Abstract
Objectives
In this study, we investigated the potential harmful effect of the exposure to silicon dioxide (SiO2) nanoparticles through in vitro toxicity assay using human bronchial epithelial cell, Beas-2B with a focus on the involvement of oxidative stress as the toxic mechanism.
Methods
SiO2-induced oxidative stress was assessed by examining formation of reactive oxygen species (ROS), the induction of superoxide dismutase (SOD) and heme oxygenase-1 (HO-1), as well as cytotoxicity effect was evaluation by cell viability. Subsequently, to understand the molecular mechanism of nanoparticle-induced oxidative stress, the involvement of oxidative stress-responding transcription factors, such as, nuclear factor-kappaB (NF-κB) and nuclear factor-E2-related factor-2 (Nrf-2), and mitogen-activated protein (MAP) kinase signal transduction pathway was also investigated.
Results
5-d i phenyltera zolium bromide (MTT) assay results show that decrease 20% in cell viability and the number of cells in the subG1 phase increased. The increase in ROS formation was observed in SiO2 nanoparticle treated cells. The expression of SOD protein was not changed, whereas that of HO-1 was increased by SiO2 nanoparticle exposure. transcription factors Nrf-2 and the expression of phosphorylated form of extracellular signal-regulating kinase (ERK) was strongly induced by SiO2 nanoparticle exposure
Conclusions
SiO2 nanoparticles exert their toxicity through oxidative stress as they cause the significant increase ROS level. SiO2 nanoparticles induce induction of HO-1 via Nrf-2-ERK MAP kinase pathway. Our tested oxidative stress parameters are rather limited in terms of allowing the full understanding of oxidative stress and cellular response by SiO2 nanoparticle exposure.
Keywords: Extracellular signal-regulating kinase, Heme oxygenase-1, Nuclear factor-E2-related factor-2, Oxidative stress, Silicon dioxide nanoparticles
INTRODUCTION
In recent years, as nanotechnology has rapidly developed, nanomaterials have been widely used in the fields of biomedicine, pharmaceutical, and other industry. Of the various manufactured nanomaterials, silicon dioxide (SiO2) nanoparticles have the potential for widespread applications. SiO2 nanoparticles are being used in these fields such as chemical mechanical polishing and as additives to drugs, cosmetics, printer toners, varnishes, and food [1,2]. In recent years, the use of SiO2 nanoparticles has been extended to biomedical and biotechnological fields, such as biosensors for simultaneous assay of glucose, lactate, L-glutamate, and hypoxanthine levels in rat striatum [3], biomarkers for leukemia [4,5], DNA delivery [6,7], targeted drug delivery [8] and controlled drug release for genes and proteins [9,10]
Considering their wide range of applications, the potential adverse effect of SiO2 nanoparticles on human health and on the environment is of great interest. In this study, we investigated the potential harmful effect of exposure to SiO2 nanoparticles by conducting an in vitro toxicity assay with a focus on the involvement of oxidative stress. Numerous previous studies on nanoparticle toxicity, with various cell types and various nanoparticle types, reported that oxidative stress is one of the most important toxicity mechanisms related to exposure to nanoparticles [11-15]. Indeed, previous studies reported oxidative stress as the toxic mechanism of SiO2 [13,16-18]
In this study, to understand the potential harmful effect of nanoparticles on human health, the oxidative stress-related toxicity was investigated by exposure to SiO2 nanoparticles. SiO2-induced oxidative stress was assessed by examining formation of reactive oxygen species (ROS), the induction of superoxide dismutase (SOD) and heme oxygenase-1 (HO-1), as well as cytotoxicity effect was evaluation by cell viability. Subsequently, to understand the molecular mechanism of nanoparticle-induced oxidative stress, the involvement of oxidative stress-responding transcription factors, such as, nuclear factor-kappaB (NF-κB) and nuclear factor-E2-related factor-2 (Nrf-2), and mitogen-activated protein (MAP) kinase signal transduction pathway was also investigated.
