Abstract
Ethanol's motivational consequences have been related to the actions of acetaldehyde, a metabolic product of ethanol oxidation. The present study assessed the role of acetaldehyde in the motivational effects of ethanol on pre-weanling rats. In Experiment 1 pups (postnatal days 13–14, PD 13–14) were given systemic administration of d-penicillamine (DP, a drug that sequesters acetaldehyde: 0, 25, 50 or 75 mg/kg) before pairings of 1.0 g/kg ethanol and a rough surface (sandpaper, conditioned stimulus, CS). At test, pups given sandpaper-ethanol pairings exhibited greater preference for the CS than unpaired controls, but this preference was not expressed by pups given DP. Pre-training administration of 25 or 50 mg/kg DP completely blocked the expression of ethanol-mediated appetitive conditioning. D-penicillamine did not alter blood ethanol levels. Subsequent experiments revealed that ethanol-induced activation was blocked by central (intra-cisterna magna injections, volume: 1 μl, dose: 0 or 75 μg) but not systemic treatment with DP (0, 25, 50 or 75 mg/kg; ip). These results indicate that: (a) pre-weanling rats are sensitive to the reinforcing effect of ethanol, and (b) that this effect is associated with the motor activating effect of the drug. These effects seem to be mediated by the first metabolite of ethanol, acetaldehyde.
Keywords: ethanol, appetitive conditioning, pre-weanling rat, acetaldehyde, motor activation, d-penicillamine
1. Introduction
Although controlled alcohol drinking is the norm in most people, for many casual drinking leads to an uncontrolled pattern of consumption (i.e., alcohol dependence). Among the several consequences of alcohol (chemically known as ethanol), its motivational effects are those primary involved in facilitating the transition from drug use to abuse and dependence (Koob and Le Moal, 2001). Exposure to ethanol early in life is yet another factor that facilitates this transition (Yates et al., 1998).
Ethanol exerts appetitive, aversive and negative reinforcing effects that can be captured, analyzed and pharmacologically dissected through the use of animal models (Pautassi et al, 2009), such as the conditioned place preference procedure (CPP). After a few pairings between an initial neutral surface (conditional stimulus, CS) and ethanol's effects (unconditional stimulus, US), animals exhibit preference for the ethanol-paired CS when tested in a preference test (Ciccocioppo et al., 1999). Another benchmark for ethanol's appetitive reinforcement involves assessing the locomotor activity evoked by the drug (Arias et al., 2008; 2009; Faria et al, 2008). Although still under discussion, the rationale is that ethanol's appetitive effects and ethanol-induced locomotor activity share a common neurobiological mechanism, the activation of an opioid-modulated, mesocorticolimbic dopaminergic system (Arias et al., 2009; Wise & Bozarth, 1987).
Ethanol-mediated CPP and ethanol-induced locomotor activity have proven useful in pinpointing several neurotransmitters (e.g., dopaminergic and opioidergic) and brain areas (e.g., ventral tegmental area, VTA) associated with ethanol's hedonics and have underscored the important role that procedural (route of administration; Nizhnikov et al., 2009) and environmental factors (stress; Matsuzawa et al., 2000) play in the expression of ethanol's appetitive effects. They have also provided data for a theoretical account suggesting that many of ethanol's motivational consequences can be attributed to the first metabolite of the drug, acetaldehyde (ACD). The initial response to the hypothesis of ethanol as a “pro-drug” was extremely controversial (Deitrich, 2004). The wealth of data accumulated since then, however, has been solid enough to suggest at least a mediational role for ACD in the effects of ethanol (Deng & Deitrich, 2008).
In animals, the intraperitoneal (ip) administration of ACD exerts biphasic effects when measured in terms of motor activity (i.e., motor activation and depression; Font et al., 2005) and motivational learning (conditioned stimulus preference and aversion; Aragon et al., 1986; Quertemont & De Witte, 2001; although its role in mediating taste aversion has been disputed, see Quertemont & De Witte, 2011). These biphasic and somehow contradictory findings have been accounted for by ascribing different consequences to ACD as a function of its site of action. ACD is peripherally produced in the liver by alcohol dehydrogenase (ADH). Whereas these blood acetaldehyde levels are believed to be aversive, the central effects of the metabolite are apparently highly reinforcing (Quertermont & Didone, 2006). Centrally administered ACD supports conditioned place preference (Quertermont & De Witte, 2001; Quintanilla & Tampier, 2003) and evokes motor activation (Arizzi et al., 2003). Moreover, it has been shown that rats selectively bred for displaying high levels of ethanol intake (P rats) will actively work to self-administer ACD into the posterior VTA (Rodd-Henricks et al., 2002). The existence of central acetaldehyde was initially dismissed because this compound rarely crosses the hematoencefalic barrier and the presence of ADH in the brain is low (Quertemont & Tambour, 2004). More recent data, however, undoubtedly suggested that ACD is produced in the brain through the catalase/H2O2 system (Aragon et al., 1992; Hamby-Mason et al., 1997). Consistent with the putative reinforcing role of centrally-produced ACD, manipulations of brain catalase activity lead to changes in ethanol's hedonics and intake. Acute lead acetate administration was associated with increased catalase activity and augmented ethanol-induced locomotion in mice (Correa et al., 2005). Conversely, the inhibition of catalase activity via administration of 3-amino-1,2,4-triazole resulted in an attenuation of ethanol-induced taste aversion, decreased ethanol intake (Aragon et al., 1985; Aragon & Amit, 1992) and facilitated ethanol-induced place preference (Font et al., 2008). Moreover, ethanol-induced motor effects are altered by the administration of a catalase inhibitor into the hypothalamic arcuate nucleus, a brain region with a high density of catalase (Sanchez-Segura et al., 2005).
Another pharmacological tool for assessing ACD's involvement in ethanol's hedonics is d-penicillamine (DP), a drug that turns off the pharmacological activity of this metabolite. DP is a thiol compound that sequesters the ACD produced by the oxidation of ethanol without altering the circulating levels of ethanol (Font et al., 2005). Systemic administration of DP blocks ethanol-induced locomotion, ethanol intake and conditioned place preference - but not aversion - in mice (Font et al., 2005; 2006a; 2006b). Few studies have assessed the role of ACD in mediating ethanol's appetitive effects in rats, most likely because adult rats rarely exhibit signs of conditioned preference to ethanol (Pautassi et al., 2009). A recent study, however, found CPP by ethanol (1.0 g/kg, IG) in adult rats and blocked this effect by administering DP or 4-methylpyrazole, a peripheral competitive inhibitor of ADH (Peana et al., 2008). A follow-up study (Enrico et al., 2009) found that either ACD or ethanol (1 g/kg IG) stimulated the activity of dopamine neurons in the nucleus accumbens. Intraperitoneal administration of DP prevented this stimulation, suggesting that DP alters ethanol's hedonics by inhibiting the stimulatory action of ACD on the mesolimbic dopamine transmission.
