Abstract
Objectives
Tetrasodium pyrophosphate (TSP) is used in processed meat products, as an emulsifier in cheese, and as a color preservative in soybean paste. However, little is known about its toxicity. This study was conducted to investigate the potential acute and repeated dose toxicity of TSP in Spraque Dawley (SD) rats.
Methods
In the acute study, animals were administered with oral or dermal doses of 2,000 mg/kg TSP. In the repeated dose study, animals were administered doses of 0, 250, 500, and 1,000 mg/kg by oral gavage five times a week for 90 days.
Results
In acute toxicity studies, no dead animals or abnormal necropsy findings were found in the control or treated group. In the repeated dose toxicity study, there were no significant changes in body weight in the 1,000 mg/kg treatment group, or food consumption, urinalysis, and hematology in any group. With regards serum biochemistry, the levels of total protein, albumin, A/G ratio, triglyceride, calcium and inorganic phosphate were altered at doses of 500 and 1,000 mg/kg. However, no changes were observed at the dose of 250 mg/kg. With regards histopathological findings, cortical tubular basophilia of the kidney increased at the dose of 1,000 mg/kg, but not at doses of 250 and 500 mg/kg. No significant changes were observed in other organs at doses of 250, 500, and 1,000 mg/kg.
Conclusions
Based on the results, TSP is unclassified according to the Globally Harmonization System, with an LD50 value of over 2,000 mg/kg. The no observed effect level (NOEL) and no observed adverse effect level (NOAEL) were 250 and 500 mg/kg /day respectively and the target organ appears to be the kidney.
Keywords: Acute, Dermal toxicity, Oral toxicity, Rat, Repeated dose, Tetrasodium pyrophosphate
INTRODUCTION
Chemicals are constantly being developed and produced to meet the needs of new applications and components. There are currently 10 million species of chemicals in circulation worldwide. Every year 2000 new species are developed and produced commercially, and continued growth of the chemical industry is expected. According to the OECD, the production of chemicals will increase by a further 80% until 2020 based on the volume produced in 1995.
Tetrasodium pyrophosphate (TSP; CAS No. 7722-88-5) is a colorless or white crystalline powder, is slightly efflorescent in air, and is insoluble in ethanol [1-3]. TSP is used in various fields, with 537 tons manufactured in korea and 125 tons imported in 2006 [4-6]. The reaction between the polyphosphate chain and protein was discovered in 1916 [7] and industrial manufacture began in 1920 [8,9]. TSP is used in processed meat and used as form of the mixture of tripolyphosphate. Pyrophosphate, glucose, and sorubin acid are added to fish and sausages. It is also used as a catalyst and as an emulsifier in cheese [10,11]. It is generally used in combination with a phosphate or metaphosphate and the amount used differs according to the pH. Addition of TSP to soybean paste prevents discoloration , and to soy sauce becomes well the harmony of colors. It is also used to restrict the acidification and fermentation of soy sauce owing to its high alkalinity, and to prevent sedimentation in soft drinks.
In humans, TSP can cause laryngopharyngitis, coughing, and breathlessness when inhaled, strong irritation, redness, pain, and edema following exposure of the skin, and eye irritation, pain, and blurriness of vision if coming into contact with the eyes [12]. Various toxicity data for TSP have recently been reported. The LD50 of rats and mice orally exposed to TSP was over 2,000 mg/kg body weight (BW). [13,14]. Mutagenic activity using the Ames test was negative [15]. However there is little information on dermal and repeated dose oral exposure toxicity. Therefore we investigated the potential acute and repeated dose toxicity, providing useful information for assessing the toxicological relevance of TSP. Tests were carried out according to the Ministry of Labor guidelines for testing the hazards and risks of industrial chemical elements and the OECD guidelines for testing chemicals, section 4.
MATERIALS AND METHODS
I. Test Substance and Preparation
TSP (Lot No. 048K0049), a colorless white powder, was chemically synthesized and provided by SIGMA-ALDRICH (Korea). TSP was dissolved in distilled water immediately before treatment. Lower doses were prepared by stepwise dilution of the highest dose.