MATERIALS AND METHODS
I. Cell Culture and Nanoparticle Treatment
Human bronchial epithelial cells, Beas-2B, were maintained in DMEM / F12 (GIBCO BRL Life Technologies, Rockville, MD, USA), supplemented with 10% (v/v) fetal bovine serum and 1% antibiotics, at 37℃ in a humidified atmosphere of air and 5% CO2. SiO2 nanoparticles were purchased from Sigma (St. Louis, MO, USA). Test solution of SiO2 nanoparticles were prepared in the culture media and dispersed for 20 minutes using a sonicator (Branson Inc., Danbury, CT, USA) to prevent aggregation. During the testing periods, the suspension of nanoparticles was stable and uniform in the culture media. For surface area measurement, Branauer, Emmett & Teller (BET) method was used with BELSORP-mini II, a volumetric adsorption apparatus (BEL Japan, Inc., Osaka, Japan). To investigate the size and shape of SiO2 nanoparticles, 20 uL of particle suspension form test media was dried on a 400 mesh carbon-coated copper grid and imaged with a JEM 1010 transmission electron microscopy (TEM; JEOL, Tokyo, Japan) at 40-100 kV. The size distribution of nanoparticles was evaluated using a Photal dynamic light scattering (DLS) spectrometer, DLS-7000 (Otsuka Electronics Co., Inc., Osaka, Japan). The concentration we used in this study was 1 mg/L to prevent aggregation and/or precipitation of particles. The cells were treated with various sizes of nanoparticles for 24 hours for toxicological studies.
II. Cell Viability Assay
Beas-2B cells were plated on 96-well plates and the plates were incubated with the nanoparticles for 24 hours. Cell viability was measured using 3-[4, 5-dimethylthiazol-2-yl]-2, 5-di phenyltera zolium bromide (MTT) reagent [19].
III. Flow Cytometry
Flow cytometry was performed on the treated and control cells for analysis of the cell cycle and apoptosis. Propidium iodide (PI) stained cells were analyzed using a flow cytometer (BD Science, San Jose, CA, USA) [20]. The effect on apoptosis was determined by the increase in the proportion of subG1 hypo-diploid cells.
IV. Formation of ROS
To detect ROS generation in nanoparticles-treated cells, a fluorometric assay using intracellular oxidation of 2,7-dichlorofluoroscein diacetate (DCFH-DA, Sigma) was performed [21,22]. Cells grown to confluence were treated with SiO2 nanoparticles for 24 hours, washed with PBS, and then incubated with DCFH-DA (40 uM) for 30 minutes. Following DCFH-DA incubation, the fluorescence of dichlorofluoroscein (DCF), which is the oxidized product of DCFH-DA, was visualized using a fluorescent microscope (Nikon, Tokyo, Japan) with excitation and emission wavelengths of 485 and 530 nm, respectively.
V. Western Blotting
For western blotting analysis, aliquots of the cell lysates were subjected to electrophoresis on a 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel, and the gel transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). The membranes were incubated with a primary antibody for 1 hours, subjected to further incubation with a secondary antibody and then exposed to X-ray film. Protein bands were detected using an enhanced chemiluminescence western blotting detection kit (Amersham, Little Chalfont, Buckinghamshire, UK). The antibodies for the detection of p38, phosphorylated p38, ERK-2, phosphorylated ERK and Nrf-2 were purchased from Santa Cruz (Santa Cruz, CA, USA), antibodies for JNK, phosphorylated JNK were from Cell Signaling (Beverly, MA, USA), antibody for HO-1 was from Stressgen (Victorya, BC, Canada) and antibody for Cu/Zn SOD was from Biodesign (Saco, ME, USA).
VI. Data Analysis
Statistical differences between the control and treated cells were examined with the aid of a parametric t test using SPSS version 12.0 KO (SPSS Inc., Chicago, Il, USA). An alpha level of 0.05 was used to determine significance in all statistical analyses.