To our knowledge, there is very little information on the role of ACD in the motivational effects of ethanol during early ontogeny of the rat. The relevance of studying this phenomenon is multiple. Unlike their adult counterparts, preweanling rats are highly sensitive to ethanol's appetitive effects, readily exhibiting first and second-order appetitive tactile conditioning to ethanol (Molina et al., 2006; 2007; Nizhnikov et al., 2009; Pautassi et al., 2008) as well as ethanol-induced motor activation (Arias et al., 2008, 2009, 2010). These effects are observed after a wide range of doses (0.5 – 2.0 g/kg) and can be blocked by dopamine and opioid antagonists (Nizhnikov et al., 2009; Arias et al., 2009). Interestingly, the pre-weanling's sensitivity to ethanol reinforcement and psychomotor activation coincides with high avidity for ethanol intake. When assessed through the consumption-off-the floor procedure (Sanders and Spear, 2007), non-initiated pre-weanling rats achieve blood alcohol levels comparable to those found in adult alcohol-preferring (P) rats (Truxell et al., 2004, 2007). It seems that the use of a preweanling animal model provides a useful preparation for analyzing determinants of ethanol's reinforcement and affinity (Pautassi et al., 2009). A feature of the rat's developing brain provides further rationale for assessing the ACD role in ethanol reinforcement during infancy. The levels of brain catalase exhibit an inverse relationship with age (i.e., greater levels at younger ages; Mavelli, 1982; Maestro & MacDonald, 1987; 1989), thus suggesting that central production of ACD is higher in pre-weanling than in adult rats.
The present study assessed the role of acetaldehyde in the motivational effects of ethanol in pre-weanling rats, as measured through conditioned tactile preference and ethanol-induced motor activation. In Experiment 1 pups were given d-penicillamine (0, 25, 50 or 75 mg/kg) before pairings of 1.0 g/kg ethanol and a rough surface (sandpaper). Pups were then tested for sandpaper preference in a two-way preference test. Experiment 2 and 3 tested ethanol-induced activation in a novel environment at PD13 following systemic or central (intra-cisterna magna injections) administration of DP. The possibility of DP altering blood ethanol levels was also analyzed.
2. Materials and Methods
2.1 General Procedures
2.1.1 Subjects
Two-hundred and sixty-nine Sprague-Dawley rat pups were employed. These animals were derived from 37 litters born and reared in an AAALAC-accredited facility (vivarium of the Center for Development and Behavioral Neuroscience, Binghamton University, Binghamton, NY, USA). Number of animals and litter representation in each experiment was as follows. Experiment 1, 96 animals (14 litters); Experiment 2, 107 animals (15 litters); Experiment 3, 66 animals (8 litters). Births were examined daily and the day of parturition was considered as postnatal day 0 (PD 0). Pups were housed with the dam in cages with free access to water and food. The colony was kept at 22 – 24 °C and a 12-h light-dark cycle was used. At the start of the experiments (PD 13) animals had a mean body weight of 31.7 ± 0.5 g. The experimental protocol was approved by the Binghamton University Institutional Review Committee for the use of Animal Subjects and all procedures are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (National Research Council, 1985).
2.1.2 Tactile Conditioning and Test Procedures (Experiment 1, PDs 13–15)
Conditioning procedures closely followed those employed by Nizhnikov et al., (2009). Briefly, Experiment 1 consisted of two phases: conditioning (two daily conditioning trials, PDs 13–14) and a test session (PD 15).
Conditioning sessions: Pups were withdrawn from their mother for sixty minutes. Then, paired (P) pups were weighed and administered d-penicillamine (DP, 0, 25, 50 or 75 mg/kg, ip), followed fifteen minutes later by an ethanol intubation (1.0 g/kg, IG). The pups were exposed to a tactile conditioned stimulus (CS, sandpaper; coarse: 50, Gatorgrit, USA) during ethanol post-administration time 20–30 min. Unpaired controls (UP) were given DP treatment and exposed to the rough CS at the same time paired animals did. UP animals, however, were not given ethanol until 90 min after the termination of CS exposure. To equate the level of maternal deprivation across groups, all animals were returned to the mother 120 min after delivery of ethanol in UP pups.
D-penicillamine doses were selected on the basis of studies showing their effectiveness in blocking the reinforcing effects of ethanol in adult mice (Font et al., 2006a) and adult Wistar rats (Peana et al., 2008). Ethanol dose, route and interval of conditioning were chosen on the basis of our previous study (Nizhnikov et al., 2009).
Test session: The test took place in a Plexiglas rectangular chamber (28 × 13 × 15.5 cm), illuminated by an overhead 40-w red bulb. Half of the floor of the chamber was covered with sandpaper, and the other half was covered with a smooth cardboard surface. Time spent over each section of the apparatus was recorded in a minute-by-minute basis by experimenters unaware of the treatment of each animal. The middle section of the apparatus (15% of the entire surface) was considered as a neutral area and not taken into account for data collection or analysis.
Tactile preference scores were expressed as total time (s) spent in contact with the sandpaper and percent time (%) spent on sandpaper. Percent time was calculated as follows: [(total time spent over sandpaper)/(total time spent over sandpaper + total time spent over smooth)× 100)]. Locomotor activity (s) during the 5-min test and frequency of wall climbing were also registered. Naïve preweanling rats exhibit equal preference for sandpaper and the smooth cardboard (Molina et al., 2006; Pautassi et al., 2008).
2.1.3 Ethanol and D-penicillamine preparation and administration procedures
Ethanol intragastric administration was conducted as described in Pautassi et al. (2002, 2005) The doses of 1.0 (Experiment 1) and 1.25 g/kg (Experiments 2 and 3) were achieved by administering 0.015 ml of an 8.4 and 10.5 % v/v ethanol solution (Pharmaco, Brookfield, CT) per gram of body weight, respectively.
In Experiments 1 and 2, d-penicillamine (Sigma–Aldrich, St. Louis, MO) was administered systemically, via IP injection and derived from two main stocks. For comparison purposes, we decided to use the same d-penicillamine doses in both Experiments. The 75 and 50 mg/kg doses were derived from a 7.5 mg/ml solution and the 25 mg/kg from a 5.0 mg/ml solution. Injection volume was 0.005 ml/g for the lowest dose, and 0.01 for the intermediate and the highest dose.
In Experiment 3 d-penicillamine was directly administered into the cisterna magna (IC administration, volume: 1 μ1, flow rate: 0.66 μ1/s, dose: 0 or 75 μg), using the procedure described in Nizhnikov et al. (2007). This DP dose was selected on the basis of a previous study that showed the effectiveness of central DP to affect ethanol intake in rats (Font et al., 2006b).
Tap water and saline (0.89 %, v/v) were employed as vehicles for the ethanol and d-penicillamine solutions, respectively. The injection volume for saline was 0.01 ml/g.
2.1.4 Assessment of ethanol-induced motor activity after systemic (Experiment 2) or central (Experiment 3) administration of d-penicillamine and determination of blood ethanol levels (BELs, Experiment 2)
On PD 13 the pups were separated from their mother and placed in pairs in a warmed holding chamber. Forty-minutes later they received either IP or IC injection of dpenicillamine (Experiments 2 and 3, respectively) followed by ethanol (0.0 or 1.25 g/kg). Ethanol administration took place 10 or 25 minutes after DP treatment, for pups given central and systemic DP injections, respectively. The rationale for selecting the ethanol dose was that, among the doses known to exert acute motor activation in preweanling rats (Arias et al., 2008, 2009, 2010), 1.25 g/kg ethanol is closest to the one employed in the conditioning section of our study. The temporal gap between DP and ethanol treatment was selected on the basis of previous studies (Font et al., 2005; Nizhnikov et al., 2007).
