Abstract
Holt-Oram syndrome (HOS) is an autosomal dominant developmental defect involving preaxial radial ray upper limb deformity and variable cardiac defects. It has been demonstrated that HOS is caused by mutations in the T-box transcription factor gene TBX5. Numerous germline mutations (more than 60) of this gene produce preterminal stop codons, which lead to synthesis of a truncated nonfunctional TBX5 protein. The haplo-insufficiency of the TBX5 gene is the most significant cause of HOS. We report on a sporadic patient with clinical features of HOS. Our patient had a cardiac anomaly – a muscular ventricular and atrial septal defect, patent ductus arteriosus and a conduction defect (a first-step atrioventricular block). Upper limb anomalies in our patient were relatively mild and unusual to HOS – distally displaced thumbs, narrow shoulders and hypotrophy of the muscles in the shoulder region. Molecular analysis identified a novel and unusual heterozygous frameshift mutation – c.1304delT (p.Leu435fsX146) – in exon 9 of the TBX5 gene, which is predicted to cause an elongated TBX5 protein with 84 miscoding amino acids and 62 supernumerary C-terminal amino acids. To the best of our knowledge, only one such type of elongation mutation has thus far been reported in the TBX5 gene.
Key Words: c.1304delT mutation, Holt-Oram syndrome, TBX5 gene mutation
Introduction
Holt-Oram syndrome (HOS, OMIM 142900) is an autosomal-dominantly inherited disorder occurring in approximately 1:100,000 live births [Elek et al., 1991], with complete penetrance and high intra- and interfamilial variability of clinical expression [Newbury-Ecob et al., 1996]. HOS is the most common of heart-hand syndromes [McDermott et al., 2005]. Most penetrant clinical features are bilateral upper limb anomalies involving the preaxial radial ray and variable cardiac defects, most commonly atrial septal defects (ASDs) and/or ventricular septal defects (VSDs); cardiac conduction disease may also occur [Basson et al., 1997]. Pulmonary vein defects are also common [Böhm et al., 2008]. The lower limbs are usually not affected in HOS [Gruenauer-Kloevekorn and Froster, 2003].
The main locus for HOS is TBX5 on chromosome 12q24. The TBX5 gene belongs to the Brachyury (T) family, which includes transcription factors with a common DNA-binding domain, the T-box [Wessels and Willems, 2010]. Seventy-four percent of patients with familial or sporadic HOS who were selected in accordance with strict diagnostic criteria had mutations in the TBX5 gene [McDermott et al., 2005]. These mutations are spread throughout the coding exons of TBX5 [Heinritz et al., 2005], and most of the TBX5 mutations are known to be truncation mutations [Basson et al., 1997].
Here, we report a boy with sporadic HOS in whom we found a novel frameshift mutation, c.1304delT, in exon 9 of the TBX5 gene, which causes the elongation of the TBX5 protein.
Case Report
The proband is a boy who is the second child of young, healthy, nonconsanguineous parents. He was born at 38 weeks of gestation by induced labor. His birth weight was 3,680 g (25–50 percentiles), length 52 cm (75–90 percentiles), head circumference 37 cm (75–90 percentiles), and Apgar score 4/7/8. At the 25th week of the pregnancy, bradycardia of the fetus was observed. Using prenatal sonography, VSD was diagnosed, and 0.2 cm of liquid was ascertained in the pericardial cavity; postnatal studies also revealed ASD and patent ductus arteriosus (PDA). From birth, sinus bradycardia and conduction defect were present. At the end of the first week of life heart failure developed, and treatment with diuretics was commenced. Dysmorphic features were dolichocephaly, dysmorphic ears, frenula of the tongue and upper lip, a structurally normal thumb with distal displacement, short distal phalanges of the fingers, a simian crease in the left hand and a sacral dimple. Radiological investigation showed no structural changes in the metacarpal bones. An operation for PDA closing was performed at the age of 3 months; the postoperative period was complicated due to conduction defect. At the age of 8 months, echocardiography revealed muscular VSD, sinus-venosus type ASD, dilatation of the right cavities, hypertrophy of the right ventricle (left ventricle 22–23 mm) and paradoxical moving of the septum. Conduction defect, sinus bradycardia, supraventricular rhythm migration and a first degree atrioventricular blockade were also present. At the age of 1 year, asthma was diagnosed.
