Abstract
Purpose
Although occasionally difficult, distinguishing abdominal neuroblastoma (NBL) from Wilms tumor (WT) at presentation is important, as surgical management differs significantly. We reviewed our 20 year experience (1987–2006) treating patients with NBL, focusing on those with an initial diagnosis of WT, to determine presenting features that would have suggested the correct pre-operative diagnosis.
Methods
Retrospective case cohort study reviewing charts and imaging of patients with NBL initially diagnosed clinically with WT. Preoperative symptoms, laboratory studies, and imaging were evaluated. Similar variables were assessed in the 20 patients with WT most recently treated at our institution.
Results
Nine patients with NBL were identified who had an exploratory laparotomy with a pre-operative diagnosis of WT;eight underwent nephrectomy at exploration. Children with NBL had symptoms such as fever and weight loss at presentation (67%) more often than patients with WT (20%). Pre-operative computed tomography (CT) demonstrated intratumoral calcifications, vascular encasement, or both in 78% of patients with NBL but were never seen in WT patients. Of interest, pre-operative urinary catecholamines were elevated in five patients ultimately diagnosed with NBL.
Conclusion
Although NBL can be mistaken for WT at presentation, the presence of constitutional symptoms, or intratumoral calcification or vascular encasement on preoperative imaging should heighten suspicion for NBL. In addition, laboratory evaluation, including urinary catecholamines, should be completed prior to surgery when the etiology of an abdominal tumor is uncertain.
Keywords: Neuroblastoma, Wilms tumor, Nephrectomy
Introduction
Neuroblastoma (NBL) and Wilms tumor (WT) represent two of the most common solid tumors of childhood1. While there are some similarities in the initial presentation of patients with these two tumor types, a thorough pre-operative work-up including a detailed history, physical examination, laboratory evaluation, and diagnostic imaging should help establish the correct diagnosis. Distinguishing between these tumor types prior to exploration and potential tumor resection is critical, as the surgical management for NBL and WT differs significantly with regard to the extent and timing of tumor resection, particularly in the setting of high-risk NBL. Treatment of WT usually begins with nephrectomy, whereas patients with high-risk abdominal neuroblastoma generally receive neoadjuvant chemotherapy, with every effort being made to preserve both kidneys at the time of delayed tumor resection. The purpose of the current investigation was to determine which of the pre-operative findings would be most useful when the distinction between these two diagnoses is uncertain. In particular, we focused on a group of patients operated on at presentation with a presumed diagnosis of WT who were subsequently found to have NBL.
Methods
We reviewed the records of nine patients treated at St. Jude Children’s Research Hospital during a 20 year period (1987–2006) for NBL, who had initially been diagnosed with WT and, as a result, had undergone an exploratory laparotomy with planned nephrectomy after presenting to their local institutions. Pre-operative factors including the presenting symptoms, physical examination, and laboratory values including complete blood count (CBC), serum lactate dehydrogenase (LDH), and urinary catecholamines/metanephrines were evaluated. In addition, the pre-operative CT reports, peri-operative progress notes, operative notes and pathology reports were reviewed. To serve as a control group, these variables were evaluated in the last 20 patients treated for WT at our institution. This retrospective review was approved by the St. Jude Children’s Research Hospital Institutional Review Board (XPD07-025).