The manipulations inherent to peripheral and central drug administration may by themselves enhance the level of motor activity in a novel environment. Hence, to properly assess ethanol-induced locomotor activity, an untreated (UT) control condition was included in Experiments 2 and 3. Untreated animals had neither d-penicillamine nor ethanol. Yet, they were assessed for motor activity similarly to the other groups.
Motor activity was registered at ethanol post-administration time 5–20 min in Experiment 2 and at post-administration time 5–10 min in Experiment 3, using a Plexiglas open field (42×42×30 cm; VersaMax Animal Activity Monitoring System, Accuscan Instruments, Columbus, OH, USA; see Arias et al., 2009). The rationale for the differences in the length of the measurement interval was that in Exp. 3 we specifically aimed at assessing if central d-penicillamine blocked the ethanol-induced motor activation that takes place during the onset of the blood ethanol curve.
In Experiment 2, those pups treated with 1.25 g/kg ethanol were sacrificed to assess if d-penicillamine altered blood ethanol levels. Blood trunk samples were obtained 25 minutes following ethanol administration, a time period that corresponds with the mid point of the tactile conditioning procedure, and then analyzed through a AM5 Alcohol Analyzer (Analox Instruments, Lunenburg, MA) (for details on the procedure, see Nizhnikov et al., 2009). BELs were expressed as milligrams of ethanol per deciliter of body fluid (mg/dl = mg %).
2.2 Experimental Designs
A 2 (sex: male or female) × 2 (conditioning procedures: CS paired or unpaired with ethanol's postabsorptive effects, P and UP groups, respectively or) × 4 (d-penicillamine treatment: 0, 25, 50 or 75 mg/kg) factorial defined the assessment of ethanol-mediated tactile conditioning (Experiment 1).
Experiment 2 was defined by the following factors: sex (male or female), dpenicillamine treatment (0, 25, 50 or 75 mg/kg, groups DP0, DP25, DP 50 and DP75, respectively) and ethanol treatment (0.0 [water vehicle] or 1.25 g/kg; groups W and E, respectively). Experiment 3 employed a 2 (sex: male or female) × 2 (central dpenicillamine treatment: 0 or 75 μg/ml) × 2 (ethanol treatment: 0.0 or 1.25 g/kg) factorial. Experiments 2 and 3 also included two untreated control conditions (one for each sex).
Across experiments, no more than two animals per litter (one male and one female) were assigned to each particular treatment. This avoided overrepresentation of litters within each specific group. In Experiments 1 and 2, each of the groups derived from the factorial design was composed by 6 animals. Experimental groups in Experiment 3 had a minimum of 5 and maximum of 8 animals. Untreated control groups had 6 animals each, except for the female group in Experiment 2, which had 5 subjects.
2.3 Data Analyses
Preliminary data analyses indicated that, across variables and Experiments, sex failed to exert significant main effects or interact with the remaining factors. Therefore, data were collapsed across sex for all subsequent analyses as well as for representation in the figures. Furthermore, the male and female untreated control groups of Experiment 2 and 3 did not differ in terms of motor activity, [F (1, 10) = 0.23; F (1, 10) = 4.37; both p's > 0.05]. They were, for each Experiment, combined in a single untreated condition.
The dependent variables registered during the 5-min tactile preference test (i.e., absolute [s] and percent time [%] in contact with the CS, locomotion [s] and wall climbing frequency) were processed through fixed factor Analysis of Variance (ANOVA; between factors: d-penicillamine treatment and conditioning procedures). The loci of significant main effects or interactions were further examined through pair-wise post-hoc comparisons (Fisher's Least Mean Significant tests, alpha level set at 0.05). To confirm that pups in a given group were exhibiting a sandpaper preference above chance (and hence exhibiting ethanol-mediated appetitive learning), percent preference scores were further analyzed. Specifically, the lower limit for the 95% confidence interval (CI) was computed and a t-test for single means against a user-defined constant was performed. The constant was a theoretical 50% percent of preference for the CS.
In Experiment 2, the data for motor activity (distance traveled, cms) during the 15-min test interval was broken down into three 5-min clusters (i.e., 5–9 min, 10–14 min and 15–19 min; further referred as bins 1, 2 and 3). Motor activity was then analyzed through a three-way mixed ANOVA with d-penicillamine treatment and ethanol treatment as between factors. Bin of evaluation (bins 1–3, bin duration: 5 min) was the repeated measures factor. The untreated group was included in the ANOVA as an isolated control condition.
Overall motor activity during the 5-min test in Experiment 3 was analyzed through a factorial ANOVA. Ethanol treatment and central d-penicillamine treatment were the between factors, whereas the untreated group served as an isolated control condition. In Experiments 2 and 3 and justified by our a priori hypothesis, planned comparisons were conducted between the UT and the W-DP0 group.
To assess if blood ethanol levels were affected by DP treatment, a one-way ANOVA was conducted (comparative factor between groups: d-penicillamine dose).
3. Results
3.1 Tactile Conditioning (Experiment 1)
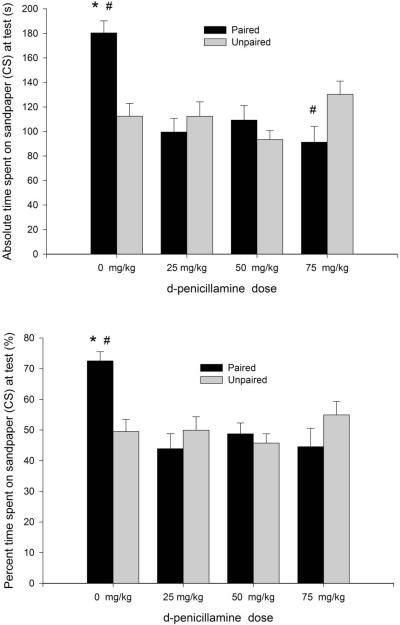
Absolute and percent preference for the CS paired with ethanol can be observed in Figure 1. Ethanol readily induced appetitive conditioning and pretreatment with 25 or 50 mg/kg of d-penicillamine blocked the expression of this conditioned response. These impressions were confirmed by the ANOVAs, which revealed very similar results for both dependent variables. There was an independent significant main effect of dpenicillamine treatment (absolute time: F3,88 = 7.15, p < 0.001; percent time: F3,88 = 4.78, p < 0.005) as well as a significant two-way interaction between ethanol and DP treatment (absolute time: F3,88 = 8.90, p < 0.001; percent time: F3,88 = 6.21, p < 0.001). The post-hoc tests revealed that paired pups given 0.0 DP spent significantly more time on the sandpaper CS than any other group, thus showing appetitive conditioning mediated by ethanol. The expression of this conditioned response was confirmed by a t-test indicating that percent sandpaper preference in group P-0 was significantly above 50%, t(11) = 53.83, p < 0.001. The lower limit for the CI was also well above 50% (65.84%).