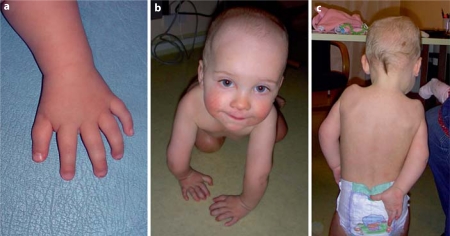
At the age of 15 months his weight was 10.3 kg (10–25 percentiles), height 80.5 cm (50 percentiles) and head circumference 47 cm (10–25 percentiles), and psychomotor development was in the normal range. He showed minimal facial dysmorphism with a prominent forehead, narrow and peculiar shoulders (‘angel wing’- like from back side), pectus excavatum, brachydactyly and a distally placed thumb (fig. 1). He did not have an index finger nipper-function, and his elbow and shoulder joints had contrariwise movement (fig. 1b, c). X-rays showed delayed bone age (the capitulum humeri corresponded to 6 months) and the existence of cubitus valgus (fig. 2). He also had bilateral hypotrophy of the muscles in the regions of the shoulder and ulna.
Fig. 1.
The patient at the age of 15 months.
Fig. 2.
X-rays of the upper limbs of the patient at the age of 15 months (R = right, L = left). See delayed bone age (the small capitulum humeri corresponded to 6 months) and the existence of cubitus valgus.
At the age of 2 years he had a radical heart operation: debanding and closing of the ASD and VSD. The post-operative period was complicated by pneumonia and cardiovascular decompensation. Due to upper limb anomaly, he has been consulted by orthopedic doctors.
Cytogenetic and FISH analyses from peripheral blood lymphocytes revealed a normal male karyotype, 46,XYish 22q11.2 (HIRA×2). Genomic DNA was isolated from the blood of the index patient and his unaffected parents using the salt-precipitation method. Eight coding exons (2–9) and their flanking regions were amplified by PCR using primers from the literature [McDermott et al., 2005]. PCR amplification was performed using Hot Smart Tag (Naxo, Estonia); DMSO (1-μl/25-μl reaction) was added to standard reagents. Products were analyzed on 1.5% agarose gels. The mutation analysis of the complete coding sequence of the TBX5 gene and exon-intron boundaries was performed using the automated bidirectional direct DNA sequencing of PCR products on an ABI PRISM® 377 autosequencer (ABI, Foster City, Calif., USA), using BigDye® Terminator Kit version 1.1 (Applied Biosystems) according to the manufacturer's instructions. Chromatograms were analyzed for TBX5 sequence alterations using specific software.
Informed consent for all genetic studies was obtained from parents according to the protocol approved by the Ethics Committee of the University of Tartu.
We identified a novel heterozygous frameshift mutation (c.1304delT, p.L435fsX146) in exon 9 of the TBX5 gene in our patient (fig. 3). The mutation was verified by resequencing. The germline origin of the mutation was confirmed by the occurrence of the same mutation in the patient's buccal mucosal epithelial cells. Molecular analysis of his parents showed normal results (fig. 3). Due to the frameshift lesion, the coding sequence is predicted to encode for an elongated protein characterized by 84 miscoding (codons 435–519) and 62 supernumerary (codons 519–581) amino acid residues at the C-terminus. This mutation was considered to be pathogenic, as it altered a region of the protein that is required for proper TBX5 function [Böhm et al., 2008].
Fig. 3.
The identification of a novel frameshift mutation predicting an elongated TBX5 protein in HOS. Chromatograms of TBX5 exon 9 showing the c.1304delT deletion (p.L435fsX146). a Unaffected mother, b the heterozygous index patient, c unaffected father.
Discussion
Here, we report on a novel frameshift mutation, c.1304delT (p.L435fsX146), in the TBX5 gene, which is predicted to produce an elongated TBX5 protein of 580 amino acids instead of a normal protein product of 518 amino acids. Most of the previously known mutations in the TBX5 gene are truncating mutations (deletions, missense, nonsense, splice site and frameshift mutations), which result in haploinsufficiency of the TBX5 protein [Gruenauer-Kloevekorn and Froster, 2003; Heinritz et al., 2005; McDermott et al., 2005]. The present finding confirms a previous report by Böhm et al. [2008] describing a single patient with HOS carrying a similar TBX5 frameshift mutation (c.1333delC, p.H445fsX136) predicted to result in an elongated and miscoded TBX5 protein.
T-box genes, including TBX5, are essential in embryogenesis, including specification of the mesoderm, as well as in limb and heart morphogenesis [Liberatore et al., 2000]. The TBX5 protein associates with other cardiac transcription factors, including GATA4 and NKX2-5, and synergistically activates different cardiac effector target genes [Hiroi et al., 2001; Garg et al., 2003; Wessels and Willems, 2010]. TBX5 has also been shown to be required for the normal cardiac conduction system [He et al., 2004]. Our patient demonstrated heart anomalies – septal defects (secondary ASD, muscular VSD) and a conduction defect (first-step atrioventricular block). As our patient was so young, it is possible that a more severe conduction defect may develop in the future. Nevertheless, our patient's upper limb anomalies were relatively mild and unusual to HOS – distally displaced thumbs, narrow shoulders and hypotrophy of the muscles in the region of the shoulders.