Results
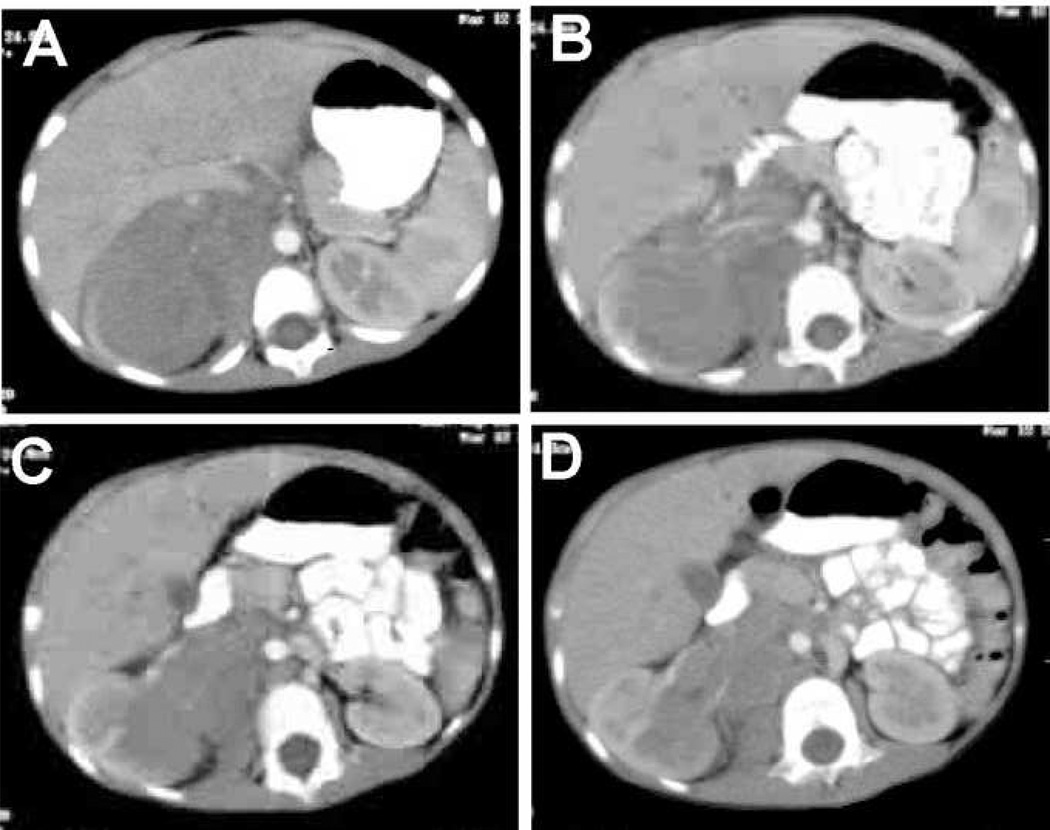
We identified 9 patients who underwent laparotomy at referring institutions with a pre-operative diagnosis of WT, who were subsequently found to have NBL. Of these, 8 had nephrectomy at their original exploration. The one patient who did not undergo nephrectomy was a 4 year old, where, at exploration, the surgeon realized that the correct diagnosis was likely NBL rather than WT, and therefore biopsied the tumor as well as contralateral lymph nodes (Figure 1). This patient turned out to have stage 3 NBL with unfavorable histology.
Figure 1.
Pre-operative CT images of a patient with a right retroperitoneal neuroblastoma who underwent an initial exploratory laparotomy with a presumed diagnosis of Wilms tumor. The correct diagnosis was confirmed intra-operatively by frozen section analysis of a biopsy of the tumor obtained when the surgeon questioned the pre-operative diagnosis.
Of the 8 patients who underwent nephrectomy for presumed WT, 6 were subsequently determined to have high-risk NBL based on age, MYCN status, and histopathology. In addition, 3 of these 8 patients were found to have disease metastatic in the bone marrow. In fact, one patient underwent bone marrow aspiration pre-operatively, but the results were not confirmed until after surgery; the other two had bone marrow examinations performed post-operatively. Sampling of contralateral lymph nodes for staging was not performed at initial surgery in any of these 8 patients.
Children presenting with neuroblastoma were more likely to have constitutional symptoms including fever and/or weight loss (67%) compared to those with WT (20%). Children with WT most often presented simply with asymptomatic abdominal distention detected by the parents or pediatrician. Pre-operative complete blood counts revealed that children with NBL had significantly lower white blood cell counts (8,800/mm3 vs 13,200/mm3, p=0.037) and platelet counts (385,000/mm3 vs 520,000/mm3, p=0.034) than children with WT. However, overall, patients were not found to have profound leukopenia or thrombocytopenia. Both groups also had decreased mean hemoglobin levels (9.7g/dL vs 10.7g/dL, p=0.072), although the difference between groups did not reach statistical significance. Serum LDH levels revealed no difference between the two groups (1390 units/L vs 1312 units/L, p=NS) (Table 1).
Table 1.
