Abstract
Objective
The recurrence rate of anti-SSA/Ro associated congenital heart block (CHB) is 17%. Reversal of 3rd degree block has never been achieved. Based on potential reduction of maternal autoantibody titers as well as fetal inflammatory responses, IVIG was evaluated as a preventative therapy for CHB.
Methods
A multicenter open-label study based on Simon’s 2-stage optimal design was initiated. Enrollment criteria included: maternal anti-SSA/Ro antibody, a previous child with CHB/rash, </= 20 mg prednisone, < 12 weeks pregnant. IVIG (400mg/kg) was given every 3 weeks from 12 to 24 weeks of gestation. The primary outcome was the development of 2nd or 3rd degree CHB.
Results
Twenty mothers completed the IVIG protocol before reaching the pre-determined stopping rule of three cases of advanced CHB. CHB was detected at 19, 20 and 25 weeks; none followed an abnormal PR interval. One of these mothers had two previous children with CHB. One child without CHB developed a transient rash consistent with neonatal lupus. Sixteen children had no manifestations of neonatal lupus at birth. No significant changes in maternal antibody titers to SSA/Ro, SSB/La, or Ro52 were detected over the course of therapy or at delivery. There were no safety issues.
Conclusions
IVIG at doses consistent with replacement does not prevent the recurrence of CHB or reduce maternal antibody titers. Having established safety with this protocol and feasibility of patient enrollment, subsequent preventative studies may be considered, perhaps to include higher doses of IVIG.
Keywords: Congenital Heart Block, Intravenous Immunoglobulin, anti-SSA/Ro antibodies, neonatal lupus
INTRODUCTION
One of the strongest clinical associations with autoantibodies directed to components of the SSA/Ro-SSB/La ribonucleoprotein complex is the development of congenital heart block (CHB) in an offspring, an alarming prospect facing 2% of mothers with these reactivities [1, 2]. The risk is 10-fold higher in women who have had a previously affected child [3–5]. CHB carries a significant mortality (20–30%, primarily fetal/ neonatal) and morbidity (67% require permanent pacing before adulthood) [5, 6]. Evidence is emerging that in addition to conduction disease, 10–15% of affected offspring will have a life-threatening cardiomyopathy [7, 8]. One of the most disturbing observations to emerge is the rapidity of disease progression, with advanced heart block detected within a week of normal sinus rhythm (NSR) [2]. Biomarkers such as prolongation of the fetal Doppler mechanical PR interval have not convincingly demonstrated utility in predicting advanced block [2]. Consistent with the fibrotic replacement of the atrioventricular node (AV) observed in autopsy studies from fetuses dying with CHB, reversal of third degree block has never been achieved [2, 9]. Current prophylactic and treatment strategies for CHB include maternal steroids, plasmapheresis, sympathomimetics, and in utero cardiac pacing [10]. None have significantly altered mortality. Accordingly, strategies aimed at preventing disease before immutable scarring ensues, assume high priority. Although it is disappointing that animal models have not fulfilled “Koch’s postulates” assuring an effect of the antibody per se, this is likely due to the fact that antibodies are necessary but insufficient. Our approach to prevention considered the necessity of maternal antibody as well as consequent fetal factors in the cascade to pathogenesis.
Intravenous gamma globulin (IVIG) has been of benefit in a variety of immune-mediated and inflammatory diseases. Rationale for its use in CHB is based on our working hypothesis of the pathogenesis of disease. Tissue injury in the fetus is presumed to depend on FcγRn-mediated transplacental passage of maternal IgG autoantibodies [11]. Anti-SSA/Ro-SSB/La antibodies, by binding to translocated antigen on the surface of apoptotic cardiocytes generated during remodeling of the conduction system and surrounding tissue, may inhibit the normal physiologic removal of these cells [12, 13]. Uncleared opsonized apoptotic cardiocytes may be subsequently efferocytosed by infiltrating macrophages with release of pro-inflammatory and profibrosing cytokines which transdifferentiate cardiac fibroblasts to a scarring phenotype [13, 14]. This scenario supports the consideration of prophylactic IVIG based on two presumed mechanisms of efficacy. The first exploits the saturation of FcγRn by IVIG. This should decrease fetal exposure to anti-SSA/Ro-SSB/La by accelerating IgG catabolism in the maternal circulation and decreasing placental transport [15, 16]. The second exploits the attenuation of anti-inflammatory responses by increasing the macrophage expression of FcγRIIB [17]. This would represent a downstream effect in the targeted organ.
