Abstract
Ellis-van Creveld (EvC) syndrome is a rare autosomal recessive malformation syndrome with the main features cardiac defects, postaxial hexadactyly, mesomelic shortening of the limbs, short ribs, dysplastic nails and teeth, oral frenula and various other abnormalities while mental function is normal. We describe 2 adult EvC patients with the cardinal skeletal features of mesomelic short stature and severe, progressive genu valgum deformity, resulting from loss of function mutations in the EVC genes. While the genu valgum was the predominating and disabling feature in patient 1, patient 2 showed acroosteolyses in the distal phalanges and a symmetrical synostosis of metacarpals in his hands. Moreover, patient 2 developed synostoses in the additional fingers in adolescence which had not been present at the age of 12 years, suggesting a further progression of skeletal disease. Joint fusion of phalanges so far has not been reported in EvC syndrome. Our data further expand the phenotypic spectrum of EvC related skeletal malformations and contribute important new information on the clinical course of EvC syndrome with increasing age.
Key Words: Ellis-van Creveld syndrome, EVC genes, Genu valgum, Skeletal manifestation, Treatment
Introduction
Ellis-van Creveld (EvC) syndrome (MIM 225500) [Ellis and van Creveld, 1940] is a rare autosomal recessive malformation syndrome mainly of the heart, the skeleton and the ectodermal derivatives. About two thirds of patients have cardiac defects, usually an atrial septal or atrioventricular septal defect which determines the overall prognosis. Characteristic skeletal features include shortening of the limbs, short ribs and postaxial polydactyly. Oral manifestations are hypodontia, dysplastic teeth, accessory frenula, and gingival hypertrophy. Other features of ectodermal dysplasia are dysplastic nails, while hair growth and sweat glands are usually normal. A variety of other abnormalities are infrequently reported [for review see Baujat and Le Merrer, 2007], including strabismus, epi- and hypospadias, cryptorchidism, pulmonary malformations, and renal anomalies. Head circumference and brain function are generally normal.
The condition is genetically heterogeneous with mutations in the EVC [Ruiz-Perez et al., 2000] and EVC2 genes [Ruiz-Perez et al., 2003] which are located head-to-head on chromosome 4q16. Reported mutation detection rates vary between 67 and 100% depending upon clinical characterization [Tompson et al., 2007; Ruiz-Perez and Goodship, 2009; Valencia et al., 2009]. The range of the clinical phenotypes, in particular in adult patients, has not yet been fully elucidated.
Here we report 2 unrelated patients diagnosed in adulthood with peculiar skeletal manifestations resulting from loss of function mutations in EVC and EVC2, respectively.
Methods
DNA samples were analyzed with informed consent for potential sequence variations within the entire coding regions and flanking splice sites of the EVC and EVC2 gene (reference sequence NM_153717.2 and NM_147127.3) using an ABI Prism BigDye Terminator Cycle Sequencing Kit version 1.1 and ABI 3100 Avant sequencer; Applied Biosystems, Foster City, Calif., USA; further details are available on request.
Case Reports
Patient 1
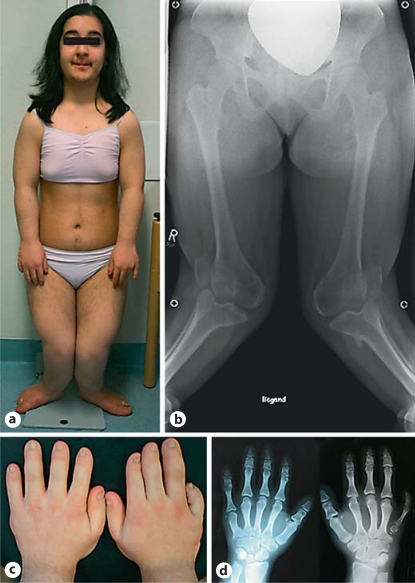
This 18-year-old woman of Turkish origin had an uneventful family history. Her parents came from the same town but were not known to be related. The patient had 2 healthy sisters. After birth postaxial heptadactyly of the right hand and hexadactyly of the left hand was noted, and the additional fingers were removed. When examined at age 17 (fig. 1a), she presented with a very short stature of 124.5 cm (−5.7 SD, www.who.int/growthref/en/), while weight and head circumference were in the low normal range (3rd to 10th centile). She was severely handicapped by her deformed legs. She had postaxial hexadactyly of both feet and short toes with dysplastic nails. X-ray radiography of the skeleton revealed mesomelic shortening of the limbs and severe genu valgum deformity of 40 degrees (fig. 1b). Her hands showed brachydactyly mainly of digits II–III and of the right digit V along with dysplastic nails (fig. 1c). X-ray investigations revealed cone-shaped epiphyses of the phalanges and fusion of the capitates and hamate bones (fig. 1d). The thorax did not show any anomalies by inspection; cardiac and lung function were normal. Ultrasonography of abdominal organs and the heart yielded normal results. She had severe hypodontia. Of her 8 incisors, only 1 was present, and 2 cuspids were missing, while there were no oral frenula or other abnormalities. She was provided with a full dental prosthesis.
