Abstract
Non-faecalis and non-faecium enterococci are an occasional cause of bacteremia, and some cases of infective endocarditis caused by these pathogens have been reported. However, the rate of infective endocarditis in non-faecalis and non-faecium enterococcal bacteremia is still undetermined. We compared the clinical features and the rate of infective endocarditis of 70 cases of non-faecalis and non-faecium enterococcal bacteremia with those of 65 cases of Enterococcus faecalis bacteremia. Non-faecalis and non-faecium enterococcal bacteremia was more frequently associated with biliary tract infection and polymicrobial bacteremia, and was less frequently associated with infective endocarditis, than was E. faecalis bacteremia (57% vs. 28%, p<0.01; 47% vs. 31%, p=0.05; 1% vs. 14%, p<0.01, respectively).
Keywords: Enterococci, Bacteremia, vanC, Endocarditis
INTRODUCTION
Enterococcus faecalis and Enterococcus faecium are significant human pathogens. Hence, the clinical features and outcomes of infections caused by these organisms have been well described.1,2 However, few clinical studies have been conducted on infections caused by non-faecalis and non-faecium enterococci such as Enterococcus avium, Enterococcus hirae, Enterococcus durans, Enterococcus gallinarum, Enterococcus casseliflavus, and Enterococcus flavescens,3-6 although these are also encountered as significant human pathogens. Among the non-faecalis and non-faecium enterococci, E. gallinarum, E. casseliflavus, and E. flavescens possessing the vanC gene are characterized by motility and intrinsic low-level resistance to vancomycin (the VanC phenotype). These species have caused concern, because treatment failure or inducible vancomycin resistance is possible during vancomycin therapy.3,5,6
Enterococci are the third common etiologic agent of infective endocarditis, accounting for 11% of cases.7 E. faecalis is a common cause of infective endocarditis, and >3% of cases of E. faecalis bacteremia have infective endocarditis. In contrast, only <1% of cases of E. faecium bacteremia have infective endocarditis. Some cases of infective endocarditis caused by non-faecalis and non-faecium enterococci have also been reported.8-13 However, the rate of infective endocarditis in non-faecalis and non-faecium enterococcal bacteremia is still undetermined.
The aim of this study was to compare the clinical features and the rate of infective endocarditis of bacteremia due to non-faecalis and non-faecium enterococci with those of E. faecalis bacteremia.
MATERIALS AND METHODS
1. Patients
The electronic medical records of all patients with positive blood cultures for enterococci between January 1999 and August 2003 at Seoul National University Hospital (Seoul, Republic of Korea) were retrospectively reviewed. Underlying diseases, the primary site of bacteremia, comorbid or predisposing conditions, antibiotic resistance, and treatment outcomes in patients with non-faecalis and non-faecium enterococcal bacteremia were compared with those in patients with E. faecalis bacteremia. Appropriate antibiotic treatment was defined as the use of one or more active antibiotics to which the organism was susceptible in vitro within 5 days of the date on which a positive blood culture was obtained.14 Antibiotics considered active included penicillin, ampicillin, piperacillin, vancomycin, teicoplanin, quinupristin-dalfopristin, and linezolid.15
2. Microbiological tests
Enterococcus species were identified on the basis of 6.5% NaCl tolerance, bile-esculin hydrolysis, and growth rate at 45℃. Species were identified from the results obtained with the Vitek system (bioMérieux, Marcy l'Etoile, France) and by tests for motility, yellow pigmentation, and methyl-α-D-glucopyranoside.16,17 Antibiotic susceptibilities were determined by the disk diffusion method, following the recommendations of the Clinical and Laboratory Standards Institute.18 The vancomycin and teicoplanin minimal inhibitory concentration (MIC) was determined by Etest® (AB BIODISK, Solna, Sweden) according to the manufacturer's manual. VanC phenotype enterococci were defined as enterococcal isolates with intrinsic low-level resistance to vancomycin (MICs 2-32 µg/ml) and susceptibility to teicoplanin.19
3. Statistical analysis
Categorical variables were compared by using the Fisher's exact test or the Pearson χ2 test, as appropriate, and continuous variables were compared by using the Mann-Whitney test or Student's t test. All tests of significance were 2-tailed, and p≤0.05 was considered to be significant. Statistical analyses of the data were performed by using SPSS for Windows (ver. 12.0; SPSS Inc., Chicago, IL).
