Abstract
Background/Aims
A phase II study of the peroxisome proliferator-activated receptor-γ agonist rosiglitazone extended release (RSG XR) in mild-to-moderate Alzheimer's disease (AD) detected a treatment benefit to cognition in apolipoprotein E (APOE)-∊4-negative subjects. The current phase III study with prospective stratification by APOE genotype was conducted to confirm the efficacy and safety of RSG XR in mild-to-moderate AD. An open-label extension study assessed the long-term safety and tolerability of 8 mg RSG XR.
Methods
This double-blind, randomized, placebo-controlled study enrolled 693 subjects. Within 2 APOE allelic strata (∊4-positive, ∊4-negative), subjects were randomized (2:2:2:1) to once-daily placebo, 2 mg RSG XR, 8 mg RSG XR or 10 mg donepezil (control). Coprimary endpoints were change from baseline to week 24 in the Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-Cog) score, and week 24 Clinician's Interview-Based Impression of Change plus caregiver input (CIBIC+).
Results
At week 24, no significant differences from placebo in change from baseline in coprimary endpoints were detected with either the RSG XR dose in APOE-∊4-negative subjects or overall. For donepezil, no significant treatment difference was detected in ADAS-Cog; however, a significant difference was detected (p = 0.009) on the CIBIC+. Peripheral edema was the most common adverse event for 8 mg RSG XR (15%) and placebo (5%), and nasopharyngitis for 2 mg RSG XR (7%).
Conclusion
No evidence of efficacy of 2 mg or 8 mg RSG XR monotherapy in cognition or global function was detected in the APOE-∊4-negative or other analysis populations. The safety and tolerability of RSG XR was consistent with its known pharmacology.
Key Words: Rosiglitazone, extended release; Monotherapy; Alzheimer's disease; Peroxisome proliferator-activated receptor-γ; Cognition; Apolipoprotein E allele ∊4; Health outcomes; Donepezil
Introduction
Alzheimer's disease (AD) is a progressive neurological condition characterized by deterioration of memory and cognition, progressive impairment of functional capacity, and various behavioral disturbances [1]. Insulin resistance is a potential underlying mechanism of metabolic pathogenesis in AD [2,3,4]. Presence of diabetes or the metabolic syndrome is associated with an increased risk for AD [5,6,7]. Cognitive impairment has been associated with biochemical and clinical features of insulin resistance syndrome [8]. Furthermore, higher insulin resistance has been demonstrated to predict subsequent cognitive impairment [9]. Hippocampal volumes were smaller in diabetic patients and those with increased insulin resistance than in normal healthy controls [10]. Insulin administration reduces neuronal accumulation of β-amyloid peptides and synaptic binding of toxic β-amyloid-derived diffusible ligands in cell culture [11]; it improves cognitive performance in rodents [12] and reportedly in patients with early AD [13,14]. These data support the development of therapeutics that target insulin pathways as potential treatments for AD.
Rosiglitazone (RSG) is an agonist of the peroxisome proliferator-activated receptor-γ (PPAR-γ) that increases glucose sensitivity, regulates lipid metabolism and promotes mitochondrial biogenesis [15,16]. PPAR-γ agonists also exhibit robust antiinflammatory actions via their ability to suppress NF-κB-dependent gene expression [17,18]. AD is typified by impaired glucose utilization in the brain and a glial-mediated inflammatory response, suggesting the utility of these agents in the treatment of AD [3,17,18]. Studies in murine models of AD demonstrated that RSG lowers amyloid plaque burden, reduces vascular and plaque-associated inflammation, attenuates loss of synaptic connectivity, and improves memory and cognition [17,19,20,21]. A pilot study of RSG found that treatment of patients with mild-to-moderate AD improved cognition, and a large phase II trial detected a beneficial effect on cognition in apolipoprotein-∊4 (APOE-∊4)-negative individuals with mild-to-moderate probable AD [22,23].
To confirm the potential efficacy and safety of RSG extended release (XR) as monotherapy in AD, a 24-week, randomized, double-blind, placebo-controlled phase III study of RSG XR was conducted in subjects with mild-to-moderate probable AD (REFLECT-1; AVA105640; NCT00428090). Efficacy and tolerability results are presented here together with safety data from an open-label extension study (REFLECT-5; AVA102677; NCT00550420) that included subjects who completed REFLECT-1.
Subjects and Methods
Study Design
REFLECT-1 was a 24-week, double-blind, double-dummy, randomized, parallel-group phase III study carried out at 134 centers in 19 countries (Austria, Bulgaria, Chile, China, Croatia, Estonia, Germany, Greece, Hungary, Mexico, New Zealand, Pakistan, Peru, Republic of the Philippines, Puerto Rico, Republic of Korea, Russian Federation, UK and USA) between February 27, 2007 and September 5, 2008. REFLECT-5 was a 52-week, open-label extension of REFLECT-1 initiated in October 2007 and terminated in February 2009 due to the lack of a significant efficacy of RSG XR in REFLECT-1.
Both studies were conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. Local institutional review boards or independent ethics committees approved the protocols. The caregiver and subject (or legal representative) provided full, written, informed consent prior to study screening.
Subject Population
In REFLECT-1, individuals between 50 and 90 years of age diagnosed with probable AD in accordance with National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer's Disease and Related Disorders Association [24] criteria for ≥3 months were eligible to enroll in AVA105640. Subjects were required to have a Mini-Mental State Examination (MMSE) [25] score of 10–23 at screening. Subjects were excluded from participation if they had: (1) possible, probable or definite vascular dementia according to National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria [26]; (2) a history or evidence of another cause of their dementia; (3) a history of seizures; (4) cardiovascular events in the last 6 months; (5) a significant psychiatric illness; (6) type 1 diabetes mellitus (DM); (7) type 2 DM (T2DM) being treated with insulin, a PPAR-γ agonist or an insulin secretagogue (e.g. a sulfonylurea or glitinide), or (8) any other clinically significant coexisting medical conditions or laboratory abnormalities.
Concomitant medications were permitted except for thiazolidinediones, PPAR-γ agonists, insulin, sulfonylureas or other insulin secretagogues (e.g. glitinides), agents with incretin effects (glucagon-like peptide-1 analogs and dipeptidyl peptidase IV inhibitors), cholinesterase inhibitors (ChEI), memantine, selegiline, conventional antipsychotic medications, barbiturates, monoamine oxidase inhibitors, benzodiazepines with half-lifes of ≥6 h taken as needed (long-term benzodiazepine use was allowed if taken at a stable dose for >2 months before screening), gemfibrozil, rifampicin, ketoconazole and trimethoprim. Antidepressants (other than monoamine oxidase inhibitors), vitamin E, Ginkgo biloba, statins, estrogen, thyroid hormones, atypical antipsychotics and chronic use of nonsteroidal antiinflammatory drugs were allowed if prescribed at a stable dose for >2 months prior to screening.
Protocol
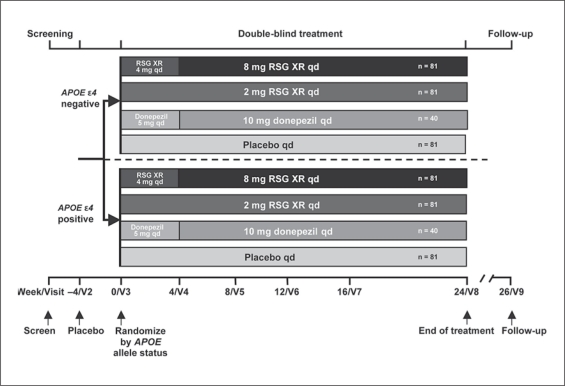
The planned duration of participation in REFLECT-1 was 32 weeks including a 2-week screening phase, a 4-week placebo single-blind run-in phase, a 24-week double-blind treatment phase, and a follow-up visit 2 weeks after the last treatment phase visit (fig. 1). Genetic assessment to determine APOE ∊4 status, as described previously [23], was mandatory for randomization. The subjects received placebo once daily (q.d.) during the 4-week run-in period. At baseline, they were stratified based on APOE status(∊4-positive: 1 or 2 copies of the ∊4 allele;∊4-negative: no copies) and then randomly assigned within each stratum in a 2:2:2:1 ratio to receive placebo, 8 mg RSG XR, 2 mg RSG XR or 10 mg donepezil, respectively. The permuted-block randomization schedule with stratification for APOE ∊4 status was generated by GlaxoSmithKline. Subjects in the 8-mg RSG XR group received RSG XR 4 mg once daily for the first 4 weeks, and 8 mg once daily for the remainder of the study. The subjects in the donepezil group received 5 mg once daily for the first 4 weeks, and 10 mg once daily for the remainder of the study.