Fig. 1.
Patient 1 at the age of 17 years with striking deformity of her legs (a, b). Brachydactyly of the patient's hands, most marked for digits II–III and of the right digit V, nail dysplasia (c). X-ray of the hands showing cone-shaped epiphyses of the phalanges and fusion of the capitates and hamate bones (d).
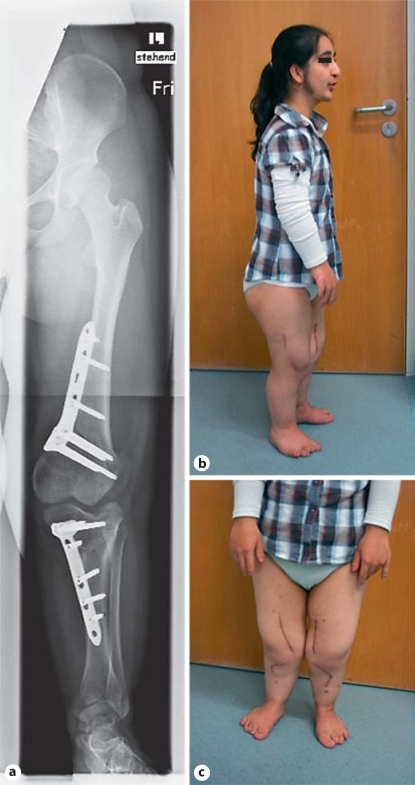
The patient underwent corrective surgery by means of a bilateral anteromedial osteotomy a few months later (fig. 2a). A medial segment of the femoral condyle was removed on both sides; control of the leg alignment and the mechanical axis was achieved with blade plates at the femur and tibia. The dislocated patella was fixated. Postoperative mobilization was possible with partial weight-bearing. Overall, the patient gained 6 cm in height (±1 SD) and had an improved gait after the operation (fig. 2b, c).
Fig. 2.
Patient 1. Radiograph of the left leg after bilateral anteromedial osteotomy at 18 years of age (a). Postoperative impression of the general appearance (b) and the legs of the patient (c).
Genetic analysis showed the previously reported homozygous nonsense mutation c.1195 C>T, p.Arg399X of EVC2 resulting in loss of function of the protein [Ruiz-Perez et al., 2003]. Her parents were both found to be heterozygous carriers of the mutation. They were of normal height and did not show minor anomalies of the EvC syndrome spectrum.
Her family moved to Germany 1 year before genetic counseling; she learnt to speak German within a single year fluently and without an accent. Her intelligence is above average; she visited the last term of a German/Turkish grammar school and was planning to go to university. She was a friendly, communicative person who coped well with her handicap and viewed the future with confidence and optimism.
