Abstract
Background
Breast milk is known to protect the infant against infectious and immuno-inflammatory diseases, but the mechanisms of this protection are poorly understood. Objectives: We hypothesized that transforming growth factor-β2 (TGF-β2), an immunoregulatory cytokine abundant in breast milk, may have a direct anti-inflammatory effect on immature human intestinal epithelial cells (IECs).
Methods
Human fetal ileal organ culture, primary human fetal IECs, and the human fetal small intestinal epithelial cell line H4 were stimulated with interleukin 1β (IL-1β) with or without TGF-β2. Pro-inflammatory cytokine secretion and mRNA expression were measured by ELISA and quantitative real-time polymerase chain reaction, respectively. Alterations in ERK signalling were detected from IECs by immunoblotting and in fetal ileal tissue culture by immunohistochemistry. SMAD6 knockdown was performed by transfecting the cells with SMAD6 siRNA.
Results
TGF-β2 significantly attenuated IL-1β-induced pro-inflammatory cytokine production in fetal intestinal organ culture and the cell culture models. In addition, TGF-β2 reduced the IL-1β-induced IL-8 and IL-6 mRNA response in H4 cells. TGF-β2 markedly inhibited IL-1β-induced phosphorylation of ERK, which was necessary for the cytokine response. The inhibitory effect of TGF-β2 on IL-1β-induced cytokine production was completely abrogated by SMAD6 siRNA knockdown.
Conclusions
TGF-β2 attenuates IL-1β-induced pro-inflammatory cytokine production in immature human IECs by inhibiting ERK signalling. The anti-inflammatory effect of TGF-β2 is dependent on SMAD6. Breast milk TGF-β2 may provide the neonate with important immunoregulatory support. TGF-β2 might provide a novel means to improve intestinal immunophysiology in premature neonates.
Key Words: Transforming growth factor-β, Immature intestinal epithelium, Modulation of immune response
Introduction
After birth, the newborn infant is exposed to colonizing bacteria and potential pathogens. During this critical transition period, the intestinal epithelial barrier has not yet reached mature competence and mucosal immune responses are markedly undeveloped [1], especially in premature infants; this renders the infant susceptible to bacterial translocation in the intestine and inflammatory intestinal conditions such as necrotizing enterocolitis (NEC). Breast milk has been observed to significantly reduce the risk of infectious and immuno-inflammatory disease, including NEC, in infants [2,3], but the precise mechanisms of this protective function are unknown.
It is well established that breast milk provides the neonate with important immunoprotection in the form of antimicrobial factors including lysozyme, lactoferrin and defensins, oligosaccharides and maternal IgA antibodies [4]. In addition to this passive protection, breast milk contains several active immunomodulatory molecules such as cytokines and growth factors, but their role in modulating developing mucosal immune function is poorly characterized. In particular, the contribution of breast milk immunomodulatory factors to the observed reduction in infectious and inflammatory disease is elusive since the mechanisms mediating the protective effects remain largely unknown.
Transforming growth factor (TGF)-β is a pleiotropic cytokine/growth factor with effects ranging from regulation of cell proliferation and differentiation to modulation of adaptive immune responses [5]. In the intestine, TGF-β is involved in regulating inflammatory responses, establishing oral tolerance by regulatory T cells, inducting IgA production and enhancing the intestinal epithelial barrier function [5,6]. TGF-β1 is the predominant TGF-β isoform produced by immune cells, whereas TGF-β2 is most abundant in secretions including breast milk. It has been suggested that breast milk TGF-β2 functions as a crucial exogenous source of TGF-β during the neonatal period when endogenous production of TGF-β1 in the intestine has not yet reached mature competence [7,8].
We have previously demonstrated that immature human intestinal epithelial cells (IECs) display an excessive inflammatory response to pro-inflammatory stimuli as compared to mature IECs [9]. Given the important role of IECs in monitoring luminal contents and controlling immune reactions in the intestine, we have proposed that this immature epithelial hyperresponsiveness may contribute to the pathogenesis of NEC [9]. To rapidly acquire immune tolerance towards colonizing bacteria, the neonatal gut is dependent on anti-inflammatory cytokines from breast milk. We hypothesized that TGF-β2 in breast milk acts as an important immunoregulatory signal to IECs immediately after birth. The aim of the present study was to determine whether TGF-β2 at a level found in breast milk has anti-inflammatory potential in the immature intestine and to elucidate the mechanisms of such anti-inflammatory effects.
Materials and Methods
Reagents
Media (DMEM/F12, CMRL, and Opti-MEM I) and other reagents for tissue and cell culture were obtained from Gibco-Invitrogen (Carlsbad, Calif., USA). M3D medium was purchased from Incell Corp. (San Antonio, Tex., USA). Hydrocortisone hemisuccinate, tricine, β-retinyl acetate, collagenase type IV, protease inhibitor cocktail, phosphatase inhibitor cocktail I and II were obtained from Sigma-Aldrich (St. Louis, Mo., USA). Fetal bovine serum was obtained from Atlanta Biologicals (Lawrenceville, Ga., USA). Recombinant human insulin (Novolin R) was obtained from Novo Nordisk A/S (Bagsvaerd, Denmark). Extracellular matrix ECL was obtained from Upstate Biotechnology (Lake Placid, N.Y., USA). The cytokines IL-1β and TGF-β2 and all ELISA reagents were obtained from R&D Systems (Minneapolis, Minn., USA). The BCA Protein Assay kit was obtained from Thermo Scientific (Rockford, Ill., USA). The LDH Cytotoxicity Detecton Kit was obtained from Roche (Mannheim, Germany). Rabbit anti-human p-ERK, ERK, p-JNK, JNK, p-p38, p-38, p-MEK1/2 and MEK1/2 antibodies were obtained from Cell Signaling Technology (Danvers, Mass., USA). Mouse anti-GAPDH was obtained from Fitzgerald (Concord, Mass., USA). SuperSignal West Femto Maximum Sensitivity Substrate and Restore Western Blot Stripping Buffer were obtained from Pierce (Rockford, Ill., USA). The Vectastain Universal Elite ABC kit and 3,3′-diaminobenzidine were obtained from Vector Laboratories (Burlingame, Calif., USA). Permount was obtained from Fisher Chemicals (Fair Lawn, N.J., USA). NuPAGE 4–12% Bis-Tris Gels, SeeBue Plus2 Prestained Standard, human recombinant epidermal growth factor (EGF), Trizol, SuperScript III Platinum SYBR Green One-Step quantitative real-time polymerase chain reaction (qRT-PCR) kits, Stealth siRNA duplex oligoribonucleotides for SMAD6, Stealth™ RNAi negative control, Lipofectamine RNAiMA and BLOCK-iT™ Alexa Fluor Red fluorescent control were obtained from Invitrogen. All other reagents were of analytical or molecular biology grade and obtained from Sigma-Aldrich.