II. Animal Husbandry and Maintenance
Young adult spraque-dawley (SD) rats (5 weeks old) of both sexes were purchased from the Orient Bio Co., Ltd. (Gyeonggi-do, Korea) and acclimated for 6 days prior to commencement of the studies. The animals were housed in groups of two to three in stainless steel wire mesh cages and were allowed sterilized tap water and commercial rodent chow (2.0 Mrad γ-ray sterilized EP pellet, Cargill Agri Purina Korea Ltd, Korea). The animals were housed in a room maintained at a temperature of 23±3℃ and a relative humidity of 55±5% with artificial lighting from 08:00 to 20:00 and with 10~18 air changes per hour. Only healthy animals were assigned to the studies. All animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (Korea Testing and Research Institute).
III. Acute Oral Toxicity Study
The study was conducted in accordance with OECD guidelines for the Testing of Chemical No. 420 'Fixed dose method' [16] and the Notification of the Ministry of Labor No. 2008-11 'Examination of toxic risk about industrial chemicals' [17]. Six-week-old female rats (5/group) were randomly assigned and fasted for approximately 12 hour prior to dosing. The test substance was formulated as a 200 mg/mL suspension in distilled water then administered to one animal at a dose of 2,000 mg/kg body weight by oral gavage; the same procedure was carried out in four animals for reconfirmation (1st, 2nd step). Each animal was observed for signs of toxicity at 1 and 3 hours after test substance administration and once daily thereafter for 14 days. Mortality and morbidity checks were performed daily. Body weight was recorded before administration, on day 1 and 7, and at termination. On day 15, surviving rats were euthanized, necropsies were performed, and any gross observations were recorded.
IV. Acute Dermal Toxicity Study
The study was conducted in accordance with the OECD guidelines for the Testing of Chemical No. 402 'Acute dermal toxicity' [16] and the Notification of the Ministry of Labor No. 2008-11 'Examination of toxic risk about industrial chemicals' [17]. Twenty rats (160-220 g) were randomly assigned. The test substance was formulated as a 400 mg/mL suspension in distilled water then administered to each animal at the dose of 2,000 mg/kg BW. The fur on the back of each animal was closely clipped 24 hour before treatment. The test substance was directly applied to a small area (4 cm-5 cm) of skin. After application of the test substance, the test area was covered with a non-occlusive dressing (a gauze patch) and then a semi-occlusive bandage for 24 hour. At the end of the exposure period, any residual test substance was removed by distilled water, and carefully dried. Each animal was observed for signs of toxicity once daily for 14 days following treatment. Mortality and morbidity checks were performed daily. Body weight was recorded before treatment, on day 1 and 7, and at termination. On day 15, surviving rats were euthanized, necropsies were performed, and any gross observations were recorded.
V. Repeated Dose 90-Day Oral Toxicity Study
A repeated dose 90-day oral toxicity study was conducted in accordance with OECD guidelines for the Testing of Chemical No. 408 'Repeated Dose 90-day Oral Toxicity Study in Rodents' [16] and the notification of the Ministry of Labor No. 2008-11 'Examination of Toxic Risk about Industrial Chemicals' [17]. Groups of 10 randomly assigned rats (6 weeks old) of each sex were injected by oral gavage with TSP suspended in distilled water at doses of 250, 500, and 1,000 mg/kg/BW/day five times a week for 90 days. The doses were selected based on the results of the acute toxicity study and a 14-day dose range study. No toxicity or mortality was observed in any of the five male and female rats treated with TSP for 14 days. The control groups received vehicle (filtered tap water) only.
VI. Mortality and Clinical Signs
All animals were observed thoroughly for the onset of any immediate signs of toxicity, and throughout the observation period to record any delayed acute effects and mortality. All animals were observed once a day for 90 days after administration.
VII. Body Weight and Food Consumption
All animals were monitored for BW changes once a week after administration. The mean BW of animals that survived up to the end of the administration period (90 days) was calculated. Food consumption by each group was measured at the start of treatment and during the 90-day administration period.
VIII. Urinalysis
During the last week of administration, urinalysis of five animals of each sex per group was conducted to determine glucose, bilirubin, ketone body, specific gravity, blood, pH, protein, urobilinogen, nitrite and leukocyte levels, using the Multistix 10SG (Bayer, U.S.A.) and urine analyzer (Clinitek 500, U.S.A.).