Patient 2
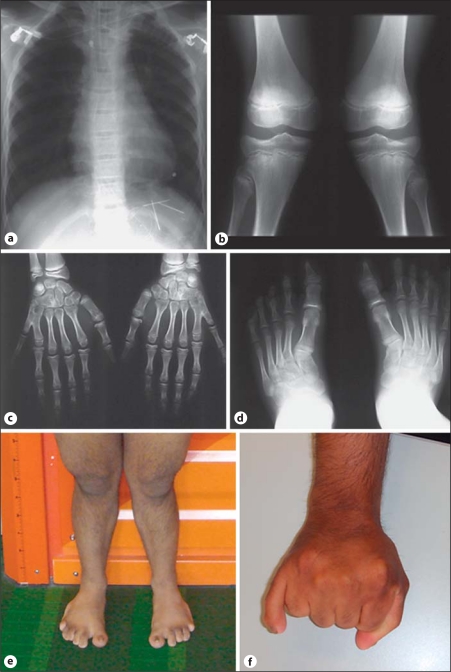
This 30-year-old man was born in Sri Lanka to unrelated parents, who were of normal size and entirely healthy. Reason for genetic counseling was that he was married to a cousin and wanted to learn about his genetic risk. His younger brother died from congenital heart disease at the age of 16 days, and he was said to have been affected by postaxial hexadactyly. In the reported patient, postaxial hexadactyly was diagnosed after birth, along with a complex cardiac defect including atrioseptal defect and a mitral valve insufficiency. He underwent surgical correction of the cardiac defect (common atrium) at the age of 8 and 12 years. At the age of 11.5 years, he measured 120 cm (−3.6 SD). Radiological examination of the skeleton 1 year later showed a normal thoracic cage (fig. 3a), shortening of the lumbar vertebrae, symmetric valgus deformity of the knees (fig. 3b), postaxial hexadacytyly of both hands with acroosteolyses in the distal phalanges II–VI and a symmetrical synostosis of metacarpals V and IV (fig. 3c), cone-shaped distal phalanges of both feet, valgus deformity of both ankles, and hexadactyly of the right foot (fig. 3d). He was mentally normal, visited a normal high school and was trained as a motor mechanic. Further cardiac examinations showed normal heart function with normal ejection fractions. Ultrasound examinations of the abdomen were normal. He had full-time employment and had no physical limitation with respect to his heart function.
Fig. 3.
Patient 2. Radiological examinations at the age of 12 years: Normal thorax configuration, enlarged heart (a). Symmetrical valgus deformity of the knees (b). Postaxial hexadactyly of both hands with acroosteolyses in the distal phalanges II–VI and a symmetrical synostosis of metacarpals V and IV (c), cone-shaped distal phalanges of both feet, valgus deformity of both ankles, and hexadactyly of the right foot (d). Examination at the age of 30 years: mesomelic shortening of the legs (e) and synostosis of the additional fingers on both hands (f).
The next time he was seen in hospital was for genetic counseling after his marriage to his first degree cousin. He showed mesomelic shortening of the legs (fig. 3e) and a synostosis of the additional fingers on both hands (fig. 3f). The patient was 151 cm tall when last examined at age 30 years (−3.5 SD). There was still a prominent valgus deformity of the knees despite surgical correction at the age of 15 years. Dental status showed 2 missing frontal teeth in the upper and lower jaw. Nail dysplasia was noticeable at the feet and at both thumbs. There was no thorax deformity, no oral frenula or other abnormalities. He did not cope well with his outer appearance despite being well integrated into his family and friends. He wanted to refrain from own children if there had been an increased recurrence risk.
Mutation analysis demonstrated the presence of 2 novel heterozygous EVC mutations c.15_16ins46bp and c.1864C>T, p.Arg622X, predicted to cause a loss of function in both alleles. Subsequently, the absence of both EVC mutations could be demonstrated for his spouse, ruling out a high recurrence risk for EvC syndrome according to autosomal recessive inheritance for the offspring in this consanguineous marriage.
Discussion
Our 2 patients add important clinical information on the long-term course of the variable EvC phenotype resulting from EVC and ECV2 gene mutations. Few adults have been reported in the English literature which are summarized in the table and compared with our patients (table 1). The skeletal manifestations of EvC syndrome are usually described in affected children and include postaxial polydactyly, syndactyly of fingers and toes, cone-shaped epiphyses of the phalanges, and carpal fusion [for review see Ruiz-Perez and Goodship, 2009]. Even within the same family, the degree of syndactyly, genu valgum, bone modeling, and polydactyly can be very variable [da Silva et al., 1980]. In the majority of patients, shortening of ribs is observed but was not seen in our patients. Progressive distal shortening of the limbs has been reported; however, the progression of skeletal disease after childhood so far has been only ill defined. Further progression of abnormal bone structure during adolescence and adulthood is suggested in patient 2, as synostosis in the additional fingers had not been present at age 12 and developed later in life. So far, joint fusion of phalanges has not been reported in EvC syndrome.