RESULTS
I. Characterization of SiO2 Nanoparticles
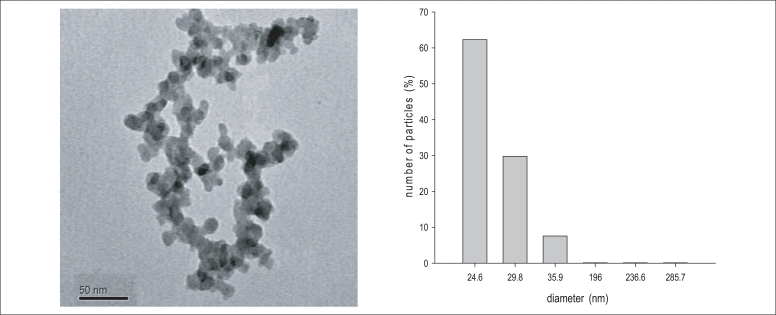
The result from characterization of SiO2 nanoparticles was summarized in Figure 1. The BET surface areas of was 201.01 m3/g, respectively. The TEM images indicated that silica nanoparticles had an even distribution with 20-40 nm individual particle sizes. The size distribution in the test medium was investigated using a DLS method, as the size of the nanoparticles distributed in the test medium were about 25 and 40 nm.
Figure 1.
Characterization of different sized SiO2 nanoparticles using TEM and DLS methods. Particles shape were analysed by TEM (A) and the size distribution in the test media were evaluated by DLS (B).
SiO2: silicon dioxide, TEM: transmission electron microsopy, DLS: dynamic light scattering.
II. Cytotoxicity of SiO2 Nanoparticles
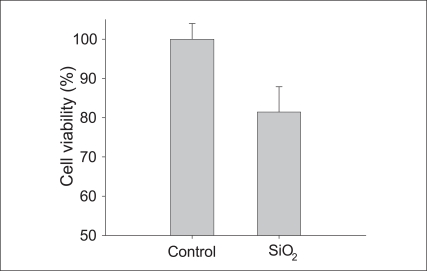
To investigate of SiO2 nanoparticle induced cytotoxicity effects on Beas-2B cells, cell viability and apoptosis were examined. MTT assay results show that decrease in cell viability was observed only about 20% compared with that of the control by SiO2 exposure (Figure 2). Flow cytometry analysis was conducted using PI staining to quantify the number of cells with a subdiploid DNA content (Figure 3). In Beas2B cells exposed to SiO2 nanoparticles, the number of cells in the subG1 phase increased. The degree of increase induced by SiO2 nanoparticles was about 3-fold compared with that of the control.
Figure 2.
The effect of 1 mg/L SiO2 nanoparticles on the cell viability investigated using MTT assay. Results were presented as relative units compared to control.
SiO2: silicon dioxide, MTT: 5-d i phenyltera zolium bromide.
Figure 3.
Flow cytometry analysis to quantify the number of cells with a subdiploid DNA content in 1 mg/L SiO2 nanoparticles exposed Beas-2B cells. H2O2 (100 uM) was used as positive control.
SiO2: silicon dioxide.
III. SiO2 Nanoparticles Induced Oxidative Stress
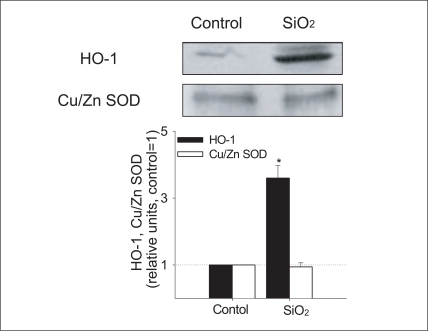
The formation of ROS was visualized in Beas2-B cells exposed to SiO2 nanoparticles by inspection under fluorescent microscope (Figure 4). DCFH-DA staining revealed increased concentration of ROS in SiO2 nanoparticles treated cells. As the increase in ROS formation was observed in SiO2 nanoparticle treated cells (Figure 4), a marker for cellular defense mechanism against oxidative stress (i.e. induction of antioxidant enzymes) was investigated in Beas2-B cells exposed to SiO2 nanoparticles (Figure 5). The expression of SOD protein was not changed, whereas that of HO-1 was increased by SiO2 nanoparticle exposure.
Figure 4.
ROS induced by nanoparticles in Beas-2B cells. The cells were incubated with 1 mg/L SiO2 nanoparticles for 24 hours and 40 uM DCFH-DA at 37℃ for 30 min and observed fluorescence microscope. H2O2 (100 uM) was used as positive control.