Precedent for the use of IVIG is the encouraging report of only 1 recurrent case of CHB in 8 mothers with previously affected children [18] and murine data demonstrating a decrease in placental transport of human anti-SSA/Ro-SSB/La antibodies following IVIG [19]. Accordingly, a prospective US based multi-center open-label trial to determine the efficacy and safety of IVIG on the prevention of CHB in women with anti-SSA/Ro antibodies and a previous child with neonatal lupus was initiated. Treatment comprised IVIG at 400 mg/kg, dosed every 3 weeks from 12 to 24 weeks of gestation. The primary outcome was the development of 2nd or 3rd degree CHB.
METHODS
Subjects
Subjects entered this prospective multi-center open label clinical trial between January 2007 and January 2009. A total of 17 women from centers across the US signed consent forms approved by the Institutional Review Board at the site of their infusion. Four mothers provided consent as participants of the Research Registry for Neonatal Lupus to release medical records and send blood specimens but received drug as prescribed by their treating physicians who elected to follow and adhere to the study protocol. Of the 21 women enrolled, there was one screen failure due to a spontaneous miscarriage at 9 weeks prior to initiation of the study protocol.
Enrollment required all of the following inclusion criteria 1) documentation of anti-SSA/Ro and/or anti-SSB/La antibodies 2) a previous child with one of the following: a) CHB (any degree) documented by EKG if live birth and/or echocardiogram and/or histology if fetal demise; b) characteristic NL rash confirmed by photograph revealing annular lesions, dermatology note, and/or biopsy; c) CHB and rash; 3) current intrauterine pregnancy ≤12 wk with normal heart beat and structure. A patient was excluded for any of the following: 1) current dose of prednisone > 20mg or any dose of dexamethasone 2) level of Ig A below normal values for laboratory 3) presence of any structural abnormalities of the fetal heart that could cause CHB, ie, L-transposition of the great arteries, AV septal defect, or heterotaxias.
Mothers could be clinically asymptomatic or have symptoms of a rheumatic disease. Rheumatologic disease was classified on the basis of case report forms filled out by the participating rheumatologists, obstetricians and cardiologists performing the echocardiograms and verified by telephone interviews and review of medical records when available (by J.P.B., C.L. and P.M.I). The following categories were assigned 1) asymptomatic if a patient denied any clinical symptoms that would be consistent with systemic lupus erythematosus (SLE) or Sjogren’s syndrome (SS); 2) undifferentiated autoimmune syndrome (UAS) if insufficient criteria for SLE or SS; 3) SLE if 4 criteria of the American College of Rheumatology were satisfied [20]; 4) possible, probable, or definite SS if a patient had at least dry eyes and dry mouth or only 1 symptom plus evidence of objective criteria in addition to autoantibodies as per the European Classification [21]; or 5) SLE and SS if criteria for both were met.
Study Design
The trial was designed as an open-label trial employing Simon’s 2-stage optimal design [22] to allow for early stopping due to absence of treatment efficacy. The first stage required 19 subjects. If 3 or more mothers had children with 2nd or 3rd degree CHB, then the study would be terminated after the first stage. If this did not occur, an additional 35 mothers would be enrolled in the second stage for a total of 54 subjects. At the end of trial, the treatment would be considered efficacious if fewer than 6 mothers of 54 had a child with advanced CHB. With this design, the study had 90% power to conclude that IVIG is efficacious if the true recurrence rate with the treatment is 5%. In addition, the probability of rejecting the treatment for further study is 95% if the true recurrence rate is 19% [3, 5]. It should be noted that the enrollment goal for the first stage was exceeded by two patients because of initial concern there might be a screen failure due to miscarriage (which occurred in one case).
Treatments and Follow-ups
IVIG infusions of 400mg/kg were given over 3–4 hrs at 12 wks, 15 wks, 18 wks, 21 wks and 24 wks of gestation. Blood samples were obtained before each infusion and at 28 wks, 34 wks and delivery (including cord blood). Fetal echocardiograms were performed weekly between 16 and 26 weeks of gestation and every two weeks thereafter until 34 weeks in accord with the protocol of PRIDE [2]. The studies were recorded on VHS videotape or DVD/optical disk by the patient’s pediatric cardiologist or obstetrician. The image copies were sent to the core fetal echocardiographic laboratory where the studies were reread by D.M.F.
Endpoints
The primary outcome was 2nd or 3rd degree AV block. The secondary outcomes were 1) sustained 1st degree AV block as defined by a prolonged mechanical PR interval [PR >150 msec, i.e., > normal mean +3 SD] [23] that did not progress to more advanced forms of AV block throughout the study and subsequently confirmed by EKG at birth 2) transiently prolonged mechanical PR interval 3) any sign of myocardial injury such as reduced contractility, tricuspid regurgitation or effusions, without change in cardiac rate or rhythm 4) echocardiographic densities consistent with endocardial fibroelastosis (EFE) confirmed postnatally 5) fetal death not related to cardiac dysfunction 6) rash consistent with neonatal lupus 7) prematurity defined as gestational age at birth < 37 weeks 8) birth weight less than 10% in the context of gestational age 9) abnormal fluid collection in the fetus consistent with hydrops.