Table 1.
Report of adult (>18 years) patients with EvC syndrome in the English literature, including our own patients
| Features | da Silva et al., 1980 Patient III.1 | da Silva et al., 1980 Patient III.4 | da Silva et al., 1980 Patient III.7 | da Silva et al., 1980 Patient III.13 | Katsouras et al., 2003 | Ulucan et al., 2008 Patient IV-15 | Ulucan et al., 2008 Patient IV-9 | Alves-Pereira et al, 2009 | Hanemann et al, 2010 | Jockel et al, 2011 | Fukuda et al, 2011 | Patient 1 | Patient 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 82 | 66 | 73 | 61 | 45 | 24 | 38 | 21 | 20 | 19 | 21 | 18 | 30 |
| Sex | female | male | male | male | female | male | male | female | female | female | female | female | male |
| Height (cm) | 142 | 149 | 158.5 | 161 | 132 | n.a. | 161.5 | 135 | 137 | n.a. | n.a. | 124.5 | 151 |
| Short limbs | + | − | − | − | + | + | + | n.a. | + | + | n.a. | + | + |
| Polydactyly | hands/feet | hands/feet | hands/feet | hands/feet | hands | hands/feet | hands/feet | hands | hands/feet | n.a. | hands | hands/feet | hands/right foot |
| Syndactyly | + | + | + | + | − | + | + | n.a. | + | n.a. | n.a. | − | − |
| Brachydactyly | + | + | − | + | + | n.a. | + | + | + | n.a. | n.a | + | + |
| Nail dysplasia | − | − | − | − | + | + | − | + | + | n.a. | n.a. | + | + |
| Genu valgum | − | − | − | − | n.a. | + | n.a. | n.a. | + | + | + | + | + |
| Cardiac malformation | + | − | + | − | ASD | ASD/VSD | VSD | − | − | n.a. | ASD | − | ASD |
| Hypodontia/dysplastic teeth | n.a. | n.a. | n.a. | n.a. | + | n.a. | + | + | + | n.a. | n.a. | + | + |
| Gene defect | n.a. | n.a. | n.a. | n.a. | n.a. | EVC (c.1868T>C) | EVC (c.1868T>C) | n.a. | n.a. | n.a. | n.a. | EVC2 (c.1195C>T) | EVC (c.l5_16ins46bp, c.1864C>T) |
n.a. = Not available; ASD = atrial septal defect; VSD = ventricular septal defect.
Little information is available on longitudinal growth assessment in EvC patients. Adult height ranges between 132 cm to 155 cm in females and 148 cm to 165 cm in males [Verbeek et al., 2011]. Growth data of 101 patients with EvC syndrome were recently summarized by Verbeek et al. [2011], including 10 patients above 20 years of age. Mean growth deviation from Dutch controls was −3.1 SD for male and −3.0 SD for female patients. The growth charts differed from controls in that there was no pubertal growth spurt and no horizontal flattening after puberty. Since the data set was small for patients beyond 10 years of age, it was not possible to decide whether there were biological or statistical reasons for this finding. Growth retardation may also be aggravated by growth hormone deficiency as reported in some EvC patients [Versteegh et al., 2007].
The genu valgum deformity in EvC syndrome is caused by a slanted proximal tibial metaphysis along with hypoplasia of the lateral part of the proximal tibial epiphysis. This was the predominating and disabling feature in our patients. Recently, 2 further patients with recurrent genu valgum deformity were reported [Fukada et al., 2011; Jöckel et al., 2011]. Another Turkish woman has been described with a similar knee deformity, who underwent a open-wedge varising osteotomy of the right knee at the age of 10 years [Jöckel et al., 2011]. Unfortunately, the authors did not report other EvC features of the patient. She developed progressive medial instability and was reoperated using a 3-step technique: (i) soft-tissue release of the knee, (ii) medialization of the tibial tuberosity (Elmslie procedure) and (iii) tibio-fibular medial derotation osteotomy with a T-plate. The authors concluded that operative treatment of knee deformities in EVC syndrome is challenging due to the progressive valgus malalignment often combined with medial instability of the knee and lateral dislocation of the patella [Jöckel et al., 2011].
