Abstract
Inhibitor of nuclear factor κB kinase-α (IKKα) is required for maintaining skin homeostasis and preventing skin tumorigenesis. However, its signaling has not been extensively investigated. In the present study, we generated two mouse lines that expressed different levels of transgenic IKKα in the basal epidermis under the control of keratin-5 promoter and further evaluated their effects on the major pathways of inflammation, proliferation, and differentiation in the skin. Regardless of the transgenic IKKα levels, the mice develop normally. Because IKKα deletion in keratinocytes blocks terminal differentiation and induces epidermal hyperplasia and skin inflammation, we depleted the endogenous IKKα in these transgenic mice and found that the transgenic IKKα represses epidermal thickness and induces terminal differentiation in a dose-dependent manner. Also, transgenic IKKα was found to elevate expression of Max dimer protein 1 (Mad1) and ovo-like 1, c-Myc antagonists, but repress activities of epidermal growth factor receptor (EGFR), extracellular signal-regulated kinase (ERK), Jun-amino-terminal kinases, c-Jun, signal transducer and activator of transcription 3 (Stat3), and growth factor levels in a dose-dependent fashion in the skin. Moreover, EGFR reduction represses IKKα deletion-induced excessive ERK, Stat3 and c-Jun activities, and skin inflammation. These new findings indicate that elevated IKKα expression not only represses epidermal thickness and induces terminal differentiation, but also suppresses skin inflammation by an integrated loop. Thus, IKKα maintains skin homeostasis through a broad range of signaling pathways.
Keywords: IKKα, Stat3, AP-1, c-Myc antagonists, MPAK, skin inflammation
The inhibitor of nuclear factor (NF) κB (IκB), IκB kinase-α (IKKα), IKKβ, and IKKγ form the IKK complex that is essential for NF-κB activation.1, 2 NF-κB is a group of protein structural-related transcription factors, and it regulates the expression of many genes encoding proteins involved in many functions, including inflammation, immunity, apoptosis, cell migration, and cell cycle regulation. IKKα and IKKβ are two highly conserved protein kinases that share many similar biochemical activities, and can form homodimers and heterodimers.2 They can phosphorylate IκBs, which are inhibitors of NF-κB. This phosphorylation event induces the degradation of IκBs, resulting in NF-κB activation. IKKβ has stronger activity for phosphorylating IκBs than IKKα does. Given the importance of the biological activities of IKK/NF-κB, deregulating their activities may elicit diseases.3, 4, 5 Studies have demonstrated that either elevating NF-κB activity or reducing NF-κB activity can provoke skin inflammation in mice.6, 7
Genetic studies have shown that IKKα regulates mouse embryonic skin development, but that IKKβ and IKKγ do not.8, 9, 10, 11, 12, 13 Ikkα−/− mice die soon after birth.8, 9, 10 At birth, these mutant newborns have shining and thickened skin. Their epidermis is hyperplastic and lacks terminally differentiating keratinocytes. The loss of water from the defective skin causes the death of the mutants. It has been shown that the overexpression of IKKα or kinase-inactive IKKα under the control of the keratin (K)-14 promoter or the K5 promoter is able to rescue the skin phenotype of Ikkα−/− mice.14, 15 Thus, IKKα is required for embryonic skin development, independent of its kinase activity.
Several laboratories have demonstrated that IKKα expression was downregulated, or that its localization was altered in human squamous cell carcinomas of the skin, lungs, and head and neck,16, 17, 18, 19, 20 highlighting the importance of IKKα in human malignancy development. We showed that induced IKKα deletion in keratinocytes causes epidermal hyperplasia and spontaneous skin tumors.15 We further identified that IKKα loss elevates an excessive autocrine loop of epidermal growth factor receptor (EGFR), Ras, extracellular signal-regulated kinase (ERK), EGFR ligands, and these ligands' activators in the epidermis of both Ikkα−/− mice and IkkαF/F/K5.Cre mice, that reintroducing IKKα or inactivating EGFR induces keratinocytes to terminal differentiation, represses epidermal hyperplasia, and prevents skin tumors. Moreover, studies have demonstrated that IKKα upregulates c-Myc antagonists by transforming growth factor-β and Smad pathways in coordinately regulating keratinocyte differentiation and proliferation.19, 20 Thus, IKKα acts as a sentry, monitoring the skin and, when necessary, halting keratinocyte hyperproliferation by multiple avenues.
It has been found that IKKα expression can be elevated in mice or cells in response to stresses, such as treatment with 12-O-tetradecanoylphorbol-13-acetate or ultraviolet light.21, 22, 23 We also found that IKKα expression levels were higher in skin papillomas, a benign form of skin tumors, compared with normal skin in C57BL6 mice.24 Approximately, only 3–5% of the papillomas progress to malignant carcinomas, but the rest eventually regress in C57BL6 mice.24 We found that C57BL6 Ikkα+/− mice developed twice as many papillomas as C57BL6 Ikkα+/+ mice. The levels of 12-O-tetradecanoylphorbol-13-acetate-induced IKKα were obviously lower in Ikkα+/− skin and papillomas than in Ikkα+/+ skin and papillomas, indicating that IKKα levels are important for preventing skin tumor development.
IKKα is one of the subunits in the IKK complex. Recently, Page et al.25 showed that the overexpression of IKKβ in the basal epidermis under the control of the K5 promoter induces constitutive NF-κB activity, and a chronic inflammation disease in the skin of K5-IKKβ transgenic mice. As mentioned above, elevated NF-κB activity, indeed, participates in the development of skin inflammation.6, 7 Inflammation is important for the development of various skin diseases and skin tumors. However, whether and how IKKα affects skin inflammation development has not been extensively investigated.
In the current study, we evaluated the effect of different levels of transgenic K5.IKKα on skin development, homeostasis, and inflammation. Unlike IKKβ in the skin, we found that the overexpression of IKKα did not cause skin inflammation and diseases, but it repressed inflammation and cell-proliferating pathways in mice. These findings suggest that IKKα may be a potential target for preventing skin diseases.