Fig. 1.
Study design.
Subjects who completed the week 24 visit were eligible to enter the open-label extension study with protocol procedures to begin immediately after the week 24 visit. Subjects who did not wish to enter the extension study had a follow-up visit at week 26.
Subjects who enrolled in the extension study (REFLECT-5) received RSG XR 4 mg once daily for the first 4 weeks, followed by 8 mg RSG XR once daily for the remainder of their participation. Clinic visits occurred at weeks 4, 8, 12, 16, 24, 36 and 52 during the first year and, with annual renewal of consent, were to occur at weeks 12, 24, 36 and 52 during each year thereafter.
Assessments in REFLECT-1
Efficacy. The primary efficacy measures were the Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-Cog) to assess cognition [27] and the Clinician's Interview-Based Impression of Change plus caregiver input (CIBIC+) score to assess global function [28]. Assessments were performed by trained and experienced independent raters. Secondary measures included: (1) the Neuropsychiatric Inventory (NPI) [29] to assess behavioral and psychological symptoms of dementia; (2) the Disability Assessment for Dementia (DAD) scale [30] to assess the ability to perform activities of daily living; (3) the MMSE to assess cognitive status; (4) Short-Term Memory Assessment scores (questions 1 and 7 from the ADAS-Cog), and (5) glycated hemoglobin (HbA1c).
Safety and Tolerability. The safety assessment included physical examinations, clinical laboratory evaluations (hematology, chemistry and urinalysis), vital signs and weight measurements, electrocardiogram (ECG) measures, and adverse events (AE). During the study, the protocols were amended to include additional safety measures for the subjects who experienced cardiovascular events. These aligned with the concurrent changes to the Avandia® label that included additional safety warnings and precautions for subjects who experience cardiovascular events [31].
Health Outcomes. Health outcome measures included: (1) change from baseline in Alzheimer's Carers’ Quality of Life Instrument (ACQLI) score [32]; (2) the Resource Utilization in Dementia instrument [33], and (3) the European Quality of Life 5 Dimensions (EQ-5D) proxy [34] to assess the subject's overall health and impairment status.
Assessments in REFLECT-5
The efficacy and safety assessments during the extension study were the same as those in REFLECT-1.
Analytical Methods in REFLECT-1
The primary efficacy endpoints were change from baseline (week 0) in the ADAS-Cog total at week 24 and the week 24 CIBIC+ score. Secondary efficacy endpoints were change from baseline in the NPI, the DAD scale, MMSE total score, the Short-Term Memory Assessment score, HbA1c and health outcome measures at week 24, and change from baseline in the ADAS-Cog and CIBIC+ scores at weeks 8, 16 and 24 (observed cases).
The planned total sample size of 566 enrolled subjects comprised 162 subjects in the 8-mg RSG XR, 2-mg RSG XR and placebo treatment groups (81 per APOE stratum) and 80 subjects on donepezil (40 per APOE stratum). The sample size was selected to allow detection of a 3-point treatment difference in change from baseline in ADAS-Cog score for either dose of RSG versus placebo in the APOE-∊4-negative stratum (88% power, assuming an SD of 5.74 and 10% dropout prior to the first postbaseline efficacy assessment), and a 0.5-point treatment difference in CIBIC+ score (80% power assuming an SD of 1.07 and 10% dropout prior to the first postbaseline efficacy assessment). The SD were based on data observed in the previous phase II study [23]. Donepezil, the most widely used of the ChEI in the treatment of AD, was used as an active control for assay sensitivity in this study. There was no expectation that the effect of donepezil would depend on the APOE ∊4 allele status; thus, a smaller number of subjects were enrolled in this group.
The full safety population included all randomized subjects who took at least 1 dose of the study drug, and it was used for evaluation of safety. The full intent-to-treat (ITT) population included those subjects in the full safety population who had at least 1 postbaseline ADAS-Cog or CIBIC+ assessment, and this population was used for efficacy analysis.
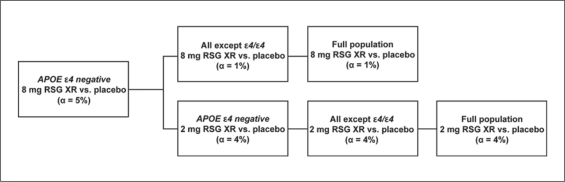
To control for family-wise type I error associated with testing of the primary endpoints for the 2 RSG XR doses in the full ITT population and 2 genetic subgroups, a testing hierarchy was employed (fig. 2). At each step of the hierarchy, if statistically significant results were observed at the specified α levels for both primary endpoints, inferential testing continued. Inferential testing stopped if statistical significance was not achieved; subsequent analyses were conducted but were considered exploratory in nature.
Fig. 2.
Primary endpoint testing hierarchy.
The primary method of analysis was mixed models for repeated measures (MMRM) [35]. The within-subject correlation over time was modeled using an unstructured covariance matrix to provide estimates of the week 24 treatment differences under the assumption that missing data occurred at random (mechanism for missingness not related to unobserved responses). Sensitivity analyses were conducted to assess the impact of missing data on study conclusions. In addition to an analysis of week 24 observed cases and last observation carried forward datasets, several scenarios for alternative missing data patterns were constructed using multiple imputations. The missing responses were imputed based on modeling of the time trends observed in the placebo arm of the trial or by inducing decline after time of withdrawal based on estimates of potential progression of AD in untreated patients (means of 1.5 and 0.28 points over 6 months for ADAS-Cog and CIBIC+ scores, respectively) [36].
Post hoc analyses were conducted to evaluate the impact of baseline glucose levels, HbA1c levels and MMSE score (mild AD defined as MMSE score of >18 points, moderate AD as MMSE score of ≤18 points) on the ADAS-Cog total scores at week 24.
For safety analyses, the on-treatment period for AE was defined as the time between the first day of study drug administration in the double-blind phase up to 1 day after the last dose of study drug. AE of special interest (AESI) were based on the pharmacologic class of RSG and included myocardial ischemia, congestive heart failure, edema, hepatic disorders, bone fractures, anemia, dyslipidemia, cerebrovascular events, hypoglycemia, neoplasms, weight gain, peripheral vascular disease, macular edema, retinopathy and parotid or salivary gland disorders. Clinical laboratory values that were identified as being of potential clinical concern were both outside of the reference range and met a change from baseline criterion prospectively defined for each parameter.
Analytical Methods in REFLECT-5
All enrolled subjects were included in the safety population. The on-treatment period for AE was defined as the time between the first day of open-label study drug administration until 1 day after the last dose of open-label study drug.
Results
Subject Disposition
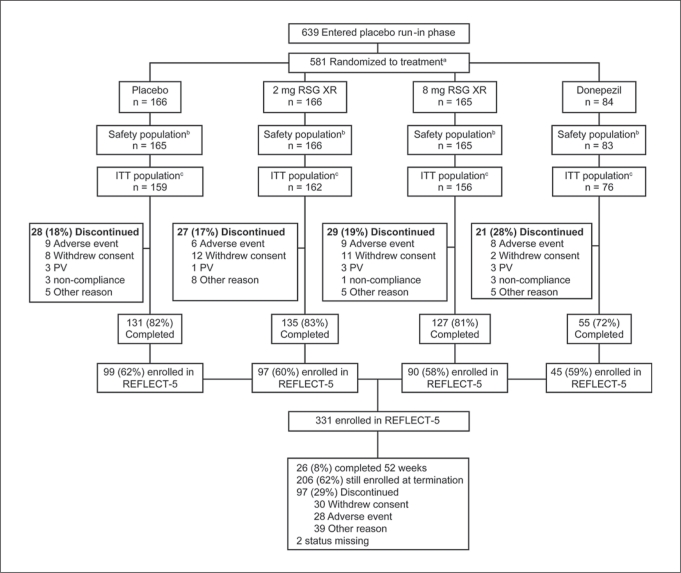
A total of 639 subjects entered the placebo run-in phase. Since 58 subjects withdrew during the placebo run-in period, 581 subjects were randomized to treatment (fig. 3). One subject in the placebo group and 1 in the donepezil group took no study medication; therefore, the safety population comprised 579 subjects. The ITT population comprised 553 subjects.