ROS: reactive oxygen species, DCFH-DA: 2,7-dichlorofluoroscein diacetate, SiO2: silicon dioxide.
Figure 5.
Expression of HO-1 and Cu/Zn SOD in Beas-2B cells exposed to 1 mg/L SiO2 nanoparticles Densitometric values of expression of HO-1 and Cu/Zn SOD were normalized using that of Actin and were presented as relative units compared to control. The control value was set to 1; data represent the mean ± standard error of the mean; n=3.
SOD: superoxide dismutase, SiO2: silicon dioxide,
HO-1: heme oxygenase-1.
*p < 0.05 (B).
IV. Oxidative Signaling Pathway
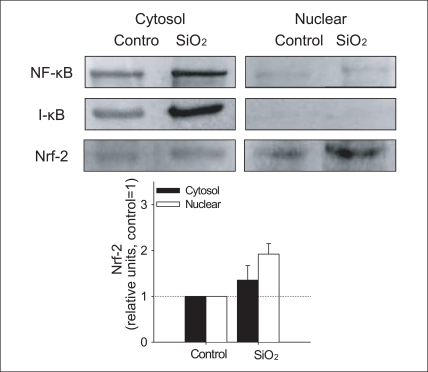
SiO2 increased formation of ROS and the induction of antioxidant enzyme, HO-1 (Figure 4,5). In order to understand the molecular mechanism of the observed oxidative stress and related physiological alteration by SiO2 nanoparticles exposure, NF-κB and Nrf-2 were examined in the cytosolic and nuclear fraction of cells treated with SiO2 nanoparticles. Neither nuclear localization of NF-κB nor the degradation of cytosolic I-κB was observed, whereas nuclear localization of Nrf-2 increased in cells treated with SiO2 nanoparticles (Figure 6). In our study, SiO2 nanoparticles induced the translocation of Nrf-2 into the nucleus as evidenced by the results of the western blot analysis (Figure 6).
Figure 6.
Expression of Nrf-2 in cytosolic and nuclear fraction of Beas-2B cells exposed to 1 mg/L SiO2 nanoparticles. Densitometric values of expression of Nrf-2 were normalized using that of Actin and were presented as relative units compared to control.
Nrf: nuclear factor-E2-related factor-2, SiO2: silicon dioxide.
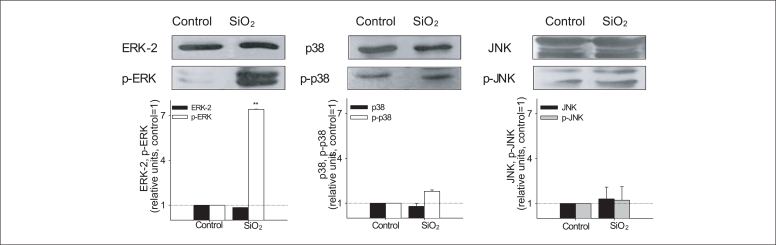
To further clarify the possible upstream oxidative signaling pathway involved in Nrf-2 activation leading to HO-1 induction, we examined the activation of MAP kinases, known as major signaling kinases, involved in cell survival against oxidative stress through the Nrf-2 signaling pathway (Figure 7). The expression of unphosphorlyated forms of MAP kinases was constant regardless of exposure of SiO2 nanoparticles, whereas, the expression of phosphorylated form of ERK was strongly induced by SiO2 nanoparticles. The phosphorylation of ERK was significant by SiO2 nanoparticles (about 5-fold compared with that of the control). The expression level of phosphorylated p38 or JNK was not changed by SiO2 nanoparticles.
Figure 7.
Expression of intact and phosphorylated ERK, p38 and JNK in Beas-2B cells exposed to 1 mg/L SiO2 nanoparticles. Densitometric values of expression of ERK, p38 and JNK were normalized using that of Actin and were presented as relative units compared to control. The control value was set to 1; data represent the mean ± standard error of the mean; n=3.