RESULTS
1. Enterococcus species in blood isolates
We identified 292 cases with enterococcal bacteremia during the study period. One hundred fifty (51.4%) were caused by E. faecium and 65 (22.3%) by E. faecalis. Seventy (24.0%) were caused by non-faecalis and non-faecium enterococci; 23 (7.8%) by E. avium, 20 (6.8%) by E. gallinarum, 19 (6.5%) by E. casseliflavus, 7 (2.4%) by E. hirae, and 1 (0.3%) by E. durans. The species responsible for seven (2.4%) isolates could not be identified. The patients from whom the isolates could not be identified were excluded from the analysis. None of patients with non-faecalis and non-faecium enterococcal bacteremia were clustered in time or place of occurrence.
2. Clinical features and the rates of infective endocarditis in non-faecalis and non-faecium enterococcal bacteremia
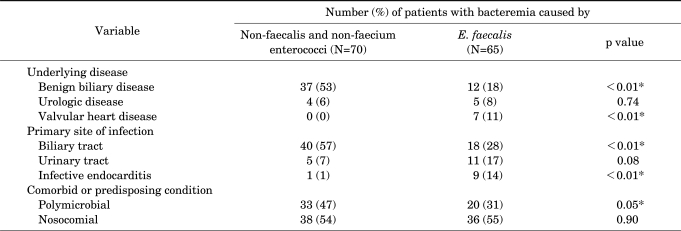
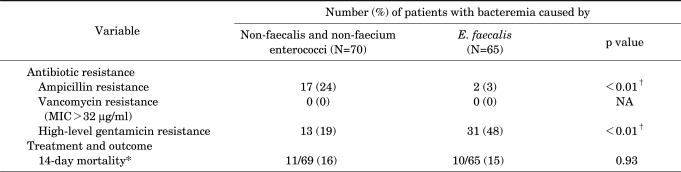
The clinical features of 135 patients with bacteremia caused by non-faecalis and non-faecium enterococci or E. faecalis are shown in Table 1. Compared with cases of E. faecalis bacteremia, underlying biliary disease and biliary tract infections were significantly more common in cases of non-faecalis and non-faecium enterococcal bacteremia. Polymicrobial bacteremia was also more common in cases of non-faecalis and non-faecium enterococcal bacteremia. E. coli (n=11), Pseudomonas species (n=6), and Klebsiella species (n=4) were the predominant blood co-isolates from cases of non-faecalis and non-faecium enterococci. Valvular heart disease and infective endocarditis were significantly less common in non-faecalis and non-faecium enterococcal bacteremia than in E. faecalis bacteremia (Table 1). However, 14-day mortality was not significantly different between patients with non-faecalis and non-faecium enterococcal bacteremia and patients with E. faecalis bacteremia (16% vs. 15%, p=0.93; Table 2).
TABLE 1.
Clinical features of 135 patients with bacteremia caused by non-faecalis and non-faecium enterococci or E. faecalis
*Statistically significant, p≤0.05.
TABLE 2.
Antibiotic resistance and outcome of 135 patients with bacteremia caused by non-faecalis and non-faecium enterococci or E. faecalis
*Expressed as number of deaths/number of patients followed up (%). †Statistically significant, p≤0.05. MIC: minimal inhibitory concentration, NA: not applicable.