Figure 1.
Ethanol-induced motivational learning. Time spent on sandpaper (conditioned stimulus, CS, top panel) and its corresponding percentage of preference (bottom panel) as a function of conditioning procedures [sandpaper paired or unpaired with intragastric administration of 1.0 g/kg ethanol] and treatment with d-penicillamine, an acetaldehyde-sequestering agent (0, 25, 75 or 75 mg/kg, ip), prior to conditioning. Each group illustrated in the figure had 12 animals. Asterisks (*) indicate significant differences between the paired-0.0 mg/kg DP group and the remaining groups (p < 0.05). Number signs (#) indicate significant differences between an ethanol-treated group and its corresponding vehicle-treated control (p < 0.05).Vertical bars represent the standard error of the means (S.E.M.).
The post-hoc tests indicated that paired pups given 25 or 50 mg/kg DP did not differ significantly from their pertinent control groups (i.e., groups UP-25 and UP-50). Thus, DP inhibited expression of ethanol reinforcement at these doses.
Also, unpaired pups treated with the highest DP dose (UP-75 group) spent significantly more absolute time in contact with the CS than their paired counterparts (P-75 group). In terms of percent preference (%), however, the post-hoc tests for these groups neared, but did not achieve, significance (p = 0.07). These results raised the possibility that DP, at 75-mg/kg, produced appetitive conditioning in the unpaired group. For both dependent variables the post-hoc tests indicated, however, the lack of significant differences across unpaired conditions. A one-way ANOVA conducted on unpaired conditions (independent between group factor: d-penicillamine treatment) confirmed this lack of significant differences: F3, 44 = 2.16 and F3, 44 = 0.92; both ps > 0.10, for absolute and percent CS preference, respectively. Moreover, a t-test revealed that % preference for sandpaper in the UP 75-mg/kg group was not significantly different from chance [t(11) = 1.12, p > 0.25] and the lower limit for the CI in this group was also below 50% (45.26%).
The ANOVAs for overall duration of locomotion and wall climbing scores during the 5-min test indicated no significant main effects or interactions. Table 1 presents mean and standard error values for these variables.
Table 1. Locomotion and wall-climbing during the two-way preference test (Experiment 1).
Behavioral activation [frequency of wall climbing and total duration of forward locomotion (s)] at test (a 5-min, two-way tactile preference test) as a function of conditioning treatment (sandpaper paired or unpaired with ethanol's pharmacological consequences; delivered intragastrically, 1 g/kg) and pretreatment with an acetaldehyde sequestering agent (d-penicillamine; 0, 25, 50 or 75 mg/kg). Values represent mean +/− SEMs.
| Wall-Climbing | Locomotion | |||
|---|---|---|---|---|
| Paired | Unpaired | Paired | Unpaired | |
| d-penicillamine 0 mg/kg | 4.75 +/− 1.14 | 4.33 +/− 1.12 | 14.33 +/− 1.86 | 14.98 +/− 1.16 |
|
| ||||
| d-penicillamine 25 mg/kg | 5.33 +/− 1.65 | 6.17 +/− 1.52 | 13.38 +/− 1.49 | 13.41 +/− 1.40 |
|
| ||||
| d-penicillamine 50 mg/kg | 8.42 +/− 1.79 | 5.83 +/− 1.45 | 12.98 +/− 1.04 | 14.3 +/− 1.32 |
|
| ||||
| d-penicillamine 75 mg/kg | 5.92 +/− 1.45 | 8.08 +/− 1.71 | 12.82 +/− 1.67 | 16.66 +/− 1.51 |
|
| ||||
3.2 Motor activity (Experiments 2 and 3)
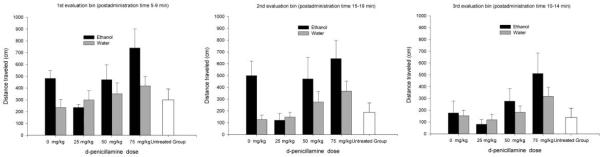
The ANOVA for motor activity after systemic (IP) DP treatment (Experiment 2) revealed independent significant main effects of ethanol treatment, d-penicillamine treatment and bin of evaluation, F1,98 = 5.62, p < 0.05; F3,99 = 5.32, p < 0.005 and F2,196 = 19.53, p < 0.001. As shown in Figure 2, ethanol-induced behavioral activation in group E-DP0 was quite clear, particularly during the first and second evaluation bins (postadministration time 5–10 and 15–20 min). Pups given the highest d-penicillamine dose (75 mg/kg) exhibited increased levels of motor activity, an effect that was essentially the same for ethanol- and vehicle-treated animals. As expected, overall locomotion across groups peaked during the first evaluation bin and then significantly decreased during each subsequent bin. Visual inspection of Figure 2 seems to suggest that DP exerted a dose-response effect on ethanol-induced motor activity, with low and high DP doses decreasing and increasing ethanol-induced motor activity, respectively. This impression, however, was not supported by the ANOVA, which indicated the absence of significant two- or three-way interactions between the factors under consideration.
Figure 2.
Ethanol-induced motor activity after systemic d-penicillamine. Locomotor activity (distance traveled, cms) during ethanol post-administration time 5–9, 10–14 and 15–19 minutes (evaluations bins 1, 2 and 3; respectively) in 13-day old male and female rats. The animals were given d-penicillamine (0, 25, 75 or 75 mg/kg, ip) 25 min prior to the administration of ethanol (1.25 g/kg, intragastric) or its vehicle. Animals in the untreated group (n = 11) were assessed for locomotor activity but did not receive intubations or injections. Each of the eight groups treated with ethanol and d-penicillamine had 12 animals. The statistical analysis (ANOVA) revealed independent significant main effects of ethanol treatment, d-penicillamine treatment and bin of evaluation, F1,98 = 5.62, p < 0.05; F3,99 = 5.32, p < 0.005 and F2,196 = 19.53, p < 0.001. The vertical bars indicate the SEM.
Planned comparisons (one for each bin) indicated that motor activity did not significantly differ between the untreated group and the basic control condition (W-DP0 group), F1, 98 = 0.22, p > 0.64; F1, 98 = 0.15, p > 0.60 and F1, 99 = 0.012, p > 0.90.
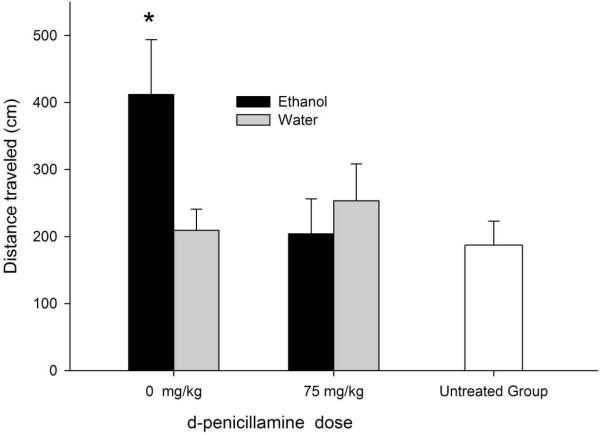
Figure 3 illustrates motor activity levels in the open field after ethanol or vehicle in pups given central injections of DP treatment (Experiment 3, tested at postadministration min 5–10). Ethanol exerted clear motor activating effects. This stimulation effect was blocked by the administration of d-decipillamine into the cisterna magna. The ANOVA indicated that the interaction between ethanol treatment and central d-penicillamine treatment achieved significance, F1, 61 = 5.38, p < 0.05. According to the post-hoc test, motor activity in animals given ethanol and only IC vehicle was significantly higher than in any of the remaining groups, which in turn did not differ between themselves. Moreover, There were no significant differences in motor activity between the UT group and the pharmacological control group (animals intubated with water and given vehicle IC); F1, 61 = 0.08, p > 0.70.
