Abstract
Trafficking of CD8 T cells, in both the steady-state and during episodes of infection or inflammation, is a highly dynamic process and involves a variety of receptor-ligand interactions. A thorough, mechanistic understanding of how this process is regulated could potentially lead to disease prevention strategies, through either enhancing (for infectious diseases or tumors) or limiting (for autoimmunity) recruitment of antigen-specific CD8 T cells to areas of tissue inflammation. As CD8 T cells transition from naive to effector to memory cells, changes in gene expression will ultimately dictate anatomical localization of these cells in vivo. In this article, we discuss recent advances in understanding how antigenic stimulation influences expression of various trafficking receptors and ligands, and how this determines the tissue localization of CD8 T cells.
Keywords: antigenic stimulation, CD8 T cell, chemokines, inflammation, integrin, selectin, trafficking, vaccination
In order to confer appropriate host protection, leukocytes must leave the circulation and enter peripheral tissues, where they can control invading pathogens. Traditionally, the progressive series of biological events that ultimately results in transmigration (the movement of leukocytes through blood vessels and into tissues) could be broken down into three individual steps that precede tissue extravation:
-
■
Rolling/tethering
-
■
Activation
-
■
Firm adhesion/attachment
Although, recent studies suggest the process may be much more complicated [1]. Cells of the innate immune system (e.g., neutrophils) are able to rapidly localize to areas of inflammation in response to invading pathogens and can provide immediate host protection. By contrast, the trafficking patterns of cells of the adaptive immune system, in particular T cells, vary considerably, and are dependent upon a number of biological variables. However, appropriate activation of T cells leads to the generation of cellular-based ‘immunological memory’ and can result in life-long protection against certain pathogens [2,3]. Therefore, a complete understanding of how both host- and cell-intrinsic genetic factors influence the trafficking patterns of T cells could ultimately result in therapeutic strategies through either enhancing or limiting antigen-specific CD8 T cell recruitment.
Following their development in the thymus, naive CD8 T cells enter the periphery and continually circulate throughout the body until they encounter foreign antigens in secondary lymphoid tissues. Once appropriately stimulated by a cognate antigenic peptide in the context of MHC I and costimulated by an antigen presenting cell (e.g., a dendritic cell [DC]), activated CD8 T cells undergo rapid and robust proliferation, in which a single naive CD8 T cell can produce as many as 10,000 daughter cells [4,5]. These newly generated cells exhibit exceptional effector functions (cytokine production and cytolosis) [6], but are short lived, with 90–95% of the population undergoing apoptosis following the peak of the expansion phase. However, the remaining 5–10% of CD8 T cells that survive the contraction phase give rise to long-lived memory populations. As CD8 T cells transition from naive to effector to memory cells, the overall gene expression profile changes, resulting in characteristics and/or functions that vary among the different populations [7]. In addition to regulating several other aspects of CD8 T-cell biology, these changes in gene expression impact CD8 T-cell trafficking patterns. As the overall molecular signature of an individual CD8 T cell strongly dictates its trafficking capabilities, this article will review important gene products that regulate cellular localization, and discuss recent advances in our understanding of naive, effector and memory CD8 T-cell trafficking patterns, during both the steady state and episodes of inflammation.
Regulators of cellular localization
As previously mentioned, individual steps that collectively result in the transmigration of a leukocyte include rolling/tethering, activation and firm attachment/adhesion. Although the entire process becomes much more complicated when factoring in intracellular signaling networks and the changes in gene expression that occur following receptor ligation, the original categorization of the cell surface receptors and ligands that regulate each of these three processes nonetheless remain critical dictators of efficient cellular trafficking (Table 1). Importantly, CD8 T cells will express different selectins–selectin ligands, chemokine receptors and integrins depending on the activation state of the cell. Therefore, matched receptor–ligand interactions between CD8 T cells and the endothelium are critical for the efficient cellular migration of CD8 T cells from circulation and into various tissues.