Fig. 3.
Subject disposition. PV = Protocol violation. a58 subjects failed run-in. bTwo randomized subjects (1 in the placebo and 1 in the donepezil group) did not take the study drug. c6 subjects in the placebo group, 4 in the 2-mg group, 9 in the 8-mg group, and 7 in the donepezil group had no postbaseline efficacy data.
The majority of randomized subjects in the placebo and RSG XR groups (81–83%) completed the study; 72% in the donepezil group completed (fig. 1). The most common reasons for withdrawal among all ITT subjects were ‘subject withdrew consent’ and ‘AE’ in the placebo and RSG XR treatment groups, and ‘AE’ in the donepezil group. Reasons for withdrawal were comparable in the APOE ∊4 analysis subgroups.
In the full ITT population, 84–90% of subjects across treatment groups were compliant, i.e. took >80% and <120% of the study medication with no interruption of >7 days. Compliance was highest in the 8-mg and 2-mg RSG XR groups (90% and 88%, respectively) and lowest in the donepezil group (84%). The results were comparable in the APOE-∊4-negative subgroups across treatment groups.
Of the 448 subjects who completed the REFLECT-1 study, 331 (74%) enrolled in the REFLECT-5 extension study (fig. 3). Among this group, 97 subjects (29%) withdrew; the most frequent reasons for withdrawal were ‘subject withdrew consent’ (9%) and ‘AE’ (8%). The median duration ± SD of exposure to RSG XR at the time of study termination was 206 ± 103 days. For 12 ± 4% of the subjects, the daily dose was reduced to 2 mg RSG XR for tolerability.
Baseline Characteristics
For REFLECT-1, the baseline demographics and disease characteristics in the full ITT population are listed in table 1. The majority of subjects were female and white. Asian subjects represented a higher proportion (31%) of the subjects in the 2-mg RSG XR group compared with the other groups (18–24%). The baseline AD characteristics were consistent with those expected in a population of individuals with mild-to-moderate AD. Approximately half of the subjects were APOE ∊4 negative. The baseline characteristics of the patients who withdrew from REFLECT-1 are listed in table 2.
Table 1.
Baseline demographics and disease characteristics (full ITT population)
| Placebo (n=159) | 2 mg RSG XR (n=162) | 8 mg RSG XR (n = 156) | 10 mg donepezil (n = 76) | |
|---|---|---|---|---|
| Age, years | ||||
| Mean ± SD | 72.5±8.56 | 71.7±7.91 | 72.6±8.63 | 72.9±7.97 |
| Range | 50–88 | 51–87 | 50–91 | 52–85 |
| Sex (female:male), % | 60:40 | 64:36 | 65:35 | 63:37 |
| Race, % | ||||
| African American/African heritage | <1 | <1 | 1 | 4 |
| American Indian or Alaskan Native | <1 | <1 | 1 | 0 |
| Asian | 21 | 31 | 24 | 18 |
| White | 77 | 67 | 72 | 75 |
| American Indian or Alaskan Native White | <1 | <1 | 2 | 3 |
| Ethnicity – Hispanic or Latino (yes:no), % | 11:89 | 8:92 | 13:87 | 14:86 |
| Body mass index | ||||
| Mean ±SD | 25.3±4.00 | 24.3±3.82 | 24.8±4.21 | 25.1±3.52 |
| Range | 16–37 | 16–37 | 14–40 | 16–33 |
| MMSE score | 19.6±4.04 | 18.9±3.98 | 19.1±4.64 | 19.4±4.01 |
| ADAS-Cog score | 25.0±10.26 | 26.3±10.30 | 25.8±11.40 | 24.9±9.68 |
| Time since first symptoms, n | 158 | 162 | 155 | 76 |
| Time since first symptoms, years | ||||
| Mean ± SD | 4.17±2.72 | 4.09±3.05 | 3.83±2.68 | 3.59±2.35 |
| Min, max | 0.6, 19.7 | 0.4, 29.0 | 0.6,23.0 | 0.5,11.4 |
| Time since first diagnosis, n | 159 | 162 | 155 | 75 |
| Time since first diagnosis, years | ||||
| Mean ± SD | 1.62±1.76 | 1.6±1.74 | 1.66±2.16 | 1.47±1.53 |
| Min, max | 0.1,11.0 | 0.1,16.0 | 0.1,22.0 | 0.1,9.8 |
| Genotype, n | ||||
| APOE ε4 negative | 82 (52%) | 84 (52%) | 80 (51%) | 38 (50%) |
| ADAS-Cog score | 24.5±10.40 | 26.2±10.44 | 24.9±10.73 | 23.3±10.29 |
| All except APOE ε4 homozygotes | 144 (91%) | 144 (89%) | 142 (91%) | 68 (89%) |
| ADAS-Cog score | 25.0±10.04 | 26.7±10.14 | 25.4±11.54 | 24.5±9.52 |
| APOE ε4 positive | 77 (48%) | 78 (48%) | 76 (49%) | 38 (50%) |
| Heterozygote (+/-) | 62 (39%) | 60 (37%) | 62 (40%) | 30 (39%) |
| Homozygote (+/+) | 15 (9%) | 18 (11%) | 14 (9%) | 8 (11%) |
Values denote means ± SD unless stated otherwise. +/- = 1 copy of the ε4 allele; +/+ = 2 copies of the ε4 allele.
Table 2.
Baseline demographics of subjects who withdrew from ITT population before completing REFLECT-1
| Placebo (n = 28) | 2 mg RSG XR (n = 27) | 8 mg RSG XR (n = 29) | 10 mg donepezil (n = 21) | |
|---|---|---|---|---|
| Age, years | ||||
| Mean ± SD | 74.8±8.08 | 71.5±7.14 | 75.2±7.86 | 75.2±7.86 |
| Range | 55–88 | 57–85 | 54–91 | 58–84 |
| Sex (female:male), % | 54:46 | 67:33 | 59:41 | 76:24 |
| Ethnicity – Hispanic or Latino (yes:no), % | 14:86 | 11:89 | 10:90 | 14:86 |
| Body mass index | ||||
| Mean ± SD | 24.6±4.14 | 24.8±4.52 | 24.2±3.86 | 25.1±3.86 |
| Range | 17–33 | 17–33 | 14–31 | 17–33 |
| MMSE score | 19.8±3.95 | 18.1±4.15 | 18.5±4.21 | 19.0±4.44 |
In REFLECT-5, the baseline and disease characteristics and APOE allele status were similar to those in REFLECT-1 (62% females; mean age: 72.8 years; mean baseline MMSE score: 19.4; 50% APOE ∊4 negative).
Primary Efficacy
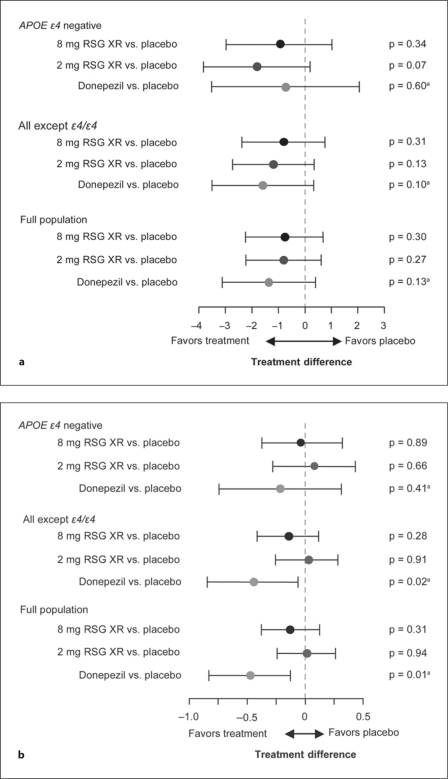
In REFLECT-1, the baseline ADAS-Cog total scores were comparable (24.9–26.3) across treatment groups in the full ITT population, and in the all-except-∊4/∊4 (24.5–26.7) and the APOE-∊4-negative (23.3–26.2) subgroups. Table 3 and figure 4a summarize the treatment comparisons for change from baseline in ADAS-Cog total scores at week 24 obtained from the primary MMRM analysis. Declines in total score at week 24 were found for both the placebo and the active treatment groups, with the exception of the 2-mg RSG XR APOE-∊4-negative and the donepezil all-except-∊4/∊4 subject groups, which both showed a minor improvement in mean score (table 3). No significant difference from placebo in change from baseline in ADAS-Cog score was detected for the primary comparisons of interest, i.e. 2 mg RSG XR and 8 mg RSG XR versus placebo in the APOE-∊4-negative subgroup (table 3; fig. 4a). No significant differences were detected in the other analysis populations.
