Abstract
Background
Huntington's disease (HD) is associated with impaired energy metabolism in the brain. Creatine kinase (CK) catalyzes ATP-dependent phosphorylation of creatine (Cr) into phosphocreatine (PCr), thereby serving as readily available high-capacity spatial and temporal ATP buffering. Objective: Substantial evidence supports a specific role of the Cr/PCr system in neurodegenerative diseases. In the brain, the Cr/PCr ATP-buffering system is established by a concerted operation of the brain-specific cytosolic enzyme BB-CK and ubiquitous mitochondrial uMt-CK. It is not yet established whether the activity of these CK isoenzymes is impaired in HD.
Methods
We measured PCr, Cr, ATP and ADP in brain extracts of 3 mouse models of HD – R6/2 mice, N171-82Q and HdhQ111 mice – and the activity of CK in cytosolic and mitochondrial brain fractions from the same mice.
Results
The PCr was significantly increased in mouse HD brain extracts as compared to nontransgenic littermates. We also found an approximately 27% decrease in CK activity in both cytosolic and mitochondrial fractions of R6/2 and N171-82Q mice, and an approximately 25% decrease in the mitochondria from HdhQ111 mice. Moreover, uMt-CK and BB-CK activities were approximately 63% lower in HD human brain samples as compared to nondiseased controls.
Conclusion
Our findings lend strong support to the role of impaired energy metabolism in HD, and point out the potential importance of impairment of the CK-catalyzed ATP-buffering system in the etiology of HD.
Key Words: Huntington's disease, Mitochondria, R6/2 mice, HdhQ111 mice, N171-82Q mice, Human
Introduction
Creatine kinase (CK) catalyzes a reversible ATP-dependent phosphorylation of creatine (Cr) into phosphocreatine (PCr), thereby establishing a readily available high-capacity spatial and temporal ATP buffering system in tissues with large and unsteady energy consumption. The PCr/Cr system can generate ATP 10 times faster than mitochondrial oxidative phosphorylation and 40 times faster than glycolysis [1]. Therefore, CK is considered an important regulator of cellular energy homeostasis. Substantial evidence supports the importance of the PCr/Cr system in neurodegenerative diseases, and the protective effect of Cr supplementation has been well documented in studies on Huntington's disease (HD), amyotrophic lateral sclerosis, Parkinsonism and brain ischemia [2,3].
Two isoenzymes of CK are expressed in brain tissues, brain-specific cytosolic enzyme BB-CK and ubiquitous mitochondrial uMt-CK. Both isoenzymes can be easily and irreversibly damaged by reactive oxygen and nitrogen species. This oxidative damage results in an aberrant intracellular partitioning of CK and its inactivation, such as observed in Alzheimer's disease and amyotrophic lateral sclerosis [2]. The extent of CK inactivation may be very substantial, e.g. BB-CK activity in Alzheimer's disease brain homogenates is decreased by 86%, whereas the expression level of CK was down by less than 14% [3].
Earlier studies found a decrease in BB-CK mRNA in striatum [4] and a significant impairment (approx. 60%) in the total activity of CK in brain homogenates of R6/2 mice [5], the most studied animal model of HD replicating many features of the disease. Surprisingly, R6/2 mice also exhibited increased striatal PCr (by 43%) and Cr (by 23%) at 12 weeks of age as compared to wild-type mice [6]. The isoenzymes of CK were not assayed separately, so it is not clear whether uMt-CK, BB-CK or both were responsible for the decrease in total CK activity. As the expression of CK isoenzymes is cell specific, such information may further advance our understanding of the severely disturbed brain energy metabolism associated with HD [7]. The specificity of the CK activity decrease in HD is also not clear, as it has so far been reported only in one mouse model (R6/2). In this study, we attempted to answer these questions by assaying the uMt-CK and BB-CK activity separately in three mouse HD models as well as in postmortem human brain specimens.
Methods
Animals and Human Tissue Samples
Our experiments were conducted in accordance with the National Institutes of Health (NIH) guidelines for the care and use of experimental animals. R6/2 mice (92 ± 2 days old) and age-matched wild-type controls, 4-month-old N171-82Q mice and their wild-type littermates, and 1-year-old HdhQ111 mice and their littermates were used in the study. Human brain specimens were obtained from the New York Brain Bank at Columbia University, Taub Institute. Two types of specimens were utilized in this study, cortex and caudate putamen samples. All of the HD specimens were collected from individuals diagnosed with HD, stage 3/4 or 2/4.