Results
Mice overexpressing different levels of the IKKα transgene in basal epidermal keratinocytes develop normally
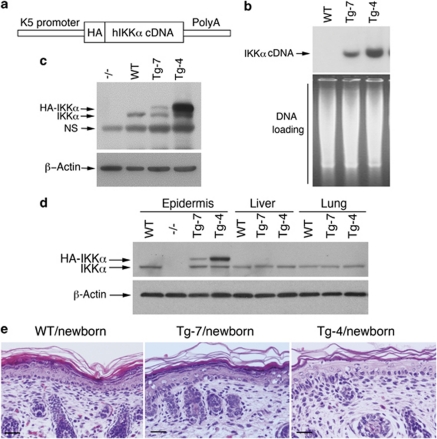
To determine the effect of IKKα doses on skin development, we generated two transgenic mouse lines, Tg-K5.IKKα-7 (Tg-7) and Tg-K5.IKKα-4 (Tg-4), with an FVB background, in which human IKKα cDNA tagged with hemagglutinin-A (HA) was driven by the K5 promoter (Figure 1a). We have not observed any pathological changes in the skin or any illnesses in these mouse lines, compared with wildtype (WT) mice over the past 6 years. Southern blotting hybridized with an N-terminal IKKα cDNA probe showed that Tg-4 mice had more copies of the IKKα transgene than Tg-7 mice (Figure 1b). Western blotting showed a much higher transgenic IKKα level in the skin and epidermis of Tg-4 mice than in those of Tg-7 mice, and these transgenes were specifically expressed in the epidermis, but not in other organs (Figures 1c and d). The transgenic Tg-4-IKKα level was higher than the endogenous IKKα level, whereas the transgenic Tg-7-IKKα level was slightly lower than the endogenous IKKα level in mice. A histological examination revealed no significant differences in the thickness of the epidermis of WT, Tg-7, and Tg-4 newborns (Figure 1e). Together, these results suggest that different levels of overexpressed IKKα in the basal epidermis do not interrupt embryonic development and skin formation in mice.
Figure 1.
Normal skin development in mice overexpressing different levels of the IKKα transgene. (a) Construct of IKKα transgenic mice. HA, hemagglutinin; hIKKα, human IKKα; K5, keratin5. (b) Southern blotting was used to determine IKKα transgene in IKKα transgenic mice. An N-terminal IKKα cDNA was used as a probe for Southern blotting. BamHI was used to digest genomic DNA, stained with ethidium bromide, isolated from the skin specimens of mice. Agarose gel image was used as the DNA loading control. (c) Western blotting was used to determine transgenic IKKα levels in the skin specimens of mice. HA-IKKα, HA-tagged transgenic IKKα; NS, nonspecific band; WT, wild-type mice; −/−, Ikkα−/− mice. (d) IKKα levels in the epidermis, liver, and lungs of Tg-4, Tg-7, WT, and Ikkα−/− (−/−) mice, as detected by western blotting. HA-IKKα, HA-tagged transgenic IKKα; −/−, Ikkα−/− mice; β-actin, a loading control. (e) Histology of hematoxylin and eosin-stained skin sections of WT, Tg-7, and Tg-4 newborn mice. Scale bars=150 μm
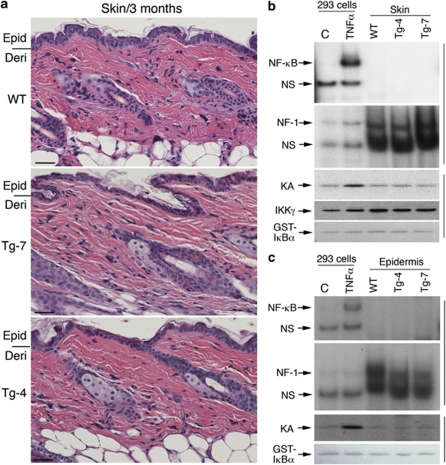
We further examined the dorsal skin morphology of WT, Tg-7, and Tg-4 mice at 3 months of age, using hematoxylin and eosin staining and found no differences among the three specimens (Figure 2a; Supplementary Figure 1). Gel-shift and IKK kinase assays revealed no elevated NF-κB DNA-binding activity or IKK kinase activity in the skins and epidermis of Tg-7 and Tg-4 mice, compared to WT mice (Figures 2b and c). These results suggest that overexpressed IKKα in the basal epidermis has no significant effect on IKK and NF-κB activity in the skin.
Figure 2.
No elevated IKK/NF-κB activation in the skin of mice overexpressing different levels of transgenic IKKα. (a) Histology of the skin from 3-month-old mice, stained with hematoxylin and eosin. Lines on the left of the panel indicate the division between the epidermis and dermis. Epi, epidermis; Der, dermis. Scale bars=150 μm. (b) NF-κB and IKK kinase activity in the skin, and (c) epidermis was detected using gel shift assay and immunoprecipitation kinase assays. HEK 293 cells treated with TNFα (10 ng/ml) for 20 min were used as the positive control. Glutathione S-transferase-IκBα, stained with Ponceau S solution, was used as a substrate of IKK. Antibody against IKKγ was used to precipitate the IKK complex. NF-1, sample loading control; NS, non-specific band
Higher levels of transgenic IKKα induce thinner epidermal layers and higher terminal differentiation levels than lower levels of transgenic IKKα in IKKα-deficient mice
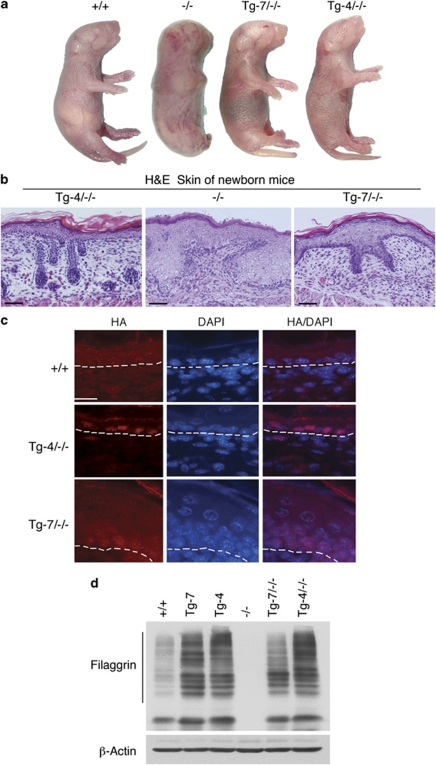
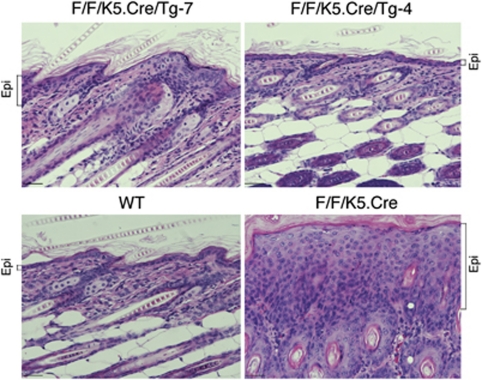
It is known that Ikkα−/− mice develop a hyperplastic epidermis that lacks terminally differentiating keratinocytes.8, 9, 10 Therefore, we compared the abilities of different transgenic IKKα levels to rescue the skin phenotypes of Ikkα−/− mice, by crossing Ikkα+/− mice with Tg-7 or Tg-4 mice to finally generate Tg-7/Ikkα−/− and Tg-4/Ikkα−/− mice. Transgenic Tg-4-IKKα completely rescued the skin phenotype in Tg-4/Ikkα−/− mice (Figure 3a). Although transgenic Tg-7-IKKα rescued Ikkα−/− mice, the skin of Tg-7/Ikkα−/− mice looked shinier than the skin of Tg-4/Ikkα−/− mice. In addition, Tg-7/Ikkα−/− mice had open eyes and short tails. It has been reported that the eyes of Ikkα−/− mice are open at birth because they lack eyelids.26 Histological examinations revealed that Tg-4-IKKα completely rescued the epidermal phenotypes of Ikkα−/− mice, but that some regions of the epidermis of Tg-7/Ikkα−/− mice retained hyperplasia, similar to the epidermis in Ikkα−/− mice, although most of the Tg-7/Ikkα−/− epidermal regions were rescued (Figure 3b). Immunofluorescence staining confirmed that the HA-tagged Tg-4-IKKα level was higher than HA-tagged Tg-7-IKKα in the nuclei of the epidermal cells in mice (Figure 3c). We did not find HA staining signals in Ikkα−/− skin (data not shown). These results indicate that the level of transgenic IKKα is important for rescuing the skin phenotype of Ikkα−/− mice. No milk was found in the stomachs of Tg-4/Ikkα−/− and Tg-7/Ikkα−/− newborns, and these mice died at birth, likely due to a defect in taking milk, as previously reported.14
Figure 3.