IX. Hematology and Coagulation
Animals were fasted overnight and, following isoflurane inhalation anesthesia, blood samples for hematology and clinical chemistry were collected from the abdominal aorta using a syringe and a 24-gauge needle. Hematology parameters in EDTA 3K-treated blood (Sewon Medical, Korea) were measured using a hematological autoanalyzer (Advia 120E, Bayer, UK), including total white blood cell (WBC) count, differential leukocyte percentage, total red blood cell (RBC) count, hemoglobin (Hb) concentration, hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), reticulocyte ratio (Retic), and platelet count (PLT). Prothrombin time (PT) and activated partial thromboplastin time (APTT) were measured in sodium citrate-treated blood using an automated hematocoagulation analyzer (Coagrex-100S, Japan).
X. Biochemistry
Blood collected for serum biochemistry was untreated and centrifuged at 3,000 rpm for 10 minutes. The serum obtained was examined for the following parameters using a biochemistry autoanalyzer (Hitachi 7060, Japan): total protein (TP), albumin (ALB), A/G ratio, total bilirubin (T-BIL), alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine (CRN), blood urea nitrogen (BUN), total cholesterol (CHOL), triglycerides (TG), glucose (GLU), calcium (Ca), phosphorus (IP) and creatine kinase (CK). Serum electrolytes such as chloride (Cl), sodium (Na), and potassium (K) were measured using an ion autoanalyzer (Bayer 644 Na/K/Cl analyzer, USA).
XI. Necropsy Findings
At scheduled termination, all surviving animals were anesthetized by isoflurane inhalation for blood sample collection, and then sacrificed by exsanguination of the abdominal aorta. Complete gross postmortem examinations were performed on all terminated animals. All organs were fixed and stored individually for histopathological examination.
XII. Organ Weights
The absolute and relative (organ-to-BW ratios) weights of the following organs were measured in all survivors following sacrifice: liver, kidneys, adrenals, testis, ovaries, and brain.
XIII. Histopathology
The following tissues were obtained from all animals: brain, pituitary, eye, thyroids, heart, lung, liver, kidney, spleen, adrenals, stomach, testis, urinary bladder, femur, and ovaries. Eyes and testes were preserved in Davidson's fixative and Bouin's fixative, respectively. Other tissues were fixed with 10% neutral buffered formalin solution. The liver, kidney, adrenal, heart, and spleen from the high dosage and control groups were routinely processed, embedded in paraffin, and sectioned to 3-5 µm. The sections were stained with hematoxylin and eosin for microscopic examination.
XIV. Statistical Analysis
The GHS class in the acute toxicity test was calculated according to the OECD guideline 420 [16]. The results are presented as the mean ± standard deviation (SD). The differences in parameters (BWs, organ weights, and the results of the blood biochemistry and hematology) between groups in the acute and sub-chronic toxicity test were assessed by a standard two-way analysis of variance (ANOVA). If these showed statistical significance, Duncan's or Dunnett's multiple range test were used to compare groups (SPSS version 12.0 [SPSS Inc., Chicago, IL, USA]). P-values < 0.05 were considered statistically significant.
RESULTS
I. Acute Oral/Dermal Toxicity Study
There were no dead animals, clinical signs, or necropsy findings in rats administered with 2,000 mg/kg of TSP via the oral or dermal route (data not shown).
II. Repeated Dose 90-Day Oral Toxicity Study
A) Mortality and clinical signs
There was no treatment-related mortality in the animals treated with the test substance during the study. Female rats treated with 500 and 1,000 mg/kg of test substance showed hair loss. No significant clinical signs were observed in any other group.
B) Body weight
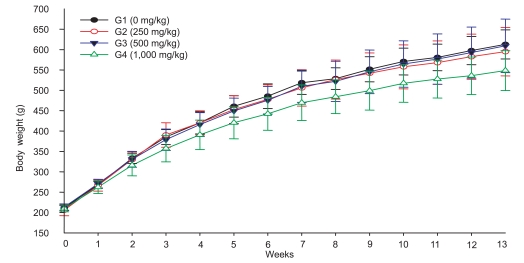
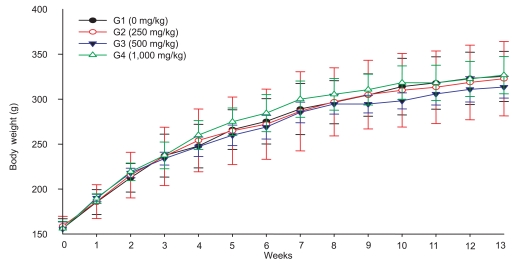
The terminal body weight of male rats treated with 1,000 mg/kg of test substance was lower than that of the control group, while there were no differences in body weight between the 250, 500 mg/kg, and control group. There was no treatment-related change in body weight in female rats (Figure 1,2).