Human Fetal Intestinal Organ Culture and IEC Culture
The study was conducted according to the NIH guidelines and Partners Human Study Committee approval (IRB No. 1999p003833). Fetal intestinal organ culture was prepared from fetal tissue obtained from therapeutic abortions as described previously [9]. Briefly, fetal small intestine was stripped of its mesentery, split longitudinally and cut into explants (5 × 5 mm) which were cultured in a Falcon organ culture dish at 37°C with 95% O2 and 5% CO2 atmosphere saturated with water vapour. The culture medium consisted of CMRL 1066 supplemented with 5% FBS, 5 g/l glucose, 20 mM tricine buffer (pH 7.4), 0.5 μg/l hydrocortisone hemisuccinate, 1 mg/l b-retinyl acetate, 50 U/ml penicillin and 50 μg/ml streptomycin. The tissues were allowed to equilibrate for 30 min before beginning the experiments. We have previously established that tissue explants remain viable up to 48 h [9]. In addition, tissue viability was indirectly assessed by mRNA recovery at the time of sample collection (data not shown).
Intestinal tissue was also used for isolation of primary IECs using a procedure modified from that described by Quaroni and Beaulieu [10]. Briefly, segments of small intestine were incubated with a 1.25% trypsin-0.5 mM EDTA solution at room temperature for 10 min. Mucus and most of the villi on the apical surface were scraped and discarded. Harder scraping was applied to yield intact crypts. Crypts were collected in serum-free medium supplemented with antibiotic-antimycotic solution and incubated with medium containing collagenase type IV (200 units/ml). The dissociated epithelial cells were spun down at 220 g at +4°C and washed in DMEM containing 10% FBS. The final pellet was suspended in OptiMEM supplemented with 20 ng/ml human EGF, 150 nM hydrocortisone 21-hemisuccinate sodium salt, 0.2 U/ml human recombinant insulin and 4% FBS and plated in tissue culture plates coated with extracellular matrix ECL and incubated with 5% CO2 at 32°C. The cells were incubated for 3 h and rinsed vigorously with PBS. Adherent cells were maintained in tissue culture for several passages (3–8) before they were used. Immunostaining for epithelial markers including E-cadherin, cytokeratin 18, mucin and ZO-1 was performed to ensure the cells were epithelial cells.
The non-transformed primary human fetal IEC line H4 was used in these studies. The cell line has been characterized in this laboratory [11]. The cells are morphologically immature and do not polarize or form tight junctions. The H4 culture medium consisted of DMEM supplemented with 5% heat-inactivated fetal bovine serum, 5% heat-inactivated neonatal bovine serum, 1% glutamine, 1% sodium pyruvate, 1% non-essential amino acids, 1% Hepes, 0.2 U/ml insulin, 50 U/ml penicillin and 50 μg/ml streptomycin. The siRNA experiments were conducted using antibiotic-free medium. The cells were cultured in culture dishes at 37°C with 95% O2 and 5% CO2 atmosphere saturated with water vapour. To determine whether TGF-β2 modulates inflammatory responses in mature IECs, the adult IEC line NCM460 was used in these studies. NCM-460 culture medium consisted of M3D medium supplemented with FBS (10%) and 50 U/ml penicillin and 50 μg/ml streptomycin.
Effect of TGF-β2 on Inflammatory Cytokine Secretion
Fetal Intestinal Organ Culture
Fetal intestinal organ culture was prepared from fetal tissue obtained from therapeutic abortions and stimulated with 1 ng/ml IL-1β with or without 3 ng/ml TGF-β2. IL-1β was used for induction of inflammatory cytokine response based on preliminary experiments comparing various inflammatory stimulators. IL-1β induces a constant inflammatory response in all the models used in this study. Unsupplemented medium served as control. All experiments were performed in triplicate at minimum. After 18 h, the culture medium was collected and centrifuged 10 min at 16,000 g at 4°C. The supernatants were stored at −20°C for subsequent determination of IL-8 and MCP-1 by ELISA.
Cell Culture Experiments
Cells were grown to 70% confluence before stimulation with 1 ng/ml IL-1β with or without 3 ng/ml TGF-β2. These concentrations were used in all experiments described in this report. Unstimulated cells were used as control. The concentration of TGF-β2 (3 ng/ml) corresponds to that found in breast milk and was found to be optimal in preliminary dose-response experiments (data not shown). All experiments were performed in triplicate or quadruplicate. After 18 h, the culture medium was collected and centrifuged, and stored at −20°C for IL-8 and IL-6 measurement by ELISA as described previously [12]. IL-8 and IL-6 secretion in IEC culture experiments was normalized to total cellular protein content. Increased cell death due to different treatments might confound cytokine secretion results, and therefore cytotoxicity was assessed using the LDH Cytotoxicity Detection Kit according to manufacturer's instructions. Treatment with IL-1β or TGF-β2 did not result in significant increase in cell death (data not shown). Following removal of culture medium, the cells were washed with PBS and lysed for 30 min on ice with 100 μl/well lysis buffer consisting of 20 mM Hepes (pH 7.6), 150 mM NaCl, 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 1% Triton-X 100, 0.5% SDS, 4 mM Na3VO4, 40 mM NaF and protease and phosphatase inhibitor cocktails I and II. Total protein content was assessed using the BCA Protein Assay Kit as per the manufacturer's protocol.