The different effects of different levels of transgenic IKKα on rescuing the skin phenotype of Ikkα−/− mice and inducing keratinocyte terminal differentiation. (a) Transgenic IKKα rescued the skin phenotype of Ikkα−/− mice at birth. +/+, Ikkα+/+; −/−, Ikkα−/−. (b) Histology of hematoxylin and eosin-stained skin sections from Tg-4/Ikkα−/− (Tg-4/−/−), Ikkα−/− (−/−), and Tg-7/Ikkα−/− (Tg-7/−/−) newborns. Scale bars =150 μm. (c) Immunofluorescence staining of HA in the skin of WT (+/+), Tg-4/−/−, and Tg-7/−/− mice. Dotted lines mark the junction of the epidermis and the dermis. Scale bars=150 μm. (d) Levels of filaggrin in the skin of newborn mice, detected by western blotting. β-Actin was used as the control
Next, we evaluated the terminal differentiation marker filaggrin using western blotting, and found that transgenic Tg-7-IKKα and Tg-4-IKKα elevated filaggrin expression in the mouse skin (Figure 3d). The level of filaggrin was slightly higher in the skin of Tg-4 mice than in the skin of Tg-7 mice. Furthermore, both transgenic Tg-7-IKKα and Tg-4-IKKα were able to induce terminal differentiation in the skin of Ikkα−/− mice, and the filaggrin levels in Tg-4-IKKα skin were slightly higher than in Tg-7-IKKα skin (Figure 3d). Thus, a higher level of transgenic IKKα has stronger activity in rescuing the Ikkα−/− skin phenotype and inducing terminal differentiation in mice when the endogenous IKKα is absent. We also noticed that the filaggrin level was higher in Tg-7 skin than in WT skin (Figure 3d). Transgenic HA-IKKα expression is under the control of a bovine K5 promoter. Although two HA-IKKα transgenes can rescue the skin phenotypes of Ikkα−/− mice, the bovine K5 promoter and endogenous Ikkα promoter may not be expressed at the same time, which may cause the inconsistence.
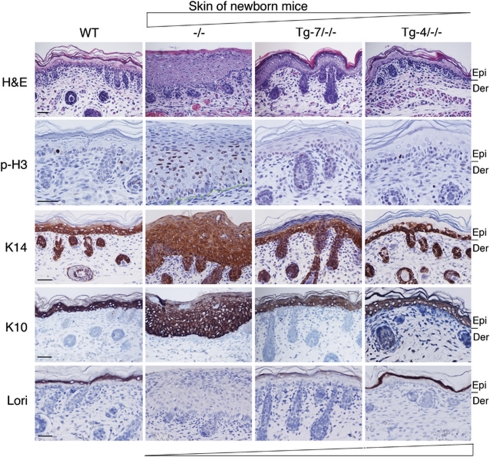
To further characterize the differentiation status induced by Tg-7-IKKα and Tg-4-IKKα in the epidermis of Tg-7/Ikkα−/− and Tg-4/Ikkα−/− newborn mice, compared with Ikkα−/− newborn mice, we performed hematoxylin and eosin and immunohistochemical staining of mitotic marker p-H3,27 basal epidermal cell marker K14, intermediate differentiation marker K10, and terminal differentiation marker loricrin. The entire thick Ikkα−/− epidermis had substantially more p-H3- and K14-stained cells than other genotype samples, and the Ikkα−/− suprabasal epidermis expressed K10, but did not express loricrin (Figure 4). Transgenic Tg-7-IKKα and Tg-4-IKKα dramatically repressed epidermal thickness and p-H3-stained cells in the epidermis of Ikkα−/− mice; however, the epidermis of Tg-7/Ikkα−/− mice had more p-H3-positive cells and was thicker than that of Tg-4/Ikkα−/− mice. Loricrin staining intensity in the epidermis of Tg-4/Ikkα−/− mice was higher than in the epidermis of Tg-7/Ikkα−/− mice. K10 staining intensity in the epidermis of Tg-4/Ikkα−/− and Tg-7/Ikkα−/− mice was similar, although K10 staining appeared to be slightly weaker in the epidermis of Tg-7/Ikkα−/− mice than in the epidermis of Tg-4/Ikkα−/− mice (Figure 4). WT mice were used as controls. These results suggest that transgenic IKKα levels are important for regulating epidermal keratinocyte proliferation and terminal differentiation in mice by a dose-dependent manner.
Figure 4.
Correlation of transgenic IKKα levels with epidermal thickness and differentiation. Paraffin-embedded skin sections of newborn mice were stained with hematoxylin and eosin, or immunohistochemically stained with phosphorylated histone H3 (p-H3), K14, K10, and loricrin (Lori). The angle sign at the top of the panel indicates the reduced thickness of the epidermis and mitotic activity. The angle sign at the bottom of the panel indicates the increased terminal differentiation and IKKα doses. The lines on the right of the panel and the green lines in the p-H3-stained skin of Ikkα−/− mice (−/−) indicated the division between the epidermis and dermis. Dark brown color indicates positive staining; blue color indicates hematoxylin counterstaining. Epi, epidermis; Der, dermis; −/−, Ikkα−/−. Scale bars=150 μm
To further determine whether transgenic IKKα also represses epidermal hyperplasia in postnatal mice lacking IKKα in the epidermis, we introduced Tg-4-IKKα and Tg-7-IKKα into IkkαF/F/K5.Cre mice.15 Previously, we showed that IKKα deletion in the epidermis causes keratinocyte proliferation and reduces cell differentiation through the EGFR/Ras/ERK pathway.15 We found that both Tg-4-IKKα and Tg-7-IKKα rescued the epidermal hyperplasia in Tg-4/IkkαF/F/K5.Cre and Tg-7/IkkαF/F/K5.Cre mice, compared with the epidermis of IkkαF/F/K5.Cre mice (Figure 5). However, some regions were thicker in the Tg-7/IkkαF/F/K5.Cre epidermis than in the Tg-4/IkkαF/F/K5.Cre epidermis. These results further support the notion that IKKα dose is important for inhibiting epidermal hyperplasia.