Table 1. Receptor–ligand pairs of selectins, chemokines and integrins involved with CD8 T-cell traffcking.
| Receptor | Ligand |
|---|---|
| Selectin | Selectin ligands |
| L-selectin (CD62L) | PNAd |
| P-selectin (CD62P) | PSGL-1 |
| E-selectin (CD62E) | CD43, CD44, ESL-1, PSGL-1 |
| Chemokine receptor | Chemokine |
| CCR7 | CCL19, CCL21 |
| CCR5 | CCL5 |
| CCR10 | CCL27, CCL28 |
| CCR9 | CCL25 |
| CCR4 | CCL17 |
| CXCR3 | CXCL9, CXCL10 |
| Integrin | Integrin ligand |
| VLA-1 (α1β1) | Collagen, laminin |
| VLA-4 (α4β1) | VCAM-1, fbronectin |
| LFA-1 (αLβ2) | ICAM-1 |
| α 4 β 7 | MAdCAM-1 |
| α E β 7 | E-cadherin |
ESL E-selectin ligand; LFA: Lymphocyte function-associated antigen; MAdCAM: Mucousal addressin cell adhesion molecule; PNAd: Peripheral node addressin; PSGL P-selectin glycoprotein ligand; VLA: Very-late antigen.
Selectins
All three members of the selectin family of proteins play important roles in regulating CD8 T-cell trafficking [8]. L-selectin (CD62L) is expressed by all naive CD8 T cells and certain subsets of memory CD8 T cells, whereas P-selectin (CD62P) and E-selectin (CD62E) are expressed by inflamed endothelial cells. Activated platelets also express P-selectin. The interaction between selectins and their ligands initiates the transmigration process, and interestingly, these selectin–ligand interactions strengthen in response to ‘shear stress’, where individual bonds between receptor and ligand actually strengthen as force is applied [9,10]. Therefore, the force exerted on cells by flowing blood actually increases the ability for selectins to interact with ligands and results in the observed ‘rolling and tethering’ behavior of immune cells on endothelium prior to transmigration. Significantly, the ability for a selectin ligand to become functional is highly dependent upon glycosylation, a post-translational modification that results in the extension of monosaccharide chains from either a serine/threonine (O-linked) or arginine (N-linked) amino acid. The process of glycosylation is complex and goes beyond the scope of this article, but has been recently discussed in great detail [11]. However, this demonstrates that regulation of selectin ligands occurs not only through control of ligand gene expression, but also through the regulation of various glycosyltransferases that are required for post-translational modification, which collectively result in a ‘functional’ selectin ligand.
Ligands for CD62L are collectively referred to as peripheral node addressins (PNAd) and react with the monoclonal antibody MECA-79 [12], which specifically identifies properly glycosylated/sulfated ligands including, but not limited to, CD34 and mucousal addressin cell adhesion molecule-1. These ligands are highly expressed in the high endothelial venule (HEV) and are critical for the homing of naive CD8 T cells to the lymph node [13]. By contrast, CD8 T cells express P-selectin glycoprotein ligand-1 (PSGL-1), which can serve as a ligand for all three selectins, although originally thought to interact primarily with P-selectin [14]. Ligands for E-Selectin on all leukocytes, including CD8 T cells, remain relatively ill defined, but could include CD44, CD43 and E-selectin ligand-1, in addition to PSGL-1 [15,16]. Therefore, the individual or collective role for expressed and appropriately glycosylated selectin ligands on CD8 T cells at various stages of activation could influence trafficking of these cells to different tissues of the body.