Pre-operative laboratory values.
| Neuroblastoma (n = 9) |
Wilms’ Tumor (n = 20) |
p value* | |
|---|---|---|---|
| WBC (mm3) | 8,800 | 13,200 | 0.037 |
| Platelet count (mm3) | 391,000 | 520,000 | 0.034 |
| Hemoglobin (g/dL) | 9.7 | 10.7 | 0.072 |
| LDH (units/L) | 1390 | 1312 | N.S. |
Student’s t-test
One clear difference in patients with NBL versus WT was the presence of elevated urinary catecholamines or metanephrines. Review of the pre-operative laboratory work up revealed that 5 patients in the NBL group actually had urinary vanillylmendalic acid (VMA) and/or homovanillic acid (HVA) evaluated; in each case these were subsequently found to be elevated. Each of the five had elevated urinary VMA (mean 300.8, range 58–806), and two had elevated urinary HVA (mean 86.5, range 47–126). Surprisingly, however, in each case, surgery preceded the availability of these results. Of the 20 patients treated for WT, only 2 underwent pre-operative urinary catecholamine analysis, and each was negative. The finding of elevated urinary catecholamines clearly suggests a diagnosis of neuroblastoma and should prompt the surgical and medical teams to complete the staging evaluation prior to proceeding with an operation.
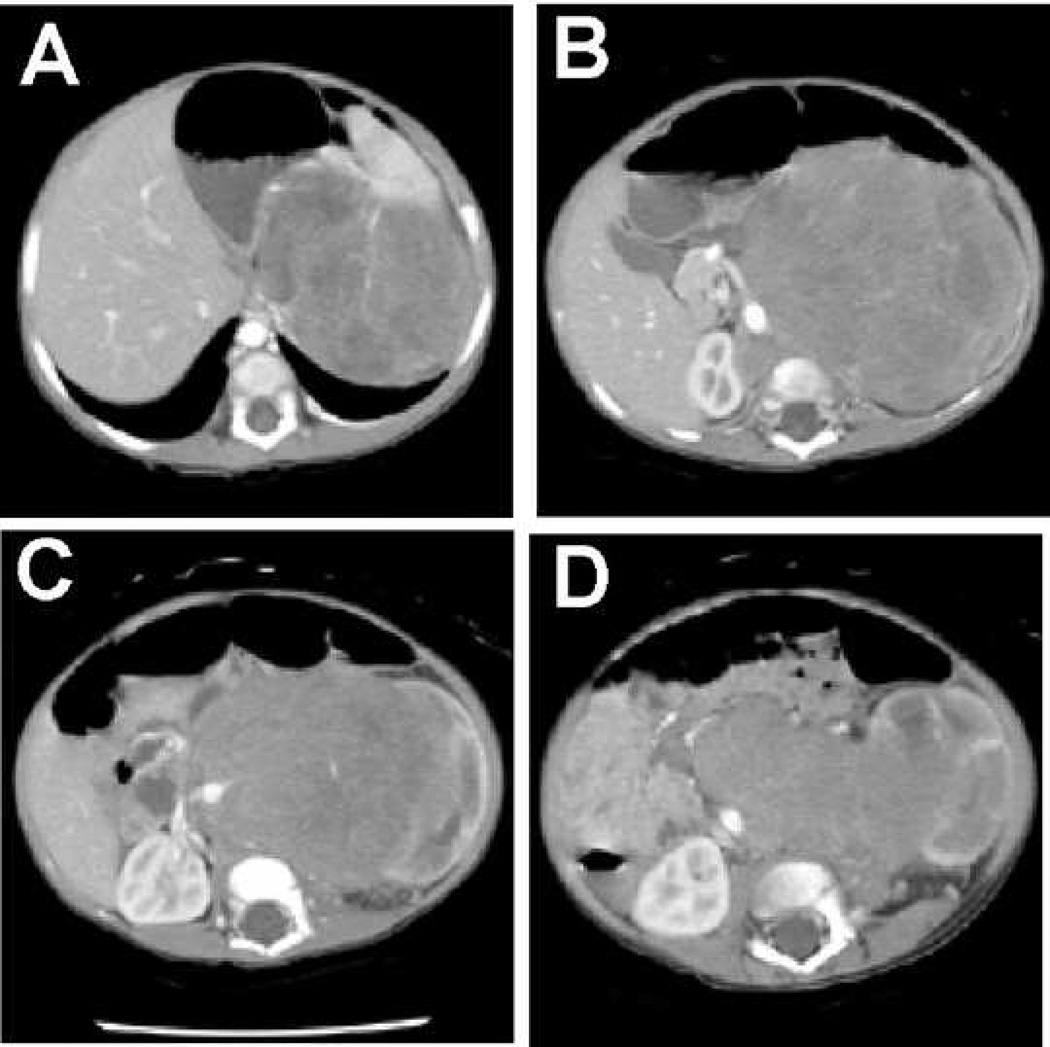
Another important component in the pre-operative evaluation of children with abdominal tumors is imaging. It is well documented that intratumoral calcifications are frequently present in NBL but are rare in patients with WT.2 The same is true for vascular encasement.3 On review of the pre-operative CT findings in children thought to have WT, and subsequently found to have NBL, 7 of 9 (78%) were found to have either intratumoral calcification, vascular encasement (i.e. celiac, superior mesenteric, or aorta) (Figure 2), or both. Conversely, none of the 20 patients most recently treated for WT at our institution had either of these finding on pre-operative CT scan.