3. Clinical features of patients with vanC phenotype enterococci
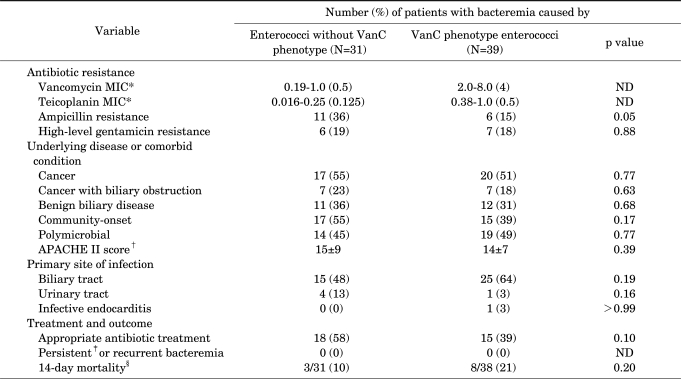
We compared the clinical features, outcomes, and microbiological data for bacteremia caused by VanC phenotype enterococci with those of bacteremia caused by non-faecalis and non-faecium enterococci without the VanC phenotype (Table 3). Biliary tract infection was the most common infection site in both groups (48% vs. 64%). No significant differences were apparent in underlying disease, infection site, or other clinical characteristics between these two groups. There was also no significant difference in outcome. Although five of the patients with bacteremia caused by VanC phenotype enterococci had undergone vancomycin therapy, no breakthrough or recurrent bacteremia was observed.
TABLE 3.
Clinical features of 70 patients with non-faecalis and non-faecium enterococcal bacteremia according to presence of the VanC phenotype
*Expressed as range (median) µg/ml. †Expressed as mean (±SD). ‡Persistent bacteremia was defined as the isolation of enterococci in blood cultures obtained from peripheral veins on ≥5 consecutive days despite appropriate antibiotic administration. §Expressed as number of deaths/number of patients followed up (%). APACHE: acute physiology and chronic health evaluation, MIC: minimal inhibitory concentration, ND: not done.
DISCUSSION
In this study, we showed that cases of non-faecalis and non-faecium enterococcal bacteremia were more likely to have biliary tract infection and polymicrobial bacteremia and were less likely to have infective endocarditis than were cases of E. faecalis bacteremia.
In the present study, biliary tract infection was significantly more common in non-faecalis and non-faecium enterococcal bacteremia than in E. faecalis bacteremia, in agreement with previous studies.2,3 The proportion of polymicrobial bacteremia (47%) in our study was similar to the findings of previous studies: 44.6% to 50%.2,4,5
The anatomical site of infection should be taken into account in treating enterococcal infection, because the appropriate treatment strategy differs for different sites. In cases of endocarditis or meningitis, combination therapy with a cell-wall active agent plus an aminoglycoside should be used.1,20 However, in cases of enterococcal bacteremia without endocarditis or meningitis, there was no statistically significant difference in outcome between monotherapy and combination therapy in a number of studies.21-25
It has been described that infective endocarditis is significantly more common in E. faecalis bacteremia than in E. faecium bacteremia.26 Previous reports showed that non-faecalis and non-faecium enterococci also have sufficient virulence to cause infective endocarditis on native heart valves in patients without predisposing valvular heart diseases.9-12 However, the rate of infective endocarditis in non-faecalis and non-faecium enterococcal bacteremia had never been evaluated. In our study, we demonstrated for the first time that the rate of infective endocarditis in non-faecalis and non-faecium enterococcal bacteremia was only about 1%, and it was significantly lower than that in E. faecalis bacteremia. Our data suggest that routine echocardiography tests or aminoglycoside combination therapy is not necessary in patients with non-faecalis and non-faecium enterococcal bacteremia, unless the patient has suspicious signs of infective endocarditis such as persistent bacteremia, predisposing heart conditions, cardiac murmur, or metastatic infection.
Some workers have suggested that bacteremia due to VanC phenotype enterococci is associated with a low risk of mortality2; however, no comparative study was ever performed on the matter. In our comparative study, however, we found that the mortality in patients with bacteremia caused by VanC phenotype enterococci was not lower than that in patients with bacteremia caused by non-VanC non-faecalis and non-faecium enterococci or E. faecalis.
VanC is known to be chromosomally encoded and constitutively expressed. However, the VanC phenotype may be inducible in some strains.27,28 Treatment failure and breakthrough bacteremia during vancomycin therapy have been reported even though the isolates were of the VanC phenotype susceptible to vancomycin.4,5 However, this phenomenon was not observed in our study. The low-level vancomycin resistance of these organisms had little influence on treatment outcomes in our study. Some studies reported high rates of vancomycin resistance (MIC >32 µg/ml) in clinical isolates of VanC phenotype enterococci,2,3 whereas others did not.4,5 In our study, vancomycin resistance was not observed in the VanC phenotype enterococci, which is consistent with the latter studies.