ERK: extracellular signal-regulating kinase, JNK: c-Jun N-terminal kinase, SiO2: silicon dioxide.
*p < 0.05, †p < 0.01.
DISCUSSION
There are many studies have reported that nanoparticle toxicity deal with oxidative stress [11-14,23-27]. However, the mechanism by which oxidative stress is involved in nanotoxicity has been poorly addressed. We measured the ROS level (Figure 4), as well as the induction of antioxidant enzyme (Figure 5), provided strong evidence for the involvement of oxidative stress in SiO2 nanoparticles-induced cytotoxicity. To understand the molecular mechanism of the observed SiO2 nanoparticle-induced cytotoxicity by oxidative stress, activation of transcription factors (i.e NF-κB, Nrf-2) and signal transduction pathway (i.e. MAP kinase pathway) investigated.
In this study, to provide cellular consequence of the oxidative stress response observed (Figure 4-7), and cytotoxicity test were conducted (Figure 2,3). Exposed to SiO2 nanoparticles seemed to affect the cell viability and apoptosis by induction of ROS. ROS production was investigated as an initial step of oxidative stress, which revealed an increase in ROS formation in the cells exposed to SiO2 compared to control.
Neither nuclear localization of NF-κB nor the degradation of cytosolic I-κB was observed, whereas nuclear localization of Nrf-2 increased in cells treated with SiO2 nanoparticles (Figure 6). In our study, SiO2 nanoparticles induced the translocation of Nrf-2 into the nucleus as evidenced by the results of the western blot analysis (Figure 6). The translocation of Nrf-2 into the nucleus following nanoparticles treatment was associated with the increase in HO-1 protein, which suggests that nanoparticles activate Nrf-2 in association with the upregulation of HO-1 in Beas2-B cells. However, in this study, SiO2 nanoparticle-induced NF-κB activation was not observed, which was unexpected, as NF-κB is the major stress response transcription factor that has been reported to respond to a wide variety of environment stressors. Therefore, although SiO2-induced NF-κB activation was not observed in this study, the relative importance of NF-κB vs Nrf-2 signaling in terms of contribution to antioxidant response, such as HO-1 upregulation, may merit further investigation with various oxidative stress-inducing nanoparticles. To confirm SiO2 nanoparticles-induced for activation of transcription factor, more direct evidence, using electrophoretic mobility shift assay (EMSA) may be needed.
The upstream signaling mechanism responsible for regulating oxidative stress is poorly defined. Most studies have focused that oxidative stress may evoke the induction of antioxidant-related transcription factors, such as Nrf-2 or NF-kB via basal signal transduction systems, such as MAP kinase cascade. The MAP kinase cascades are multifunctional signaling pathways that are evolutionally well conserved in all eukaryotic cells. Three MAP kinase cascades that converge on ERKs, JNKs, and p38 MAP kinases have already been characterized [28,29]. Two of those three MAP kinase cascades converge on JNKs and p38 MAP kinases are preferentially activated by cytotoxic stresses, such as X-ray/UV irradiation, heat/osmotic shock, and oxidative/nitrosative stress [30-33]. To further clarify the possible upstream oxidative signaling pathway involved in Nrf-2 activation leading to HO-1 induction, we examined the activation of MAP kinases, known as major signaling kinases, involved in cell survival against oxidative stress through the Nrf-2 signaling pathway (Figure 7). The expression of unphosphorlyated forms of MAP kinases was constant regardless of exposure of SiO2 nanoparticles, whereas, the expression of phosphorylated form of ERK was strongly induced by SiO2 nanoparticles.
Activation of ERK MAP kinase pathway by SiO2nanoparticles suggests that the induction of HO-1 may be mediated through Nrf-2-ERK MAP kinase signaling pathway. ERK has been known to respond to internal stimuli, such as growth factors. However, our study revealed that SiO2 nanoparticles strongly induce the phosphorylation of ERK. Increase expression of phosphorylated ERK was also observed in our previous study with CeO2 nanoparticles exposure [34]. These results suggest that the ERK signaling pathway also responds to environmental stressors. The activation of the ERK signaling pathway by external stimuli has already been reported [30-33].