Figure 3.
Ethanol-induced motor activity after central d-penicillamine. Locomotor activity (distance traveled, cms) during ethanol post-administration time 5–9 minutes in 13-day old male and female rats. The animals were given intra-cisterna magna injections of d-penicillamine (volume: 1 μl, dose: 0 or 75 μg; vehicle: saline) 10 min before the administration of ethanol (1.25 g/kg, intragastric) or its vehicle. Animals in the untreated group (n=12) had neither d-penicillamine nor ethanol treatment. Yet, they were assessed for motor activity similarly to the other groups. Each of the four groups treated with ethanol and d-penicillamine had 12–15 animals. The asterisk (*) indicates significant differences between the ethanol-0.0 mg/kg DP group and the remaining groups (p < 0.05). The vertical bars indicate the SEM.
3.3 Blood Ethanol Levels (Experiment 2)
Blood samples were taken from animals given ethanol and then treated with IP d-penicillamine. The ANOVA indicated that treatment with DP did not affect BELs. Mean and SEM for each group was as follows: DP0, 111.98 +/− 4.74 mg %; DP25, 109.03 +/− 2.98 mg %; DP50, 111.94 +/− 3.28 mg %; DP75, 102.67 +/− 3.80 mg %.
4. Discussion
According to the present study ethanol induces, at moderate doses and in preweanling rats, conditioned tactile preference and locomotor activating effects. These results, congruent with previous studies (Nizhnikov et al., 2009; Arias et al., 2009), are important when considering that adult rats often express opposite effects, ethanol-induced suppression of motor activity (Sanchis-Segura et al., 2005) and conditioned place aversion (Cunningham et al., 1993). Rats selectively bred for alcohol-preference exhibit behavioral stimulation following low-dose ethanol (Rodd et al., 2004), yet they fail to express conditioned preferences (Stewart et al., 1996).
Interesting information emerged when the time course of the behavioral assessments was considered. Ethanol induced motor activation shortly after the intubation (5–14 min post administration) but not at a later interval. The sandpaper-ethanol pairings that resulted in increased sandpaper preference at test also began at an early post administration time (20 min). It seems that the expression of ethanol-induced activation and conditioned preference in pre-weanling rats is associated with the rising limb of the blood ethanol curve (Nizhnikov et al., 2009).
These results fit well with postulates that consider the motor-stimulating and reinforcing effects of ethanol as related phenomena (Arias et al., 2009), likely dependent on the integrity of the opioid (Frohelich et al., 1991) and dopaminergic systems (Meyer et al., 2009). The latter work revealed a genetic correlation between activity of the mesolimbic dopamine system and sensitivity to ethanol-induced locomotion. Likewise, there is a negative association between ethanol-induced motor locomotor activity and genetic sensitivity to the ataxic and hypnotic effects of ethanol (Dudek et al., 1984; Dudek & Philips, 1990). Ethanol-induced taste aversion is also stronger in mice selectively bred for insensitivity to ethanol-induced locomotor stimulation than in mice bred for sensitivity to this effect of ethanol (Risinger et al., 1994). The ataxic, hypnotic and aversive effects of ethanol presumably serve as deterrents to escalated ethanol intake (Spear & Varlinskaya, 2010).
The novel findings of the present study are those that relate the first metabolite of ethanol, acetaldehyde, with the expression of ethanol-induced motor activation and conditioned preference in pre-weanling rats. Our strategy, similar to that employed by Font et al. (2005; 2006; 2006a) and Peana et al. (2008), involved altering the bioavailability of acetaldehyde by administering d-penicillamine, an ACD-chelating agent that selectively sequesters ACD and creates a pharmacologically-inactive condensation product that is excreted in urine (Cohen et al., 2000). Systemic (IP) dpenicillamine blocked ethanol-induced conditioned tactile preference without affecting blood ethanol levels. Specifically, pups given sandpaper-ethanol pairings displayed enhanced preference for this cue at test, but this preference was not expressed by pups given the acetaldehyde-chelating agent. Pre-training administration of 25 or 50 mg/kg DP completely blocked the expression of ethanol-mediated appetitive conditioning. DP inhibited ethanol-induced motor activation as well, when administered centrally, via cisterna magna injections.
The finding that DP blunted ethanol-induced conditioned tactile preference and motor activation is consistent with previous data gathered in mice (Font et al., 2005; 2006; 2008) and adult rats (Enrico et al., 2009; Peana et al., 2008). In conjunction with those and other studies (Diana et al., 2008; Rodd et al., 2005), the present results favor the hypothesis that acetaldehyde mediates ethanol's appetitive motivational effects.
No indication of conditioned behavioral activation was found during the two-way tactile test, thus ruling out the possibility that the measure of sandpaper preference was contaminated by competing ethanol-mediated conditioned motor responses (Cunningham & Noble, 1992). Moreover, blood ethanol concentrations were not altered by DP and unpaired subjects given pairings of sandpaper and varying doses of d-penicillamine did not differ from vehicle-treated counterparts. These results indicate that d-penicillamine did not induce conditioned preference or aversion by itself and that its effect on ethanol's behavioral consequences was not confounded by pharmacokinetic differences. It could be argued that the motor activity induced by ethanol in Exp. 2 and 3 was a consequence of alcohol enhancing behavioral stimulation derived from the administration and injection procedures. This possibility, however, is unlikely since in these experiments untreated and vehicle-treated animals exhibited similar motor activity levels.
DP clearly inhibited ethanol-induced conditioned preference at the lower doses (25 and 50 mg/kg) although at the highest dose there were signs of DP actually reversing the learning outcome, with paired pups given 75 mg/kg DP spending less time in contact with the sandpaper surface than unpaired controls also treated with this dose of DP. The magnitude of this effect was small, albeit statistically significant in terms of absolute preference scores. It was conceivable that DP induced appetitive conditioning in these unpaired animals. Statistical analyses, however, indicated similar level of sandpaper preference in unpaired animals, regardless treatment with DP. It is known that, after a single drug administration, ethanol (Pautassi et al., 2002) and other psychoactive agents (e.g., amphetamine; Wang et al., 2010) exert simultaneous appetitive and aversive effects, with the final behavioral outcome likely depending on the net difference between them. Interestingly, a previous study found that DP blocked ethanol-induced conditioned place preference but did not affect ethanol-induced conditioned place aversion (Font et al., 2006a). Furthermore, some evidence suggests that acetaldehyde is not involved in ethanol-mediated conditioned taste aversion (Quertemont, 2003; Escarabajal et al., 2003). These results may help understand the present data. If DP blocked only the appetitive motivational effects of ethanol, then the aversive motivational effects of ethanol (Hunt et al., 1990; Pautassi et al., 2005) should have been available to support conditioned aversion. More research will be needed to test these tentative explanations.