The present data have some limitations. First, we used only biochemical methods, not a molecular method for the species level identification of enterococci. Seven (2.4%) of the isolates were not species identified, and therefore infections caused by these isolates were not included in the analysis. Second, this was a single-center study and factors such as the rate of referrals or surgery for complex disorders could have impacted the results.
In conclusion, compared with patients with E. faecalis bacteremia, patients with non-faecalis and non-faecium enterococcal bacteremia were more likely to have biliary tract infection and polymicrobial bacteremia and were less likely to have infective endocarditis. The outcome of non-faecalis and non-faecium enterococcal bacteremia was not different from that of E. faecalis bacteremia. VanC phenotype did not affect mortality in patients with enterococcal bacteremia.
ACKNOWLEDGEMENTS
We express our gratitude to the Medical Research Collaborating Center (MRCC) of Seoul National University Hospital for statistical review and consultation.
References
- 1.Murray BE. The life and times of the Enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noskin GA, Peterson LR, Warren JR. Enterococcus faecium and Enterococcus faecalis bacteremia: acquisition and outcome. Clin Infect Dis. 1995;20:296–301. doi: 10.1093/clinids/20.2.296. [DOI] [PubMed] [Google Scholar]
- 3.Choi SH, Lee SO, Kim TH, Chung JW, Choo EJ, Kwak YG, et al. Clinical features and outcomes of bacteremia caused by Enterococcus casseliflavus and Enterococcus gallinarum: analysis of 56 cases. Clin Infect Dis. 2004;38:53–61. doi: 10.1086/380452. [DOI] [PubMed] [Google Scholar]
- 4.de Perio MA, Yarnold PR, Warren J, Noskin GA. Risk factors and outcomes associated with non-Enterococcus faecalis, non-Enterococcus faecium enterococcal bacteremia. Infect Control Hosp Epidemiol. 2006;27:28–33. doi: 10.1086/500000. [DOI] [PubMed] [Google Scholar]
- 5.Ratanasuwan W, Iwen PC, Hinrichs SH, Rupp ME. Bacteremia due to motile Enterococcus species: clinical features and outcomes. Clin Infect Dis. 1999;28:1175–1177. doi: 10.1086/517774. [DOI] [PubMed] [Google Scholar]
- 6.Reid KC, Cockerill FR, III, Patel R. Clinical and epidemiological features of Enterococcus casseliflavus/flavescens and Enterococcus gallinarum bacteremia: a report of 20 cases. Clin Infect Dis. 2001;32:1540–1546. doi: 10.1086/320542. [DOI] [PubMed] [Google Scholar]
- 7.Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG, Jr, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talarmin JP, Pineau S, Guillouzouic A, Boutoille D, Giraudeau C, Reynaud A, et al. Relapse of Enterococcus hirae prosthetic valve endocarditis. J Clin Microbiol. 2011;49:1182–1184. doi: 10.1128/JCM.02049-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poyart C, Lambert T, Morand P, Abassade P, Quesne G, Baudouy Y, et al. Native valve endocarditis due to Enterococcus hirae. J Clin Microbiol. 2002;40:2689–2690. doi: 10.1128/JCM.40.7.2689-2690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortu M, Gabrielli E, Caramma I, Rossotti R, Gambirasio M, Gervasoni C. Enterococcus gallinarum endocarditis in a diabetic patient. Diabetes Res Clin Pract. 2008;81:e18–e20. doi: 10.1016/j.diabres.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Dargere S, Vergnaud M, Verdon R, Saloux E, Le Page O, Leclercq R, et al. Enterococcus gallinarum endocarditis occurring on native heart valves. J Clin Microbiol. 2002;40:2308–2310. doi: 10.1128/JCM.40.6.2308-2310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stepanović S, Jovanović M, Lavadinović L, Stosović B, Pelemis M. Enterococcus durans endocarditis in a patient with transposition of the great vessels. J Med Microbiol. 2004;53:259–261. doi: 10.1099/jmm.0.05382-0. [DOI] [PubMed] [Google Scholar]
