in IHH men in terms of serum testosterone, spermatogenesis, and testicular growth. They reported that hCG treatment increased the testicular volume from 5.5 (pretreatment) to 10.8 mL (maximum) during treatment and that all men attained normal serum testosterone levels after hCG treatment. During hCG treatment, 14 of the 22 men had positive sperm appearance in their semen. Ley and Leonard13 also reported that hCG treatment is sufficient to both initiate and maintain spermatogenesis. Although the studies mentioned above reported the effect of gonadotropin therapy in males with IHH, all studied heterogeneous groups of males with complete or partial gonadotropin deficiency, which may be one reason for the good response to the hCG treatment.
The spectrum of gonadotropin deficiency is manifested by the men's initial testis volume.14 The initial testicular volume of men with IHH is highly correlated with maximum testicular volume and sperm production.12 A testicular volume of less than 4 mL is considered to indicate complete gonadotropin deficiency, whereas a testicular volume of at least 4 mL is considered to indicate some degree of gonadotropin stimulation.15,16 In this study, we included patients with mean testicular volumes less than 6 mL at the initial diagnosis, and the patients showed a good response to hCG therapy. Therefore, we suggest that hCG treatment may have a beneficial effect on gonadal function in patients with a small testicular volume and on penile growth in patients presenting with micropenis.
A limitation of our study is that we could not measure semen parameters before and after surgery, because most of our patients were of an adolescent age and did not agree to provide a semen sample. Another limitation is the small sample size and the absence of a control group treated with combined human menopausal gonadotropin (hMG) or testosterone. The appropriate timing and dosage of hCG therapy and its mode of action has not been conclusively determined, and controversy currently exists in the literature. Furthermore, our study population was mixed with both adolescent and adult patients, and we could not evaluate the outcomes separately for these different age groups of prepubertal and postpubertal patients. It is uncertain whether the initial gain in penile length will be maintained into adulthood. Further study is needed with a prospective design, large sample size, long-term follow-up in terms of penile growth, and a control group of patients with another treatment modality.
In conclusion, hCG treatment seemed to be effective in IHH, because it successfully increased the serum testosterone level and testicular volume and stimulated penile growth. Our data also imply that hCG treatment for patients with IHH presenting with micropenis results in a satisfactory gain in penile length.
References
- 1.Kallmann FJ, Schoenfeld WA, Barrera SE. The genetic aspects of primary eunuchoidism. Am J Ment Defic. 1994;48:203–236. [Google Scholar]
- 2.Kirk JM, Savage MO, Grant DB, Bouloux PM, Besser GM. Gonadal function and response to human chorionic and menopausal gonadotrophin therapy in male patients with idiopathic hypogonadotrophic hypogonadism. Clin Endocrinol (Oxf) 1994;41:57–63. doi: 10.1111/j.1365-2265.1994.tb03785.x. [DOI] [PubMed] [Google Scholar]
- 3.Bistritzer T, Lunenfeld B, Passwell JH, Theodor R. Hormonal therapy and pubertal development in boys with selective hypogonadotropic hypogonadism. Fertil Steril. 1989;52:302–306. doi: 10.1016/s0015-0282(16)60859-2. [DOI] [PubMed] [Google Scholar]
- 4.Lee PA, Mazur T, Danish R, Amrhein J, Blizzard RM, Money J, et al. Micropenis. I. Criteria, etiologies and classification. Johns Hopkins Med J. 1980;146:156–163. [PubMed] [Google Scholar]
- 5.Yoshimoto Y, Moridera K, Imura H. Restoration of normal pituitary gonadotropin reserve by administration of luteinizing-hormone-releasing hormone in patients with hypogonadotropic hypogonadism. N Engl J Med. 1975;292:242–245. doi: 10.1056/NEJM197501302920505. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman AR, Crowley WF., Jr Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med. 1982;307:1237–1241. doi: 10.1056/NEJM198211113072003. [DOI] [PubMed] [Google Scholar]
- 7.Reitano JF, Caminos-Torres R, Snyder PJ. Serum LH and FSH responses to the repetitive administration of gonadotropin-releasing hormone in patients with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1975;41:1035–1042. doi: 10.1210/jcem-41-6-1035. [DOI] [PubMed] [Google Scholar]
- 8.Snyder PJ, Rudenstein RS, Gardner DF, Rothman JG. Repetitive infusion of gonadotropin-releasing hormone distinguishes hypothalamic from pituitary hypogonadism. J Clin Endocrinol Metab. 1979;48:864–868. doi: 10.1210/jcem-48-5-864. [DOI] [PubMed] [Google Scholar]
- 9.Menon PS, Khatwa UA. The child with micropenis. Indian J Pediatr. 2000;67:455–460. doi: 10.1007/BF02859468. [DOI] [PubMed] [Google Scholar]
- 10.Bistritzer T, Lunenfeld B, Passwell JH, Theodor R. Hormonal therapy and pubertal development in boys with selective hypogonadotropic hypogonadism. Fertil Steril. 1989;52:302–306. doi: 10.1016/s0015-0282(16)60859-2. [DOI] [PubMed] [Google Scholar]
- 11.Schopohl J, Mehltretter G, von Zumbusch R, Eversmann T, von Werder K. Comparison of gonadotropin-releasing hormone and gonadotropin therapy in male patients with idiopathic hypothalamic hypogonadism. Fertil Steril. 1991;56:1143–1150. [PubMed] [Google Scholar]
- 12.Burris AS, Rodbard HW, Winters SJ, Sherins RJ. Gonadotropin therapy in men with isolated hypogonadotropic hypogonadism: the response to human chorionic gonadotropin is predicted by initial testicular size. J Clin Endocrinol Metab. 1988;66:1144–1151. doi: 10.1210/jcem-66-6-1144. [DOI] [PubMed] [Google Scholar]
- 13.Ley SB, Leonard JM. Male hypogonadotropic hypogonadism: factors influencing response to human chorionic gonadotropin and human menopausal gonadotropin, including prior exogenous androgens. J Clin Endocrinol Metab. 1985;61:746–752. doi: 10.1210/jcem-61-4-746. [DOI] [PubMed] [Google Scholar]
- 14.Van Wagenen G, Simpson ME. Embryology of the ovary and testis, Homo sapiens and Macaca mulatta. New Haven: Yale University Press; 1965. [Google Scholar]
- 15.Zachmann M, Prader A, Kind HP, Häfliger H, Budliger H. Testicular volume during adolescence. Cross-sectional and longitudinal studies. Helv Paediatr Acta. 1974;29:61–72. [PubMed] [Google Scholar]
- 16.Waaler PE, Thorsen T, Stoa KF, Aarskog D. Studies in normal male puberty. Acta Paediatr Scand Suppl. 1974;249:1–36. doi: 10.1111/j.1651-2227.1974.tb07587.x. [DOI] [PubMed] [Google Scholar]