CONCLUSIONS
SiO2 nanoparticles exert their toxicity through oxidative stress as they cause the significant increase in cellular H2O2 concentrations. SiO2 nanoparticles induce induction of HO-1 via Nrf-2-ERK MAP kinase pathway. Our tested oxidative stress parameters are rather limited in terms of allowing the full understanding of oxidative stress and cellular response by SiO2 nanoparticle exposure. Further studies on the mechanism by which SiO2 nanoparticles induce the Nrf2-ERK MAP kinase pathway are warranted to better understand the nanoparticle-induced cytotoxicity by oxidative stress, as are studies with dose-response and time-course studies.
ACKNOWLEDGEMENTS
This work was supported by the Midcareer Researcher Program through National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0027722)
Footnotes
The authors have no conflict of interest to declare on this study.
This article is available from: http://e-eht.org/
References
- 1.Lu J, Liong M, Zink JI, Tamanoi F. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small. 2007;3(8):1341–1346. doi: 10.1002/smll.200700005. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J, Wu P, Brancewicz C, Li Y. A liposome-containing slurry for tungsten chemical mechanical polishing. J Electrochem Soc. 2007;154(3):H225–H230. [Google Scholar]
- 3.Zhang FF, Wan Q, Li CX, Wang XL, Zhu ZQ, Xian YZ, et al. Simultaneous assay of glucose, lactate, L-glutamate and hypoxanthine levels in a rat striatum using enzyme electrodes based on neutral red-doped silica nanoparticles. Anal Bioanal Chem. 2004;380(4):637–642. doi: 10.1007/s00216-004-2804-x. [DOI] [PubMed] [Google Scholar]
- 4.Santra S, Zhang P, Wang K, Tapec R, Tan W. Conjugation of biomolecules with luminophore-doped silica nanoparticles for photostable biomarkers. Anal Chem. 2001;73(20):4988–4993. doi: 10.1021/ac010406+. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci U S A. 2003;100(23):13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, et al. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci U S A. 2005;102(32):11539–11544. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gemeinhart RA, Luo D, Saltzman WM. Cellular fate of a modular DNA delivery system mediated by silica nanoparticles. Biotechnol Prog. 2005;21(2):532–537. doi: 10.1021/bp049648w. [DOI] [PubMed] [Google Scholar]
- 8.Zhao W, Gu J, Zhang L, Chen H, Shi J. Fabrication of uniform magnetic nanocomposite spheres with a magnetic core/mesoporous silica shell structure. J Am Chem Soc. 2005;127(25):8916–8917. doi: 10.1021/ja051113r. [DOI] [PubMed] [Google Scholar]
- 9.Roy I, Ohulchanskyy TY, Bharali DJ, Pudavar HE, Mistretta RA, Kaur N, et al. Optical tracking of organically modified silica nanoparticles as DNA carriers: a nonviral, nanomedicine approach for gene delivery. Proc Natl Acad Sci U S A. 2005;102(2):279–284. doi: 10.1073/pnas.0408039101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slowing II, Vivero-Escoto JL, Wu CW, Lin VS. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev. 2008;60(11):1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Shvedova AA, Castranova V, Kisin ER, Schwegler-Berry D, Murray AR, Gandelsman VZ, et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003;66(20):1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 12.Green M, Howman E. Semiconductor quantum dots and free radical induced DNA nicking. Chem Commun (Camb) 2005;(1):121–123. doi: 10.1039/b413175d. [DOI] [PubMed] [Google Scholar]
- 13.Lin W, Huang YW, Zhou XD, Ma Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol. 2006;217(3):252–259. doi: 10.1016/j.taap.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Monteiller C, Tran L, MacNee W, Faux S, Jones A, Miller B, et al. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup Environ Med. 2007;64(9):609–615. doi: 10.1136/oem.2005.024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eom HJ, Choi J. p38 MAPK activation, DNA damage, cell cycle arrest and apoptosis as mechanisms of toxicity of silver nanoparticles in Jurkat T cells. Environ Sci Technol. 2010;44(21):8337–8342. doi: 10.1021/es1020668. [DOI] [PubMed] [Google Scholar]