Figure 2.
Pre-operative CT images of a patient with a left retroperitoneal neuroblastoma who underwent an initial nephrectomy because of a presumed diagnosis of Wilms tumor. Although the image in panel A might be consistent with Wilms tumor, the displacement of the aorta with encasement of the SMA (B) and both renal arteries (C), and the relation to the kidney (D), suggest the correct diagnosis.
Discussion
Distinguishing abdominal NBL from WT is critical when evaluating children with abdominal tumors as the surgical management for these tumors differs significantly. While surgical extirpation of the primary tumor is a critical component of the treatment of both malignancies, nephrectomy is rarely necessary in the treatment of neuroblastoma.4 In fact, preservation of renal function is important as increasingly nephrotoxic regimens are used to control high-risk neuroblastoma.5 In addition, when a diagnosis of NBL is made, the optimal timing of surgery to remove the tumor is often dependent on risk stratification.6,7 In patients with high-risk NBL, initial surgery is usually limited to obtaining tissue for determining the diagnosis and tumor biology, staging, and placement of a central venous access device.6 Tumor resection is then performed following cytoreductive neoadjuvant therapy.6,7 On the other hand, in patients with WT, primary resection is generally attempted at presentation, at least in the United States. In the current study, we reviewed our 20 year experience in treating high-risk NBL and identified a subset of patients originally thought to have WT. All but one of these patients underwent nephrectomy at the time of original laparotomy.
Children with NBL may present with a variety of clinical features including abdominal distension/pain, anemia, fever, weight loss, hypertension, or symptoms related to metastatic disease such a bone pain.8,9 In contrast, children with WT often only present with abdominal distension that is detected by the parents or primary care physician.10 Accordingly, in review of the history and physical examination of patients in our study, children with NBL tended to have findings of fever and or weight loss more frequently than children with WT (67% vs 20%). Thus, ill-appearing children or those having a broad spectrum of constitutional symptoms during presentation for an abdominal mass should heighten suspicion for NBL. Nearly half of all patients with NBL have metastatic disease at the time of presentation11, often to the bone marrow which may lead to pancytopenia. Our review of complete blood counts in patients diagnosed with NBL versus WT revealed that patients with NBL had lower mean white blood cell counts and lower mean platelet counts, although these children were generally not leukopenic or thrombocytopenic. Children in both groups were slightly anemic.
One staple in the diagnosis of NBL is a finding of elevated urinary catecholamines or metanephrines which will be present in over 90% of patients.12 If there is any question as to the diagnosis, this test should be performed and the results reviewed prior to surgery. The most surprising finding in the current investigation was that in children undergoing laparotomy for presumed WT, five patients actually had urinary VMA and/or HVA collected pre-operatively, indicating that NBL was entertained in the differential diagnosis. In each case these levels were elevated. Remarkably, however, surgery had proceeded without waiting for the results. If urinary catecholamines or metanephrines are collected because of a questionable diagnosis of NBL, clearly these results should be reviewed prior to proceeding with tumor resection, and particularly nephrectomy.
Currently, children with an abdominal mass invariably undergo CT scanning to evaluate the nature of the mass. It is well documented that stippled calcifications, which can often be picked up on plain radiographs, and are routinely present on CT are suggestive although not diagnostic of NBL.2,13 In addition, the finding of vascular encasement by a tumor is highly predictive of NBL over WT.3 On review of pre-operative CT scans in the current study, of nine patients eventually diagnosed with NBL, 6 (67%) had calcifications, and 4 (44%) demonstrated vascular encasement, with 7 of 9 (78%) demonstrated either one or both of these findings. These results suggest that when the diagnosis is in question, the finding of tumor calcification or vascular encasement on pre-operative imaging should heighten suspicion for NBL and prompt completion of the appropriate pre-operative laboratory work and staging. In cases where CT is equivocal, MRI may add information with regard to the primary tumor, as well as yield staging information for both WT and NBL.