Effect of TGF-β2 on Inflammatory Cytokine Gene Expression
H4 cells were grown to 70% confluence before stimulation with IL-1β with or without TGF-β2. Untreated cells served as control. All experiments were performed in triplicate. After 6 h, total cellular RNA was extracted by the Trizol-chloroform extraction method as described previously [9]. This time point was chosen based on a preliminary time-course experiment (data not shown). The level of IL-8 and IL-6 mRNA was measured in duplicate for each sample by qRT-PCR using the SuperScript III Platinum SYBR Green One-Step qRT-PCR kit with MJ Opticon 2 DNA engine (MJ Research Inc., Waltham, Mass., USA) according to the manufacturer's instructions. OpticonMONITOR analysis software version 2.01 (MJ Research Inc.) was implemented to normalize the levels of IL-8 and IL-6 mRNA to the standard GAPDH level for each sample.
Involvement of Intracellular Signalling Pathways
Immunoblotting
H4 cells were grown to 70% confluence and treated for 5, 15, 30, 60, 90 and 120 min with IL-1β with or without 3 ng/ml TGF-β2. Untreated cells were used to determine baseline. The cells were then washed with PBS, lysed and protein content was assessed and changing levels of activated kinases were determined by immunoblotting. Equal amounts (30 μg) of total protein were fractionated by electrophoresis using NuPAGE 4–12% Bis-Tris Gels along with protein standards. Fractionated proteins were transferred onto a PDVF membrane, then blocked with 5% non-fat dry milk in TBS with 0.05% Tween 20 (TBST) before incubation overnight with the primary antibody at the recommended concentration. After washing the membrane with 5% milk in TBST, it was incubated with the appropriate horseradish peroxidase-conjugated secondary antibody. The amount of specific protein was visualized by enhanced chemiluminesence using the SuperSignal West Femto Maximum Sensitivity Substrate. To confirm equal loading of the lanes, the membranes were stripped and reprobed with anti-GAPDH antibody.
Immunohistochemistry
Fetal intestinal organ culture was prepared as described above and the explants were stimulated with IL-1β with or without TGF-β2. After 30 min, the explants were rapidly frozen in liquid nitrogen and stored in −80°C. Immunohistochemical staining was performed as described previously [13]. Briefly, series of 5-μm-thick sections were cut from frozen tissue samples embedded in OCT. Immunostaining was performed by incubation with p-ERK antibody (Cell Signalling Technologies, Mass., USA) diluted 1:100 for 60 min followed by incubation with Vectastain Universal Elite ABC kit with 3,3′-diaminobenzidine as chromogen for 60 min employing the protocol and reagents recommended by the manufacturer for frozen sections. Tissues were counterstained in Harris haematoxylin and mounted with Permount. All incubations were performed at room temperature. The specificity of the immunoreaction was monitored by replacing the primary antibody with non-immune sera.
Inhibition of the ERK Pathway
To determine the role of ERK activation in IL-1β-induced secretion of IL-8 and IL-6, the specific MEK1/2 inhibitor PD 98059 was used. H4 cells were grown to 70% confluence and treated with 100 μM PD 98059 1 h prior to challenge with IL-1β with or without TGF-β2. Untreated cells served as control. Dimethyl sulphoxide, in which PD 98059 is solved, was detected to have no effect on IL-1β-induced secretion of IL-8 or IL-6 (data not shown).
SMAD6 siRNA Knockdown in H4 Cells
To determine whether SMAD6 mediates the effects of TGF-β2, experiments using specific siRNA oligonucleotide-mediated knockdown of SMAD6 were carried out. Three potential Stealth™ Select siRNA duplex oligoribonucleotides for SMAD6 constructed using the BLOCK-iT™ RNA designer were obtained from Invitrogen. The optimal siRNA duplex, concentration and time point were determined in a series of preliminary experiments (data not shown). H4 cells were grown in antibiotic-free medium to 50% confluence. After 1 day, the cells were transfected with 10 nM SMAD6 siRNA or 10 nM Stealth RNAi-negative control using Lipofectamine RNAiMAX according to the manufacturer's recommendations. Transfection efficiency was assessed by transfecting the cell with 10 nM BLOCK-iT Alexa Fluor Red fluorescent control and detecting fluorescence 24 h after transfection. Specific knockdown efficiency of SMAD6 was measured by qRT-PCR for SMAD6 mRNA.
Statistical Analyses
Secretion of IL-8 and IL-6 in cell culture experiments was expressed as means ± standard error (SE); comparisons between groups were performed using Student's two-tailed t test. Gene expression data obtained by qRT-PCR and cytokine secretion data from fetal organ culture experiments are expressed as geometric means with SE after logarithmic transformation; comparisons between groups were performed using Student's two-tailed t test after logarithmic transformation. A p value <0.05 was considered statistically significant and indicated by an asterisk (∗). Two (∗∗) and three (∗∗∗) asterisks denote a p value <0.01 and <0.001, respectively.