- 16.Kaewamatawong T, Shimada A, Okajima M, Inoue H, Morita T, Inoue K, et al. Acute and subacute pulmonary toxicity of low dose of ultrafine colloidal silica particles in mice after intratracheal instillation. Toxicol Pathol. 2006;34(7):958–965. doi: 10.1080/01926230601094552. [DOI] [PubMed] [Google Scholar]
- 17.Park EJ, Park K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol Lett. 2009;184(1):18–25. doi: 10.1016/j.toxlet.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Liu J, He H, Zhou L, Gong C, Wang X, et al. SiO2 nanoparticles induce cytotoxicity and protein expression alteration in HaCaT cells. Part Fibre Toxicol. 2010;7:1. doi: 10.1186/1743-8977-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 21.Fotakis G, Cemeli E, Anderson D, Timbrell JA. Cadmium chloride-induced DNA and lysosomal damage in a hepatoma cell line. Toxicol In Vitro. 2005;19(4):481–489. doi: 10.1016/j.tiv.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Elbekai RH, El-Kadi AO. The role of oxidative stress in the modulation of aryl hydrocarbon receptor-regulated genes by As3+, Cd2+, and Cr6+ Free Radic Biol Med. 2005;39(11):1499–1511. doi: 10.1016/j.freeradbiomed.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Xia T, Korge P, Weiss JN, Li N, Venkatesen MI, Sioutas C, et al. Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: implications for ultrafine particle toxicity. Environ Health Perspect. 2004;112(14):1347–1358. doi: 10.1289/ehp.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro. 2005;19(7):975–983. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 25.Sayes CM, Gobin AM, Ausman KD, Mendez J, West JL, Colvin VL. Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials. 2005;26(36):7587–7595. doi: 10.1016/j.biomaterials.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Foster KA, Galeffi F, Gerich FJ, Turner DA, Müller M. Optical and pharmacological tools to investigate the role of mitochondria during oxidative stress and neurodegeneration. Prog Neurobiol. 2006;79(3):136–171. doi: 10.1016/j.pneurobio.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limbach LK, Wick P, Manser P, Grass RN, Bruinink A, Stark WJ. Exposure of engineered nanoparticles to human lung epithelial cells: influence of chemical composition and catalytic activity on oxidative stress. Environ Sci Technol. 2007;41(11):4158–4163. doi: 10.1021/es062629t. [DOI] [PubMed] [Google Scholar]
- 28.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81(2):807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 29.Takeda K, Matsuzawa A, Nishitoh H, Ichijo H. Roles of MAPKKK ASK1 in stress-induced cell death. Cell Struct Funct. 2003;28(1):23–29. doi: 10.1247/csf.28.23. [DOI] [PubMed] [Google Scholar]
- 30.Roberts ES, Richards JH, Jaskot R, Dreher KL. Oxidative stress mediates air pollution particle-induced acute lung injury and molecular pathology. Inhal Toxicol. 2003;15(13):1327–1346. doi: 10.1080/08958370390241795. [DOI] [PubMed] [Google Scholar]
- 31.Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Lui L, et al. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 2004;79(6):393–403. [PubMed] [Google Scholar]
- 32.Kim J, Sharma RP. Cadmium-induced apoptosis in murine macrophages is antagonized by antioxidants and caspase inhibitors. J Toxicol Environ Health A. 2006;69(12):1181–1201. doi: 10.1080/15287390600631144. [DOI] [PubMed] [Google Scholar]
- 33.Iles KE, Dickinson DA, Wigley AF, Welty NE, Blank V, Forman HJ. HNE increases HO-1 through activation of the ERK pathway in pulmonary epithelial cells. Free Radic Biol Med. 2005;39(3):355–364. doi: 10.1016/j.freeradbiomed.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eom HJ, Choi J. Oxidative stress of CeO2 nanoparticles via p38-Nrf-2 signaling pathway in human bronchial epithelial cell, Beas-2B. Toxicol Lett. 2009;187(2):77–83. doi: 10.1016/j.toxlet.2009.01.028. [DOI] [PubMed] [Google Scholar]