Laboratory Studies
Samples were aliquoted and maintained at −70°C. For each subject, serial samples obtained as per the protocol were stored until the pregnancy was completed. The evaluation of antibody titers and total IgG in all maternal samples and corresponding cord bloods was completed on the same day. Titers of antibodies to anti-SSA/Ro and/or anti-SSB/La were done by ELISA (Diamedix Corp, Miami, FL). In this commercial test, the cutoff for normal has been established at 19 EU for both SSA/Ro and SSB/La. Titers of antibodies to Ro52 were evaluated by ELISA using recombinant Ro52 as previously described [24]. IgG levels were determined by radial immunodiffusion using the BN ProSpec® System, Dade Behring-Siemens Health Care.
Statistics
Changes from baseline in maternal antibody titers measured at specific time points during pregnancy were evaluated with the paired t-test. A two sided P-value < 0.05 was considered statistically significant.
RESULTS
Demographics and Study Population
The demographic characteristics of the patients including health and antibody status and obstetrical history are summarized in Table 1. Twenty mothers have completed IVIG treatments with 19 having given birth. The majority of enrollees were Caucasian (80%). The maternal diagnoses at the time of enrollment in the study were as follows: 3 (15%) were classified as asymptomatic, 7 (35%) as UAS, 5 (25%) SS, 4 (20%) SLE with secondary SS, and 1 patient with Rheumatoid Arthritis and secondary SS. Sixteen (80%) of the patients had antibodies to SSB/La in addition to SSA/Ro. Eighteen (90%) of the enrolled women had a prior pregnancy complicated by CHB (1 with two CHB children) and two had a previous child with a neonatal lupus rash. In 8 (40%) of the mothers, the prior pregnancy complicated by CHB ended in a fetal demise. Four (20%) of the patients were on prednisone <20 mg/day (mean dose 9.5 mg/day; range 8–10 mg/day) during the study and 2 mothers were taking hydroxychloroquine 400 mg/day throughout pregnancy.
Table 1.
Demographic characteristics of the 20 mothers enrolled in the PITCH study
| N (%) | |
|---|---|
| Maternal Race/Ethnicity | |
| Caucasian | 16 (80) |
| Asian | 2 (10) |
| African-American | 1 (5) |
| Hispanic | 1 (5) |
| Maternal Diagnosis | |
| Asym/UAS | 10 (50) |
| SS | 5 (25) |
| SS/SLE | 4 (20) |
| RA/SS | 1 (5) |
| Maternal Obstetric History | |
| Prior cardiac NL | |
| - CHB alive | 10 (50) |
| -Fatal CHB | 6 (30) |
| -Fatal Cardiomyopathy | 2 (10) |
| Prior Rash | 2 (10) |
| Maternal Antibody Status | |
| Anti SSA/Ro-SSB/La | 16 (80) |
| Anti SSA/Ro only | 4 (20) |
| Anti Ro52 | 20 (100) |
| Maternal Medications | |
| Prednisone (<20 mg/day) | 4 (20) |
| Hydroxychloroquine (400 mg/day) | 2 (10) |
Fetal Outcomes
Twenty mothers have completed the IVIG infusions (1 woman only received 4 doses of IVIG) and 19 mothers have given birth. Seventeen fetuses have completed the serial echocardiograms with normal PR intervals (Figure 1) and normal birth EKGs. Of these 17, one neonate presented with a rash consistent with neonatal lupus several days after birth.
Figure 1. Mechanical Doppler PR interval for CHB and non-CHB pregnancies.