Table 3.
Change from baseline in ADAS-Cog total scores at week 24 – MMRM1 (ITT population)
| Subject group | Treatment group | Number2 | LSM | SE | Treatment comparison |
||
|---|---|---|---|---|---|---|---|
| difference3 | 95% CI | P | |||||
| APOE ε4 negative | placebo | 63 | 1.6 | 0.78 | _ | _ | _ |
| 2 mg RSG XR | 69 | −0.2 | 0.67 | −1.8 | −3.8,0.2 | 0.074 | |
| 8 mg RSG XR | 65 | 0.6 | 0.67 | −1.0 | −3.0, 1.0 | 0.338 | |
| 10 mg donepezil | 28 | 0.9 | 1.16 | −0.7 | −3.5,2.1 | 0.6024 | |
| All except ε4/ε4 | placebo | 117 | 1.5 | 0.58 | - | - | - |
| 2 mg RSG XR | 115 | 0.3 | 0.54 | −1.2 | −2.7, 0.4 | 0.131 | |
| 8 mg RSG XR | 112 | 0.7 | 0.57 | −0.8 | −2.4, 0.8 | 0.315 | |
| 10 mg donepezil | 49 | −0.1 | 0.79 | −1.6 | −3.5,0.3 | 0.1054 | |
| Full population | placebo | 131 | 2.0 | 0.56 | - | - | - |
| 2 mg RSG XR | 130 | 1.2 | 0.53 | −0.8 | −2.2, 0.6 | 0.272 | |
| 8 mg RSG XR | 125 | 1.2 | 0.55 | −0.8 | −2.2, 0.7 | 0.297 | |
| 10 mg donepezil | 56 | 0.6 | 0.74 | −1.3 | −3.1,0.4 | 0.1314 | |
ADAS-Cog total scores range from 0 to 70, with negative change from baseline indicating clinical improvement. LSM = Least squares mean; SE = standard error for LSM.
Model includes terms for country group, visit, APOE ε4 gene dose, treatment, baseline body mass index, baseline MMSE score, baseline MMSE score by visit, and treatment by visit
Number of subjects with an ADAS-Cog score at week 24.
Difference (active treatment minus placebo).
Unadjusted p.
Fig. 4.
Adjusted means (95% CI) for the coprimary endpoints. a ADAS-Cog score change from baseline at week 24. b CIBIC+ score at week 24. aUnadjusted p.
Table 4 and figure 4b provide outcomes for CIBIC+ scores. The mean treatment differences relative to placebo were close to 0 for 2 mg and 8 mg RSG XR in both APOE subgroups and in the full ITT population. No significant difference from placebo in CIBIC+ score at week 24 was detected with RSG XR 2 mg or 8 mg in the APOE-∊4-negative subgroup or the other analysis populations.
Table 4.
CIBIC+ scores at week 24 – MMRM1 (ITT population)
| Subject group | Treatment group | Number2 | LSM | SE | Treatment comparison |
||
|---|---|---|---|---|---|---|---|
| difference3 | 95% CI | P | |||||
| APOE ε4 negative | placebo | 63 | 4.2 | 0.14 | _ | _ | _ |
| 2 mg RSG XR | 71 | 4.2 | 0.12 | 0.1 | −0.3,0.4 | 0.663 | |
| 8 mg RSG XR | 66 | 4.1 | 0.11 | 0.0 | −0.4, 0.3 | 0.891 | |
| 10 mgdonepezil | 28 | 3.9 | 0.22 | −0.2 | −0.7,0.3 | 0.4144 | |
| All except ε4/ε4 | placebo | 117 | 4.3 | 0.10 | - | - | - |
| 2 mg RSG XR | 117 | 4.3 | 0.10 | 0.0 | −0.3,0.3 | 0.913 | |
| 8 mg RSG XR | 114 | 4.1 | 0.10 | −0.1 | −0.4,0.1 | 0.276 | |
| 10 mgdonepezil | 49 | 3.8 | 0.17 | −0.5 | −0.8,-0.1 | 0.0254 | |
| Full population | placebo | 131 | 4.3 | 0.09 | _ | _ | _ |
| 2 mg RSG XR | 133 | 4.3 | 0.09 | 0.0 | −0.2,0.3 | 0.939 | |
| 8 mg RSG XR | 127 | 4.2 | 0.10 | −0.1 | −0.4,0.1 | 0.307 | |
| 10 mgdonepezil | 56 | 3.8 | 0.16 | −0.5 | −0.8,-0.1 | 0.0094 | |
The CIBIC+ rates global functioning with scores ranging from 1 (markedly improved) to 7 (markedly worse). A score of 4 represents no change and negative treatment differences reflect benefit in the active treatment over placebo. LSM = Least squares mean; SE = standard error for LSM.
Model includes terms for country group, visit, APOE ε4 gene dose, treatment, baseline body mass index, baseline MMSE score, baseline MMSE score by visit, and treatment by visit.
Number of subjects with a CIBIC+ score at week 24.
Difference (active treatment minus placebo).
Unadjusted p.
The comparison of donepezil with placebo was not part of the testing hierarchy, and the p values calculated for the primary endpoints are unadjusted (fig. 4; tables 3, 4). No significant difference was found for donepezil versus placebo in change in ADAS-Cog score at week 24 in the 3 analysis populations. However, for CIBIC+, statistically significant differences between donepezil and placebo were detected in the full ITT population (p = 0.009) and the all-except-∊4/∊4 subjects (p = 0.025). Treatment differences derived from imputation-based sensitivity analysis (data not presented) were very similar to the results of the primary repeated measures analysis.
In REFLECT-5, at week 24, the mean change ± SD from the open-label study baseline in ADAS-Cog score was 1.9 ± 5.23 points (data from 243 subjects assessed), and the mean CIBIC+ score was 4.3 ± 1.05 (data from 241 subjects assessed).
Secondary Efficacy Outcomes
In REFLECT-1, no statistically significant treatment effects were observed with 2 mg or 8 mg RSG XR on DAD, NPI or MMSE scores, with effect sizes being very small. A statistically significant effect (difference of −1.1, 95% CI of −2.2 to −0.1, p = 0.037) on the Short-Term Memory Assessment was detected with 2 mg RSG XR in the APOE-∊4-negative subgroup. For donepezil, in the all-except-∊4/∊4 subgroup and the full ITT population, a trend toward significance (all-except-∊4/∊4: difference of 3.7, 95% CI of −0.3 to 7.7, p = 0.067; full ITT population: difference of 3.6, 95% CI of −0.4 to 7.5, p = 0.078) on DAD, and a statistically significant treatment effect on MMSE (all-except-∊4/∊4: difference of 1.1, 95% CI of 0.1–2.0, p = 0.023; full ITT population: difference of 1.0, 95% CI of 0.1–1.8, p = 0.030) were observed. HbA1c levels remained fairly constant throughout the study across all treatment groups and in both APOE subgroups and the full ITT population.
In REFLECT-5, subjects experienced no appreciable change relative to their REFLECT-1 baseline scores in their MMSE, DAD or NPI scores at weeks 24 or 48, and no differences based on parent study treatment assignment or APOE ∊4 status were noted.
Post hoc Analyses
Evaluation of the effect of baseline, nonfasted glucose or HbA1c levels on ADAS-Cog outcomes showed no correlations. In the analysis of outcome by MMSE, ADAS-Cog treatment difference for 2 mg RSG XR versus placebo was similar in the mild and moderate subgroups (mean differences of −2.0 points for both). For the 8-mg dose, the treatment difference was larger in the moderate (−3.0 points) than in the mild (0.1 points) subgroup. This was also observed in the donepezil subgroups (moderate: −1.8 points; mild: 0.2 points).