Previous studies found conditioned tactile preference in preweanling rats after 1.0 but not 0.5 or 2.0 g/kg ethanol (Nizhnikov et al., 2009; Pautassi et al., 2005). The present study assumed that inhibition of ethanol-induced conditioning by DP reflects an attenuation of ethanol's appetitive effects. The data, however, could also be interpreted as DP enhancing ethanol's effects (i.e., causing a shift to the right in the ethanol dose response curve). This is an interesting, empirical question that would require assessing the effect of DP on ethanol doses that usually do not exert conditioning. The evidence derived from previous studies, however, seems to indicate that DP's effects on ethanol's motivational sensitivity are due to the inhibition of central acetaldehyde and its resulting positive effects. In these studies DP has been found to prevent ethanol and acetaldehyde-induced stimulation of the dopamine mesolimbic system without affecting morphine stimulatory action (Enrico et al., 2009), reduce ethanol's anxiolytic effects (Correa et al., 2008), prevent the acquisition of CPP by ethanol in adult rats (Peana et al., 2008) and prevent ethanol-induced conditioned place preference -- but not aversion -- in mice, without producing rewarding or aversive effects by itself nor affecting CPP by morphine or cocaine (Font et al., 2006a). Moreover, Font et al. (2005) treated mice with a wide range of ethanol and d-penicillamine doses and invariably found a DP-related shift to the left in the ethanol dose effect curve.
Previous studies indicate also that the interaction between acetaldehyde and d-penicillamine takes place in the brain (Font et al. 2005; 2006a; 2006b), particularly in areas with high levels of catalase expression (Sanchis-Segura et al., 2005). The data gathered in Experiments 2 and 3 support this claim. Central but not systemic administration of DP suppressed ethanol-induced motor activation, without altering motor activity in control pups. It has been proposed that ACD has a key role in the neurochemical chain that leads to activation of the mesolimbic dopamine system (Diana et al., 2008), which in turn is thought to underlie the expression of ethanol reinforcement (Bechtholt & Cunningham, 2005). The differences between IC and IP administration of d-penicillamine can also relate to the latter inactivating the circulating levels of acetaldehyde, which cause motor depression (Quertermont & Didone, 2006) and hypothermia (Closon et al., 2009). Support for the latter hypothesis, however, is somewhat weakened by the fact that, following moderate ethanol consumption, blood acetaldehyde levels are usually very low (Quertermont & Didone, 2006).
When given IP the higher (75 mg/kg), but not the lower doses of d-penicillamine (25 and 50 mg/kg) had the nonspecific effect of enhancing motor activity with or without ethanol administration. Font et al. (2005) similarly observed that DP doses higher than 75 mg/kg altered spontaneous behavior in mice. The mechanisms by which high-dose DP causes this nonspecific effect may relate to its inhibitory action on production of nitric oxide. We cannot exclude that this side effect also participated in blocking ethanol-induced conditioned reinforcement. Previous work found that inhibition of nitric oxide production blocked appetitive reinforcement by morphine (Manzanedo et al., 2004).
One obvious weakness of the present study, albeit shared by most of the literature on the motivational effects of ACD (Deitrich 2004; Quertermont & Didone, 2006), is that we did not assess acetaldehyde concentrations but instead assumed their decrease was induced by the ACD-sequestering agent. Despite this constraint, the present study adds further support for the idea that acetaldehyde, at doses that do not alter blood ethanol levels nor exert nonspecific motor alterations, mediate important ethanol-induced effects.
The main conclusion of the study is that in the heterogeneous rat prior to weaning, acetaldehyde contributes to the expression of ethanol-induced behavioral activation and appetitive conditioning. The confirmation that preweanling -- as well as adolescent (Pautassi et al., 2008; Philpot et al., 2003) -- rats develop conditioned tactile preference is also important, given the difficulty in demonstrating CPP in adult rats (Cunningham et al., 1993). Some recent papers, however, suggest an opposite age-related pattern in mice. Song et al. (2007) observed CPP by 2 g/kg ethanol in adult, but not in adolescent, mice. Similarly, Dickinson et al. (2009) found low sensitivity to ethanol reinforcement in adolescent mice. These results suggest a species-related difference in the expression of CPP by ethanol, with young rats but not young mice exhibiting conditioned tactile preference. An explicit testing of this hypothesis would require training both species under the same parameters and behavioral preparation. The simple and apparently reliable tactile conditioning employed in the current study may prove useful towards assessing this apparent species-related difference in motivational sensitivity to ethanol.
Acknowledgments and Funding sources
This work was supported by National Institute on Alcohol Abuse and Alcoholism grants AA011960, AA01309 and AA017823 to NES, AA018164 to MN and grants PIP CONICET 2010–2012 (Argentina) to RMP. We would like to thank Teri Tanenhaus and Pouyan Rhamani for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appel SB, Liu Z, McElvain MA, Brodie MS. Ethanol excitation of dopaminergic ventral tegmental area neurons is blocked by quinidine. Journal of Pharmacology and Experimental Therapeutics. 1986;306:437–46. doi: 10.1124/jpet.103.050963. [DOI] [PubMed] [Google Scholar]
- Aragon CM, Abitbol M, Amit Z. Acetaldehyde may mediate reinforcement and aversion produced by ethanol. An examination using a conditioned taste-aversion paradigm. Neuropharmacology. 2003;25:79–83. doi: 10.1016/0028-3908(86)90062-6. [DOI] [PubMed] [Google Scholar]
- Aragon CM, Rogan F, Amit Z. Ethanol metabolism in rat brain homogenates by a catalase-H2O2 system. Biochem Pharmacol. 1992;44:93–98. doi: 10.1016/0006-2952(92)90042-h. [DOI] [PubMed] [Google Scholar]
- Aragon CM, Spivak K, Amit Z. Blockade of ethanol induced conditioned taste aversion by 3-amino-1,2,4-triazole: evidence for catalase mediated synthesis of acetaldehyde in rat brain. Life Sci. 1985;37:2077–84. doi: 10.1016/0024-3205(85)90579-x. [DOI] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Ethanol induces locomotor activating effects in preweanling Sprague-Dawley rats. Alcohol. 2009;43:13–23. doi: 10.1016/j.alcohol.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Molina JC, Spear NE. Differential role of micro, delta and kappa opioid receptors in ethanol-mediated locomotor activation and ethanol intake in preweanling rats. Physiol Behav. 2010;99:348–354. doi: 10.1016/j.physbeh.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Molina JC, Mlewski EC, Pautassi RM, Spear N. Acute sensitivity and acute tolerance to ethanol in preweanling rats with or without prenatal experience with the drug. Pharmacol Biochem Behav. 2008;89:608–22. doi: 10.1016/j.pbb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arizzi M, Correa M, Betz A, Wisniecki A, Salamone J. Behavioral effects of intraventricular injections of low doses of ethanol, acetaldehyde, and acetate in rats: studies with low and high rate operant schedules. Behav Brain Res. 2003;147:203–10. doi: 10.1016/s0166-4328(03)00158-x. [DOI] [PubMed] [Google Scholar]