Although abdominal NBL can occasionally be mistaken for WT at presentation, constitutional symptoms such as fever and weight loss and the finding of intratumoral calcification or vascular encasement on pre-operative imaging should alert the surgeon to the correct diagnosis. In addition, a complete laboratory evaluation, including measurement of urinary catecholamines, should be completed prior to surgery when the etiology of an abdominal tumor is uncertain. If there is still diagnostic uncertainty after completion of this work-up, it is recommended that biopsy rather than resection of the primary tumor be performed so that appropriate treatment algorithms are followed. While this review encompasses a small group of patients referred to a single institution, it serves an important reminder that the work-up of children with abdominal tumors should be thorough and complete prior to surgical intervention. Certainly, a future study which queries a larger database would provide further insight into the frequency of misdiagnosed abdominal tumors and may point to additional potential pitfalls the clinician should avoid.
Acknowledgments
Supported by the US Public Health Service Childhood Solid Tumor Program Project Grant No. CA 23099, by Cancer Center Support Grant No. 21766 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kim S, Chung DH. Pediatric solid malignancies: neuroblastoma and Wilms' tumor. Surg Clin North Am. 2006;86:469–487. doi: 10.1016/j.suc.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman RA, Holt JF, Heidelberger KP. Calcification in primary and metastatic Wilms' tumor. AJR Am J Roentgenol. 1978;130:783–785. doi: 10.2214/ajr.130.4.783. [DOI] [PubMed] [Google Scholar]
- 3.Scott DJ, Wallace WH, Hendry GM. With advances in medical imaging can the radiologist reliably diagnose Wilms' tumours? Clin Radiol. 1999;54:321–327. doi: 10.1016/s0009-9260(99)90563-9. [DOI] [PubMed] [Google Scholar]
- 4.Grosfeld JL. Neuroblastoma. In: Grosfeld JL, O’Neill JA, Fonkalsrud EW, Coran AG, editors. Pediatric Surgery. 6th Edition. Elsevier Science; 2006. pp. 467–494. [Google Scholar]
- 5.Shamberger RC, Smith EI, Joshi VV, et al. The risk of nephrectomy during local control in abdominal neuroblastoma. J Pediatr Surg. 1998;33:161–164. doi: 10.1016/s0022-3468(98)90424-9. [DOI] [PubMed] [Google Scholar]
- 6.Henry MC, Tashjian DB, Breuer CK. Neuroblastoma update. Curr Opin Oncol. 2005;17:19–23. doi: 10.1097/01.cco.0000147901.12325.90. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein JL, Katzenstein HM, Cohn SL. Advances in the diagnosis and treatment of neuroblastoma. Oncologist. 2003;8:278–292. doi: 10.1634/theoncologist.8-3-278. [DOI] [PubMed] [Google Scholar]
- 8.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 9.Grosfeld J. Neuroblastoma: a 1990 overview. Pediatr Surg Int. 1991;6:9–13. [Google Scholar]
- 10.Green DM. The diagnosis and management of Wilms' tumor. Pediatr Clin North Am. 1985;32:735–754. doi: 10.1016/s0031-3955(16)34834-9. [DOI] [PubMed] [Google Scholar]
- 11.Ninane J, Pearson A, Andrassy R. In: Pediatric Surgical Oncology. Andrassy R, editor. 1. W.B. Saunders; 1998. pp. 175–212. [Google Scholar]
- 12.Strenger V, Kerbl R, Dornbusch HJ, et al. Diagnostic and prognostic impact of urinary catecholamines in neuroblastoma patients. Pediatr Blood Cancer. 2007;48:504–509. doi: 10.1002/pbc.20888. [DOI] [PubMed] [Google Scholar]
- 13.Peretz GS, Lam AH. Distinguishing neuroblastoma from Wilms tumor by computed tomography. J Comput Assist Tomogr. 1985;9:889–893. doi: 10.1097/00004728-198509000-00009. [DOI] [PubMed] [Google Scholar]