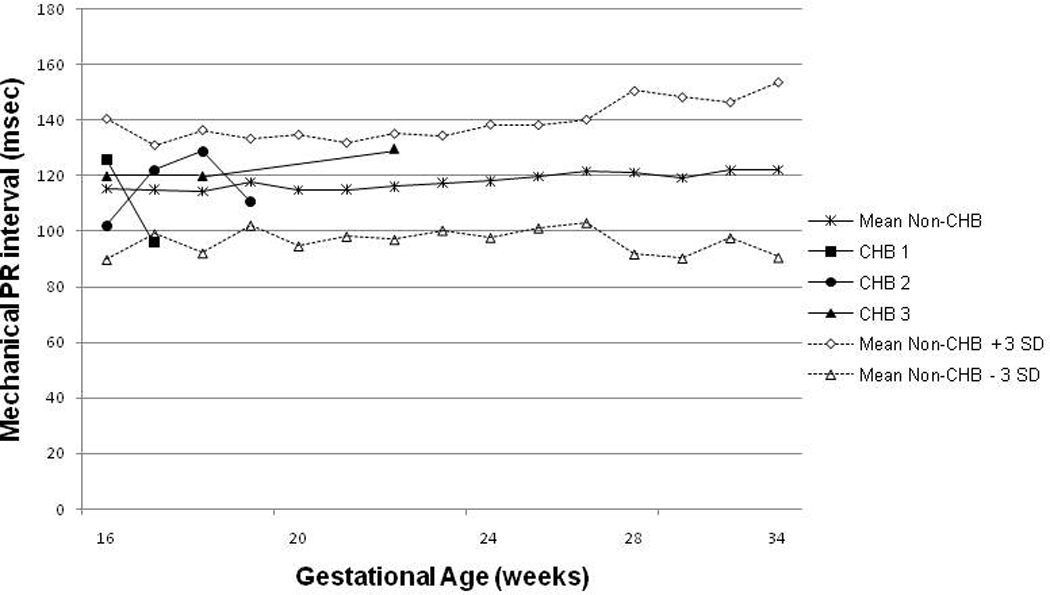
The figure illustrates the mean and SD calculated for each gestational week. For the non-CHB group, values between 16 and 34 weeks are shown. For the CHB group, only values prior to CHB detection are shown. The dashed lines represent the Mean + 3SD and the Mean − 3SD values respectively. The PR intervals for the CHB fetuses are all within normal range (Mean+/− 3SD) and thus 1st degree block did not precede advanced CHB.
Three fetuses were diagnosed with advanced block. The first case of CHB was detected at 19 weeks of gestation. The patient had received 3 doses of IVIG at weeks 12, 15 and 18. The fetal echocardiogram at detection revealed third degree block with mild tricuspid regurgitation and no hydrops. The previous fetal echocardiogram at 17 weeks of gestation revealed a normal PR interval and no signs of valvular dysfunction. Once CHB was diagnosed, the patient began dexamethasone at 4mg/day for 10 days with no effect on the heart rhythm. The patient delivered at 37 weeks of gestation. The neonate was small for gestational age and received a permanent pacemaker at birth. In addition, the postnatal echocardiogram showed a moderate secundum atrial septal defect corrected by surgical closure at 2 months of age.
The second fetus with CHB was diagnosed at 20 weeks of gestation after 3 doses of IVIG. Routine weekly fetal echocardiograms were done as per protocol from 16 weeks of gestation and each showed a normal PR interval. Two weeks after the third infusion the fetal echocardiogram revealed 2nd degree Wenckebach with occasional dropped beats. The mother was prescribed one oral dose of 4 mg of dexamethasone and received a total of 2g/kg IVIG plus intravenous dexamethasone 4mg/day for 2 days. However, 2nd degree CHB progressed to 3rd degree CHB within 2 days. There was no evidence of tricuspid regurgitation, effusions or hydropic changes. The baby was born at 37 weeks of gestation and was small for gestational age. The postnatal echocardiogram confirmed 3rd degree CHB with normal systolic function. A pacemaker was implanted at 7 days of age.
A third CHB case was detected at 25 weeks of gestation after the mother received 4 IVIG infusions. Previous PR intervals during pregnancy had been normal up to 22 weeks; however the mother missed the 23 and 24 week fetal echocardiograms and third degree block was detected at 25 weeks of gestation. After discussion with her physician, the patient decided to take only one dose of 4 mg dexamethasone. The fetal echocardiogram performed during her last evaluation at 30 weeks of gestation, showed no signs of hydrops, TR regurgitation or effusions. The ventricular rates remain above 96 beats per minute.
Two of the mothers whose pregnancies were complicated by CHB were classified as SLE with secondary SS at the time of enrollment and one of them as UAS. Two of the mothers had, in addition to anti-SSA/Ro, anti-SSB/La antibodies and each had anti-Ro52 antibodies. One of these mothers had two previous children with CHB. The other two had one prior pregnancy complicated by CHB, one of which ended in fetal demise. The study was stopped at this point according with the pre-determined rule.
Maternal Antibody Titers
Maternal sera obtained from mothers completing the study were available for evaluation of anti-SSA/Ro-SSB/La titers. Antibody titers assessed before every IVIG infusion, and at 28 wks, 34 wks and delivery were compared with values obtained at baseline. As shown in Figure 2, treatment with IVIG did not significantly alter the titers of anti-SSA/Ro, anti-Ro52, or anti-SSB/La antibodies (P>0.05 for all comparisons).
Figure 2. Antibody titers and IgG levels during pregnancy.