- Bechtholt A, Cunningham C. Ethanol-induced conditioned place preference is expressed through a ventral tegmental area dependent mechanism. Behav Neurosci. 2005;119:213–23. doi: 10.1037/0735-7044.119.1.213. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Froldi R, Quitadamo E, Massi M. Ethanol induces conditioned place preference in genetically selected alcohol-preferring rats. Psychopharmacol. 1999;141:235–41. doi: 10.1007/s002130050830. [DOI] [PubMed] [Google Scholar]
- Closon C, Didone V, Tirelli E, Quertemont E. Acetaldehyde and the hypothermic effects of ethanol in mice. Alcohol Clin Exp Res. 2009;33:2005–14. doi: 10.1111/j.1530-0277.2009.01039.x. [DOI] [PubMed] [Google Scholar]
- Cohen JF, Elberling JA, DeMaster EG, Lin RC, Nagasawa HT. N-Terminal dipeptides of D(−)-penicillamine as sequestration agents for acetaldehyde. J Med Chem. 2000;43:1029–33. doi: 10.1021/jm9902741. [DOI] [PubMed] [Google Scholar]
- Correa M, Manrique HM, Font L, Escrig MA, Aragon CMG. Reduction in the anxiolytic effects of ethanol by centrally formed acetaldehyde: the role of catalase inhibitors and acetaldehyde-sequestering agents. Psychopharmacol. 2008;200:455–64. doi: 10.1007/s00213-008-1219-3. [DOI] [PubMed] [Google Scholar]
- Correa M, Pascual M, Sanchis-Segura C, Guerri C, Aragon CMG. Lead-induced catalase activity differentially modulates behaviors induced by short-chain alcohols. Pharmacol Biochem Behav. 2005;82:443–52. doi: 10.1016/j.pbb.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Noble D. Conditioned activation induced by ethanol: role in sensitization and conditioned place preference. Pharmacol Biochem Behav. 1992;43:307–13. doi: 10.1016/0091-3057(92)90673-4. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Niehus J, Noble D. Species difference in sensitivity to ethanol's hedonic effects. Alcohol. 1993;10:97–102. doi: 10.1016/0741-8329(93)90087-5. [DOI] [PubMed] [Google Scholar]
- Deitrich RA. Acetaldehyde: déjà vu du jour. J Stud Alcohol. 2004;65:557–72. doi: 10.15288/jsa.2004.65.557. [DOI] [PubMed] [Google Scholar]
- Dickinson SD, Kashawny SK, Thiebes KP, Charles DY. Decreased sensitivity to ethanol reward in adolescent mice as measured by conditioned place preference. Alcohol Clin Exp Res. 2009;33:1246–51. doi: 10.1111/j.1530-0277.2009.00950.x. [DOI] [PubMed] [Google Scholar]
- Dudek BC, Abbott ME, Phillips TJ. Stimulant and depressant properties of sedative-hypnotics in mice selectively bred for differential sensitivity to ethanol. Psychopharmacol. 1984;82:46–51. doi: 10.1007/BF00426379. [DOI] [PubMed] [Google Scholar]
- Dudek BC, Phillips TJ. Distinctions among sedative, disinhibitory, and ataxic properties of ethanol in inbred and selectively bred mice. Psychopharmacol. 1990;101:93–99. doi: 10.1007/BF02253724. [DOI] [PubMed] [Google Scholar]
- Diana M, Peana AT, Sirca D, Lintas A, Melis M, Enrico P. Crucial role of acetaldehyde in alcohol activation of the mesolimbic dopamine system. Ann N Y Acad Sci. 2008;1139:307–317. doi: 10.1196/annals.1432.009. [DOI] [PubMed] [Google Scholar]
- Enrico P, Sirca D, Mereu M, Peana AT, Lintas A, Golosio A, et al. Acetaldehyde sequestering prevents ethanol-induced stimulation of mesolimbic dopamine transmission. Drug alcohol depen. 2009;100:265–71. doi: 10.1016/j.drugalcdep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Escarabajal MD, Witte PD, Quertemont E. Role of acetaldehyde in ethanol-induced conditioned taste aversion in rats. Psychopharmacol. 2003;167:130–36. doi: 10.1007/s00213-003-1427-9. [DOI] [PubMed] [Google Scholar]
- Faria RR, Lima Rueda AV, Sayuri C, Soares SL, Malta MB, Carrara-Nascimento PF, et al. Environmental modulation of ethanol-induced locomotor activity: Correlation with neuronal activity in distinct brain regions of adolescent and adult Swiss mice. Brain Res. 2008;1239:127–40. doi: 10.1016/j.brainres.2008.08.056. [DOI] [PubMed] [Google Scholar]
- Font L, Aragon C, Miquel M. Ethanol-induced conditioned place preference, but not aversion, is blocked by treatment with D-penicillamine, an inactivation agent for acetaldehyde. Psychopharmacol. 2006a;184:56–64. doi: 10.1007/s00213-005-0224-z. [DOI] [PubMed] [Google Scholar]
- Font L, Aragon CMG, Miquel M. Voluntary ethanol consumption decreases after the inactivation of central acetaldehyde by d-penicillamine. Behav Brain Res. 2006b;171:78–86. doi: 10.1016/j.bbr.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Font L, Miquel M, Aragon CMG. Prevention of ethanol-induced behavioral stimulation by D-penicillamine: a sequestration agent for acetaldehyde. Alcohol Clin Exp Res. 2005;29:1156–64. doi: 10.1097/01.alc.0000171945.30494.af. [DOI] [PubMed] [Google Scholar]
- Font L, Miquel M, Aragon CMG. Involvement of brain catalase activity in the acquisition of ethanol-induced conditioned place preference. Physiol Behav. 2008;93:733–41. doi: 10.1016/j.physbeh.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Zweifel M, Harts J, Lumeng L, Li TK. Importance of delta opioid receptors in maintaining high alcohol drinking. Psychopharmacol. 1991;103:467–72. doi: 10.1007/BF02244246. [DOI] [PubMed] [Google Scholar]
- Koob G, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Maestro RD, McDonald W. Distribution of superoxide dismutase, glutathione peroxidase and catalase in developing rat brain. Mech Ageing Dev. 1987;41:29–38. doi: 10.1016/0047-6374(87)90051-0. [DOI] [PubMed] [Google Scholar]
- Maestro RD, McDonald W. Subcellular localization of superoxide dismutases, glutathione peroxidase and catalase in developing rat cerebral cortex. Mech Ageing Dev. 1989;48:15–31. doi: 10.1016/0047-6374(89)90022-5. [DOI] [PubMed] [Google Scholar]
- Manzanedo C, Aguilar MA, Rodríguez-Arias M, Navarro M, Miñarro J. 7-Nitroindazole blocks conditioned place preference but not hyperactivity induced by morphine. Behav Brain Res. 2004;150:73–82. doi: 10.1016/S0166-4328(03)00225-0. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M. Ethanol, but not the anxiolytic drugs buspirone and diazepam, produces a conditioned place preference in rats exposed to conditioned fear stress. Pharmacol Biochem Behav. 2000;65:281–88. doi: 10.1016/s0091-3057(99)00224-5. [DOI] [PubMed] [Google Scholar]