Sample Preparation and HPLC Assay of Adenine Nucleotides, Cr and PCr
Mice were sacrificed by decapitation immediately followed by head immersion into liquid nitrogen. The brain was dissected from frozen heads on a cold plate chilled with dry ice. One half of a mouse brain was stored at −80°C to be used in the CK assay. Mouse brain tissue (approx. 20 mg) excised without thawing from another brain half was homogenized in 0.2 ml of dry-ice-chilled acetonitrile and mixed with 0.1 ml of ice-cold deionized (MilliQ) H2O. The homogenate was centrifuged at 18,000 g for 15 min at 4°C; 0.15 ml of the supernatant was mixed with 0.15 ml of deionized H2O and centrifuged at 18,000 g for 15 min at 4°C. Measurements of Cr, PCr, ADP and ATP were performed as previously described [8].
Sample Preparation for CK Assay
Frozen Brain Samples. Brain tissue from the same frozen mouse brains as used in the HPLC assays was used. Brain tissue (approx. 50–60 mg) from R6/2 and N171-82Q mice, or dissected tissue samples from human brain specimens (approx. 30–50 mg) were added to 1.2 ml of ice-cold MSEGTA buffer (225 mM mannitol, 75 mM sucrose, 5 mM HEPES at pH 7.4 and 1 mM EGTA) and thawed to approx. 0°C in an ice bath. After that, tissue samples were manually homogenized with a 2-ml Dounce-type glass pestle/glass body tissue grinder (Contes, USA) with 50–60 strokes, and centrifuged at 6,000 g for 4 min at 4°C. The pellets were discarded, and the supernatants (approx. 1 ml) were further centrifuged at 18,000 g for 20 min at 4°C. The 0.8-ml aliquots of cytosol-enriched supernatants were collected and stored at −20°C overnight until assayed for CK activity (‘cytosol’ fractions). The pellets were resuspended in 1.7 ml of MSEGTA buffer and centrifuged at 18,000 g for 10 min at 4°C; the supernatants were discarded and the pellets were resuspended in 1.7 ml and centrifuged at 18,000 g for 10 min at 4°C. The mitochondria-enriched pellets were vigorously resuspended in 0.2 ml of a 0.5% Triton X-100 and 20 mM HEPES (pH 7.8) 1:1 mixture, and centrifuged at 18,000 g for 10 min at 4°C. The supernatant was stored at −20°C overnight until assayed for CK activity (‘mitochondria’ fractions).
Fresh Brain Tissue Samples. HdhQ111 mice and their littermates were sacrificed by cervical dislocation followed by decapitation; brains were excised, dissected clear of olfactory bulbs and the brain stem, and placed in 15 ml of ice-cold MSEGTA buffer. For cytosol extraction, approximately 1/2 of the anterior left or right brain hemisphere was dissected such that it included the frontal cortex and all other underlying brain structures. This dissected part was manually homogenized in 8 ml of ice-cold MSEGTA buffer with a 15-ml Dounce-type glass pestle/glass body tissue grinder (Contes). Brain homogenate was centrifuged at 30,000 g for 15 min at 4°C. The pellets were discarded, and the cytosol-enriched supernatants collected and stored at −20°C overnight until assayed for CK activity (‘cytosol’ fractions). For the isolation of mitochondria, the rest of the brain was manually homogenized in 10 ml of ice-cold MSEGTA buffer with a 15-ml Dounce-type glass pestle/glass body tissue grinder (Contes). The mitochondria were isolated and purified by Percoll gradient, essentially as described by Sims [9], with modifications. Briefly, brain homogenate was diluted to 20 ml with MSEGTA buffer and centrifuged at 6,000 g for 4 min at 4°C. The pellets were discarded, and the supernatants centrifuged at 12,000 g for 10 min at 4°C. The pellets were resuspended in MSEGTA buffer, layered over 7 ml of 23% Percoll gradient (225 mM mannitol, 75 mM sucrose, 5 mM HEPES at pH 7.4, and 1 mM EGTA dissolved in 100% Percoll solution and diluted to 23% with MSEGTA buffer), and centrifuged at 30,000 g for 15 min at 4°C. The lower band was collected and washed 3 times with MSEGTA buffer by centrifuging at 12,000 g for 11 min at 4°C. Finally, the mitochondrial pellets were resuspended in 0.1 ml of MSEGTA buffer and stored at −20°C overnight until assayed for CK activity (‘mitochondria’ fractions).