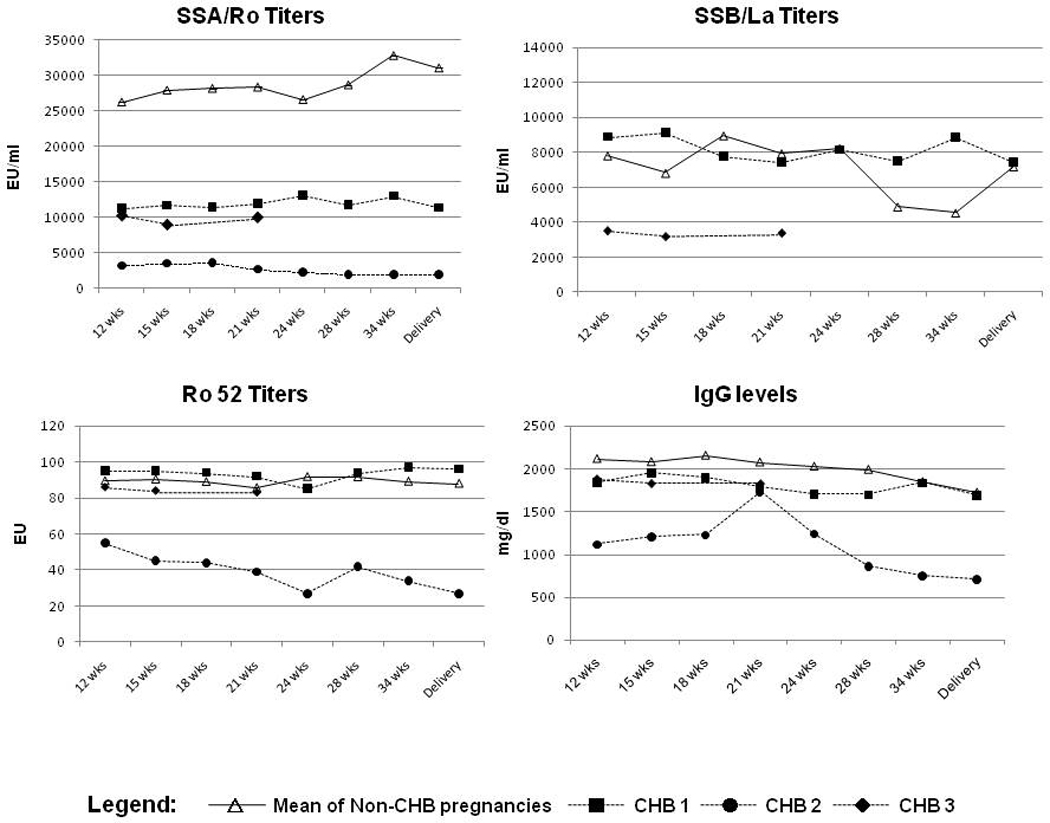
There were no significant decreases in the titers of anti-SSA/Ro-SSB/La, anti-Ro52 or IgG levels in the mothers that received IVIG. Two of the women with affected pregnancies received only 3 IVIG doses and the third mother with an affected pregnancy only 4 doses; results are shown separately.
Safety Data
There were no changes in maternal blood pressure, severe headaches, rashes, fever or any other adverse effects related to the infusions. There were no serious adverse events. Neonatal growth data are presented in Table 2. Weight, height, and head circumference were derived from gestational age -specific growth curves to correct for prematurity when necessary. Four (21%) of the newborns, two with CHB and two healthy, were small for gestational age (<10%) and 3 (16%) were born prematurely (<37 weeks of gestation). One of the mothers with a healthy baby small for gestational age and two of the mothers with premature babies were taking prednisone during pregnancy.
Table 2.
Neonatal growth and safety data
| N (%) | |
|---|---|
| Weight at birth | |
| <10% | 4 (22) |
| 10–50 % | 7 (39) |
| 51–90 % | 7 (39) |
| Length at birth | |
| <10% | 3 (17) |
| 10–50 % | 6 (33) |
| 51–90 % | 9 (50) |
| Head Circumference at birth | |
| <10% | 3 (17) |
| 10–50 % | 5 (28) |
| 51–90 % | 8 (44) |
| unknown | 2 (11) |
DISCUSSION
This study recruited 21 mothers, 20 of whom received prophylactic IVIG to prevent autoimmune associated CHB. The study was discontinued after reaching the stopping rule of detecting 3 CHB cases in the first 19 patients enrolled. There were no significant changes in the titers of antibodies to SSA/Ro or SSB/La done by commercial ELISA in a central laboratory or in the titer of antibodies to Ro52. No safety issues were raised in this pilot study. In parallel with the enrollment of the PITCH study, a European study was initiated in December 2004. The treatment protocol was identical to that utilized in PITCH. That study was terminated after 3 cases of CHB were identified following enrollment of 15 mothers who had previously affected children with CHB (unpublished data, with permission of Dr. Munther Khamashta, U.K.). Combining data generated from the two studies in which the prior pregnancy was CHB, not rash, there were 6 cases of recurrent CHB in 33 mothers (15 UK and 18 US) which is consistent with the recently reported recurrence rate of 17.4% [4] and confirms that IVIG at 400mg/kg dosed every three weeks from 12 gestational weeks is not effective in reducing the incidence of recurrent disease.