Figure 1.
Changes in body weight of male rats orally treated with tetrasodium pyrophosphate for 90 days.
Figure 2.
Changes in body weight of female rats orally treated with tetrasodium pyrophosphate for 90 days.
C) Food consumption
A significant decrease in food consumption was observed in the 1,000 mg/kg group of males at week 8 and 9. Food consumption in females increased significantly in the 1,000 mg/kg group at week 4. There were no significant changes in food consumption in the other groups (Table 1).
Table 1.
Food consumption of SD rats orally treated with tetrasodium pyrophosphate for 90 days
*p<0.05, †p<0.01.
D) Urinalysis and hematology
There were no specific symptoms in urinalysis at the doses of 250, 500, and 1,000 mg/kg. There were no significant changes in hematology in the 250 mg/kg group.
WBC and neutrophil counts were significantly increased in males and females of the 1,000 mg/kg group, whereas the lymphocyte count was significantly lower in males and females of the 1,000 mg/kg group. RBC, HB, HCT, PT, and APTT were significantly reduced in the males of the 1,000 mg/kg group, and PT was significantly reduced in males of the 500 mg/kg group (Table 2).
Table 2.
Hematological values of SD rats orally treated with tetrasodium pyrophosphate for 90 days
WBC: white blood cell, RBC: red blood cell, Hb: hemoglobin, Hct: hematocrit, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin , MCHC: mean corpuscular hemoglobin concentration, PLT: platelet. PT: prothrombin time, APTT: activated partial thromboplastin time.
*p<0.05, †p<0.01.
E) Biochemistry
There were no significant changes in the serum biochemistry of males and females in the 250 mg/kg group.
Total protein was significantly reduced in both sexes in the 500 and 1,000 mg/kg group. Albumin was significantly reduced in males of the 500 and 1,000 mg/kg group, and in females of the 1,000 mg/kg group. The A/G ratio was significantly increased in females of the 500 and 1,000 mg/kg group. T-BIL was significantly increased in females of the 1,000 mg/kg group, and ALP was significantly reduced in females of the 1,000 mg/kg group. AST was significantly increased in males of the 1,000 mg/kg group, but ALT was significantly reduced in females of the 1,000 mg/kg group. TG were significantly increased in both sexes in the 1,000 mg/kg group, while Ca, IP, Na, K, and Cl were significantly reduced in both sexes in this group. IP was also reduced in males of the 500 mg/kg group (Table 3).
Table 3.
Biochemical values of SD rats orally treated with tetrasodium pyrophosphate for 90 days
TP: total protein, T-BIL: total bilirubin, ALP: alkaline phosphatase, AST: aspartate aminotransferase, ALT: alanine aminotransferase, CRN: creatinine, BUN: blood urea nitrogen , CHOL: total cholesterol, TG: triglycerides, GLU: glucose, Ca: calcium, IP: phosphorus, CK: creatine kinase, Na: sodium, K: potassium, Cl: chloride.
*p<0.05, †p<0.01.
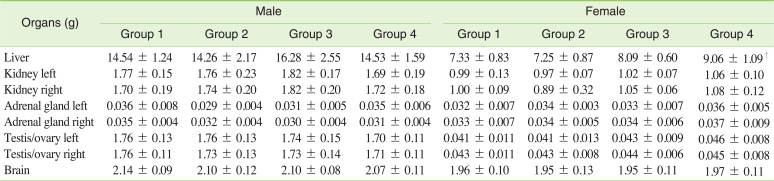
F) Necropsy findings and organ weight
There were no grossly visible findings or lesions in any group. There were no significant changes in organ weight in males and females of the 250 mg/kg group. The relative weight of the liver in the males of the 500 mg/kg and 1,000 mg/kg groups showed a significant increase. The absolute and relative weight of the liver in females of the 500 mg/kg and 1,000 mg/kg groups were also significantly increased (Table 4,5).
Table 4.