- 13.Mirzoyev Z, Anavekar N, Wilson F, Uslan D, Baddour L, Mookadam F. Enterococcus avium endocarditis. Scand J Infect Dis. 2004;36:876–878. doi: 10.1080/00365540410024754. [DOI] [PubMed] [Google Scholar]
- 14.Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585–590. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 15.Vergis EN, Hayden MK, Chow JW, Snydman DR, Zervos MJ, Linden PK, et al. Determinants of vancomycin resistance and mortality rates in enterococcal bacteremia. a prospective multicenter study. Ann Intern Med. 2001;135:484–492. doi: 10.7326/0003-4819-135-7-200110020-00007. [DOI] [PubMed] [Google Scholar]
- 16.Devriese LA, Pot B, Kersters K, Lauwers S, Haesebrouck F. Acidification of methyl-alpha-D-glucopyranoside: a useful test to differentiate Enterococcus casseliflavus and Enterococcus gallinarum from Enterococcus faecium species group and from Enterococcus faecalis. J Clin Microbiol. 1996;34:2607–2608. doi: 10.1128/jcm.34.10.2607-2608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Facklam RR, Collins MD. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989;27:731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing; eighteenth informational supplement. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI); 2008. p. M100-S18. [Google Scholar]
- 19.Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis. 2006;42(Suppl 1):S25–S34. doi: 10.1086/491711. [DOI] [PubMed] [Google Scholar]
- 20.Elliott TS, Foweraker J, Gould FK, Perry JD, Sandoe JA Working Party of the British Society for Antimicrobial Chemotherapy. Guidelines for the antibiotic treatment of endocarditis in adults: report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother. 2004;54:971–981. doi: 10.1093/jac/dkh474. [DOI] [PubMed] [Google Scholar]
- 21.Watanakunakorn C, Patel R. Comparison of patients with enterococcal bacteremia due to strains with and without high-level resistance to gentamicin. Clin Infect Dis. 1993;17:74–78. doi: 10.1093/clinids/17.1.74. [DOI] [PubMed] [Google Scholar]
- 22.Graninger W, Ragette R. Nosocomial bacteremia due to Enterococcus faecalis without endocarditis. Clin Infect Dis. 1992;15:49–57. doi: 10.1093/clinids/15.1.49. [DOI] [PubMed] [Google Scholar]
- 23.Gullberg RM, Homann SR, Phair JP. Enterococcal bacteremia: analysis of 75 episodes. Rev Infect Dis. 1989;11:74–85. doi: 10.1093/clinids/11.1.74. [DOI] [PubMed] [Google Scholar]
- 24.Maki DG, Agger WA. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine (Baltimore) 1988;67:248–269. [PubMed] [Google Scholar]
- 25.Jang HC, Lee S, Song KH, Jeon JH, Park WB, Park SW, et al. Clinical features, risk factors and outcomes of bacteremia due to enterococci with high-level gentamicin resistance: comparison with bacteremia due to enterococci without high-level gentamicin resistance. J Korean Med Sci. 2010;25:3–8. doi: 10.3346/jkms.2010.25.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson DJ, Murdoch DR, Sexton DJ, Reller LB, Stout JE, Cabell CH, et al. Risk factors for infective endocarditis in patients with enterococcal bacteremia: a case-control study. Infection. 2004;32:72–77. doi: 10.1007/s15010-004-2036-1. [DOI] [PubMed] [Google Scholar]
- 27.Billot-Klein D, Gutmann L, Sablé S, Guittet E, van Heijenoort J. Modification of peptidoglycan precursors is a common feature of the low-level vancomycin-resistant VANB-type Enterococcus D366 and of the naturally glycopeptide-resistant species Lactobacillus casei, Pediococcus pentosaceus, Leuconostoc mesenteroides, and Enterococcus gallinarum. J Bacteriol. 1994;176:2398–2405. doi: 10.1128/jb.176.8.2398-2405.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahm DF, Free L, Handwerger S. Inducible and constitutive expression of vanC-1-encoded resistance to vancomycin in Enterococcus gallinarum. Antimicrob Agents Chemother. 1995;39:1480–1484. doi: 10.1128/aac.39.7.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]