IVIG is approved by the FDA for primary immunodeficiency, idiopathic thrombocytopenic purpura, Kawasaki disease, B cell-chronic lymphocytic leukemia with hypogammaglobulinemia, pediatric HIV infection, and allogenic bone marrow transplant in adults [25]. Several mechanisms have been posited to account for the effects of IVIG in autoantibody-mediated disorders, but none satisfactorily explains all clinical situations. Immunomodulatory properties attributed to IVIG include downregulation by antiidiotypic antibodies and effects on receptors for cytokines and complement [26]. Administration of IVIG in a murine model [27] inhibits autoantibody-mediated thrombocytopenia by inducing inhibitory FcγRIIB receptors on macrophages. Recent data support that a structure within the Fc portion of IVIG, a N-linked glycan terminating in sialic acid in a 2,6 linkage to the penultimate galactose, is a requisite for activity [28]. The 2,6-sialylated Fc interacts with a lectin identified as SIGN-R1, expressed on marginal zone macrophages to initiate an anti-inflammatory pathway that ultimately modifies the ability of effector macrophages to trigger activation FcγRs in response to autoantibody deposition. One pathway by which this may be accomplished is through enhanced expression of the inhibitory FcR, FcγRIIB, on these effector macrophages. Although the applicability of these findings to the pathogenesis of CHB is unknown, it is notable that the fully processed glycan is found in only 1–3% of IgG in IVIG. Further experimental evidence from murine models demonstrates that maternal administration of IVIG significantly inhibits the transplacental passage of maternal anti-SSA/Ro-SSB/La antibodies and decreases the level of pathogenic antibody in both the maternal and fetal circulation in fetal/neonatal alloimmune thrombocytopenia [19, 27]. In addition, a highly emphasized explanation unifying the beneficial action of IVIG is accelerated catabolism of IgG which is mediated by saturation of FcγRn receptors [15, 16].
Accordingly, intravenous IgG (IVIG) was considered a promising candidate for the prevention of CHB based on several potential mechanisms, the first two relate to lowering or even eliminating maternal antibody in the fetal circulation (maternal perspective): 1) increased catabolism of maternal antibody; 2) decreased placental transport of maternal antibody. Unknown however is the threshold level of antibody needed to cause injury and whether IVIG can effectively lower the level below the threshold needed to bind apoptotic cells and trigger an inflammatory/fibrosing sequence. The third consideration is an effect of IVIG transported into the fetal circulation where it might act to upregulate surface expression of the inhibitory FcγRIIB receptors on fetal macrophages thereby decreasing secretion of proinflammatory and profibrosing cytokines (fetal perspective). Accordingly, modulation of inhibitory signaling could be a potent therapeutic strategy for attenuating autoantibody-triggered inflammatory diseases. Highly speculative would be an anti-apoptotic effect of IVIG, which would certainly be relevant to the pathogenesis of CHB, in which there is evidence that apoptosis of cardiocytes provides an essential link between antibody and fibrosis [29].
The decision to dose the IVIG at 400mg/kg was based on safety, efficacy and cost issues. The replacement dose of IVIG is generally considered to be between 300 and 500mg/kg. Consistent with the serum half life of IgG, this dosing is repeated every 3–4 weeks to maintain a protective serum level [30]. It is unknown to what extent and when IVIG might decrease placental transport of the autoantibodies since it is acknowledged that antibody transport across the human placenta is minimal at 12 weeks of gestation. In addition, the dose was based on body weight of the mother but clearly the effect was not necessarily intended to achieve a lowering of the mother’s total autoantibody burden. It was also hypothesized that this dose might achieve an anti-inflammatory effect in the fetus which would support the lower dose if based on the weight of the fetus, not the mother. The Finnish study, which was the only published work on preventive IVIG in CHB, comprised only 2 doses of 1g/kg [18], thus the cumulative doses used herein were identical.
A potential reason for the absence of observed efficacy relates to underdosing of the IVIG. Anti-inflammatory doses of IVIG have been reported in the range of 1–3 gm/kg body weight [30]. The absence of a change in maternal antibody titers could be a reflection of the low dose. Precedent for IVIG lowering of antibody titers as a biomarker of effect in humans is the report of serologic (decreased antibody) and clinical remission in patients with bullous pemphigus treated with IVIG [31]. Whether a significant decrease in antibody titer can be employed as a reliable biomarker of drug coverage has not been established. Precedent for the use of higher doses and presumed safety during pregnancy are studies on anti-phospholipid associated fetal loss and fetal alloimmune thrombocytopenia [32, 33].