Results
TGF-β2 Attenuates IL-1β-Induced Inflammatory Cytokine Secretion in the Immature Human Intestine
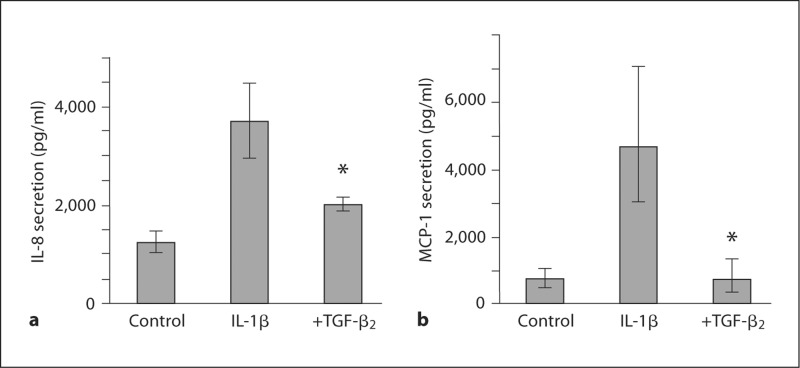
The potential of TGF-β2 to attenuate inflammatory responses in the immature intestinal mucosa was investigated in fetal intestinal organ culture prepared from 18-week-old human fetal intestinal tissue. Baseline IL-8 secretion in unstimulated explants was 1,244 pg/ml (1,055–1,465) and increased to 3,706 pg/ml (3,073–4,470) in response to IL-1β (p = 0.012; fig. 1a). TGF-β2 significantly reduced IL-1β-induced secretion of IL-8 to 2,012 pg/ml (1,883–2,151) (p = 0.037; fig. 1a). In an independent experiment, IL-1β induced MCP-1 secretion from 722 pg/ml (493–1,056) to 7,315 pg/ml (5,968–8,966) (p = 0.0011), which was reduced to 709 pg/ml (372–1,349) by TGF-β2 (p = 0.038; fig. 1b).
Fig. 1.
TGF-β2 attenuates IL-1β-induced inflammatory cytokine responses in the immature human intestine. IL-1β induced a significant increase in IL-8 secretion in fetal intestinal organ culture prepared from an 18-week-old fetus, and this response was significantly reduced by TGF-β2 (a). In a similar independent experiment, MCP-1 secretion was significantly increased in response to IL-1β, and the response was significantly attenuated by TGF-β2 (∗ p < 0.05) (b). a ∗ p < 0.05 compared with IL-1β-induced secretion of IL-8. b ∗ p < 0.05 compared with IL-1β-induced secretion of MCP-1.
TGF-β2 Attenuates IL-1β-Induced Inflammatory Cytokine Secretion in Immature Human Epithelial Cells
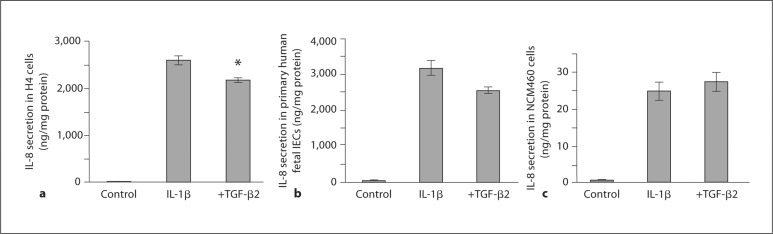
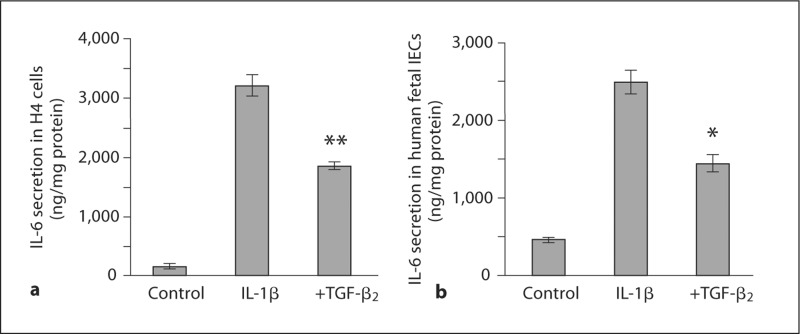
To investigate whether the effect of TGF-β2 observed in the immature intestinal mucosa is reflected at the level of the immature human IECs, H4 cells were stimulated with IL-1β with and without TGF-β2 and the secretion of IL-8 (fig. 2a) and IL-6 (fig. 3a) was measured. IL-1β increased IL-8 secretion from 3 ± 2 to 2,605 ± 98 ng/mg protein (p < 0.0001) and this response was significantly attenuated by TGF-β2 to 2,183 ± 48 ng/mg protein (p = 0.018). IL-1β increased IL-6 secretion from 143 ± 36 to 3,189 ± 176 ng/mg protein (p = 0.0019) and this response was attenuated to 1,835 ± 63 ng/mg protein (p = 0.0019) by TGF-β2.
Fig. 2.
TGF-β2 attenuates IL-1β-induced IL-8 secretion in immature human IECs. IL-1β induced a significant increase in IL-8 secretion in H4 cells, and this response was significantly reduced by TGF-β2 (a). This finding was confirmed in a similar experiment using primary fetal IECs obtained from an 18-week-old fetus (b). However, in the non-transformed adult intestinal epithelial cell line NCM460 (c), IL-1β significantly increased IL-8 secretion, but TGF-β2 failed to attenuate this response. a ∗ p < 0.05 compared with IL-1β-induced secretion of IL-8.
Fig. 3.
TGF-β2 attenuates IL-1β-induced IL-6 secretion in im mature human IECs. IL-1β induced a significant increase in IL-6 secretion in H4 cells, and this response was significantly reduced by TGF-β2 (a). This finding was confirmed in a similar experiment using primary fetal IECs obtained from an 18-week-old fetus (b). a ∗∗ p < 0.01 compared with IL-1β-induced secretion of IL-6. b ∗ p < 0.05 compared with IL-1β-induced secretion of IL-6.
The results obtained from H4 cells were confirmed by conducting a similar experiment using primary fetal IECs isolated from intestinal tissue obtained from an 18-week-old human fetus (fig. 2b, 3b). IL-1β induced a significant increase in IL-8 secretion from 42 ± 5 to 3,189 ± 208 ng/mg protein (p = 0.001). TGF-β2 attenuated IL-1β-induced IL-8 secretion to 2,568 ± 89 ng/mg protein (p = 0.051; fig. 2b). IL-1β increased IL-6 secretion from 66 ± 4 to 2,687 ± 144 ng/mg protein (p = 0.0008), which was reduced to 1,850 ± 115 ng/mg protein by TGF-β2 (p = 0.010; fig. 3b). In a similar experiment with the adult untransformed IEC line NCM460 (fig. 2c), IL-1β induced a significant increase in IL-8 secretion, but unlike immature IECs, the mature enterocytes were unaffected by TGF-β2.