Figure 5.
Inhibitory effects of transgenic IKKα doses on epidermal hyperplasia of floxed Ikkα mice with IKKα deletion in keratinocytes. Hematoxylin and eosin-stained skin sections from indicated 9-day–old mice. Epi, epidermis; F/F, IkkαF/F. Scale bars=150 μm
Transgenic IKKα-mediated molecular alterations involved in keratinocyte proliferation and differentiation are IKKα dose-dependent
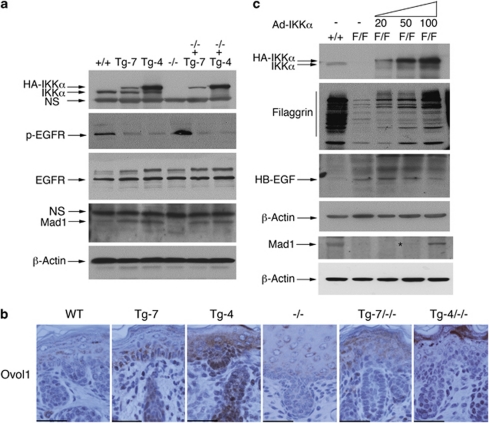
Previously, we detected elevated autocrine activity of EGFR and growth factors through a transcriptional regulation mechanism in IKKα-deficient keratinocytes.15 Also, it has been reported that IKKα regulates the expression of Max dimer protein 1 (Mad1) and ovo-like 1 (Ovol1), which are differentiation inducers and c-Myc antagonists.20 Thus, we evaluated whether different levels of transgenic IKKα have different effects on EGFR activity, and on Mad1 and Ovol1 expression levels. Western blotting showed that Tg-7-IKKα and Tg-4-IKKα not only repressed EGFR activity in the skins of mice with a WT background, but also strongly repressed IKKα loss-induced EGFR activity in the skin of Ikkα−/− mice in an IKKα dose-dependent manner (Figure 6a). On the other hand, IKKα induced Mad1 and Ovol1 expression in a dose-dependent fashion (Figures 6a and b).
Figure 6.
Effects of IKKα doses on EGFR activity, HB-EGF, Mad1 and Ovol1 levels, and terminal differentiation in the skin. (a) Protein levels from the epidermis of WT (+/+), Tg-7, Tg-4, Ikkα−/− (−/−), Tg-7/Ikkα−/− (−/−+Tg-7), and Tg-4/Ikkα−/− (−/−+Tg-4) newborn mice were detected using western blotting. β-Actin was used as protein loading control. (b) Ovol1 expression levels in the paraffin-embedded skin sections of indicated newborn mice were detected with immunohistochemical staining. Dark brown color indicates positive staining; blue color indicates hematoxylin counterstaining. Scale bars=150 μm. (c) Primary cultured keratinocytes were infected with different amounts of adenoviruses expressing IKKα. After 5 days of viral infection, protein levels of HB-EGF, filaggrin, and Mad1 were determined using western blotting. +/+, keratinocytes isolated from WT mice; F/F, keratinocytes isolated from IkkαF/F/K5.Cre mice 15; 20, 50, and 100 μl of cell culture supernatant containing the adenovirus expressing IKKα (Ad-IKKα) was added into cultured keratinocytes. The angle sign at the top of the panel indicates increased Ad-IKKα doses
To further verify the effect of IKKα on Mad1 expression in keratinocytes, we re-expressed a series of IKKα doses in keratinocytes lacking the endogenous IKKα isolated from IkkαF/F/K5.Cre mice15 and found that Mad1 expression was induced by IKKα in a dose-dependent fashion. Filaggrin levels were used as controls for IKKα introduction,28 because IKKα induces terminal differentiation in keratinocytes (Figure 6c). We have reported that IKKα loss upregulates the expression of heparin-binding epidermal growth factor (HB-EGF), an EGFR ligand, which forms an autocrine loop with EGFR, Ras, and ERK.15 Western blotting also showed that re-expression of IKKα repressed levels of HB-EGF, but induced expression of filaggrin in an IKKα dose-dependent manner (Figure 6c). Together, these results indicate that IKKα can regulate keratinocyte proliferation and differentiation through the EGFR and Mad1/Ovol1 pathways.
Transgenic IKKα and reduced EGFR repress IKKα loss-mediated mitogen-activated protein kinase, activator protein 1 (AP-1), and signal transducer and activator of transcription 3 (Stat3) activities and skin inflammation
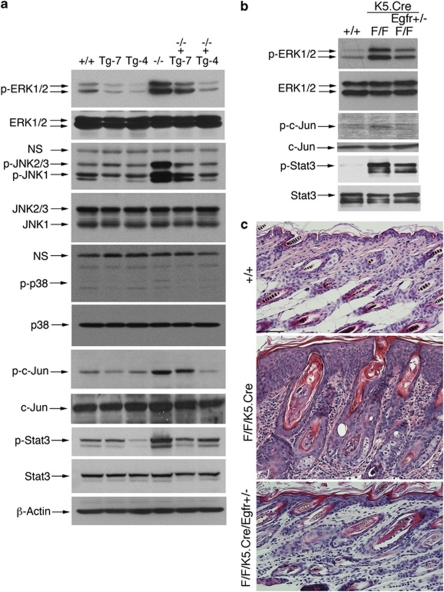
To explore those important pathways involved in inflammation, proliferation, and migration in IKKα-deficient skin, we examined ERK, Jun-amino-terminal kinase (JNK) 1/2, p38, c-Jun, and Stat3 activity in the skin of WT, Tg-4, Tg-7, Ikkα−/−, Tg-4/Ikkα−/−, and Tg-7/Ikkα−/− mice using western blotting. We found elevated ERK, JNK1/2, c-Jun, and Stat3 activity in Ikkα−/− skin (Figure 7a). Tg-4-IKKα and Tg-7-IKKα repressed ERK, JNKs, and Stat3 activity in the transgenic skin, compared with WT skin in an IKKα dose-dependent manner. Tg-4-IKKα and Tg-7-IKKα also repressed IKKα loss-elevated ERK, JNKs, c-Jun, and Stat3 activities in Tg-4/Ikkα−/− and Tg-7/Ikkα−/− skins, compared with Ikkα−/− skin. We did not observe a significant effect of IKKα levels on p38 activity in the skin (Figure 7a).