A clear limitation of the study is the open label design. The decision against randomization was made based on several assumptions. The recurrence rate was estimated at 19% based on available publications at the time of study design [3,5]. After discussion among the co-investigators, it was decided that a clinically meaningful outcome of therapy would be at least a 50% decrease in the predicted recurrence rate. Based on power calculations, if the improvement from 19% to 10% was the case, 261 mothers with a previous CHB child per group would be required to complete a randomized control study. Even if IVIG could decrease the recurrence rate to 5%, it would require 97 mothers per group. Given the 2% frequency of CHB in anti-SSA/Ro positive mothers unselected for prior disease and the average yearly enrollment of the RRNL at 2 patients per month, it was deemed highly unlikely these enrollment goals could be achieved. Indeed, the current U.S. study took 2 years to achieve enrollment for the first phase of our study, and the European study required 4 years to enroll 15 patients. Furthermore, the likelihood that all patients would agree to randomization knowing a potentially effective therapy was being studied was deemed exceedingly rare.
In summary, this study employing Simon’s optimal design showed that IVIG at 400mg/kg on a triweekly schedule from 12 weeks to 24 weeks of gestation is ineffective as a prevention of CHB in pregnancies at risk of recurrence. This finding was corroborated by the European study which used an identical protocol. However, the results support the feasibility of recruitment of high risk patients and the safety of IVIG and thus consideration might be given to higher anti-inflammatory doses.
Acknowledgments
We thank the members of the independent Advisory Board for this study: Joan Merrill, M.D; Ware Branch, M.D.; and Bonnie Bermas, M.D. We also thank Amy Lawless for the help preparing the manuscript.
This work was funded by the Alliance for Lupus Research and NIH, Contract NO1-AR-4-2220 (Research Registry for Neonatal Lupus). Clinical trial registration information-URL: http://www.clinicaltrials.gov. Unique identifier: NCT00460928
REFERENCES
- 1.Brucato A, Frassi M, Franceschini F, Cimaz R, Faden D, Pisoni MP, et al. Risk of Congenital Complete Heart Block in Newborns of Mothers with Anti-Ro/SSA Antibodies Detected by Counterimmunoelectrophoresis. A prospective Study of 100 Women. Arthritis Rheum. 2001;44:1832–1835. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DM, Kim MY, Copel JA, Davis C, Phoon CK, Glickstein JS, et al. Utility of Cardiac Monitoring in Fetuses at Risk for Congenital Heart Block. The PR interval and Dexamethasone evaluation (PRIDE) Prospective Study. Circulation. 2008;117:485–493. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 3.Julkunen H, Eronen M. The Rate of Recurrence of Isolated Congenital Heart Block: A Population Based Study. Arthritis Rheum. 2001;44:487–488. doi: 10.1002/1529-0131(200102)44:2<487::AID-ANR70>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 4.Llanos C, Izmirly PM, Katholi M, Clancy RM, Friedman DM, Kim MY, et al. Recurrence Rates of Cardiac Manifestations Associated with Neonatal Lupus and Maternal/Fetal Risk Factors. Arthritis Rheum. doi: 10.1002/art.24768. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, et al. Autoimmune-associated congenital heart block: Mortality, morbidity, and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–1666. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 6.Waltuck J, Buyon J. Autoantibody-associated congenital heart block: Outcome in mothers and children. Annals Int Med. 1994;120:544–551. doi: 10.7326/0003-4819-120-7-199404010-00003. [DOI] [PubMed] [Google Scholar]
- 7.Nield LE, Silverman ED, Smallhorn JF, Taylor GP, Brendan J, Mullen M, Benson LN, Hornberger LK. Endocardial fibroelastosis associated with maternal anti-Ro and anti-La antibodies in the absence of atrioventricular block. Circulation. 2002;40:796–802. doi: 10.1016/s0735-1097(02)02004-1. [DOI] [PubMed] [Google Scholar]
- 8.Moak JP, Barron KS, Hougen TJ, Wiles HB, Balaji S, Sreeram N, et al. Congenital heart block: development of late-onset cardiomyopathy, a previously underappreciated sequela. J Am Coll Cardiol. 2001;37:238–242. doi: 10.1016/s0735-1097(00)01048-2. [DOI] [PubMed] [Google Scholar]
- 9.Friedman DM, Kim KY, Copel JA, Llanos C, Davis C, Buyon JP. Prospective Evaluation of Fetuses with Autoimmune Associated Congenital Heart Block Followed in the PR Interval and Dexamethasone Evaluation (PRIDE) Study. Am J Cardiol. 2009;103:1102–1106. doi: 10.1016/j.amjcard.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buyon JP, Clancy RM, Friedman DM. Cardiac Manifestations of Neonatal Lupus Erythematosus: guidelines to management integrating integrating clues from the bench and bedside. Nat Clin Pract Rheumatol. 2009;5:139–148. doi: 10.1038/ncprheum1018. [DOI] [PubMed] [Google Scholar]