Safety and Tolerability Outcomes
In the REFLECT-1 safety population, the proportion of subjects in the 2-mg RSG XR group reporting at least 1 AE was comparable with the placebo group (36 vs. 38%), and the proportion in the 8-mg RSG XR group was higher than in the placebo group (42 vs. 38%) (table 5). The AE incidence was highest (51%) in the donepezil group. Incidences of severe AE, serious AE (SAE) and AE leading to discontinuation for both RSG XR doses were comparable to those for placebo. The incidences of drug-related AE with onset during the treatment phase were similar for 2 mg RSG XR and placebo (14% for both) and higher for both 8 mg RSG XR (24%) and donepezil (23%) (table 5). Dizziness and peripheral edema, both drug related, were the only AE leading to premature discontinuation in >1 subject in the RSG XR groups; 2 subjects in the 8-mg dose group discontinued for each of these AE. On-treatment SAE considered to be drug related were hip fracture (1 subject; placebo), congestive heart failure and myocardial infarction (1 subject; 2 mg RSG XR), muscle spasms (1 subject; donepezil) and acute myocardial infarction (1 subject; donepezil).
Table 5.
Overview of AE incidence during the treatment phase (safety population: full and APOE-e4-negative subgroup)
| Number of subjects reporting event |
||||||||
|---|---|---|---|---|---|---|---|---|
| placebo |
2 mg RSG XR |
8 mg RSG XR |
10 mgdonepezil |
|||||
| full (n = 165) | APOE ε4 negative (n = 86) | full (n=166) | APOE ε4 negative (n = 85) | full (n = 165) | APOE ε4 negative (n = 86) | full (n = 83) | APOE ε4 negative (n = 42) | |
| AnyAE | 62 (38) | 27 (31) | 60 (36) | 30 (35) | 69 (42) | 36 (42) | 42 (51) | 19 (45) |
| Severe AE | 5 (3) | 0 (0) | 3 (2) | 2 (2) | 2 (1) | 1 (1) | 5 (6) | 1 (2) |
| Drug-related AE | 23 (14) | 11 (13) | 23 (14) | 11 (13) | 40 (24) | 21 (24) | 19 (23) | 10 (24) |
| SAE (fatal or nonfatal) | 10 (6) | 5 (6) | 7 (4) | 4 (5) | 8 (5) | 3 (3) | 6 (7) | 2 (5) |
| AE leading to discontinuation | 8 (5) | 6 (7) | 8 (5) | 3 (4) | 10 (6) | 7 (8) | 11 (13) | 5 (12) |
Values in parentheses denote percentages. SAE = Serious AE.
In the 8-mg RSG XR group, peripheral edema was the most common AE and increased in a dose-dependent manner (table 6). No other frequently reported AE in the RSG XR groups was dose related. In the 2-mg RSG XR group, nasopharyngitis and hyperlipidemia were the most common AE.
Table 6.
AE occurring during the treatment phase in >5% of any treatment group (safety population: full and APOE-e4-negative subgroup)
| Number of subjects reporting event |
||||||||
|---|---|---|---|---|---|---|---|---|
| placebo |
2 mg RSG XR |
8 mg RSG XR |
10 mgdonepezil |
|||||
| full (n = 165) | APOE ε4 negative (n = 86) | full (n=166) | APOE ε4 negative (n = 85) | full (n = 165) | APOE ε4 negative (n = 86) | full (n = 83) | APOE ε4 negative (n = 42) | |
| Peripheral edema | 9 (5) | 4 (5) | 6 (4) | 2 (2) | 24 (15) | 11 (13) | 3 (4) | 2 (5) |
| Diarrhea | 1 (<1) | 0 | 2 (1) | 1 (1) | 4 (2) | 3 (3) | 4 (5) | 1 (2) |
| Nasopharyngitis | 4 (2) | 1 (1) | 11 (7) | 6 (7) | 4 (2) | 2 (2) | 1 (1) | 0 |
| Headache | 0 | 0 | 2 (1) | 1 (1) | 2 (1) | 1 (1) | 7 (8) | 7 (17) |
| Hyperlipidemia | 1 (<1) | 0 | 9 (5) | 3 (4) | 1 (<1) | 0 | 2 (2) | 0 |
| Nausea | 0 | 0 | 1 (<1) | 0 | 1 (<1) | 1 (1) | 4 (5) | 3 (7) |
| Gastroesophageal reflux | 1 (<1) | 1 (1) | 0 | 0 | 0 | 0 | 2 (2) | 2 (5) |
| Insomnia | 1 (<1) | 0 | 0 | 0 | 0 | 0 | 3 (4) | 2 (5) |
Values in parentheses denote percentages.
AE findings for the APOE-∊4-negative safety populations were similar to those in the full safety population (tables 5, 6).
Regarding REFLECT-1 deaths, nonfatal SAE and AESI, 2 (<1%) deaths were reported during the study (1 cardiac failure in the placebo group, and 1 aspiration and respiratory failure in the 8-mg RSG XR group). Neither event was considered to be related to the study drug. No SAE was reported in >1 subject in any treatment group.
Table 7 summarizes AESI with onset during the double-blind treatment phase. In the full ITT safety population, these were more frequent in the 8-mg RSG XR group compared with the other treatment groups. Edema was considered drug related in 24 subjects (15%) in the 8-mg RSG XR group. The incidence of other AESI that were attributed to study treatment was similar across treatment groups. The results in the APOE-∊4-negative subgroup were consistent with those in the full safety population.
Table 7.
AESI with onset during the treatment phase (safety population: full and APOE-e4-negative subgroup)
| Number of subjects with event |
||||||||
|---|---|---|---|---|---|---|---|---|
| placebo |
2 mg RSG XR |
8 mg RSG XR |
10 mg donepezil |
|||||
| full (n = 165) | APOE ε4 negative (n = 86) | full (n = 166) | APOE ε4 negative (n = 85) | full (n = 165) | APOE ε4 negative (n = 86) | full (n = 83) | APOE ε4 negative (n = 42) | |
| AnyAESI | 28 (17) | 9 (10) | 26 (16) | 10 (12) | 39 (24) | 17 (20) | 12 (14) | 4 (10) |
| Edema | 9 (5) | 4 (5) | 6 (4) | 2 (2) | 28 (17) | 13 (15) | 3 (4) | 2 (5) |
| Anemia | 1 (<1) | 0 | 1 (<1) | 0 | 6 (4) | 4 (5) | 0 | 0 |
| Dyslipidemia | 8 (5) | 1 (1) | 12 (7) | 5 (6) | 6 (4) | 1 (1) | 3 (4) | 1 (2) |
| CHF/PE | 1 (<1) | 0 | 1 (<1) | 1 (1) | 1 (<1) | 1 (1) | 0 | 0 |
| Weight gain | 1 (<1) | 0 | 0 | 0 | 1 (<1) | 1 (1) | 0 | 0 |
| Cerebrovascular | 1 (<1) | 1 (1) | 2 (1) | 0 | 0 | 0 | 1 (1) | 0 |
| Fractures | 3 (2) | 1 (1) | 2 (1) | 2 (2) | 0 | 0 | 1 (1) | 1 (2) |
| Hepatic disorders | 3 (2) | 1 (1) | 2 (1) | 0 | 0 | 0 | 2 (2) | 0 |
| Myocardial ischemia | 0 | 0 | 1 (<1) | 1 (1) | 0 | 0 | 1 (1) | 0 |
| Neoplasms/cancer – malignant | 1 (<1) | 0 | 1 (<1) | 0 | 0 | 0 | 1 (1) | 0 |
| Neoplasms/cancer – unspecified | 1 (<1) | 1 (1) | 0 | 0 | 0 | 0 | 1 (1) | 1 (2) |
| Peripheral vascular disease | 0 | 1 (<1) | 0 | 0 | 0 | 0 | 0 | 0 |
Values in parentheses denote percentages. CHF = Congestive heart failure; PE = peripheral edema.
The AESI of dyslipidemia was reported for 29 subjects: 8 (5%) for placebo, 12 (7%) for 2 mg RSG XR, 6 (4%) for 8 mg RSG XR and 3 (4%) for donepezil (table 7). Six of the 29 subjects did not have associated lipid laboratory abnormalities during the study.