- Mavelli I, Rigo A, Federico R, Ciriolo MR, Rotilio G. Superoxide dismutase, glutathione peroxidase and catalase in developing rat brain. Biochem J. 1982;204:535–40. doi: 10.1042/bj2040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Meshul CK, Phillips TJ. Ethanol- and cocaine-induced locomotion are genetically related to increases in accumbal dopamine. Genes, Brain Behav. 2009;8:346–55. doi: 10.1111/j.1601-183X.2009.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear NE. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41:41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JC, Ponce LF, Truxell E, Spear NE. Infantile sensitivity to ethanol's motivational effects: Ethanol reinforcement during the third postnatal week. Alcohol Clin Exp Res. 2006;30:1506–19. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- National Research Council Guide for the Care and Use of Laboratory Animals. National Institute of Health Publication. 1985 (Revised), No. 85-23. [Google Scholar]
- Nizhnikov ME, Pautassi RM, Truxell E, Spear NE. Opioid antagonists block the acquisition of ethanol-mediated conditioned tactile preference in infant rats. Alcohol. 2009;43(5):347–358. doi: 10.1016/j.alcohol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol Clin Exp Res. 2008;32:2016–27. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov ME, Spear NE. Assessing appetitive, aversive, and negative ethanol-mediated reinforcement through an immature rat model. Neurosci Biobehav Rev. 2009;33:953–74. doi: 10.1016/j.neubiorev.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Godoy JC, Spear NE, Molina JC. Early responsiveness to stimuli paired with different stages within the state of alcohol intoxication. Alcohol Clin Exp Res. 2002;26:644–54. [PubMed] [Google Scholar]
- Pautassi RM, Ponce LF, Molina JC. Effects of early exposure to ethanol on subsequent learning mediated by the unconditional attributes of the drug. Latinamerican J Psychol. 2005;37:149–166. [Google Scholar]
- Pautassi RM, Truxell E, Molina JC, Spear NE. Motivational effects of intraorally-infused ethanol in rat pups in an operant self-administration task. Physiol Behav. 2008;93:118–29. doi: 10.1016/j.physbeh.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peana AT, Enrico P, Assaretti AR, Pulighe E, Muggironi G, Nieddu M, et al. Key role of ethanol-derived acetaldehyde in the motivational properties induced by intragastric ethanol: a conditioned place preference study in the rat. Alcohol Clin Exp Res. 2008;32:249–58. doi: 10.1111/j.1530-0277.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin Exp Res. 2003;27:593–99. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Quertemont E. Discriminative stimulus effects of ethanol with a conditioned taste aversion procedure: lack of acetaldehyde substitution. Behav Pharmacol. 2003;14:343–350. doi: 10.1097/01.fbp.0000082130.08343.47. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Tambour S. Is ethanol a pro-drug? The role of acetaldehyde in the central effects of ethanol. Trends Pharmacol Sci. 2004;25:130–34. doi: 10.1016/j.tips.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Quertemont E, De Witte P. Conditioned stimulus preference after acetaldehyde but not ethanol injections. Pharmacol Biochem Behav. 2001;68:449–54. doi: 10.1016/s0091-3057(00)00486-x. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Didone V. Role of acetaldehyde in mediating the pharmacological and behavioral effects of alcohol. Alcohol Res Health. 2006;29:258–265. [PMC free article] [PubMed] [Google Scholar]
- Quintanilla ME, Tampier L. Acetaldehyde-reinforcing effects: differences in low-alcohol-drinking (UChA) and high-alcohol-drinking (UChB) rats. Alcohol. 2003;31:63–9. doi: 10.1016/j.alcohol.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Risinger F, Malott D, Prather L, Niehus D, Cunningham C. Motivational properties of ethanol in mice selectively bred for ethanol-induced locomotor differences. Psychopharmacology. 1994;116:207–16. doi: 10.1007/BF02245064. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McKinzie DL, Webster AA, Murphy JM, Lumeng L, et al. Low-dose stimulatory effects of ethanol during adolescence in rat lines selectively bred for high alcohol intake. Alcohol Clin Exp Res. 2004;28:535–43. doi: 10.1097/01.alc.0000122107.08417.d0. [DOI] [PubMed] [Google Scholar]
- Rodd Z, Bell R, Zhang Y, Murphy J, Goldstein A, Zaffaroni A, et al. Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: Involvement of dopamine and serotonin. Neuropsychopharmacol. 2005;30:330–38. doi: 10.1038/sj.npp.1300561. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks Z, Melendez R, Zaffaroni A, Goldstein A, McBride W, Li T. The reinforcing effects of acetaldehyde in the posterior ventral tegmental area of alcohol-preferring rats. Pharmacol Biochem Behav. 2002;72:55–64. doi: 10.1016/s0091-3057(01)00733-x. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Correa M, Miquel M, Aragon CMG. Catalase inhibition in the Arcuate nucleus blocks ethanol effects on the locomotor activity of rats. Neurosci Lett. 2005;376:66–70. doi: 10.1016/j.neulet.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Sanders S, Spear N. Ethanol acceptance is high during early infancy and becomes still higher after previous ethanol ingestion. Alcohol Clin Exp Res. 2007;31:1148–58. doi: 10.1111/j.1530-0277.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- Sheng Deng X, Deitrich RA. Putative role of brain acetaldehyde in ethanol addiction. Curr Drug Abuse Rev. 2008;1:3–8. doi: 10.2174/1874473710801010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Wang XY, Zhao M, Wang XY, Zhai HF, Lu L. Role of stress in acquisition of alcohol-conditioned place preference in adolescent and adult mice. Alcohol Clin Exp Res. 2007;31:2001–5. doi: 10.1111/j.1530-0277.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science. Dev Psychobiol. 2010;52:236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Murphy J, McBride W, Lumeng L, Li T. Place conditioning with alcohol in alcohol-preferring and -nonpreferring rats. Pharmacol Biochem Behav. 1996;53:487–91. doi: 10.1016/0091-3057(95)02102-7. [DOI] [PubMed] [Google Scholar]
- Truxell E, Spear NE. Immediate acceptance of ethanol in infant rats: ontogenetic differences with moderate but not high ethanol concentration. Alcohol Clin Exp Res. 2004;28:1200–11. doi: 10.1097/01.alc.0000134220.34842.18. [DOI] [PubMed] [Google Scholar]
- Truxell E, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31:755–65. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Wang YC, Huang ACW, Hsiao S. Paradoxical simultaneous occurrence of amphetamine-induced conditioned taste aversion and conditioned place preference with the same single drug injection: a new “pre- and post-association” experimental paradigm. Pharmacol Biochem Behav. 2010;95:80–7. doi: 10.1016/j.pbb.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta TS. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol, and drug dependence. Alcohol Clin Exp Res. 1998;22:914–20. [PubMed] [Google Scholar]