Absolute organ weights of SD rats orally treated with tetrasodium pyrophosphate for 90 days
*p<0.01.
Table 5.
Relative organ weights of SD rats orally treated with tetrasodium pyrophosphate for 90 days
*p<0.05, †p<0.01.
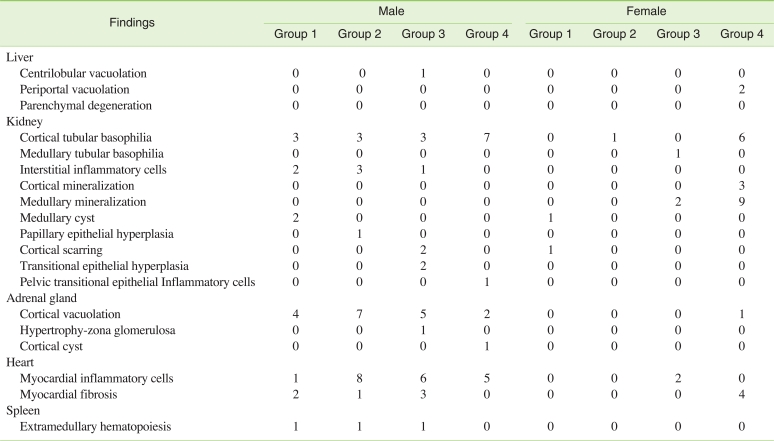
G) Histopathology
No exposure-related histopathological changes were observed in any of the organs examined in SD rats, with the exception of kidney lesions. Cortical tubular basophilia of the renal tubule was more evident in males of the 1,000 mg/kg group and mineralization of the kidney was evident in females of the 1,000 mg/kg group. Other background lesions were observed in the 0, 250, 500, and 1,000 mg/kg groups in both sexes (Table 6).
Table 6.
Incidence of histopathological findings in SD rats orally treated with tetrasodium pyrophosphate for 90 days (n=10)
DISCUSSION
No acute toxicity of TSP was observed at the dose of 2,000 mg/kg. No abnormal clinical signs were observed following acute oral or dermal administration of TSP. In the repeated dose toxicity study, significantly differences in parameters were observed such as body weight, hematological and serum biochemical analysis and histopathological lesions at the medium and high doses of TSP. TSP may be toxic to humans when exposed orally or via inhalation [12]. According to acute oral toxicity studies, the LD50 in rats is over 2,000 mg/kg BW [18] and the dermal LD50 in rabbits is 7,940 mg/kg [18]. Dermal administration of TSP at doses of up to 2,000 mg/kg showed no signs of toxicity.
In the repeated dose study, body weight and terminal BW were reduced compared to controls in males of the 1,000 mg/kg group, but not the females. However, females of the 500 mg/kg group showed a slight reduction in BW, suggesting that TSP affects the BW of female rats at doses of over 1,000 mg/kg.
Food consumption varied temporally in a dose-independent manner, with significant changes in some groups. The increase in WBCs such as neutrophil suggests that there was an inflammatory reaction to TSP administration, but microscopically there was no neutrophil infiltration of the target organ. A decrease in RBC-related parameters indicates anemia, but there was no clinical sign of this, and the level of parameters were on normal physiological change level of rat. So there was no evidence that TSP induces anemia in rats at the dose of 1,000 mg/kg. PT and APTT were also reduced, but the level of parameters was on background data level of normal rat and was biologically meaningless things. Therefore, TSP had no toxic effect on the hematological parameters of SD rats at the dose of 1,000 mg/kg.
TP and albumin decreased in this experiment, which may be related to the reduction in BW [19]. A decrease in BW may be related to malnutrition, the lack of vitamins, and a decrease in body electrolytes [20,21]. So it maybe adopt change according to the BW change, and it is toxicologically meaningless things. T-BIL and ALP were also reduced in females of the 1,000 mg/kg group, and may be linked to the kidney lesions. The males of the 1,000 mg/kg group showed a tendency for a reduction in these parameters. This explains why kidney lesions were more common in the high dose group. Therefore, these changes of T-BIL and ALP were related to toxicity to the kidney at the dose of 1,000 mg/kg TSP. AST and relative liver organ weight showed an increase in males of the 1,000 mg/kg group, while AST showed no increase and ALT was reduced in females of the 1,000 mg/kg group. Microscopic examination found no obvious liver lesions associated with TSP treatment, which means that TSP treatment had no effect on AST or ALT. TG was increased in both sexes in the 1,000 mg/kg group, but it is temporally meaningless change. Decrease of Ca, IP and Na at dose of 1000 mg/kg group and the level of those parameters were on background data of normal rat.