- 11.Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J Immunol. 1996;157:3317–3322. [PubMed] [Google Scholar]
- 12.Clancy RM, Neufing PJ, Zheng P, O’Mahony M, Nimmerjahn F, Gordon TP, et al. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–2422. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miranda-Carus ME, Askanase AD, Clancy RM, Di Donato F, Chou TM, Libera RM, et al. Anti-SSA/Ro and anti-SSB/La autoantibodies bind the surface of apoptotic fetal cardiocytes and promote secretion of TNF-alpha by macrophages. J Immunol. 2000;165:5345–5351. doi: 10.4049/jimmunol.165.9.5345. [DOI] [PubMed] [Google Scholar]
- 14.Clancy RM, Askanase AD, Kapur RP, Chiopelas E, Azar N, Miranda-Carus ME, et al. Transdifferentiation of cardiac fibroblasts, a fetal factor in anti-SSA/Ro-SSB/La antibody-mediated congenital heart block. J Immunol. 2002;169:2156–2163. doi: 10.4049/jimmunol.169.4.2156. [DOI] [PubMed] [Google Scholar]
- 15.Hansen RJ, Balthasar JP. Effects of intravenous immunoglobulin on platelet count and antiplatelet antibody disposition in a rat model of autoimmune thrombocytopenia. Blood. 2002;100:2087–2093. [PubMed] [Google Scholar]
- 16.Hansen RJ, Balthasar JP. Intravenous immunoglobulin mediates an increase in anti-platelet antibody clearance via the FcRn receptor. Thromb Haemost. 2002;88:898–899. [PubMed] [Google Scholar]
- 17.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 18.Kaaja R, Julkunen H. Prevention of Recurrence of Congenital Heart Block with Intravenous Immunoglobulin and Corticosteroid Therapy: comment on the editorial by Buyon et al. Arthritis Rheum. 2004;50:280–281. doi: 10.1002/art.10716. [DOI] [PubMed] [Google Scholar]
- 19.Tran HB, Cavill D, Buyon JP, Gordon TP. Intravenous Immunoglobulin and placental transport of anti-Ro/La antibodies: comment on the letter by Kaaja and Julkunen. Arthritis Rheum. 2004;50:337–338. doi: 10.1002/art.11498. [DOI] [PubMed] [Google Scholar]
- 20.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 21.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. for the European Study Group on Classification Criteria for Sjogren’s Syndrome. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 23.Glickstein JS, Buyon J, Friedman D. Pulsed Doppler echocardiographic assessment of the fetal PR interval. Am J Cardiol. 2000;86:236–239. doi: 10.1016/s0002-9149(00)00867-5. [DOI] [PubMed] [Google Scholar]
- 24.Tseng CE, Caldwell K, Feit S, Chan EK, Buyon JP. Subclass distribution of maternal and neonatal anti-Ro (SSA) and La (SSB) antibodies in congenital heart block. J Rheumatol. 1996;23:925–932. [PubMed] [Google Scholar]
- 25.Looney RJ, Huggins J. Use of intravenous immunoglobulin (IVIG) Best Practice Res Clin Haematol. 2006;18:3–25. doi: 10.1016/j.beha.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 26.Yu Z, Lennon VA. Mechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. New Eng J Med. 1999;340:227–228. doi: 10.1056/NEJM199901213400311. [DOI] [PubMed] [Google Scholar]
- 27.Ni H, Chen P, Spring CM, Sayeh E, Semple JW, Lazarus AH, et al. A novel murine model of fetal and neonatal alloimmune thrombocytopenia: response to intravenous IgG therapy. Blood. 2006;107:2976–2983. doi: 10.1182/blood-2005-06-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 2008;105 doi: 10.1073/pnas.0810163105. 19571-18578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–182. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 30.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–533. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 31.Li N, Zhao M, Hilario-Vargas J, Prisayanh P, Warren S, Diaz LA, et al. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest. 2005;115:3440–3450. doi: 10.1172/JCI24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Branch WD, Peaceman AM, Druzin M, Silver RK, El-Sayed Y, Silver RM, et al. A multicenter, placebo-controlled pilot study of intravenous immune globulin treatment of antiphospholipid syndrome during pregnancy. Am J Obstet Gynecol. 2000;182:122–127. doi: 10.1016/s0002-9378(00)70500-x. [DOI] [PubMed] [Google Scholar]
- 33.Berkowitz RL, Lesser ML, McFarland JG, Wissert M, Primiani A, Hung, et al. Antepartum Treatment without Early Cordocentesis for Standard-Risk Alloimmune Thrombocytopenia. Obstet Gynecol. 2007;110:249–255. doi: 10.1097/01.AOG.0000270302.80336.dd. [DOI] [PubMed] [Google Scholar]