CK Assay
Cr phosphokinase activity was assayed by a classical procedure [10]. Reaction buffer (t = 37°C; pH 6.5) was composed of 20 mM D-glucose, 2 mM NADP+, 2 mM ADP, 5 mM AMP, 10 mM magnesium acetate, 2 μg/ml oligomycin (inhibitor of mitochondrial ATPase), 15 μM P3,P5-di(adenosine-5′) pentaphosphate (inhibitor of mitochondrial adenylate kinase), 3,500 U/l hexokinase, 2,000 U/l glucose-6-phosphate dehydrogenase, 2 mM DL-Dithiothreitol, 2 mM EDTA, 100 mM imidazole acetate and 20 mM PCr. The reduction of NADP+ to NADPH was followed for 5 min spectrophotometrically as an absorbance change at 340 nm. The reaction rate was calculated from the linear part of the absorbance curve. CK activity was expressed in nanomoles of NADPH reduced per minute per milligram of protein using the extinction coefficient for NADPH (E340 = 6.22 × mM−1).
Immunoblot Analysis
Samples of cytosolic or mitochondrial fractions were diluted to equal protein content (measured with a bicinchoninic acid kit; Pierce, USA) and separated on a 4–20% Tris-glycine gradient SDS-PAGE system (Invitrogen Life Technologies, USA), electroblotted onto a polyvinylidene difluoride membrane (BioRad, USA) and immunoreacted with an appropriate primary antibody followed by horseradish-peroxidase-conjugated secondary antibodies (Kirkegaard Perry Labs Inc., USA). The following antibodies and dilutions were used: Creatine Kinase BB (rabbit polyclonal, 1:1,000; Abcam Inc., USA), Creatine Kinase MT (rabbit polyclonal, 1:1,000; Abcam), Mn-SOD (mouse monoclonal, 1:7,000; Abcam). The immunoreactive proteins were visualized by incubating the blots in the chemiluminescence substrate (Pierce) and developed on a Fuji Medical X-Ray Film (Crystalgen Inc.) by SRX-101A (Ewen-Parker X-Ray Corporation, Elmsford, USA). The developed films were scanned and quantified using ImageJ software, version 1.37 (Wayne Rasband, NIH, USA).
Data Analysis
The data were analyzed by using the MS Office Excel (Microsoft, USA) statistical analysis package with t test function, 2 tails and equal variances.
Results
HPLC Determination of Cr, PCr and Adenine Nucleotides
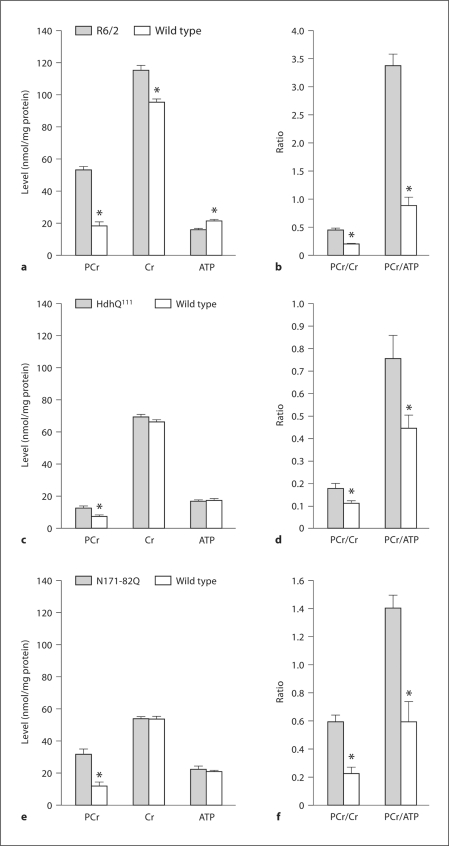
One previous study reported a significant increase in striatal PCr and Cr in 12-week-old R6/2 mice as determined by nuclear magnetic resonance spectroscopy [6]. We confirmed and extended these findings. Figures 1 and 2 show that PCr was significantly increased in R6/2 (fig. 1a), HdhQ111 (fig. 1c) and N171-82Q (fig. 1e) mice as compared to their wild-type controls. However, the Cr level was elevated only in R6/2 mice, and the ATP level was significantly depressed only in R6/2 mice. The PCr/Cr and PCr/ATP ratios were significantly elevated in all HD mouse brain samples (fig. 1b, d, f). Although ATP levels were similar in the wild-type controls in all mouse lines, the amounts of Cr and PCr were 2–3 times higher in brains from R6/2 mice and their wild-type controls as compared to those in HdhQ111 and N171-82Q mice and their littermates. The reason for such a difference is not clear as all samples were processed identically as described in the Methods section; it may therefore be mouse strain related. We also measured AMP and ADP levels but found no difference between HD and wild-type mice in all strains, and no difference between strains (data not presented). Altogether, these data suggest that R6/2, HdhQ111 and N171-82Q HD mice are less capable of CK-catalyzed PCr-to-ATP conversion than their wild-type controls, and that such conversion is more inhibited in R6/2 mice, as evidenced by a much higher PCr/ATP ratio in their brain (PCr/ATP ratio: approx. 