The AESI of bone fracture with onset during double-blind treatment was reported for 6 subjects (1%; all were single fractures in females): 3 in the placebo group (wrist fracture, upper arm fracture and hip fracture); 2 in the 2-mg RSG XR group (hand fracture and hip fracture), and 1 in the donepezil group (upper arm fracture).
The AESI of myocardial ischemia was reported in 2 subjects. The first subject was a female in the 2-mg RGS XR group with a history of type 2 DM, pneumonia and hypertension. At study screening, the subject's ECG showed inverted T waves. Her concomitant medications included amlodipine and metformin. The second subject was a male in the 10-mg donepezil group with a history of hypertension and an unspecified cardiac event in 1985. Approximately 2 months prior to randomization, an ECG revealed a probable septal necrosis. His concomitant medications included atenolol, hydrochlorothiazide, aspirin, losartan potassium, risperidone, passion flower and mirtazapine.
With regard to REFLECT-1 abnormal laboratory parameter, vital sign and ECG findings, RSG XR treatment was associated with dose-dependent decreases in hemoglobin levels, hematocrits and other cell counts, consistent with hemodilution [37]. RSG XR was associated with increases in total cholesterol and low-density lipoprotein cholesterol. Mean creatine kinase (CK) and lactate dehydrogenase (LDH) levels were increased in both RSG XR dose groups. For CK, shifts from normal at baseline to levels of potential clinical concern (>1.25 × upper limit of normal, ULN) at any time on treatment occurred in 2% (placebo), 7% (2 mg RSG XR), 7% (8 mg RSG XR) and 6% (donepezil) of the subjects. For LDH, shifts to levels of potential clinical concern (>2 × ULN) occurred in 0–1% of the subjects across the treatment groups. Shifts from normal glucose at baseline to low glucose (<3.9 mmol/l) anytime on treatment were more common in the 8-mg RSG XR group (7%) relative to the other treatments (3–4%); however, shifts to low values that were of potentially clinical concern (<3.6 mmol/l) were similar across treatments (2, 3, 3 and 1% for placebo, 2 mg RSG XR, 8 mg RSG XR and donepezil, respectively), and no AE of hypoglycemia were reported. Changes in vital signs were small and within normal ranges, and changes in ECG values were relatively small and consistent across treatment groups. Weight increases of potential clinical concern (i.e. ≥7% increase) were more common for both 2 mg (13%) and 8 mg (16%) RSG XR compared with placebo (6%); weight increase was reported as an AE in only 2 subjects (1 in the placebo and 1 in the 8-mg RSG XR group).
Concerning REFLECT-5 safety and tolerability findings, among the 331 subjects in the safety population, 3 deaths (causes listed as unknown, carbon monoxide poisoning and circulatory collapse) were reported. All deaths occurred in the follow-up phase, approximately 1 month after the last dose of RSG XR; the case of circulatory collapse was considered by the investigator to be possibly related to study drug treatment. Five subjects (2%) experienced nonfatal SAE during open-label treatment. The most frequent AE were: peripheral edema in 44 subjects (13%); headache and anemia, each in 9 subjects (3%); dizziness in 8 subjects (2%); nasopharyngitis in 6 subjects (2%); cough, dyslipidemia and hyperlipidemia, each in 5 subjects (2%), and diarrhea in 2 subjects (<1%). AESI occurred in 79 subjects (24%): edema in 44 (13%); anemia in 16 (5%); dyslipidemia in 15 (5%), and fractures in 7 (2%). All other AESI occurred in ≤1% of the subjects.
Health Outcomes
In REFLECT-1, caregiver time spent assisting subjects with basic activities was significantly reduced by 1 h per day with 2 mg RSG XR (p = 0.026) and with donepezil (p = 0.034) relative to placebo in the full ITT population. This difference was not observed for the 8-mg RSG XR group. A trend toward less time spent assisting subjects with instrumental activities (e.g. shopping, food preparation) and toward improvement in caregiver quality of life (ACQLI score) at week 24 was detected in all active treatment groups.
Discussion
The effects of 24 weeks of treatment with 2-mg or 8-mg RSG XR monotherapies on cognition and global function in APOE-∊4-negative subjects were not statistically or clinically different from placebo. In a previous exploratory analysis of RSG XR monotherapy in mild-to-moderate AD, a treatment-by-genotype interaction was detected for change from baseline versus placebo in ADAS-Cog score at week 24 and in week 24 CIBIC+ score [23]. In that study, ADAS-Cog treatment differences ranged from −2.3 to −2.9 across RSG XR doses (2, 4, 8 mg) in APOE-∊4-negative subjects, and trends toward improvements in this genetic subgroup were also observed on CIBIC+. In contrast, no beneficial effects of RSG XR were observed in APOE-∊4-positive subjects or in the overall ITT population. In this larger study, which was prospectively designed to enable rigorous assessment of changes in ADAS-Cog and CIBIC+ scores in the total study population and in APOE ∊4 subgroups, there was adequate power to enable detection of modest effects, and the findings from the exploratory study were not replicated. The SD of the treatment differences and the dropout rate were consistent with the sample size calculations.
A donepezil treatment group was included in this study as an active control. Since there was no expectation for an interaction between the standard recommended daily dose of donepezil and APOE allele status, analysis for this group was powered at the main effect level (full ITT population). Significant efficacy versus placebo was not detected for ADAS-Cog performance in the full ITT donepezil-treated population, a result that was unexpected based on previous donepezil trial outcomes in the mild-to-moderate AD population [38,39]. However, a significant effect was detected with donepezil on CIBIC+ scores at 24 weeks in the full ITT population as well as in the all-except-∊4/∊4 subgroup, although for both groups, the improvement was less robust than that seen in prior donepezil studies [38,39]. Nevertheless, the CIBIC+ outcomes in the donepezil control analysis suggest that the assay sensitivity was adequate to allow interpretation of the RSG XR versus placebo differences. Also in support of the assay sensitivity, ADAS-Cog values in the placebo group showed that the mean scores declined over time (as expected), although the magnitude of the decline was slightly smaller than reported in historical studies with ChEI [38,39,40,41,42].
The first step in the testing hierarchy (8 mg versus placebo in the APOE-∊4-negative group) (fig. 2) did not fulfill the statistical requirements for significance. Analysis results for the other prospectively defined subgroups (full ITT and all-except-∊4/∊4 populations) are reported for completeness, but should be considered exploratory. No efficacy was identified for either RSG XR dose in the analyses of the all-except-∊4/∊4 group or full ITT population. The effects of RSG XR were evaluated in the population that excluded the homozygote group (i.e. in the all-except-∊4/∊4 group) since a potential for slower disease progression in ∊4/∊4 homozygotes has been reported [43,44,45]. The results for the all-except-∊4/∊4 group, which comprised 90% of the population in this study, were very similar to those for the full ITT population, indicating that ∊4 homozygous status did not affect outcomes in our sample.
The ADAS-Cog may be more sensitive to disease progression and treatment effects in moderate versus mild AD [46], and a post hoc analysis was performed to analyze whether treatment effects in this study differed by baseline MMSE scores (i.e. in subjects with moderate versus mild AD). For 2 mg RSG XR versus placebo, the treatment effect was similar and nonsignificant for both subjects with mild (MMSE score ≥18) and more moderate (MMSE score <18) AD; however, for 8 mg RSG XR and donepezil, the ADAS-Cog treatment benefit in the moderate AD subgroup was numerically greater than that in the mild subgroup. No clear differences were detected in the magnitude of effects for CIBIC+ scores between mild and moderate subgroups. Since group sizes were reduced for this analysis and lacked statistical power, caution is needed in interpreting these findings. Nevertheless, this finding is consonant with results from studies with other agents showing that the ADAS-Cog has greater sensitivity to detect cognitive decline and treatment effects in moderately severe versus milder AD [47,48,49].
Although a correlation between cognitive performance and markers of glycemia in patients with DM has been identified in numerous studies [50], correlations between cognition and glucose control in AD regardless of diabetic status are poorly defined [51,52]. Our post hoc analysis detected no correlation between ADAS-Cog performance and baseline glucose or HbA1c levels. It should be noted that our analysis of glucose levels was limited because no fasting samples were collected in this study.