The main phenotypic impairments of upper body skeletal structures and the heart in our patient were similar to those of a patient with a c.1333delC mutation, which also causes an elongation of the TBX5 protein (table 1) [Böhm et al., 2008]. However, our patient did not have any structural skeletal changes in the upper limb region. Unfortunately, no functional data are available regarding our patient. Therefore, the next step of this work will aim at generating functional data on the TBX5 C-terminal mutation c.1304delT, e.g. by analyzing the TBX5 protein from biological material or by molecular modeling. To date, we agree with Böhm et al. [2008] that the elongation of the TBX5 tail could alter function by modulating the protein configuration.
Table 1.
Comparison of clinical features of patients with frameshift mutations resulting in elongated and miscoded TBX5 proteins
| Patient | Age years | Sex | Heart anomalies | Limb anomalies | Other features |
|---|---|---|---|---|---|
| c.1333delC [Böhm et al., 2008] | 4 | M | bradycardia due to severe atrioventricular block; muscular VSD; total ASD; right hypoplastic lung and pulmonary veins | bilateral triphalangeal thumbs; bilateral hypoplastic clavicles and radii | micrognathia; long philtrum |
| c.1304delT [present case] | 1.3 | M | bradycardia due to first-step atrioventricular block; conduction defect; muscular VSD; ASD; PDA | bilateral structurally normal thumbs with distal displacement; narrow shoulders; hypotrophy of muscles in region of shoulders | Prominent forehead; frenula of tongue and lip; simian crease in the left hand; cubitus valgus hands; pectus excavatum |
Our observation confirms that mutations resulting in an elongated TBX5 protein underlie HOS. Our results support the idea that any TBX5 mutation not prominently in the T-box region has the potential to affect the development of the heart and the limbs in HOS. The severity of impairments seems, however, to depend on the precise location of the mutation. There may, of course, be additional genetic factors that could synergistically modulate the individual HOS phenotype.
Acknowledgements
We would like to thank the family and the patient for their kind cooperation. This study was supported by Estonian Science Foundation grants GARMP 6573 and GARLA 8175 and by target financing grant SF 0180096s08.
References
- Basson CT, Bachinsky DR, Lin RC, Levi T, Elkins JA, et al. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet. 1997;15:30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- Böhm J, Heinritz W, Craig A, Vujic M, Ekman-Joelsson BM, et al. Functional analysis of the novel TBX5 c.1333delC mutation resulting in an extended TBX5 protein. BMC Med Genet. 2008;9:88. doi: 10.1186/1471-2350-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elek C, Vitéz M, Czeizel E. [Holt-Oram syndrome] Orv Hetil. 1991;132:73–74. 77–78, (in Hungarian) [PubMed] [Google Scholar]
- Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424:443–447. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- Gruenauer-Kloevekorn C, Froster UG. Holt-Oram syndrome: a new mutation in the TBX5 gene in two unrelated families. Ann Genet. 2003;46:19–23. doi: 10.1016/s0003-3995(03)00006-6. [DOI] [PubMed] [Google Scholar]
- He J, McDermott DA, Song Y, Gilbert F, Kligman I, Basson CT. Preimplantation genetic diagnosis of human congenital heart malformation and Holt-Oram syndrome. Am J Med Genet A. 2004;126A:93–98. doi: 10.1002/ajmg.a.20487. [DOI] [PubMed] [Google Scholar]
- Heinritz W, Shou L, Moschik A, Froster UG. The human TBX5 gene mutation database. Hum Mutat. 2005;26:397. doi: 10.1002/humu.9375. [DOI] [PubMed] [Google Scholar]
- Hiroi Y, Kudoh S, Monzen K, Ikeda Y, Yazaki Y, et al. Tbx5 associates with Nkx2–5 and synergistically promotes cardiomyocyte differentiation. Nat Genet. 2001;28:276–280. doi: 10.1038/90123. [DOI] [PubMed] [Google Scholar]
- Liberatore CM, Searcy-Schrick RD, Yutzey KE. Ventricular expression of TBX5 inhibits normal heart chamber development. Dev Biol. 2000;223:169–180. doi: 10.1006/dbio.2000.9748. [DOI] [PubMed] [Google Scholar]
- McDermott DA, Bressan MC, He J, Lee JS, Aftimos S, et al. TBX5 genetic testing validates strict clinical criteria for Holt-Oram syndrome. Pediatr Res. 2005;58:981–986. doi: 10.1203/01.PDR.0000182593.95441.64. [DOI] [PubMed] [Google Scholar]
- Newbury-Ecob RA, Leanage R, Raeburn JA, Young ID. Holt-Oram syndrome: a clinical genetic study. J Med Genet. 1996;33:300–307. doi: 10.1136/jmg.33.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels MW, Willems PJ. Genetic factors in non-syndromic congenital heart malformations. Clin Genet. 2010;78:103–123. doi: 10.1111/j.1399-0004.2010.01435.x. [DOI] [PubMed] [Google Scholar]