Abstract
We screened a cohort of 5 male and 20 female patients with a Rett spectrum disorder for mutations in the coding region of FOXG1, previously shown to cause the congenital variant of Rett syndrome. Two de novo mutations were identified. The first was a novel missense mutation, p.Ala193Thr (c.577G>A), in a male patient with congenital Rett syndrome, and the second was the p.Glu154GlyfsX301 (c.460dupG) truncating mutation in a female with classical Rett syndrome, a mutation that was previously reported in an independent patient. The overall rate of FOXG1 mutations in our cohort is 8%. Our findings stress the importance of FOXG1 analysis in male patients with Rett syndrome and in female patients when mutations in the MECP2 and CDKL5 genes have been excluded.
Key Words: Congenital variant, FOXG1 gene, Male patient, Rett syndrome
Introduction
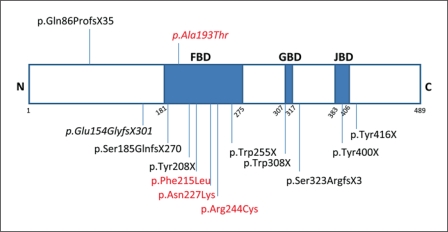
Rett syndrome is a severe neurodevelopmental disorder characterized by psychomotor regression and loss of speech, mental retardation, acquired microcephaly, seizures, and stereotypical hand movements [Rett, 1966]. For a long time, Rett syndrome was thought to be an X-linked dominant condition occurring almost exclusively in females. The clinical phenotype can be variable, and distinction between classical Rett syndrome and variant Rett syndrome has been made by delineation of 5 clinical variants: the infantile onset seizure variant, the congenital variant, the ‘forme fruste’, the late childhood regression variant, and the preserved speech variant [Hagberg et al., 2002]. The first Rett syndrome gene identified was MECP2 on Xq28, encoding methyl-CpG binding protein 2 [Amir et al., 1999]. In classical Rett syndrome, mutations in this gene are found in over 95% of affected girls, but the percentage of mutations identified in patients with variant Rett is much lower [Webb and Latif, 2001; Neul et al., 2010]. Male patients with mutations in MECP2 usually have a phenotype different from Rett syndrome, including severe early postnatal encephalopathy and early death [Kankirawatana et al., 2006]. The cyclin-dependent kinase-like 5 gene (CDKL5), also located on the X-chromosome, was the second Rett gene identified, and mutations in this gene have been identified in both females and males with the infantile seizure variant of the disorder [Mari et al., 2005; Scala et al., 2005]. The FOXG1 gene, located at 14q12 and encoding the forkhead-box protein G1, a brain-specific transcriptional repressor, was recently reported to be responsible for the congenital variant of Rett syndrome [Ariani et al., 2008]. So far, 5 inactivating microdeletions and 12 different point mutations, including 9 truncating and 3 missense have been reported [Ariani et al., 2008; Jacob et al., 2009; Bahi-Buisson et al., 2010; Mencarelli et al., 2010; Philippe et al., 2010; Le Guen et al., 2011a, b]. The reported mutations are scattered throughout the 3 functional domains of the gene (fig 1). Strikingly, all of the patients except one exhibit the congenital variant of Rett syndrome with hypotonia and abnormal development from birth. Most cohorts consisted exclusively of female patients. Recently, however, since FOXG1 is an autosomal gene, a cohort of 50 male patients with Rett-like encephalopathy was screened and a FOXG1 frameshift mutation was identified in a male patient with congenital Rett syndrome [Le Guen et al., 2011a].
Fig. 1.
Mutations in FOXG1 identified to date. The 2 mutations found in male patients are indicated at the top. Missense mutations are indicated in red, the mutations identified in this study are indicated in italics. FBD = Forkhead-binding domain; GBD = Groucho-binding domain; JBD = JARID1B-binding domain.
In this study, we screened a cohort consisting of male and female patients with Rett spectrum disorders and identified de novo mutations in 1 of 5 male and 1 of 20 female patients.
Patients and Methods
Our patient cohort of 20 females and 5 males was recruited from the Child Neurology Department of the University Hospital of Ant- werp. This cohort was selected from a large cohort of approximately 350 patients that was sent in for mutation analysis because of Rett-like features or developmental delay. In all female patients, MECP2 rearrangements and mutations were excluded by Multiplex ligation-dependent probe amplification (MLPA) and sequence analysis. Male patients with a mutation in the MECP2 gene usually have a phenotype clearly distinct from Rett syndrome and were therefore not screened for mutations in this gene. In addition, mutations in the CDKL5 gene were excluded by sequence analysis in patients of both sexes with variant Rett syndrome. In total, 12 MECP2 and 1 CDKL5 mutation have been identified in this group of patients. From this large, clinically heterogeneous cohort, we created a cohort for mutation analysis of the FOXG1 gene of 25 patients by selecting the patients most suspicious for Rett syndrome based on clinical criteria. In this cohort, 11 patients were diagnosed with classical Rett, 5 with congenital Rett, 4 with the early-onset seizure variant, and 5 patients with encephalopathy with seizures and postnatal microcephaly. The whole coding sequence of the FOXG1 gene was screened for mutations by direct sequencing in all patients. Primer sequences and PCR conditions are described in the online supplement data (www.karger.com/doi/10.1159/000330755).