RSG XR monotherapy was generally well tolerated during 24 weeks of treatment in this study. The most common AE reported in the RSG XR groups were consistent with the profile of RSG immediate release (IR) in T2DM [31]. The proportion of subjects with AE was lower for 2 mg RSG XR compared with 8 mg RSG XR and was generally comparable across the APOE ∊4 subgroups and the full safety population.
As expected, the incidence of PPAR-γ-agonist-related AE was higher with RSG XR, although the rates were relatively low across treatment groups. The most noteworthy AE with RSG XR treatment were nasopharyngitis and hyperlipidemia at the 2-mg dose, and peripheral edema at the 8-mg dose; these were the most common AE, and the only AE occurring at a rate more than twice that for the placebo. The incidence of edema was not unexpected as fluid retention is a well-documented side effect of this class of hypoglycemic drugs, mediated by actions at PPAR-γ receptors in the renal collecting ducts by increases in vascular permeability and reduced systemic vascular resistance [53,54,55]. The absolute incidence of AE of edema for RSG XR was consistent with that for RSG IR in patients with T2DM and with results from recent metabolic studies [36,37]. The absolute difference between the 8-mg RSG XR and placebo groups in the incidence of edema was approximately 10%, which is consistent with previous reports on elderly subjects with T2DM [31,37]. Interestingly, the incidence of edema in the placebo group (5%) was higher than that seen in previous studies. The higher absolute rates across all treatments may be due to closer surveillance, given an increasing level of education among clinicians related to the reporting of edema as an AE.
Other AE related to fluid retention (e.g. anemia, cardiac failure, acute cardiac failure), elevated transaminases, depression and bone fractures were uncommon at both RSG XR doses. It is noteworthy that not all investigators reported hyperlipidemia as an AE; therefore, the incidence of hyperlipidemia based on clinical laboratory data was higher than that reported as an AE. For the AE of dyslipidemia, reported in 5% of the subjects, associated laboratory values, relevant history and/or evidence of elevated baseline lipids were often lacking. In light of findings for hyperlipidemia in this study, laboratory data would likely provide a more consistent basis for determining the true incidence of dyslipidemia.
Mean changes in hematology and clinical chemistry parameters were generally small and comparable across treatments. Dose-dependent declines in hemoglobin levels, hematocrits and other cell counts observed in the RSG treatment groups were consistent with hemodilution [37]. Dose-dependent increases in total cholesterol and low-density lipoprotein observed with RSG XR treatment were consistent with the known-effects profile of RSG IR in T2DM [31]. A higher proportion of subjects in the RSG XR versus placebo or donepezil treatment groups experienced CK elevation greater than the ULN. The numbers of subjects across treatment groups with CK elevations of 2× ULN (1–4/group) and 3× ULN (0–1/group) were small, with no notable differences between groups. Changes in mean LDH levels were observed for both RSG XR doses, but shifts of potential clinical concern were similar to those for donepezil or placebo.
Although the duration of RSG exposure was lengthened during the open-label extension study, the safety profile was very similar to that in the 8-mg RSG XR dose group in the blinded REFLECT-1 study. The rates of treatment discontinuation were similar in the studies, and there were no major differences between APOE-∊4-negative and APOE-∊4-positive subjects in the overall incidence of SAE or AE, including AESI, during the study. In both studies, the most common AE overall, as well as the most common drug-related AE, was peripheral edema. Based on mean values, cognitive decline and slightly worse global change were evident during the open-label extension study. No differences in results were noted for any efficacy endpoint based on APOE ∊4 status.
The limitations of this study include those inherent in investigations of AD: a limitation on treatment duration for a placebo-controlled study in a significantly ill population [36]; variability in AD progression [56], and the use of assessment tools that may be inadequate for detecting drugs with a modest treatment effect in a mild-to-moderate AD population [46,57]. The ADAS-Cog was chosen as an assessment in this study, based on published outcomes for the active control donepezil, and on its widespread use in evaluating new therapies for AD. Our results for donepezil effects and the post hoc analysis by baseline severity were consistent with other reports, suggesting that the ADAS-Cog is most sensitive as a primary endpoint in more moderate AD populations. Another limitation may be that effective levels of RSG may not be achieved in target tissues in the brain since RSG is a substrate for the multidrug-resistant gene product permeability glycoprotein [22,58]. In addition, evidence suggests that the permeability glycoprotein efflux transporter is upregulated in the presence of inflammatory cytokines [59], raising the possibility that neuroinflammation common in AD could limit RSG exposure and obviate its potential benefit [60]. A PPAR-γ with high penetration of the blood-brain barrier should be investigated to further evaluate the role of this class of agents in AD.
Although efficacy was not demonstrated with RSG treatment in this study, the abundance of data on the role of underlying metabolic disorders remains of central concern in AD, and agents such as RSG, which modify insulin sensitivity, remain as therapeutic options worthy of further inquiry. This study illustrates approaches to managing the complexities of AD clinical trials and the measures needed to recruit optimal control groups, such as incorporating genetic stratification and a donepezil-treated positive control arm. However, cognitive instruments that are more sensitive to cognitive decline in mild AD are required for studies of short duration.
Conflicts of Interest
M.G., C.A., M.Z.-H., S.E., M.I. and S.S. are current employees of GlaxoSmithKline. A.M.S. was an employee of GlaxoSmithKline when the study was conducted. S.C. is a consultant to, and receives grant support from, GlaxoSmithKline. G.L. and Ü.L. are consultants to GlaxoSmithKline.
Acknowledgements
Funding for these studies was provided by GlaxoSmithKline (NCT00428090 and NCT00550420). The authors wish to acknowledge the following: the participating subjects and their caregivers and the REFLECT-1 and REFLECT-5 study investigators; David Hosford, Marc Risner, Carl Chiang, Conn Harrington, Carly Donovan and Barbara Jeter of GlaxoSmithKline for their contributions during the study and the study analysis, and Mary Richardson of GlaxoSmithKline for critical review during the development of the manuscript. Editorial support in the form of editorial suggestions on draft versions of this paper, assembling tables and figures, collating author comments and copyediting was provided by Ann Garvey, PhD, at UBC Evidence Solutions and was funded by GlaxoSmithKline.
References
- 1.Cummings JL. Alzheimer's disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 2.Watson GS, Craft S. The role of insulin resistance in the pathogenesis of Alzheimer's disease: implications for treatment. CNS Drugs. 2003;17:27–45. doi: 10.2165/00023210-200317010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Craft S. Insulin resistance and Alzheimer's disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 4.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Solfrizzi V, Scafato E, Capurso C, et al. Metabolic syndrome, mild cognitive impairment, and progression to dementia: the Italian Longitudinal Study on Aging. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.12.012. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Forti P, Pisacane N, Rietti E, et al. Metabolic syndrome and risk of dementia in older adults. J Am Geriatr Soc. 2010;58:487–492. doi: 10.1111/j.1532-5415.2010.02731.x. [DOI] [PubMed] [Google Scholar]
- 8.Geroldi C, Frisoni GB, Paolisso G, et al. Insulin resistance in cognitive impairment: the InCHIANTI study. Arch Neurol. 2005;62:1067–1072. doi: 10.1001/archneur.62.7.1067. [DOI] [PubMed] [Google Scholar]
- 9.Bourdel-Marchasson I, Lapre E, Laksir H, Puget E. Insulin resistance, diabetes and cognitive function: consequences for preventative strategies. Diabetes Metab. 2010;36:173–181. doi: 10.1016/j.diabet.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Rasgon NL, Kenna HA, Wroolie TE, et al. Insulin resistance and hippocampal volume in women at risk for Alzheimer's disease. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.12.005. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Felice FG, Vieira MN, Bomfim TR, et al. Protection of synapses against Alzheimer's-linked toxins: insulin signaling prevents the pathogenic binding of Aβ oligomers. Proc Natl Acad Sci USA. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 13.Craft S, Asthana S, Cook DG, et al. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer's disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28:809–822. doi: 10.1016/s0306-4530(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 14.Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 15.Balfour JA, Plosker GL. Rosiglitazone. Drugs. 1999;57:921–930. doi: 10.2165/00003495-199957060-00007. [DOI] [PubMed] [Google Scholar]
- 16.Strum JC, Shehee R, Virley D, et al. Rosiglitazone induces mitochondrial biogenesis in mouse brain. J Alzheimers Dis. 2007;11:45–51. doi: 10.3233/jad-2007-11108. [DOI] [PubMed] [Google Scholar]
- 17.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Ghisletti S, Huang W, Ogawa S, et al. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPAR-γ. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicolakakis N, Aboulkassim T, Ongali B, et al. Complete rescue of cerebrovascular function in aged Alzheimer's disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor-γ agonist. J Neurosci. 2008;28:9287–9296. doi: 10.1523/JNEUROSCI.3348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006;199:265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1ΔE9 mouse model of Alzheimer's disease. Mol Psychiatry. 2010;15:272–285. doi: 10.1038/mp.2009.72. 228. [DOI] [PubMed] [Google Scholar]
- 22.Watson GS, Cholerton BA, Reger MA, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 23.Risner ME, Saunders AM, Altman JF, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 27.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 28.Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change: the Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S22–S32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 29.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 30.Gélinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer's disease: the disability assessment for dementia. Am J Occup Ther. 1999;53:471–481. doi: 10.5014/ajot.53.5.471. [DOI] [PubMed] [Google Scholar]
- 31.GlaxoSmithKline. AVANDIA® prescribing information. Research Triangle Park, GlaxoSmithKline.