TGF-β2 Attenuates IL-1β-Induced Inflammatory Cytokine Secretion in Immature IECs by Reducing Gene Transcription
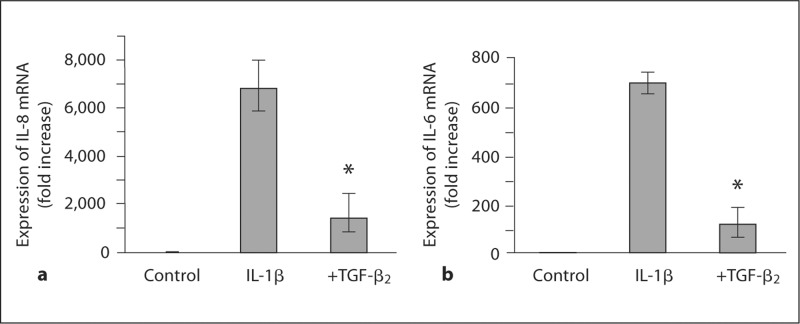
To determine whether TGF-β2 inhibits IL-1β-induced inflammatory cytokine gene expression, H4 cells were stimulated with IL-1β with and without TGF-β2and the expression of mRNA for IL-8 (fig. 4a) and IL-6 (fig. 4b) was measured. Stimulation with IL-1β resulted in a 6,849-fold increase in IL-8 mRNA (p < 0.0001) but was reduced to 1,438-fold by TGF-β2 (p = 0.048). In a similar fashion, IL-6 mRNA expression was increased 691-fold by IL-1β (p < 0.0001) and the response was attenuated to 116-fold by TGF-β2 (p = 0.025).
Fig. 4.
TGF-β2 reduces IL-1β-induced inflammatory cytokine gene expression in H4 cells. IL-1β induced a significant increase in the expression of IL-8 (a) and IL-6 (b) mRNA 6 h after stimulation, and this was significantly reduced by TGF-β2. These data suggest that the immunomodulatory effect of TGF-β2 results from transcriptional regulation of pro-inflammatory gene expression. a ∗ p < 0.05 compared with IL-1β-induced IL-8 mRNA expression. b ∗ p < 0.05 compared with IL-1β-induced IL-6 mRNA expression.
TGF-β2 Attenuates IL-1β-Induced Inflammatory Cytokine Secretion in Immature IECs by Inhibiting the ERK Signalling Pathway
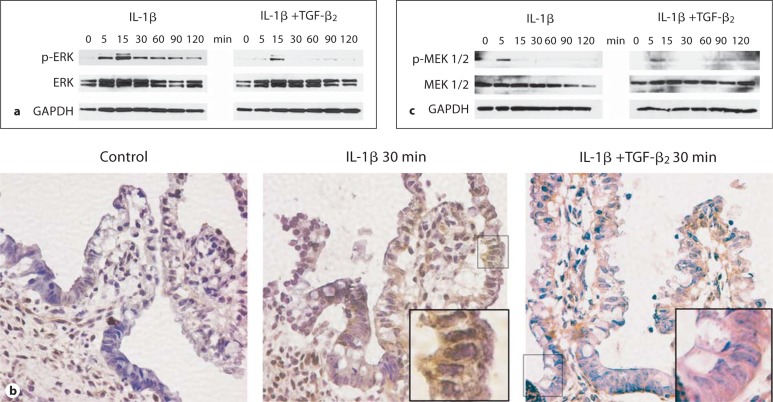
To dissect which intracellular signal transduction pathways are involved in mediating IL-1β-induced IL-8 and IL-6 secretion and attenuation of these responses by TGF-β2, H4 cells were stimulated with IL-1β with or without TGF-β2 and the rate of IκB-α degradation and activation of specific MAP kinase pathways were assessed by immunoblotting. The rate of IL-1β-induced degradation of IκB-α was unaffected by TGF-β2 (data not shown), suggesting no effect on NF-κB signalling. However, specific activation of ERK was detected starting 5 min and up to 120 min after stimulation with IL-1β (fig. 5a). TGF-β2 markedly inhibited IL-1β-induced activation of ERK (fig. 5a). In a similar fashion, activation detected by phosphorylated ERK protein was also detected by immunohistochemistry 30 min after stimulation with IL-1β in the epithelial layer of fetal ileal organ culture explants from a 14-week-old fetus, and this ERK activation was markedly reduced by TGF-β2 (fig. 5b).
Fig. 5.
TGF-β2 inhibits IL-1β-induced ERK activation in H4 cells. Stimulation with IL-1β rapidly induced activation of ERK, which was inhibited by TGF-β2 as demonstrated by immunoblotting for p-ERK, ERK and GAPDH at the indicated time points in H4 cells (a). In a similar fashion, IL-1β induced phosphorylation of ERK after 30 min detected by immunohistochemistry in fetal intestinal organ culture explants obtained from a 14-week-old fetus (b). Phosphorylation of the ERK kinase MEK1/2 was detected 5 min after stimulation with IL-1β in H4 cells by immunoblotting (c). Interestingly, MEK1/2 phosphorylation in response to IL-1β was not affected by TGF-β2.
The rate of MEK1/2 phosphorylation was assayed to determine whether attenuation of IL-1β-induced ERK phosphorylation by TGF-β2 resulted from inhibition of the upstream activating kinase MEK1/2 (fig. 5c). Stimulation with IL-1β resulted in activation of MEK1/2 in 5 min, which was unaffected by TGF-β2 (fig. 5c). The rate of IL-1β-induced activation of JNK or p38 MAP kinases was unaffected by TGF-β2 (data not shown).