3.5) (fig. 1b) as compared to that in HdhQ111 brains (PCr/ATP ratio: approx. 0.8) (fig. 1d) or N171-82Q brains (PCr/ATP ratio: approx. 1.4) (fig. 1f). Therefore, we examined the activity and the protein levels of uMt-CK and BB-CK in R6/2, N171-82Q and HdhQ111 mice.
Fig. 1.
PCr, CR and ATP levels in brains of HD mice. Asterisk: significant difference between HD and wild-type values; p < 0.001 (R6/2), p < 0.05 (HdhQ111) and p < 0.03 (N171-82Q). Number of experiments: n = 14 for R6/2 and their wild-type littermates; n = 10 for HdhQ111 and n = 6 for their wild-type littermates; n = 10 for N171-82Q and their wild-type littermates. a, c, e PCr, Cr and ATP levels in the brains of R6/2, HdhQ111 and N171-82Q mice, respectively. b, d, f PCr/Cr and PCr/ATP ratios in the brains of R6/2, HdhQ111 and N171-82Q mice, respectively.
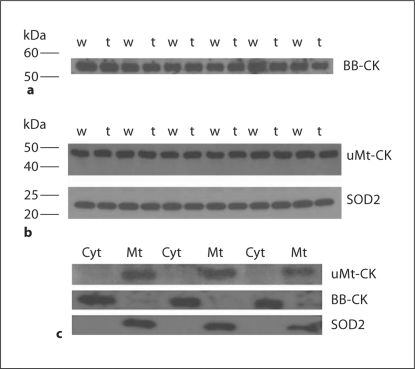
Fig. 2.
Evaluation of brain fractionation procedure. a BB-CK content in cytosol samples extracted from brains of HdhQ111 (labeled ‘t’) and their wild-type littermates (labeled ‘w’). b uMt-CK and Mn-SOD (superoxide dismutase 2, SOD2) content in mitochondrial samples of the same mice. c Cytosol and mitochondria samples from 3 randomly selected pairs of brain samples loaded on gel at the same protein content and stained for uMt-CK, BB-CK and Mn-SOD.
Activity and Protein Levels of CK Isoenzymes in R6/2 and HdhQ111 Mouse Brains
To measure the specific activities of uMt-CK and BB-CK, brain samples were fractionated into cytosol-enriched and mitochondria-enriched fractions, as described in the Methods section. After fractionation, the samples were diluted to equalize their protein content, and the efficiency of fractionation was evaluated by Western blotting with isoenzyme-specific antibodies; the amount of contamination of cytosolic fractions was also evaluated by SOD2-specific antibody, which detects Mn-SOD, a marker enzyme for mitochondrial matrix. Figure 3 presents a typical result of such an evaluation. The cytosolic fractions obtained from brains of HD mice and their littermates were devoid of detectable SOD2 and uMt-CK (fig. 2c), whereas the amount of BB-CK protein was very similar in all samples (fig. 2a presents 6 randomly selected pairs of samples of a total of 9 pairs used in the CK assay). The mitochondria samples were essentially free of detectable BB-CK (fig. 2c presents 3 randomly selected pairs of samples of a total of 9 pairs used in the CK assay), whereas Mn-SOD and uMt-CK amounts were very similar in the mitochondrial fractions from HD mice and their littermates (fig. 2b presents 6 randomly selected pairs of samples of a total of 9 pairs used in the CK assay). This confirms the efficiency of our procedure in separating the mitochondria-localized uMt-CK and cytosol-localized BB-CK.