An increase of liver organ weight was usually accompanied by a decrease in BW. However the liver weight of females receiving the 1,000 mg/kg dose increased without a decrease in BW.
Tubular basophilia of the renal tubule was evident in males and females, with mineralization of the kidney in females. Tubular basophilia is a relatively frequent finding in young control rats [22], and can be induced by nephrotoxic agents; the lesion is regenerative in nature. L-cystein induced tubular basophilia at the dose of 1000 mg/kg after 4 weeks of administration in rats [23], and methotrexate caused an increase in tubular basophilia in mice [24]. The high incidence of tubular basophilia showed that the target organ of TSP may be the kidney. Focal mineralization of renal tubules is a frequent finding in the rat [25]. In one report, 100% of female Wistar rats, but no males, exhibited the lesion. Cam deposits may be induced in female rats by administering estrogen [26]. Thus female hormones may have some effect on mineralization of the renal tubule. The results in females in the current study were similar to previous research on another chemical, in which mineralization was increased compared to the control, meaning that TSP may be related to mineralization toxicity in females [26]. Pyrophosphate is a potent inhibitor of vascular calcification [27], however for its tetrasodium salt, TSP induced renal mineralization. Thus it is future investigation whether TSP was inhibitor of vascular calcification or not.
In summary, T-BIL and ALP decreased, and tubular basophilia of the kidney increased in both sexes at the dose of 1,000 mg/kg, while mineralization increased in females at this dose. Hematological parameters such as TP, ALB, A/G ratio, AST, IP and Na showed significant changes in males and females of the 500 mg/kg and 1000 mg/kg groups, but these may not have been toxicological changes.
Based on these results, the no observed effect level (NOEL) and no observed adverse effect level (NOAEL) were considered to be 250 and 500 mg/kg /day respectively under the conditions of this study.
ACKNOWLEDGEMENTS
This work was supported by the Korea Occupational Safety & Health Agency, Ministry of Labor, Republic of Korea, and Grant-in-Aid for chemical hazard assessment, 2009.
Footnotes
The authors have no conflict of interest to declare on this study.
This article is available from: http://e-eht.org/
References
- 1.Ashford RD. Ashford's Dictionary of Industrial Chemicals; Properties, Production, Uses. London: Wavelength Publications; 1994. [Google Scholar]
- 2.Windholz M. Merck Index. Whitehouse Station, NJ: Merck & Co.; 1996. [Google Scholar]
- 3.IUCLID dataset. European Commission-European Chemicals Bureau. [cited 2011 Aug 31]. Available from: http://esis.jrc.ec.europa.eu/doc/existing-chemicals/IUCLID/data_sheets/7722885.pdf.
- 4.National organic standards board handling committee recommendation for §205.605(b) United States Department of Agriculture. [cited 2011 Aug 31]. Available from: http://www.ams.usda.gov/AMSv1.0/getfile?dDocName=STELPRDC5079516&acct=nosb.
- 5.Molins RA. Phosphates in Food. Boca Raton: CRC Press; 1991. [Google Scholar]
- 6.Ministry of Environment. 3rd Report to the Amount of Chemicals in Circulation. Seoul: Ministry of Environment; 2006. (Korean) [Google Scholar]
- 7.Ellinger RH. Phosphates in food processing. In: Furia TE, Chemical Rubber Company, editor. CRC Handbook of Food Additives. 2nd ed. Cleveland: CRC Press; 1972. pp. 617–780. [Google Scholar]
- 8.Dickerson WH. United States patent office. [cited 2011 Aug 31]. Available from: http://www.freepatentsonline.com/1654283.pdf.
- 9.Horvath AA. United States patent office. [cited 2011 Aug 31]. Available from: http://www.freepatentsonline.com/2429579.pdf.