A Japanese woman, aged 21, underwent bilateral varus dome osteotomies of the proximal tibiae with K-wire at the age of 8 years [Fukada et al., 2011]. However, valgusdeformity and patella dislocation worsened, and a surgical correction including bilateral varus closed-wedge osteotomies of the distal femora and lateral retinacular release with medial reefing was undertaken at 13 years. Final follow-up at 21 years of age showed a significant improvement in the alignment and function of both knee joints. The authorssuggested that a one-stage osteotomy at both femoral and tibial sites should be considered to prevent recurrence of the valgus deformity [Fukada et al., 2011].
EVC and EVC2 gene mutations each account for about half of the patients with genetically confirmed patients, and it has been speculated that additional genes will be responsible for only a small proportion of cases [Ruiz-Perez and Goodship, 2009; Valencia et al., 2009]. Differential diagnosis of EvC syndrome includes the orofaciodigital syndromes, other short rib polydactylies, the Jeune dystrophy, and Weyers acrodental dysostosis [Baujat and Le Merrer, 2007]. EvC syndrome can normally be distinguished from other syndromes by the presence of dysplastic nails and typical deformation of fingers, short limbs with genu valgum deformity, normal mental function, and by its favorable prognosis. Weyers dysostosis is a mild autosomal dominant condition allelic to EvC syndrome and caused by heterozygous mutations of the EVC2 gene removing the final 43 amino acids of the protein [Ruiz-Perez and Goodship, 2009].
Both genes, EVC as well as EVC2, code for a protein with a leucine zipper and a transmembrane domain and have been shown to be involved in the transduction of Sonic hedgehog signaling [Ruiz-Perez and Goodship, 2009]. It is likely that an aberrant response to hedgehog signaling leads to chondrodysplasia similar to mutations in other genes of cilia-related proteins. In cell cultures, Evc and Evc2 are both positive regulators of the hedgehog pathway and interact directly with each other [Blair et al., 2011]. Furthermore, mice homozygous for an Evc null allele reveal remarkable overlap with EvC syndrome including short limbs, short ribs and dental abnormalities, but do not have polydactyly or cardiovascular malformations [Ruiz-Perez and Goodship, 2009]. Histological studies of the growth plate show epiphyseal shortening and defective periostal induction compatible with a defect in Indian hedgehog signaling. The precise role of EVC and EVC2 in hedgehog signal transduction remains to be elucidated, as Evc is not required for ciliogenesis in mouse mutants, despite the fact that both proteins localize to the base of primary cilia [Ruiz-Perez and Goodship, 2009]. Other human disorders of the Indian hedgehog pathway include autosomal recessive acrocapitofemoral dysplasia (MIM 607778) and milder autosomal dominant brachydactyly type A1 (MIM 112500).
At present, there is no clear genotype-phenotype correlation for the 2 EVC genes, most EvC patients have nonsense or frameshift mutations predicting a non-sense-mediated decay of transcripts [Tompson et al., 2007]. Only few missense changes or in-frame deletions are reported which warrant further studies as regards protein function. Furthermore, an unusually mild phenotype with near normal height was seen in a Turkish family with a homozygous missense mutation in EVC [Ulucan et al., 2008], suggesting a less severe clinical manifestation in patients homozygous or compound heterozygous for mutations with residual protein function of the mutant gene product.
Our data further contribute to the phenotypic manifestation and the clinical evolution of EvC syndrome in adulthood. The observations are important for genetic counseling as well as for the clinical management of patients with EvC syndrome.