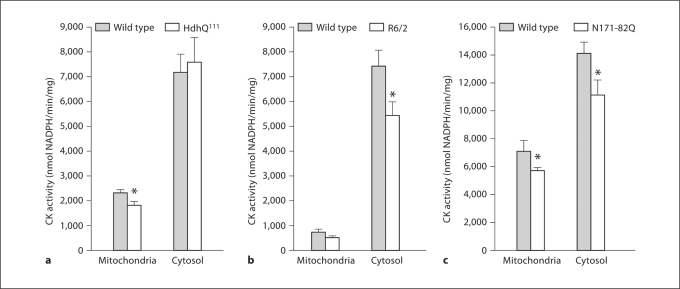
Fig. 3.
CK activity in mitochondrial and cytosolic fractions of HD mice. Asterisk: significant difference between HD and wild-type CK activities; p < 0.01 (HdhQ111), p < 0.03 (R6/2), p < 0.03 (N171-82Q). Number of experiments: n = 9 for HdhQ111 and their wild-type littermates; n = 10 for R6/2 and n = 12 for their wild-type littermates; n = 6 for N171-82Q and n = 5 for their wild-type littermates. a HdhQ111. b R6/2. c N171-82Q.
The activity of uMt-CK was clearly lower in HdhQ111 mice than in their wild-type littermates (1,802.7 ± 132.4 in HD vs. 2,335.7 ± 125.1 in wild type; p < 0.01) (fig. 3a), whereas there was no difference in BB-CK activity (fig. 3a). In R6/2 mice, the BB-CK activity was significantly decreased (7,503.2 ± 659.9 in wild type vs. 5,468.6 ± 554.4 in HD; p < 0.03) (fig. 3b), and the activity of uMt-CK was also lower by approximately 28% (728.9 ± 105.9 in wild type vs. 520.9 ± 90.3 in HD), but the difference did not reach statistical significance. In N171-82Q mice (fig. 3c), both uMt-CK and BB-CK activities were significantly decreased by approximately 25–27% (7,191.3 ± 627.2 in wild type vs. 5,768.2 ± 204.0 in HD, p < 0.03, and 14,129.9 ± 755.4 in wild type vs. 11,234.6 ± 997.1 in HD, p < 0.03, for uMt-CK and BB-CK, respectively).
Summarizing, these data indicate that either cytosolic or mitochondrial CK activity is substantially decreased in all 3 HD mouse models, irrespectively of whether they express N-terminal or full knock-in mutated htt protein.
Activity of CK Isoenzymes in Human HD Brains
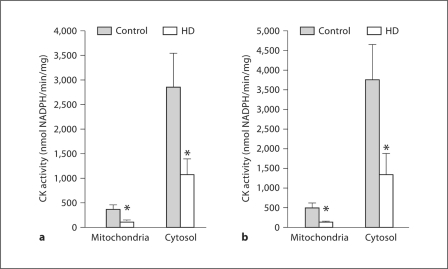
The BB-CK- and uMt-CK-enriched fractions were extracted from human brain specimens as described in the Methods section. Similar to the mouse brain samples, the cytosolic fractions were essentially devoid of uMt-CK and Mn-SOD and rich in BB-CK, whereas the mitochondrial fractions were rich in Mn-SOD and uMt-CK but devoid of BB-CK (data not presented). We found significant decreases in uMt-CK (approx. 64%) (fig. 4a) and BB-CK activity (approx. 62%) (fig. 4a) in caudate putamen HD samples as compared to nondiseased controls. The statistically significant decreases in uMt-CK activity (approx. 72%) and BB-CK activity (approx. 64%) were also apparent in the cortex samples (fig. 4b). Thus, both uMt-CK and BB-CK activities are severely decreased in human HD brain samples as compared to nondiseased controls.
Fig. 4.