Results
Two unrelated patients out of 25 carried a de novo mutation in the FOXG1 gene. A novel missense mutation, p.Ala193Thr (c.577G>A), was identified in a boy with congenital Rett syndrome. The nucleotide change lies within the DNA-binding forkhead domain and affects a residue highly conserved between different species. It was found neither in 100 normal control chromosomes, nor in the parents. Paternity was confirmed by Powerplex®16 analysis (Promega Corporation, Madison, Wisc.). A truncating mutation, p.Glu154GlyfsX301 (c.460dupG), that has previously been reported as pathogenic was identified in a girl with classical Rett syndrome [Bahi-Buisson et al., 2010]. This 1-bp duplication results in the loss of the 3 functional domains: the forkhead-binding domain, the Groucho-binding domain and the JARID-1B-binding domain. In one female patient with an encephalopathy and secondary microcephaly, we identified the variant p.His57dup (c.159_161dupCCA) that was inherited from an unaffected father.
Clinical Description of Case 1
This first patient is a boy who was born at term to healthy unrelated parents after an uneventful pregnancy. His birth weight was 4 kg (75th centile) and his head circumference (OFC) was 34 cm (10th–25th centile). Length at birth was not recorded. Early developmental problems were observed as there was a delay in eye contact. Head control seemed initially adequate but within weeks after obtaining normal control, regression was noted leading to a loss of head control. The boy had feeding difficulties and excessive crying. Deceleration of head growth and microcephaly was evident within the first 3 months. Seizures started at 4 months of age with infantile spasms followed by consecutively generalized tonic-clonic, myoclonic and atonic seizures. MRI of the brain showed delayed myelination. At the age of 10 years, he had daily drop attacks despite anticonvulsant multitherapy. There was no speech, and he had bursts of inappropriate laughter. He walked with support but had spasticity in all limbs and a dyskinetic movement disorder. Stereotypic hand movements such as hand washing, wringing and mouthing were continuously present. Voluntary hand movement was limited to touching and grasping. He had severe strabismus and teeth grinding.
Clinical Description of Case 2
Case 2 is a woman aged 32 years and first child of healthy unrelated parents after an uneventful pregnancy. She was born at 38 weeks with normal auxological parameters. Initial development was normal according to the parents, and the baby had normal social skills and sat with support at the age of 5 months. A period of regression was described by the mother from the age of 7 months, where loss of voluntary hand use and a decline in social skills rapidly evolved over a few days or weeks. This acute episode coincided with a febrile illness and was considered as an acute infectious encephalopathy, at that time. Irritability with inconsolable crying was constant in this period. By 18 months, she was severely delayed with global hypotonia, no speech, strabismus, and stereotypic hand movements such as hand pulling and mouthing. She never had epileptic seizures. At evaluation, at 32 years of age, she was severely retarded and wheelchair-bound with spastic quadriplegia, scoliosis, hand stereotypies, and dystonia. She had no speech. She continued to have feeding problems and had a gastrostomy and severe reflux oesophagitis. We noted sialorrhea, bruxism and convergent strabismus. An EEG did not show any epileptic abnormalities (fig 2).
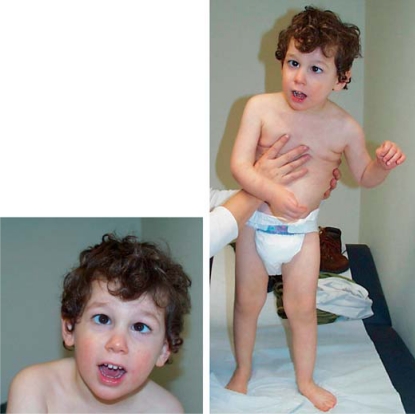
Fig. 2.
Clinical pictures of our male patient with congenital Rett syndrome and a missense mutation in FOXG1 at the age of 2.5 years.
Discussion
This study reports on the identification of 2 de novo FOXG1 mutations, one novel missense mutation and one previously described frameshift mutation, in 2 unrelat- ed patients. This brings the total number of published FOXG1 patients to 14, with 13 different mutations. We report the second male patient with congenital Rett syndrome. Together with an earlier report of the discovery of a frameshift mutation in one patient out of a cohort of 50 males with congenital encephalopathy, microcephaly and movement disorders, our case stresses the importance of screening the FOXG1 gene not only in females, but also in male patients with clinical features of Rett syndrome.
Our male patient carries a de novo p.Ala193Thr missense mutation. To date, only 3 missense mutations in FOXG1 have been reported [Mencarelli et al., 2010; Le Guen, 2011b]. Interestingly, all 4 missense mutations, including the one identified in our patient, are located within the highly conserved DNA-binding forkhead domain of the protein (amino acids 181–275). The clinical phenotype in all 4 patients with missense mutations is comparable and consistent with the congenital variant of Rett syndrome.