Figure 7.
IKKα or reduction of EGFR represses inflammation pathways and skin thickness and inflammation. (a) IKKα inhibits ERK, JNK, c-Jun, and Stat3 activity in an IKKα dose-dependent manner in the skin. Western blotting examined indicated proteins in the skin specimens of WT (+/+), Tg-4, Tg-7, Ikkα−/− (−/−), Tg-4/Ikkα−/− (−/−+Tg-4), and Tg-7/Ikkα−/− (−/−+Tg-7) newborns. p, phosphorylated; NS, non-specific bands; β-Actin, protein loading control. (b) Western blotting shows protein levels in WT, IkkαF/F/K5.Cre (F/F/K5.Cre), and IkkαF/F/K5.Cre/Egfr+/− with 16-day-old mice. (c) Comparison of hematoxylin and eosin-stained skin histology of in WT (+/+), IkkαF/F/K5.Cre (F/F/K5.Cre), and IkkαF/F/K5.Cre/Egfr+/− (F/F/K5.Cre/Egfr+/−) with 16-day-old mice. Scale bars=100 μm
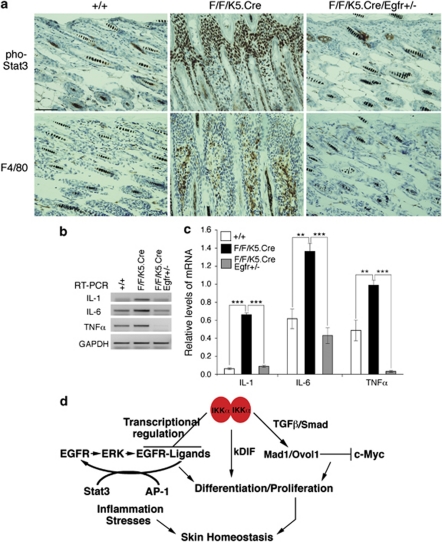
Because inactivating EGFR prevented skin tumor development in IkkαF/F/K15CrePR1 mice,15 we further tested the effect of EGFR on the ERK, Stat3, and activator protein 1 (Ap-1) pathways in IkkαF/F/K5.Cre mice, and we found that reducing EGFR repressed ERK, c-Jun, and Stat3 activities, epidermal hyperplasia, and infiltrating cells in the skin stroma (as an inflammation indicator; Figures 7b and c). Because IKKα inhibits EGFR activity in the epidermis,15 we further determined whether EGFR reduction affects Stat3 activity and skin inflammation in IkkαF/F/K5.Cre mice. We used IkkαF/F/K5.Cre/Egfr+/− mice for this study, because IkkαF/F/K5.Cre/Egfr−/− mice die during embryonic development or soon after birth. Immunohistochemical staining showed increased Stat3 activity and macrophages in IkkαF/F/K5.Cre skin compared with WT skin, and that reduced levels of EGFR repressed Stat3 activity and inflammation (Figure 8a). Interestingly, we did not observe increased granulocyte-differentiation antigen-1-positive cells in IkkαF/F/K5.Cre skin (Supplementary Figure 2). Further analysis for cytokine expression consistently showed elevated interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (TNFα) in IkkαF/F/K5.Cre skin, compared with those in WT and IkkαF/F/K5.Cre/Egfr+/− skin samples (Figures 8b and c). Thus, IKKα deletion induces chronic skin inflammation and Stat3 activity; reduced EGFR or elevated IKKα can downregulate Stat3 activity and abolish skin inflammation. In Figure 8d, we summarized those pathways that IKKα utilizes in regulating keratinocyte differentiation, proliferation, and skin inflammation while maintaining skin homeostasis.
Figure 8.
IKKα deletions cause skin inflammation in mice. (a) Stat3 activity and macrophages (F4/80) in the skin sections of WT (+/+), IkkαF/F/K5.Cre (F/F/K5.Cre), and IkkαF/F/K5.Cre/Egfr+/− (F/F/K5.Cre/Egfr+/−) mice were detected by immunohistochemical staining. pho-, phosphorylated; brown color indicates positive stained cells; blue color indicates hematoxylin counterstaining. Scale bars=200 μm. (b) Relative levels of TNFα, IL-6, and IL-1 with mRNA WT (+/+), IkkαF/F/K5.Cre (F/F/K5.Cre), and IkkαF/F/K5.Cre/Egfr+/− (F/F/K5.Cre/Egfr+/−) skin samples detected using reverse transcriptase-PCR detected by gel. Levels of glyceraldehyde-3-phosphate dehydrogenase mRNA were used to normalize the expression levels of these cytokines. (c) The statistic results for (b). **P<0.01 (t-test); ***P<0.001 (t-test). Each point (n=3) used. (d) A working model of how IKKα maintains skin homeostasis. Transforming growth factor-β and transcription regulation were previously described.15, 20 kIDF, keratinocyte-inducible differentiation factor(s).28 Transcriptional regulation was previously described.15 Arrow indicates upregulation. Line with vertical line at the end indicates downregulation
Furthermore, we evaluated the protein levels of IKKα, IKKβ, IKKγ, RelA (p65), p-p65, NF-κB2 p100/p52, IκBα, p-IκBα, and RelB in the skin of WT, Tg-7, Tg-4, Ikkα−/−, Tg-7/Ikkα−/−, and Tg-4/Ikkα−/− mice. No significant differences in the levels of most of these tested molecules were detected in the skin specimens (Supplementary Figure 3). Although p52 levels in Tg-7 and Tg-4 skin were elevated compared with WT skin, there were no increased inflammation and no morphological changes in the skin of these transgenic mice (Figure 2a; Supplementary Figure 1). Furthermore, p52 levels were also elevated in Tg-7/−/− and Tg-4/−/−, compared with Ikkα−/− (−/−) skin (Supplementary Figure 3). These results suggest that there was not a close relationship between p52 levels and skin phenotypes in these mice. Thus, the elevated p52 levels may not be a major player in IKKα-mediated activity during embryonic skin development.
Discussion
In this study, we found that the overexpression of IKKα did not interrupt skin development, but rather induced an inhibitory effect on the epidermis thickness and skin inflammation in mice in an IKKα dose-dependent manner through a broad range of pathways. This finding provides insight into the mechanism by which IKKα prevents skin diseases.