CK activity in mitochondrial and cytosolic fractions of human HD and nondiseased control brain specimens. a CK activity in mitochondria-enriched and cytosol samples isolated from the caudate putamen. Asterisk: significant difference in CK activity between HD and nondiseased samples; p < 0.01 for the mitochondria-enriched fractions, p < 0.02 for the cytosolic fractions. Number of experiments: n = 17 for nondiseased mitochondria-enriched samples; n = 18 for HD mitochondria-enriched samples; n = 19 for both HD and nondiseased cytosol samples. b CK activity in mitochondria-enriched and cytosol samples isolated from cortex. Asterisk: significant difference in CK activity between HD and nondiseased samples; p < 0.02 for the mitochondria-enriched fractions, p < 0.035 for the cytosolic fractions. Number of experiments: n = 12 for nondiseased samples; n = 11 for HD samples.
Discussion
The major findings of this study are the significant decrease in CK activity in mouse and human HD samples and significantly higher PCr levels in brains of HD mice as compared to wild-type controls. The elevated PCr may seem an odd but not unprecedented finding in HD mouse brains. Earlier, increased striatal PCr and Cr in 12-week-old R6/2 mice – about the same age as those used in this study – was shown by in vivo 1H-nuclear magnetic resonance spectroscopy [6]. Several studies have indicated that the relationship between CK activity and PCr level in tissues may be complicated and tissue dependent. For example, PCr and Cr levels were only marginally decreased in skeletal muscles of mice deficient in both cytosolic and mitochondrial CK activity [11,12], whereas in the brain tissue of mice genetically rendered free of BB-CK and uMt-CK, the PCr was undetectable without changes in total ATP [13]. The origin of PCr in cells devoid of CK activity is not clear; for the muscle tissue, it was proposed that PCr may accumulate via its uptake from neighboring cells having residual CK activity. Indeed, some PCr uptake from the circulation seems possible [14], and exogenous PCr was reported to protect cultured neurons from 3-nitropropionic acid even better than Cr [15], which is accumulated in the neurons via a specific Cr transporter.
Elevated levels of PCr may indicate that it is metabolically inert, so that cells are not able to use it as an ATP buffer, as earlier suggested [11,12]. This conclusion seems to be in agreement with our findings of decreased CK activity in HD mouse brain.
We also observed a different specificity of the decrease in activity of the CK isoenzymes. While only uMt-CK was decreased in HdhQ111 mice, both isoenzymes were decreased in human HD samples, and both isoenzymes were decreased in N171-82Q mice and in R6/2 mice (although only BB-CK changes reached statistical significance; see figure 3b and commentary in the description of these results). We hypothesize that the selective changes in one or another CK isoenzyme activity might be related to the severity of the disease phenotype. The HD-like phenotype is overt and clear in ≥90-day-old R6/2 mice and human postmortem HD brain samples, which show the decreased activity of both uMt-CK and BB-CK; yet it is very mild (if existent at all) in approximately 1-year-old HdhQ111 mice, which exhibit a decrease in uMt-CK only. It is known that a deficiency in either uMt-CK or BB-CK alone does not result in a severe neurological phenotype; however, a genetic ablation of both isoenzymes (CK–/– double-knockout mice) results in reduced body weight, a 7% reduction in brain weight and hippocampal size, and severely impaired spatial learning in both a dry and a wet maze, lower nest-building activity and diminished acoustic startle reflex responses when compared to age-matched wild-type mice [16].
Another aspect that should be considered is the cell specificity of expression of CK isoenzymes in the brain. In the mouse cerebral cortex, uMt-CK is expressed selectively in excitatory and inhibitory neurons, and localized in their mitochondria in dendrites, cell bodies, axons and terminals [17]. In primary cultures of rat astrocytes, the BB-CK mRNA level is up to 15 times higher than in neurons [18]. In the adult brain, the strongest uMt-CK expression is reported in neurons from layer Va pyramidal cells, thalamic nuclei, cerebellar Purkinje cells, olfactory mitral cells and hippocampal interneurons [19]. The expression of BB-CK is exclusive to inhibitory neurons only in neuronal populations in the mouse brain, and to astrocytes among glial populations [17]. Therefore, a selective damage and degeneration of inhibitory neurons associated with HD might result in a severe decrease in both uMt-CK and BB-CK. The striatal spiny projection neurons which degenerate in HD are inhibitory and utilize γ-aminobutyric acid as a neurotransmitter. Although general atrophy of the cortex and striatum had been observed in 12-week-old R6/2 mice, neither cell loss nor signs of enhanced cell death were detectable [20]. This suggests that any observed CK activity loss in our mouse HD samples was likely due to oxidative damage to CK proteins, to which they are extremely sensitive [2], caused by severe oxidative stress associated with HD [21]. To note, CK was found amongst the most oxidatively damaged proteins in R6/2 brains [5]. We are planning to further examine the relationship between the damage to CK isoenzymes and their enzymatic activity in different HD mouse models and in human HD brain specimens.