In our second female patient, we found, for the first time as far as we know, a frameshift mutation that was previously reported in an unrelated patient [Bahi-Buisson et al., 2010]. This enables comparison of clinical phenotypes in patients with the same genetic abnormality. Both patients with the 1-bp duplication frameshift mutation p.Glu154GlyfsX301 are females. Clinically there is some overlap in features, but the overall clinical picture is different in the sense that our patient fits the classical Rett criteria, and the previously described patient has congenital Rett syndrome. Similarity is found in the following: both had a normal pre- and perinatal period, both are now profoundly mentally retarded and both have convergent strabismus, jerky arm movements, hand stereotypies, bruxism, and gastro-oesophageal reflux. This is not remarkable as these are common features of Rett syndrome and variant Rett. However, noteworthy is that both patients do not have epilepsy. Our patient never had any seizures and is now an adult woman. The previously described patient was 5.8 years, whereas usually in Rett syndrome epilepsy starts at a mean age of 4 years.
In conclusion, our data give additional support to the implication of FOXG1 in the molecular etiology of Rett phenotypes in boys. We therefore highly recommend screening of FOXG1 in all boys with congenital or variant Rett. Reports of further cases are necessary to delineate genotype-phenotype correlations.
Supplementary Material
Supplementary data
Acknowledgements
We thank the patients and their families for their cooperation, and Frank Kooy and Liesbeth Rooms for critical reading of the manuscript. Our research was supported by grants from the Belgian National Fund for Scientific Research-Flanders (FWO).
References
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Ariani F, Hayek G, Rondinella D, Artuso R, Mencarelli MA, et al. FOXG1 is responsible for the congenital variant of Rett syndrome. Am J Hum Genet. 2008;83:89–93. doi: 10.1016/j.ajhg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi-Buisson N, Nectoux J, Girard B, Van Esch H, De Ravel T, et al. Revisiting the phenotype associated with FOXG1 mutations: two novel cases of congenital Rett variant. Neurogenetics. 2010;11:241–249. doi: 10.1007/s10048-009-0220-2. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Hanefeld F, Percy A, Skjeldal O. An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. Eur J Paediatr Neurol. 2002;6:293–297. doi: 10.1053/ejpn.2002.0612. [DOI] [PubMed] [Google Scholar]
- Jacob FD, Ramaswamy V, Andersen J, Bolduc FV. Atypical Rett syndrome with selective FOXG1 deletion detected by comparative genomic hybridization: case report and review of literature. Eur J Hum Genet. 2009;17:1577–1581. doi: 10.1038/ejhg.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankirawatana P, Leonard H, Ellaway C, Scurlock J, Mansour A, et al. Early progressive encephalopathy in boys and MECP2 mutations. Neurology. 2006;67:164–166. doi: 10.1212/01.wnl.0000223318.28938.45. [DOI] [PubMed] [Google Scholar]
- Le Guen T, Bahi-Buisson N, Nectoux J, Bod- daert N, Fichou Y, et al. A FOXG1 mutation in a boy with congenital variant of Rett syndrome. Neurogenetics. 2011a;12:1–8. doi: 10.1007/s10048-010-0255-4. [DOI] [PubMed] [Google Scholar]
- Le Guen T, Fichou Y, Nectoux J, Bahi-Buisson N, Rivier F, et al. A missense mutation within the fork-head domain of the forkhead box g1 gene (FOXG1) affects its nuclear localization. Hum Mutat. 2011b;32:E2026–2035. doi: 10.1002/humu.21422. [DOI] [PubMed] [Google Scholar]
- Mari F, Azimonti S, Bertani I, Bolognese F, Colombo E, et al. CDKL5 belongs to the same molecular pathway of MECP2 and it is responsible for the early-onset seizure variant of Rett syndrome. Hum Mol Genet. 2005;14:1935–1946. doi: 10.1093/hmg/ddi198. [DOI] [PubMed] [Google Scholar]
- Mencarelli MA, Spanhol-Rosseto A, Artuso R, Rondinella D, De Filippis R, et al. Novel FOXG1 mutations associated with the congenital variant of Rett syndrome. J Med Genet. 2010;47:49–53. doi: 10.1136/jmg.2009.067884. [DOI] [PubMed] [Google Scholar]
- Neul JL, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, et al. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68:944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe C, Amsallem D, Francannet C, Lambert L, Saunier A, et al. Phenotypic variability in Rett syndrome associated with FOXG1 mutations in females. J Med Genet. 2010;47:59–65. doi: 10.1136/jmg.2009.067355. [DOI] [PubMed] [Google Scholar]
- Rett A. On an unusual brain atrophy syndrome in hyperammonemia in childhood (in German) Wien Med Wochenschr. 1966;116:723–736. [PubMed] [Google Scholar]
- Scala E, Ariani F, Mari F, Caselli R, Pescucci C, et al. CDKL5/STK9 is mutated in Rett syndrome variant with infantile spasms. J Med Genet. 2005;42:103–107. doi: 10.1136/jmg.2004.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb T, Latif F. Rett syndrome and the MECP2 gene. J Med Genet. 2001;38:217–223. doi: 10.1136/jmg.38.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data