Many studies have shown that the overexpression of IKKα elevates IKK and NF-κB activity in cell lines.29, 30 Overexpressed NF-κB p65 or p50, or deletion of IκBα in the basal epidermis has been shown to cause neonatal lethality or skin inflammation in mice.6, 7, 31 Because depleting TNFR can rescue skin phenotypes of K5-mIκBα transgenic mice, the different cytokine profiles of NF-κB activity in the epidermis and the dermis have been thought to be a cause for the skin phenotypes in mice overexpressing mIκBα in the epidermis.32 However, depleting TNFR does not rescue the skin phenotypes in mice with IKKα deletion in the epidermis.15 Overexpressed IKKβ elevates IKK/NF-κB activity and causes an inflammatory skin disease in K5-IKKβ mice.25 Although IKKα and IKKβ are highly conserved protein kinases, our results here clearly showed that elevated IKKα expression in the epidermis did not provoke IKK/NF-κB activity and skin inflammation, and did not affect skin development in mice. Thus, overexpressed IKKα and IKKβ/NF-κB may have different physiological roles in the skin. Also, it is possible that some cell lines and tissues may respond differently to the IKKα-led signaling pathways.
In this study, we found that the Tg-4/Ikkα−/− newborns had a normal appearance, but that the Tg-7/Ikkα−/− newborns retained some phenotypes from Ikkα−/− mice, indicating that Tg-4-IKKα, but not Tg-7-IKKα, completely rescued phenotypes of Ikkα−/− mice, and IKKα dose is important for mouse embryonic development. In addition, Tg-4-IKKα showed stronger activity in inhibiting ERK and EGFR activity, and epidermal mitotic activities in the epidermis of Ikkα−/− mice than Tg-7-IKKα. Previously, we reported that EGFR and ERK activities, and HB-EGF levels are involved in IKKα-mediated keratinocyte differentiation and proliferation regulation.15 Here, we detected an IKKα dose-dependent inhibitory effect on IKKα loss-induced EGFR and ERK activities, and HB-EGF levels in the skin and keratinocytes. Thus, IKKα doses are also important for suppressing excessive mitosis of keratinocytes. On the other hand, Descargues et al.20 and Marinari et al.19 have shown that IKKα regulates the expression of Mad1 and Ovol1, c-Myc antagonists, through transforming growth factor-β/smad, in keratinocytes, skin, and skin tumors in human and mice, and that Mad1 and Ovol1 are required to induce keratinocyte differentiation and inhibit keratinocyte proliferation. In the present study, we found that transgenic IKKα substantially induced the expression of Mad1 and Ovol1 in a dose-dependent manner, and that the expression levels of Mad1 and Ovol1 were well correlated with IKKα levels and differentiation levels in the skin and keratinocytes. Together, these results suggest that IKKα regulates keratinocyte differentiation and proliferation through the two antagonizing pathways of EGFR-ligands/ERK/EGFR and Mad1/Ovol1 in maintaining skin homeostasis in a dose-dependent manner.
Interestingly, although Tg-4 mice expressed much higher levels of transgenic IKKα than Tg-7 mice, the level of filaggrin in Tg-4 mice was slightly higher than that in Tg-7 mice. However, when endogenous IKKα was absent, the epidermis of Tg-4/Ikkα−/− mice expressed much higher levels of filaggrin and loricrin than Tg-7/Ikkα−/− mice. The expression of the intermediate differentiation marker K10 did not differ significantly between Tg-7/Ikkα−/− and Tg-4/Ikkα−/− mice. It appears that IKKα is important for inducing terminal differentiation; however, there is a saturated state of overexpressed IKKα in the induction of terminal differentiation. Because our data showed that an increased IKKα dose had a biologically significant function in maintaining epidermal homeostasis, a higher level of IKKα may be better at antagonizing excessive mitotic activity in the skin.
We also noticed that although the transgenic IKKα generally affects these tested molecules in a dose-dependent manner, it appears that the impact of IKKα doses on the individual target has differences, compared with the transgenic IKKα levels between Tg-7 and Tg-4 mice. On the other hand, IKKα doses well induce or inhibit its targets in cultured keratinocytes in a dose-dependent fashion (Figure 6c). Together, these results suggest that the altered levels of IKKα's targets are not only caused by IKKα doses, but also affected by IKKα-mediated multi-changes in vivo (cross-talking). Thus, the impact of IKKα on these tested pathways may not equal in animals.
c-Jun is one of the AP-1 proteins, which are prototypic oncogenes, and it regulates keratinocyte proliferation and differentiation, skin inflammation, and skin cancer.33, 34 The EGFR/HB-EGF pathway is important for the AP-1 pathway in maintaining skin homeostasis. Jun/AP-1 proteins also regulate the expression of cytokines, such as IL-6 and TNFα. JNKs can activate Jun proteins.34 In the present study, we found elevated JNKs and c-Jun activities in the skin of Ikkα−/− mice, and we found that the transgenic IKKα or reduced EGFR repressed these activities in an IKKα dose-dependent manner. Thus, the enhanced JNK and c-Jun activities may contribute to the epidermal hyperplasia, skin inflammation, and tumorigenesis in IKKα-deficient mice.
Many cytokines and growth factors, including IL-6 and EGF, can activate Stat3.35, 36 Stat3 induces the expression of a large array of inflammatory mediators. In the skin, excessive Stat3 activity has a crucial role in inducing and maintaining a pro-carcinogenic inflammatory microenvironment and promoting skin carcinogenesis.36 Here, we showed that IKKα loss elevated Stat3 activity and that transgenic IKKα repressed Stat3 activity in the skin in a dose-dependent fashion. It has been shown that deleting Stat3 in keratinocytes reduces chemical carcinogen and ultraviolet B-induced skin carcinogenesis, and that overexpressed Stat3 in the epidermis promotes skin carcinogenesis.36, 37, 38 Conversely, deleting IKKα in keratinocytes causes spontaneous skin tumors,15 reducing IKKα expression promotes chemical carcinogen and UVB-induced skin carcinogenesis,24, 27 and overexpressing IKKα in the epidermis inhibits the development of malignant skin tumors and metastases induced by chemical carcinogens.16 It appears that Stat3 and IKKα have converse roles in skin carcinogenesis, although IKKα seems to have a more specific effect on embryonic skin development and keratinocyte differentiation than Stat3 does.8, 9, 10, 28, 39 Furthermore, we showed that reducing EGFR repressed Stat3 activity, epidermis thickness, and inflammation in IKKα-deficient skin. Previously, we showed that IKKα suppresses EGFR activity in keratinocytes.15 Thus, Stat3, EGFR, and IKKα pathways crosstalk in regulating skin proliferation, differentiation, and inflammation. Although we did not find that IKKα significantly affects IKK/NF-κB activity, a major player in inducing inflammation, in the skin, the elevated Stat3 activity may promote skin inflammation and carcinogenesis in IKKα-deficient mice. Importantly, overexpressed IKKα can repress Stat3 activity, which may have a key role in preventing skin diseases.