- 10.Grad DR. Phosphoric acids and phosphates. In: Kroschwitz JI, Howe-Grant M, Kirk RE, editors. Encyclopedia of Chemical Technology. 4th ed. Vol. 19. New York: Wiley; 1996. pp. 669–718. Pigments to Powders, Handling. [Google Scholar]
- 11.Heidolph BB, Gard DR. Phosphates and food processing. In: Francis FJ, Hui YH, editors. Encyclopedia of Food Science and Technology/ 3, [In-R] New York: Wiley; 2000. pp. 1881–1885. [Google Scholar]
- 12.Hawley GG, N Irving Sax NI, Lewis RJ, Sr, Hawley GG. Hawley's Condensed Chemical Dictionary. 11th ed. New York: Van Nostrand Reinold; 1987. [Google Scholar]
- 13.Datta PK, Frazer AC, Sharratt M, Sammons HG. Biological effects of food additives. II.--sodium pyrophosphate. J Sci Food Agric. 1962;13(11):556–566. [Google Scholar]
- 14.European Commission European Chemicals Bureau IUCLID dataset of tetrasodium pyrophosphate. [cited 2011 Aug 31]. Available from: http://esis.jrc.ec.europa.eu/doc/existing-chemicals/IUCLID/data_sheets/7722885.pdf.
- 15.Kim SJ, Rim KT, Kim HY, Yang JS. Mutagenicity of octane and tetrasodium pyrophosphate in bacterial reverse mutation (Ames) test. J Toxicol Sci. 2010;35(4):555–562. doi: 10.2131/jts.35.555. [DOI] [PubMed] [Google Scholar]
- 16.Organisation for Economic Co-operation and Development (OECD) Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents. Paris: OECD Publishing; 1998. [Google Scholar]
- 17.Examination of toxic risk about industrial chemicals. Ministry of Labor. [cited 2011 Aug 31]. Available from: http://www.moel.go.kr/view.jsp?cate=4&sec=4&smenu=1&div_cd=&mode=view&bbs_cd=116&seq=1203498938807&page=1&state=A.
- 18.Material safety data sheet. ICL Performanance Products LP. [cited 2011 Aug 31]. Available from: http://www.iclperfproductslp.com/mm/files/Tetrasodium_Pyrophosphate_Anhydrous_EN.pdf.
- 19.Li JB, Wassner SJ. Effects of food deprivation and refeeding on total protein and actomyosin degradation. Am J Physiol. 1984;246(1 Pt 1):E32–E37. doi: 10.1152/ajpendo.1984.246.1.E32. [DOI] [PubMed] [Google Scholar]
- 20.Philbrick DJ, Hill DC. Development of malnutrition in rats. Am J Clin Nutr. 1974;27(8):813–818. doi: 10.1093/ajcn/27.8.813. [DOI] [PubMed] [Google Scholar]
- 21.Zemel MB. The role of dairy foods in weight management. J Am Coll Nutr. 2005;24(6 Suppl):537S–546S. doi: 10.1080/07315724.2005.10719502. [DOI] [PubMed] [Google Scholar]
- 22.Hard GC, Khan KN. A contemporary overview of chronic progressive nephropathy in the laboratory rat, and its significance for human risk assessment. Toxicol Pathol. 2004;32(2):171–180. doi: 10.1080/01926230490422574. [DOI] [PubMed] [Google Scholar]
- 23.Sawamoto O, Kyo S, Kaneda S, Harada M, Kishimoto S, Koshitani O, et al. Four-week intravenous repeated dose toxicity study of L-cysteine in male rats. J Toxicol Sci. 2003;28(2):95–107. doi: 10.2131/jts.28.95. [DOI] [PubMed] [Google Scholar]
- 24.Chelab KG, Majeed SK. Methotrexate-induced histopathological changes in the kidneys of mice. Iraqi J Vet Sci. 2009;23(Suppl 2):219–222. [Google Scholar]
- 25.Peter CP, Burek JD, van Zwieten MJ. Spontaneous nephropathies in rats. Toxicol Pathol. 1986;14(1):91–100. doi: 10.1177/019262338601400111. [DOI] [PubMed] [Google Scholar]
- 26.Cousins FB, Geary CP. A sex-determined renal calcification in rats. Nature. 1966;211(5052):980–981. doi: 10.1038/211980b0. [DOI] [PubMed] [Google Scholar]
- 27.O'Neill WC, Lomashvili KA, Malluche HH, Faugere MC, Riser BL. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int. 2011;79(5):512–517. doi: 10.1038/ki.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]