Summarizing, our findings lend strong support to the hypothesis of impaired energy metabolism in HD and point out the potential importance of the CK-catalyzed ATP buffering system in the etiology of HD.
Acknowledgment
Some of the data reported in this manuscript were presented at the 2007 annual Society of Neuroscience Meeting [22]. This study was supported by NIH grants AG01493 (A.A.S., M.F.B.) and NS39258 (M.F.B.), and the Huntington Disease Society Coalition for the Cure. We thank the New York Brain Bank at Columbia University, Taub Institute, for supplying human HD samples.
Note Added in Proof
During the submission and review of our manuscript, Kim et al. [1] reported that BB-CK is severely decreased in brain and blood buffy coat specimens of R6/2 mice (18–68%, depending on the age of mice) and human HD patients (28–63%, depending on the severity of disease), which is in a remarkable quantitative agreement with our findings reported here.
References
- 1.Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlattner U, Tokarska-Schlattner M, Wallimann T. Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta. 2006;1762:164–180. doi: 10.1016/j.bbadis.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Bürklen TS, Schlattner U, Homayouni R, Gough K, Rak M, Szeghalmi A, Wallimann T. The creatine kinase/creatine connection to Alzheimer's disease: CK-inactivation, APP-CK complexes and focal creatine deposits. J Biomed Biotechnol. 2006;2006:35936. doi: 10.1155/JBB/2006/35936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luthi-Carter R, Hanson SA, Strand AD, Bergstrom DA, Chun W, Peters NL, Woods AM, Chan EY, Kooperberg C, Krainc D, Young AB, Tapscott SJ, Olson JM. Dysregulation of gene expression in the R6/2 model of polyglutamine disease: parallel changes in muscle and brain. Hum Mol Genet. 2002;11:1911–1926. doi: 10.1093/hmg/11.17.1911. [DOI] [PubMed] [Google Scholar]
- 5.Perluigi M, Poon HF, Maragos W, Pierce WM, Klein JB, Calabrese V, Cini C, de Marco C, Butterfield DA. Proteomic analysis of protein expression and oxidative modification in R6/2 transgenic mice: a model of Huntington disease. Mol Cell Proteomics. 2005;4:1849–1861. doi: 10.1074/mcp.M500090-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Tkac I, Dubinsky JM, Keene CD, Gruetter R, Low WC. Neurochemical changes in Huntington R6/2 mouse striatum detected by in vivo 1H-NMR spectroscopy. J Neurochem. 2007;100:1397–1406. doi: 10.1111/j.1471-4159.2006.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browne SE, Beal MF. The energetics of Huntington's disease. Neurochem Res. 2004;29:531–546. doi: 10.1023/b:nere.0000014824.04728.dd. [DOI] [PubMed] [Google Scholar]
- 8.Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington's disease. J Neurosci. 1998;18:156–163. doi: 10.1523/JNEUROSCI.18-01-00156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem. 1990;55:698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- 10.H⊘rder M, Magid E, Pitkänen E, Härkönen M, Strömme JH, Theodorsen L, Gerhardt W, Waldenström J. Recommended method for the determination of creatine kinase in blood modified by the inclusion of EDTA. The Committee on Enzymes of the Scandinavian Society for Clinical Chemistry and Clinical Physiology (SCE) Scand J Clin Lab Invest. 1979;39:1–5. doi: 10.3109/00365517909104932. [DOI] [PubMed] [Google Scholar]
- 11.Steeghs K, Benders A, Oerlemans F, de Haan A, Heerschap A, Ruitenbeek W, Jost C, van Deursen J, Perryman B, Pette D, Bruckwilder M, Koudijs J, Jap P, Veerkamp J, Wieringa B. Altered Ca2+ responses in muscles with combined mitochondrial and cytosolic creatine kinase deficiencies. Cell. 1997;89:93–103. doi: 10.1016/s0092-8674(00)80186-5. [DOI] [PubMed] [Google Scholar]