- 32.Doward LC. The development of the Alzheimer's Carers’ Quality of Life Instrument (ACQLI) Qual Life Res. 1997;6:639. [Google Scholar]
- 33.Wimo A, Wetterholm AL, Mastey V, Winblad B. Evaluation of the healthcare resource utilization and caregiver time in anti-dementia drug trials: a quantitative battery; In: Wimo A, Karlsson G, Jonsson B, Winblad B, editors. The Health Economics of Dementia. London: John Wiley & Sons; 1998. pp. 465–499. [Google Scholar]
- 34.EuroQol Group EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 35.Lane P. Handling drop-out in longitudinal clinical trials: a comparison of the LOCF and MMRM approaches. Pharm Stat. 2008;7:93–106. doi: 10.1002/pst.267. [DOI] [PubMed] [Google Scholar]
- 36.Gold M. Study design factors and patient demographics and their effect on the decline of placebo-treated subjects in randomized clinical trials in Alzheimer's disease. J Clin Psychiatry. 2007;68:430–438. doi: 10.4088/jcp.v68n0313. [DOI] [PubMed] [Google Scholar]
- 37.Kreider M, Heise M. Rosiglitazone in the management of older patients with type 2 diabetes mellitus. Int J Clin Pract. 2002;56:538–541. [PubMed] [Google Scholar]
- 38.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Group. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 39.Burns A, Rossor M, Hecker J, et al. The effects of donepezil in Alzheimer's disease: results from a multinational trial. Dement Geriatr Cogn Disord. 1999;10:237–244. doi: 10.1159/000017126. [DOI] [PubMed] [Google Scholar]
- 40.Farlow M, Anand R, Messina J, Jr, Hartman R, Veach J. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer's disease. Eur Neurol. 2000;44:236–241. doi: 10.1159/000008243. [DOI] [PubMed] [Google Scholar]
- 41.Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: a 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000;54:2261–2268. doi: 10.1212/wnl.54.12.2261. [DOI] [PubMed] [Google Scholar]
- 42.Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild-to-moderate Alzheimer's disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. BMJ. 2000;321:1445–1449. doi: 10.1136/bmj.321.7274.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoyt BD, Massman PJ, Schatschneider C, Cooke N, Doody RS. Individual growth curve analysis of APOE ∊4-associated cognitive decline in Alzheimer disease. Arch Neurol. 2005;62:454–459. doi: 10.1001/archneur.62.3.454. [DOI] [PubMed] [Google Scholar]
- 44.Frisoni GB, Govoni S, Geroldi C, et al. Gene dose of the ∊4 allele of apolipoprotein E and disease progression in sporadic late-onset Alzheimer's disease. Ann Neurol. 1995;37:596–604. doi: 10.1002/ana.410370509. [DOI] [PubMed] [Google Scholar]
- 45.Stern Y, Brandt J, Albert M, et al. The absence of an apolipoprotein ∊4 allele is associated with a more aggressive form of Alzheimer's disease. Ann Neurol. 1997;41:615–620. doi: 10.1002/ana.410410510. [DOI] [PubMed] [Google Scholar]
- 46.Irizarry MC, Webb DJ, Bains C, et al. Predictors of placebo group decline in the Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-Cog) in 24-week clinical trials of Alzheimer's disease. J Alzheimers Dis. 2008;14:301–311. doi: 10.3233/jad-2008-14304. [DOI] [PubMed] [Google Scholar]
- 47.Doraiswamy PM, Krishnan KR, Anand R, et al. Long-term effects of rivastigmine in moderately severe Alzheimer's disease: does early initiation of therapy offer sustained benefits? Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:705–712. doi: 10.1016/s0278-5846(01)00326-8. [DOI] [PubMed] [Google Scholar]
- 48.Kurz A, Farlow M, Quarg P, Spiegel R. Disease stage in Alzheimer disease and treatment effects of rivastigmine. Alzheimer Dis Assoc Disord. 2004;18:123–128. doi: 10.1097/01.wad.0000127445.00442.a1. [DOI] [PubMed] [Google Scholar]
- 49.Maher-Edwards G, Zvartau-Hind M, Hunter AJ, et al. Double-blind, controlled phase II study of a 5-HT6 receptor antagonist, SB-742457, in Alzheimer's disease. Curr Alzheimer Res 2009, E-pub ahead of print. [DOI] [PubMed]
- 50.Cukierman-Yaffee T. The relationship between dysglycemia and cognitive dysfunction. Curr Opin Investig Drugs. 2009;10:70–74. [PubMed] [Google Scholar]
- 51.Craft S, Dagogo-Jack SE, Wiethop BV, et al. Effects of hyperglycemia on memory and hormone levels in dementia of the Alzheimer type: a longitudinal study. Behav Neurosci. 1993;107:926–940. doi: 10.1037//0735-7044.107.6.926. [DOI] [PubMed] [Google Scholar]
- 52.Domínguez RO, Marschoff ER, Guareschi EM, et al. Insulin, glucose and glycated hemoglobin in Alzheimer's and vascular dementia with and without superimposed type II diabetes mellitus condition. J Neural Transm. 2008;115:77–84. doi: 10.1007/s00702-007-0804-7. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T. Collecting duct-specific deletion of peroxisome proliferator-activated receptor-γ blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci USA. 2005;102:9406–9411. doi: 10.1073/pnas.0501744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang T, Soodvilai S. Renal and vascular mechanisms of thiazolidinedione-induced fluid retention. PPAR Res. 2008;2008:943614. doi: 10.1155/2008/943614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karalliedde J, Buckingham RE. Thiazolidinediones and their fluid-related adverse effects: facts, fiction and putative management strategies. Drug Saf. 2007;30:741–753. doi: 10.2165/00002018-200730090-00002. [DOI] [PubMed] [Google Scholar]
- 56.Cortes F, Nourhashémi F, Guérin O, et al. Prognosis of Alzheimer's disease today: a two-year prospective study in 686 patients from the REAL-FR Study. Alzheimers Dement. 2008;4:22–29. doi: 10.1016/j.jalz.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 57.Schneider LS, Sano M. Current Alzheimer's disease clinical trials: methods and placebo outcomes. Alzheimers Dement. 2009;5:388–397. doi: 10.1016/j.jalz.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gendelman HE. Biomarkers, laboratory, and animal models for the design and development of adjunctive therapies for HIV-1 dementia and other neuroinflammatory disorders. J Neuroimmune Pharmacol. 2007;2:8–13. doi: 10.1007/s11481-006-9050-2. [DOI] [PubMed] [Google Scholar]
- 59.Bauer B, Hartz AM, Miller DS. Tumor necrosis factor-α and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71:667–675. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- 60.Yu C, Argyropoulos G, Zhang Y, Kastin AJ, Hsuchou H, Pan W. Neuroinflammation activates Mdr1b efflux transport through NF-κB: promoter analysis in BBB endothelia. Cell Physiol Biochem. 2008;22:745–756. doi: 10.1159/000185558. [DOI] [PMC free article] [PubMed] [Google Scholar]