Materials and Methods
Generation of IKKα transgenic mice
The mice used in this study were cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee of The University of Texas, MD Anderson Cancer Center (animal protocol 04-01-05732) and the National Cancer Institute, Smithville, TX, USA (animal protocols 08-074 and 08-075). To generate K5-IKKα transgenic lines, a human IKKα cDNA fragment tagged with HA was subcloned into the NotI and SnaB1 sites of a BK5 vector containing a bovine K5 promoter as previously described.15 The constructed DNA was linearized by XhoI digestion and used to generate Tg-K5-IKKα transgenic mice with an FVB background in the transgenic core located at the Science Park Research Division of The University of Texas, MD Anderson Cancer Center. The polymerase chain reaction primers 5′-AAAGTGTGGGCTGAAGCAGTG-3′ and 5′-GCCCAACAACTTGCTCAAATG-3′ (1273–1819 bp) were used to generate a 546-bp DNA fragment for genotyping. Ikkα−/− and K5-IKKα transgenic mice were crossed to obtain K5-IKKα/Ikkα−/− mice, which were identified by genotyping.8
Gel shift assay
Electrophoretic mobility shift assays of NF-κB DNA-binding activity were performed using a kit (E3300; Promega, Madison, WI, USA). The NF-1 probe was purchased from Santa Cruz Biotechnology Inc. (sc-2553; Santa Cruz, CA, USA). A reaction containing 7.5 μl of 2 × electrophoretic mobility shift assay buffer, 1.5 μg of poly dI-dC, 10 μg of protein lysates, 5 × 103 cpm NF-κB probe, and up to 15 μl of water was incubated at room temperature for 30 min. The protein from HEK 293 cells (5 μg) and the protein from skin samples (30 μg) for each reaction was tested. A 5% non-denatured polyacrylamide gel was pre-run in 0.5 × tris borate EDTA for 1.5 h at 100 V, and the reactions were subjected into the gel. The gel was dried and exposed to film.
Western blot analysis, immunohistochemical staining, and immunoinflorescence staining
Cell lysates (40 μg) or protein extracts from tissues (50 μg) were applied to an acrylamide gel, and the levels of protein expression were measured using western blotting with specific antibodies, as previously described.8, 15 The antibodies used included antibodies against IKKα (IMG 136, Imgenex, San Diego, CA, USA), p-ERK (9101, Cell Signaling Inc., Dancers, MA, USA), ERK1 (SC-93, Santa Cruz Biotechnology Inc., ERK2 (SC-1647, Santa Cruz Biotechnology Inc.), P100/p52 (4882, Cell Signaling Inc.), phosphorylated histone H3 (p-H3, 06-570, Upstate, Millipore, Temecula, CA, USA), p65 (SC-372, Santa Cruz Biotechnology Inc.), phosphorylated p65 (p-65, 3031S, Cell Signaling Inc.), HB-EGF (M-18, Santa Cruz Biotechnology Inc.), HA (F-7, Santa Cruz Biotechnology Inc.; 3724, Cell Signaling Inc.), IκBα (C-21, Santa Cruz Biotechnology Inc.), phosphorylated IκBα (p-IκBα, 9241S, Cell Signaling Inc.), Mad1 (4682, Cell Signaling Inc.), Ovol1 (W-18, sc-102053, Santa Cruz Biotechnology Inc.), β-Actin (A-5441, Sigma-Aldrich, St. Louis, MO, USA), Stat3 (9132, Cell Signaling Inc.), p-Stat3 (9145, Cell Signaling Inc.), RelB (C-19, Santa Cruz Biotechnology Inc.), c-Jun (H-79, Santa Cruz Biotechnology Inc.), p-c-Jun (9261S, Santa Cruz Biotechnology Inc.), p38 (9212, Cell Signaling Inc.), and p-p38 (9211S, Cell Signaling Inc.). Paraffin-embedded skin sections were immunohistochemically stained with K10 and loricrin in the tissue and histology core at Science Park Research Division of The University of Texas, MD Anderson Cancer Center. K14, p-H3, phosphorylated Stat3, granulocyte-differentiation antigen-1, F4/80, and Ovol1 were immunohistochemically stained in the histology and pathology laboratory, National Cancer Institute (Frederick, MD, USA). Immunofluorescent staining of HA was performed as previously described.28
IKK kinase assay
For the immunocomplex IKK assay, glutathione S-transferase-IκBα (amino acids 1−54) was used as a kinase substrate.1 An anti-IKKγ antibody was used to immunoprecipitate the IKK complex from 100 μg of protein lysates.
Southern blot assay
Genomic DNA was isolated from normal skin with an extraction kit (Promega). Southern blotting was performed according to the manufacturer's instructions (Bio-Rad, Hercules, CA, USA). In brief, DNA (10 μg) was digested overnight with BamHI, applied to a 0.9% agarose gel, transferred to Zeta-Probe GT blotting membranes (162-0197, Bio-Rad), and fixed by ultraviolet light. The DNA-blotting membranes were hybridized with an N-terminal IKKα cDNA (nucleotides 1−575) probe.
Keratinocyte culture
Mouse primary keratinocytes were isolated and cultured, as previously described.28 In brief, skin specimens isolated from newborn mice or E19 embryos were treated with 0.25% trypsin (15050-065; Invitrogen, Rockville, MD, USA) for 8 to 10 h at 4 °C; the epidermis was separated from the dermis. The isolated keratinocytes were plated onto 60-cm cell dishes containing a keratinocyte serum-free medium (10785; GIBCO, Rockville, MD, USA). The reintroduction of different IKKα doses in cultured keratinocytes was performed, as previously described.28
Reverse transcriptase-PCR
Total RNA was isolated from the epidermis or keratinocytes using TRI Reagent (Molecular Research Center).24 cDNA was synthesized by a RETROscript kit (Ambion, Inc., Austin, TX, USA). PCR primers used included TNFα (5′-CACCACGCTCTTCTGTCTACTGAAC-3′ and 5′-TGAGATAGCAAATCGGCTGACG-3′), IL-6 (5′-TCTGGGAAATCGTGGAAATGAG-3′ and 5′-TCTCTGAAGGACTCTGGCTTTGTC-3′), IL-1 (5′-CCAGGATGAGGACATGAGCACC-3′ and 5′-TTCTCTGCAGACTCAAACTCCAC-3′), and glyceraldehyde-3-phosphate dehydrogenase.16
Acknowledgments
This work was supported by National Cancer Institute (NCI) grants CA102510, CA117314 (to YH), and CA105345 (to SMF), and by NCI intramural funding.