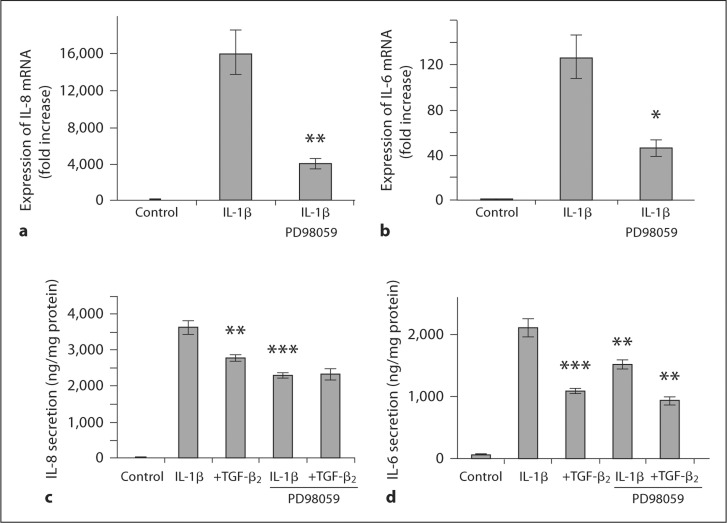
To determine whether the observed inhibition of the ERK pathway by TGF-β2 is causally related to attenuated IL-8 and IL-6 induction by IL-1β, experiments using the specific ERK kinase inhibitor PD 98059 were undertaken. Inhibition of ERK activation by PD 98059 resulted in a significant reduction in IL-1β-induced expression of IL-8 (p = 0.002; fig. 6a) and IL-6 mRNA (p = 0.011; fig. 6b) as well as decreased secretion of IL-8 (p = 0.0006; fig. 6c) and IL-6 protein (p = 0.007; fig. 6d).
Fig. 6.
ERK activation contributes to IL-1β-induced inflammatory cytokine secretion in H4 cells. ERK activation was inhibited in H4 cells by the specific inhibitor PD 98059, which significantly reduced IL-1β-induced expression of IL-8 (a) and IL-6 (b) mRNA. In a similar fashion, secretion of IL-8 (c) and IL-6 (d) protein in H4 cells in response to IL-1β was significantly reduced by PD 98059. These data suggest that ERK activation is necessary for optimal IL-8 and IL-6 responses to IL-1β in H4 cells. a ∗∗ p < 0.01 compared with IL-1β-induced IL-8 mRNA expression. b ∗ p < 0.05, compared with IL-1β-induced IL-6 mRNA expression. c ∗∗ p < 0.01, ∗∗∗ p < 0.001 compared with IL-1β-induced secretion of IL-8. d ∗∗ p < 0.01, ∗∗∗ p < 0.001, compared with IL-1β-induced secretion of IL-6
Inhibition of IL-1β-Induced Inflammatory Cytokine Secretion by TGF-β2 Is Dependent on SMAD6
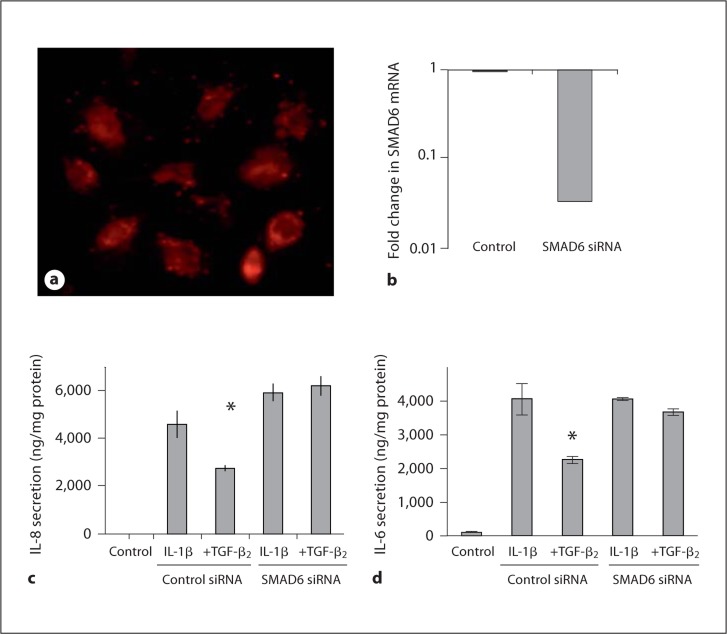
To determine whether SMAD6, an inhibitory signalling molecule activated by TGF-β, is involved in mediating the anti-inflammatory effects of TGF-β2, experiments were carried out after SMAD6 expression in H4 cells was reduced by siRNA (fig. 7). Acceptable transfection efficiency as assessed by the incorporation of Alexa Fluor was achieved (fig. 7a) and a knockdown efficiency of 96% of SMAD6 mRNA was detected 48 h after transfection (fig. 7b). Stimulation with IL-1β resulted in a significant increase in IL-8 and IL-6 secretion in both control and SMAD6 siRNA-treated cells (fig. 7c, d). However, TGF-β2 attenuated IL-8 and IL-6 responses in the control experiment (p = 0.034 and p = 0.018, respectively) whereas SMAD6 siRNA completely abrogated TGF-β2-mediated attenuation of IL-1β-induced IL-8 (p = 0.61) and IL-6 (p = 0.39) secretion by TGF-β2 (fig. 7c, d). These data indicate that activation of inhibitory SMAD6 by TGF-β2 is necessary for the observed immunoregulatory effects of TGF-β2 in immature human IECs.
Fig. 7.
SMAD6 is necessary for TGF-β2-mediated inhibition of IL-1β-induced IL-8 and IL-6 secretion in H4 cells. To determine whether SMAD6 mediates the immunomodulatory effect of TGF-β2 in H4 cells, the cells were transfected with siRNA oligonucleotide specific for SMAD6 or control siRNA. Acceptable transfection efficiency was achieved as assessed by detecting incorporation of Alexa Fluor into cells 24 h after transfection (a). SMAD6 siRNA resulted in 96% reduction in SMAD6 mRNA expression 48 h after transfection as compared to cells transfected with control siRNA (b). IL-1β induced a significant increase in both IL-8 (c) and IL-6 (d) secretion, and this was attenuated by TGF-β2 in control cells. However, SMAD6 siRNA abolished the inhibition of IL-1β-induced production of IL-8 (c) and IL-6 (d) by TGF-β2. These data suggest that SMAD6 is necessary for an anti-inflammatory effect of TGF-β2. c ∗ p < 0.05 compared with IL-1β-induced secretion of IL-8 in control. d ∗ p < 0.05 compared with IL-1β-induced secretion of IL-6 in control.