- 12.in 't Zandt HJ, de Groof AJ, Renema WK, Oerlemans FT, Klomp DW, Wieringa B, Heerschap A. Presence of (phospho)creatine in developing and adult skeletal muscle of mice without mitochondrial and cytosolic muscle creatine kinase isoforms. J Physiol. 2003;548:847–858. doi: 10.1113/jphysiol.2002.034538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.in ‘t Zandt HJ, Renema WK, Streijger F, Jost C, Klomp DW, Oerlemans F, van der Zee CE, Wieringa B, Heerschap A. Cerebral creatine kinase deficiency influences metabolite levels and morphology in the mouse brain: a quantitative in vivo 1H and 31P magnetic resonance study. J Neurochem. 2004;90:1321–1330. doi: 10.1111/j.1471-4159.2004.02599.x. [DOI] [PubMed] [Google Scholar]
- 14.Down WH, Chasseaud LF, Ballard SA. The effect of intravenously administered phosphocreatine on ATP and phosphocreatine concentrations in the cardiac muscle of the rat. Arzneimittelforschung. 1983;33:552–554. [PubMed] [Google Scholar]
- 15.Brustovetsky N, Brustovetsky T, Dubinsky JM. On the mechanisms of neuroprotection by creatine and phosphocreatine. J Neurochem. 2001;76:425–434. doi: 10.1046/j.1471-4159.2001.00052.x. [DOI] [PubMed] [Google Scholar]
- 16.Streijger F, Oerlemans F, Ellenbroek BA, Jost CR, Wieringa B, van der Zee CE. Structural and behavioural consequences of double deficiency for creatine kinases BCK and UbCKmit. Behav Brain Res. 2005;157:219–234. doi: 10.1016/j.bbr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Tachikawa M, Fukaya M, Terasaki T, Ohtsuki S, Watanabe M. Distinct cellular expressions of creatine synthetic enzyme GAMT and creatine kinases uCK-Mi and CK-B suggest a novel neuron-glial relationship for brain energy homeostasis. Eur J Neurosci. 2004;20:144–160. doi: 10.1111/j.1460-9568.2004.03478.x. [DOI] [PubMed] [Google Scholar]
- 18.Molloy GR, Wilson CD, Benfield P, de Vellis J, Kumar S. Rat brain creatine kinase messenger RNA levels are high in primary cultures of brain astrocytes and oligodendrocytes and low in neurons. J Neurochem. 1992;59:1925–1932. doi: 10.1111/j.1471-4159.1992.tb11028.x. [DOI] [PubMed] [Google Scholar]
- 19.Boero J, Qin W, Cheng J, Woolsey TA, Strauss AW, Khuchua Z. Restricted neuronal expression of ubiquitous mitochondrial creatine kinase: changing patterns in development and with increased activity. Mol Cell Biochem. 2003;244:69–76. [PubMed] [Google Scholar]
- 20.Turmaine M, Raza A, Mahal A, Mangiarini L, Bates GP, Davies SW. Nonapoptotic neurodegeneration in a transgenic mouse model of Huntington's disease. Proc Natl Acad Sci USA. 2000;97:8093–8097. doi: 10.1073/pnas.110078997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browne SE, Beal MF. Oxidative damage in Huntington's disease pathogenesis. Antioxid Redox Signal. 2006;8:2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- 22.Zhang SF, Yang L, Beal M, Starkov AA: Brain creatine kinase activity is decreased in mouse models of Huntington's disease. Society for Neuroscience. Neuroscience Meeting Planner. Program No. 255.12/M26. San Diego, 2007.
Reference
- 1.Kim J, Amante DJ, Moody JP, Edgerly CK, Bordiuk OL, Smith K, Matson SA, Matson WR, Scherzer CR, Rosas HD, Hersch SM, Ferrante RJ. Reduced creatine kinase as a central and peripheral biomarker in Huntington's disease. Biochim Biophys Acta. 2010;1802:673–681. doi: 10.1016/j.bbadis.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]