Glossary
- IKKα
inhibitor of nuclear factor κB kinase-α
- Mad1
Max dimer protein 1
- EGFR
epidermal growth factor receptor
- Stat3
signal transducer and activator of transcription 3
- HB-EGF
heparin-binding epidermal growth factor
- NF-κB
nuclear factor κB
- IκB
inhibitor of NF-κB
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by M Karin
Supplementary Material
References
- DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 (Suppl:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Park E, Liu B, Xia X, Fischer SM, Hu Y. Critical role of IkB kinase alpha in embryonic development and skin carcinogenesis. Histol Histopathol. 2009;24:265–271. doi: 10.14670/HH-24.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski RZ, Baldwin AS., Jr NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–389. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- Rebholz B, Haase I, Eckelt B, Paxian S, Flaig MJ, Ghoreschi K, et al. Crosstalk between keratinocytes and adaptive immune cells in an IkappaBalpha protein-mediated inflammatory disease of the skin. Immunity. 2007;27:296–307. doi: 10.1016/j.immuni.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Baltimore D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, et al. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, et al. Limb and skin abnormalities in mice lacking IKKα. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- Li Q, Lu Q, Hwang JY, Buscher D, Lee KF, Izpisua-Belmonte JC, et al. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 1999;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, et al. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris C, Godfrey VL, Krahn-Senftleben G, Takahashi T, Roberts JL, Schwarz T, et al. Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell. 2000;5:969–979. doi: 10.1016/s1097-2765(00)80262-2. [DOI] [PubMed] [Google Scholar]
- Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J, et al. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 2000;14:854–862. [PMC free article] [PubMed] [Google Scholar]
- Sil AK, Maeda S, Sano Y, Roop DR, Karin M. IKKα acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004;428:660–664. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- Liu B, Xia X, Zhu F, Park E, Carbajal S, Kiguchi K, et al. IKKalpha is required to maintain skin homeostasis and prevent skin cancer. Cancer Cell. 2008;14:212–225. doi: 10.1016/j.ccr.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Park E, Zhu F, Bustos T, Liu J, Shen J, et al. A critical role for IkappaB kinase alpha in the development of human and mouse squamous cell carcinomas. Proc Natl Acad Sci USA. 2006;103:17202–17207. doi: 10.1073/pnas.0604481103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda G, Chiba T, Kawashiri S, Satoh T, Imai K. Epigenetic inactivation of IkappaB kinase-alpha in oral carcinomas and tumor progression. Clin Cancer Res. 2007;13:5041–5047. doi: 10.1158/1078-0432.CCR-07-0463. [DOI] [PubMed] [Google Scholar]
- Van Waes C, Yu M, Nottingham L, Karin M. Inhibitor-kappaB kinase in tumor promotion and suppression during progression of squamous cell carcinoma. Clin Cancer Res. 2007;13:4956–4959. doi: 10.1158/1078-0432.CCR-07-1287. [DOI] [PubMed] [Google Scholar]
- Marinari B, Moretti F, Botti E, Giustizieri ML, Descargues P, Giunta A, et al. The tumor suppressor activity of IKKalpha in stratified epithelia is exerted in part via the TGF-beta antiproliferative pathway. Proc Natl Acad Sci USA. 2008;105:17091–17096. doi: 10.1073/pnas.0809288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descargues P, Sil AK, Sano Y, Korchynskyi O, Han G, Owens P, et al. IKKalpha is a critical coregulator of a Smad4-independent TGFbeta-Smad2/3 signaling pathway that controls keratinocyte differentiation. Proc Natl Acad Sci USA. 2008;105:2487–2492. doi: 10.1073/pnas.0712044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc Natl Acad Sci USA. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M, Afaq F, Adhami VM, Mukhtar H. Lupeol modulates NF-kappaB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene. 2004;23:5203–5214. doi: 10.1038/sj.onc.1207641. [DOI] [PubMed] [Google Scholar]
- Liu B, Yang Y, Chernishof V, Loo RR, Jang H, Tahk S, et al. Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell. 2007;129:903–914. doi: 10.1016/j.cell.2007.03.056. [DOI] [PubMed] [Google Scholar]
- Park E, Zhu F, Liu B, Xia X, Shen J, Bustos T, et al. Reduction in IkappaB kinase alpha expression promotes the development of skin papillomas and carcinomas. Cancer Res. 2007;67:9158–9168. doi: 10.1158/0008-5472.CAN-07-0590. [DOI] [PubMed] [Google Scholar]
- Page A, Navarro M, Garin M, Perez P, Casanova ML, Moreno R, et al. IKKbeta leads to an inflammatory skin disease resembling interface dermatitis. J Invest Dermatol. 2010;130:1598–1610. doi: 10.1038/jid.2010.28. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Hu Y, Karin M. IkappaB kinase alpha is essential for development of the mammalian cornea and conjunctiva. Invest Ophthalmol Vis Sci. 2000;41:3665–3669. [PubMed] [Google Scholar]
- Xia X, Park E, Liu B, Willette-Brown J, Gong W, Wang J, et al. Reduction of IKKalpha expression promotes chronic ultraviolet B exposure-induced skin inflammation and carcinogenesis. Am J Pathol. 2010;176:2500–2508. doi: 10.2353/ajpath.2010.091041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M. IKKα controls formation of the epidermis independently of NF-κB. Nature. 2001;410:710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, et al. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- Seitz CS, Lin Q, Deng H, Khavari PA. Alterations in NF-κB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-κB. Proc Natl Acad Sci USA. 1998;95:2307–2312. doi: 10.1073/pnas.95.5.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind MH, Rozell B, Wallin RP, van Hogerlinden M, Ljunggren HG, Toftgard R, et al. Tumor necrosis factor receptor 1-mediated signaling is required for skin cancer development induced by NF-kappaB inhibition. Proc Natl Acad Sci USA. 2004;101:4972–4977. doi: 10.1073/pnas.0307106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenz R, Scheuch H, Martin P, Frank C, Eferl R, Kenner L, et al. c-Jun regulates eyelid closure and skin tumor development through EGFR signaling. Dev Cell. 2003;4:879–889. doi: 10.1016/s1534-5807(03)00161-8. [DOI] [PubMed] [Google Scholar]
- Zenz R, Eferl R, Scheinecker C, Redlich K, Smolen J, Schonthaler HB, et al. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res Ther. 2008;10:201–211. doi: 10.1186/ar2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano S, Chan KS, DiGiovanni J. Impact of Stat3 activation upon skin biology: a dichotomy of its role between homeostasis and diseases. J Dermatol Sci. 2008;50:1–14. doi: 10.1016/j.jdermsci.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Chan KS, Sano S, Kataoka K, Abel E, Carbajal S, Beltran L, et al. Forced expression of a constitutively active form of Stat3 in mouse epidermis enhances malignant progression of skin tumors induced by two-stage carcinogenesis. Oncogene. 2008;27:1087–1094. doi: 10.1038/sj.onc.1210726. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Angel JM, Sano S, DiGiovanni J. Constitutive activation and targeted disruption of signal transducer and activator of transcription 3 (Stat3) in mouse epidermis reveal its critical role in UVB-induced skin carcinogenesis. Oncogene. 2009;28:950–960. doi: 10.1038/onc.2008.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H, et al. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 1999;18:4657–4668. doi: 10.1093/emboj/18.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.