Discussion
This series of experiments provides evidence for the first time that TGF-β2 attenuates inflammatory responses in the immature human gut at a concentration found in breast milk. Importantly, an anti-inflammatory effect was also seen in cell culture experiments conducted using the fetal intestinal epithelial cell line H4 and primary IECs from an 18-week-old fetus. Breast milk TGF-β2 may thus directly inhibit excessive inflammatory responses in immature IECs. These cells are in direct contact with breast milk TGF-β2 during the neonatal period and also the initial site of the inductive phase of inflammation in the intestine. We therefore conclude that these in vitro observations may reflect the events taking place in the neonatal gut and that IECs are one of the primary targets for immunomodulation by TGF-β2 in breast milk.
TGF-β1 has been reported to inhibit ERK phosphorylation in naïve CD4+ T cells activated through the T cell receptor [14]. Here we describe for the first time that TGF-β2 inhibits IL-1β-induced ERK phosphorylation in immature human IECs. Importantly, the ERK pathway was shown to be necessary for an optimal pro-inflammatory response. Expression of both IL-8 and IL-6 mRNA and secreted protein in response to IL-1β were significantly reduced when ERK signalling was inhibited by PD 98059, a compound which specifically prevents activation of ERK by inhibiting the upstream kinase MEK1/2. The reduction in IL-1β-induced IL-8 secretion by PD 98059 was comparable to that resulting from TGF-β2 and no further reduction in IL-8 secretion was achieved by TGF-β2 in cells treated with PD 98059. This suggests that inhibition of the ERK pathway may be the predominant mechanism by which TGF-β2 attenuates IL-1β-induced IL-8 secretion. In the case of IL-1β-induced IL-6 secretion, however, TGF-β2 appears to have a more complex inhibitory mechanism since secretion of IL-6 was reduced even further by TGF-β2 in cells treated with PD 98059. We found TGF-β2 to have no effect on MEK1/2 phosphorylation, which suggests that TGF-β2 inhibits ERK phosphorylation by interfering with signalling events downstream of activated MEK1/2.
TGF-β signalling from the TGF-β receptor complex is mediated by members of the SMAD signalling molecule family which includes the inhibitory factors SMAD6 and SMAD7 [15]. SMAD6 has recently been detected to inhibit the IκB-α pathway in HEK293 cells [16]. In this model, SMAD6 was activated by TGF-β1 and interrupted IL-1β signalling downstream of its receptor by binding to the complex consisting of Pellino-1, IRAK1, TRAF6 and MyD88. We did not detect reduced IL-1β-induced IκB-α signalling after treatment with TGF-β2 in the present study. Nonetheless, since IRAK1, TRAF6 and MyD88 are all also necessary for activation of the ERK pathway in response to IL-1β [17], we sought to investigate whether SMAD6 plays a role in mediating the effects of TGF-β2 in H4 cells. By knocking down SMAD6 using siRNA, we were able to abolish the TGF-β2-mediated reduction of IL-1β-induced IL-8 and IL-6 secretion. We conclude that SMAD6 is necessary for modulation of the IL-1β-induced inflammatory cytokine production by TGF-β2 through a previously unknown mechanism, the characterization of which is underway but beyond the scope of the present study.
Breast milk TGF-β has been implicated in healthy immune maturation in both experimental animal models [18] and human infants and children [19,20]. It is also well established that TGF-β modulates adaptive immune responses and is involved in the induction and function of regulatory T cells [5]. Less is known about the effects of TGF-β on innate immunity. However, data from an experimental animal model suggest that breast milk TGF-β2 has a direct impact on innate immune responses [21]. Our observations suggest a novel immunomodulatory function of breast milk, with TGF-β2 directly modulating innate immune responses by immature IECs. These cells are in continuous contact with intestinal microbes and constitute the first line of cellular innate immune defence and therefore play a crucial role in launching or withholding from inflammatory responses. As alluded to above, immature IEC hyperresponsiveness may contribute to the detrimental inflammatory vicious cycle central in the pathogenesis of NEC. We therefore interpret our data to suggest that the protective effect of breast milk compared to cow's milk-based infant formula against the development of inflammatory conditions such as NEC may in part be mediated by TGF-β2, perhaps working synergistically with other immunomodulatory factors in breast milk. Despite a recent report according to which commercially available pasteurized cow's milk may contain significant amounts (up to 1.5 ng/ml) of bovine TGF-β2 which remains immunologically active in vitro and in vivo[22], it is not known whether bovine TGF-β is present in infant formula. In experiments conducted using a rat model, it has been demonstrated that maternal milk protects rat pups from developing detrimental immune responses to orally administered bovine β-lactoglobulin [23]. In line with this, feeding infant formula to rat pups results in an allergic-type immune response which is significantly reduced if the formula is supplemented with breast milk levels of TGF-β [24]. These data suggest that infant formula does not contain sufficient amounts of biologically active TGF-β. Taken together, these observations not only emphasize the importance of breast milk to the developing intestine but also provide a rationale for further research into the use of oral TGF-β2 to modulate neonatal intestinal immune responses and novel interventions such as probiotics which have the potential to increase breast milk TGF-β2 concentration [25].
Acknowledgements
We would like to thank Dr. C. Pothoulakis and Dr. P. Moyer for providing the NCM460 cells. Alex Theventhiran is acknowledged for technical assistance.
W.A.W. is supported by grants NIH R01-HD12437; R01-DK70260; P01-DK33506; P30-DK40561. N.N.N. is supported by grants NIH R01-HD059126. S.R. is supported by the Academy of Finland, Finnish Society for Pediatric Research, Foundation for Medical Research in Finland and the Helsingin Sanomat Foundation.