References
- Alves-Pereira D, Berini-Aytés L, Gay-Escoda C. Ellis-van Creveld syndrome. Case report and literature review. Med Oral Patol Oral Cir Bucal. 2009;14:E340–343. [PubMed] [Google Scholar]
- Baujat G, Le Merrer M. Ellis-van Creveld syndrome. Orphanet J Rare Dis. 2007;2:27. doi: 10.1186/1750-1172-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HJ, Tompson S, Liu Y-N, Campbell J, MacArthur K, et al. Evc2 is a positive modulator of Hedgehog signaling that interacts with Evc at the cilia membrane and is also found in the nucleus. BMC Biology. 2011;9:14. doi: 10.1186/1741-7007-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva EO, Janovitz D, de Albuquerque SC. Ellis-van Creveld syndrome: report of 15 cases in an inbred family. J Med Genet. 1980;17:349–356. doi: 10.1136/jmg.17.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RW, van Creveld S. A syndrome characterized by ectodermal dysplasia, polydactyly, chondrodysplasia, and congenital morbus cordis. Arch Dis Childh. 1940;5:65–84. doi: 10.1136/adc.15.82.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada A, Kato K, Hasegawa M, Nishimura A, Sudo A, Uchida A. Recurrent knee valgus deformity in Ellis-van Creveld syndrome. J Pediatr Orthop B. 2011 doi: 10.1097/BPB.0b013e328345d929. E-pubahead of print. [DOI] [PubMed] [Google Scholar]
- Hanemann JA, de Carvalho BC, Franco EC. Oral manifestations in Ellis-van Creveld syndrome: report of a case and review of the literature. J Oral Maxillofac Surg. 2010;68:456–460. doi: 10.1016/j.joms.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Jöckel JA, Reichel H, Nelitz M. Correction of knee deformity in patients with Ellis-van Creveld syndrome: a case report and review of the literature. Knee. 2011 doi: 10.1016/j.knee.2011.03.003. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Katsouras CS, Thomadakis C, Michalis LK. Cardiac Ellis-van Creveld syndrome. Int J Cardiol. 2003;87:315–316. doi: 10.1016/s0167-5273(02)00289-9. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Goodship JA. Ellis-van Creveld syndrome and Weyers acrodental dysostosis are caused by cilia-mediated diminished response to hedgehog ligands. Am J Med Genet C Semin Med Genet. 2009;151C:341–351. doi: 10.1002/ajmg.c.30226. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Ide SE, Strom TM, Lorenz B, Wilson D, et al. Mutations in a new gene in Ellis-van Crefeld syndrome and Weyers acrodental dysostosis. Nat Genet. 2000;24:283–286. doi: 10.1038/73508. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Tompson SW, Blair HJ, Espinoza-Valdez C, Lapunzina P, et al. Mutations in two nonhomologous genes in a head-to-head configuration cause Ellis-van Creveld syndrome. Am J Hum Genet. 2003;72:728–732. doi: 10.1086/368063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompson SW, Ruiz-Perez VL, Blair HJ, Barton S, Navarro V, et al. Sequencing EVC and EVC2 identifies mutations in two-thirds of Ellis-van Creveld syndrome patients. Hum Genet. 2007;120:663–670. doi: 10.1007/s00439-006-0237-7. [DOI] [PubMed] [Google Scholar]
- Ulucan H, Gül D, Sapp JC, Cockerham J, Johnston JJ, Biesecker LG. Extending the spectrum of Ellis van Creveld syndrome: a large family with a mild mutation in the EVC gene. BMC Med Genet. 2008;9:92–103. doi: 10.1186/1471-2350-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia M, Lapunzina P, Lim D, Zannolli R, Bartholdi D, et al. Widening the mutation spectrum of EVC and EVC2: ectopic expression of Weyer variants in NIH 3T3 fibroblasts disrupts Hedgehog signaling. Hum Mutat. 2009;30:1667–1675. doi: 10.1002/humu.21117. [DOI] [PubMed] [Google Scholar]
- Verbeek S, Eilers PH, Lawrence K, Hennekam RC, Versteegh FG. Growth charts for children with Ellis-van Creveld syndrome. Eur J Pediatr. 2011;170:207–211. doi: 10.1007/s00431-010-1287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteegh FG, Buma SA, Costin G, de Jong WC, Hennekam RC, EvC Working Party Growth hormone analysis and treatment in Ellis-van Creveld syndrome. Am J Med Genet A. 2007;143A:2113–2121. doi: 10.1002/ajmg.a.31891. [DOI] [PubMed] [Google Scholar]