References
- 1.Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. 2000;55:688–697. doi: 10.1034/j.1398-9995.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 2.Quigley MA, Henderson G, Anthony MY, McGuire W. Formula milk versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD002971.pub2. CD002971. [DOI] [PubMed] [Google Scholar]
- 3.Rautava S, Walker WA. Academy of Breastfeeding Medicine founder's lecture 2008: breastfeeding – an extrauterine link between mother and child. Breastfeed Med. 2009;4:3–10. doi: 10.1089/bfm.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007;61:2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 5.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 6.Planchon SM, Martins CA, Guerrant RL, Roche JK. Regulation of intestinal epithelial barrier function by TGF-β1. Evidence for its role in abrogating the effect of a T cell cytokine. J Immunol. 1994;153:5730–5739. [PubMed] [Google Scholar]
- 7.Penttila IA, van Spriel AB, Zhang MF, Xian CJ, Steeb CB, Cummins AG, Zola H, Read LC. Transforming growth factor-β levels in maternal milk and expression in postnatal rat duodenum and ileum. Pediatr Res. 1998;44:524–531. doi: 10.1203/00006450-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Zhang MF, Zola H, Read LC, Penttila IA. Localization of transforming growth factor-β receptor types I, II, and III in the postnatal rat small intestine. Pediatr Res. 1999;46:657–665. doi: 10.1203/00006450-199912000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: a possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci USA. 2000;97:6043–6048. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quaroni A, Beaulieu JF. Cell dynamics and differentiation of conditionally immortalized human intestinal epithelial cells. Gastroenterology. 1997;113:1198–1213. doi: 10.1053/gast.1997.v113.pm9322515. [DOI] [PubMed] [Google Scholar]
- 11.Sanderson IR, Ezzell RM, Kedinger M, Erlanger M, Xu ZX, Pringault E, Leon-Robine S, Louvard D, Walker WA. Human fetal enterocytes in vitro: modulation of the phenotype by extracellular matrix. Proc Natl Acad Sci USA. 1996;93:7717–7722. doi: 10.1073/pnas.93.15.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claud EC, Savidge T, Walker WA. Modulation of human intestinal epithelial cell IL-8 secretion by human milk factors. Pediatr Res. 2003;53:419–425. doi: 10.1203/01.PDR.0000050141.73528.AD. [DOI] [PubMed] [Google Scholar]
- 13.Rautava J, Jee KJ, Miettinen PJ, Nagy B, Myllykangas S, Odell EW, Soukka T, Morgan PR, Heikinheimo K. ERBB receptors in developing, dysplastic and malignant oral epithelia. Oral Oncol. 2008;44:227–235. doi: 10.1016/j.oraloncology.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Luo X, Zhang Q, Liu V, Xia Z, Pothoven KL, Lee C. Cutting Edge: TGF-β-induced expression of Foxp3 in T cells is mediated through inactivation of ERK. J Immunol. 2008;180:2757–2761. doi: 10.4049/jimmunol.180.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmierer B, Hill CS. TGFβ-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 16.Choi KC, Lee YS, Lim S, Choi HK, Lee CH, Lee EK, Hong S, Kim IH, Kim SJ, Park SH. Smad6 negatively regulates interleukin 1-receptor-Toll-like receptor signaling through direct interaction with the adaptor Pellino-1. Nat Immunol. 2006;7:1057–1065. doi: 10.1038/ni1383. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad R, Sylvester J, Zafarullah M. MyD88, IRAK1 and TRAF6 knockdown in human chondrocytes inhibits interleukin-1-induced matrix metalloproteinase-13 gene expression and promoter activity by impairing MAP kinase activation. Cell Signal. 2007;19:2549–2557. doi: 10.1016/j.cellsig.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D, Glaichenhaus N, Julia V. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med. 2008;14:170–175. doi: 10.1038/nm1718. [DOI] [PubMed] [Google Scholar]
- 19.Kalliomaki M, Ouwehand A, Arvilommi H, Kero P, Isolauri E. Transforming growth factor-β in breast milk: a potential regulator of atopic disease at an early age. J Allergy Clin Immunol. 1999;104:1251–1257. doi: 10.1016/s0091-6749(99)70021-7. [DOI] [PubMed] [Google Scholar]
- 20.Oddy WH, Halonen M, Martinez FD, Lohman IC, Stern DA, Kurzius-Spencer M, Guerra S, Wright AL. TGF-β in human milk is associated with wheeze in infancy. J Allergy Clin Immunol. 2003;112:723–728. doi: 10.1016/s0091-6749(03)01941-9. [DOI] [PubMed] [Google Scholar]
- 21.Penttila IA, Flesch IE, McCue AL, Powell BC, Zhou FH, Read LC, Zola H. Maternal milk regulation of cell infiltration and interleukin 18 in the intestine of suckling rat pups. Gut. 2003;52:1579–1586. doi: 10.1136/gut.52.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozawa T, Miyata M, Nishimura M, Ando T, Ouyang Y, Ohba T, Shimokawa N, Ohnuma Y, Katoh R, Ogawa H, Nakao A. Transforming growth factor-β activity in commercially available pasteurized cow milk provides protection against inflammation in mice. J Nutr. 2009;139:69–75. doi: 10.3945/jn.108.092528. [DOI] [PubMed] [Google Scholar]
- 23.Tooley KL, El-Merhibi A, Cummins AG, Grose RH, Lymn KA, DeNichilo M, Penttila IA. Maternal milk, but not formula, regulates the immune response to β-lactoglobulin in allergy-prone rat pups. J Nutr. 2009;139:2145–2151. doi: 10.3945/jn.109.108845. [DOI] [PubMed] [Google Scholar]
- 24.Penttila I. Effects of transforming growth factor-β and formula feeding on systemic immune responses to dietary β-lactoglobulin in allergy-prone rats. Pediatr Res. 2006;59:650–655. doi: 10.1203/01.pdr.0000203149.75465.74. [DOI] [PubMed] [Google Scholar]
- 25.Rautava S, Kalliomaki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol. 2002;109:119–121. doi: 10.1067/mai.2002.120273. [DOI] [PubMed] [Google Scholar]