Chemokines
Chemokines are a family of small, structurally related molecules that can strongly dictate immune cell trafficking to specific tissues. In humans, a large number of chemokines (~50) have been identified [17,18]. Chemokines trigger biological activity through interaction with chemokine receptors, which are seven-transmembrane cell surface G-protein-coupled receptors (GPCRs) [19]. Following GPCR ligation, the receptor associated Gα subunit from the heterotrimeric G-protein complex undergoes GDP–GTP exchange and then dissociates from the Gβγ portion. The resulting two complexes (Gα and Gβγ) initiate the activation of a number of signaling pathways that result in changes in gene transcription, actin reorganization, establishment of cell polarity and activation of cell-surface integrins. Thus, interactions with chemokines ‘activate’ an immune cell for subsequent transendothial extravasation and then ‘direct’ cellular migration into tissues.
In immunological terms, chemokines can be broadly defined as being either homeostatic (present in the steady state) or inflammatory [20]. For example, some chemokines are known to play important roles in processes such as T-cell development and the trafficking of naive T cells into lymph nodes [21-23]. By contrast, other chemo kines are produced in response to inflammatory stimuli and assist in rapidly recruiting immune cells to areas of inflammation, often in a tissue-specific manner. Importantly, the repertoire of chemokine receptors expressed by activated CD8 T cells significantly changes compared with naive cells, presumably to direct these activated T cells toward the sites of infection. Importantly, various subpopulations of memory CD8 T cells express different combinations of chemokine receptors for both homeostatic and inflammatory cytokines, which can, at least partially, dictate the localization of distinct memory CD8 T-cell populations toward different tissues.
Integrins
Integrins are heterodimeric cell surface receptors, containing both an α and β chain. The ability for an integrin to interact with a ligand is greatly enhanced following appropriate cellular stimulation. Therefore, integrins can become further ‘activated’ following the interaction of a T cell with an extracellular stimulus, such as ligation of chemokine receptors or the T-cell receptor (TCR). This process is collectively known as ‘inside-out signaling’ since intracellular signaling pathways act upon the cytoplasmic side of the integrin to increase its binding potential [24]. Inside-out signaling results in both changes in affinity of an individual integrin, as well as changes in avidity, which occurs through the clustering of integrins on the cell surface. Once properly activated, integrins regulate the firm adhesion of leukocytes to endothelial cells and are also critical during the trans-endothelial process.
Naive, effector and memory CD8 T cells express the αLβ2 (CD11a/CD18) integrin, lympho cyte function associated-antigen (LFA)-1. Through its interaction with ICAM-1 (CD54), LFA-1 is not only critical for localizing CD8 T cells during migration, but also facilitates T cell–antigen presenting cell interactions during activation [25-27]. Activated CD8 T cells also express the α4β1 (CD49d/CD29) integrin, very-late antigen (VLA)-4, which interacts with VCAM-1 (CD106) expressed on inflamed endothelium [28]. At various stages of activation, CD8 T cells also express other integrins, such as VLA-1 (α1β1), αEβ7 and α4β7, which also potentially contribute to localization and/or retention of CD8 T cells in various tissues. Therefore, expression and activation of specific integrins may dictate both trafficking potential and retention of CD8 T cells in v arious tissues throughout the body.
Trafficking of CD8 T cells
The localization of different subpopulations of CD8 T cells to various anatomical locations largely reflects the desired immunological function that these cells provide. Naive CD8 T cells are found predominately in the circulation, spleen and lymph nodes where they survey the entire body for DCs presenting cognate antigens that will result in their activation. By contrast, recently activated effector CD8 T cells rapidly localize to areas of inflammation in order to combat infection [29]. Finally, memory CD8 T cells can be found in both lymph nodes (to become reactivated) and tissues (to provide immediate protective immunity against pathogens expressing cognate antigens) [30]. Importantly, it is believed that the steps leading to cellular transmigration in secondary lymphoid organs and inflamed tissues are similar, although the individual gene products that regulate each step may differ depending on the activation state of the CD8 T cell (Figure 1). Indeed, changes in the expression of various selectin and selectin ligands, chemokine receptors and integrins all occur following CD8 T-cell activation and subsequent memory formation, resulting in the localization of these cells to areas of the body that will most efficiently improve host protection.
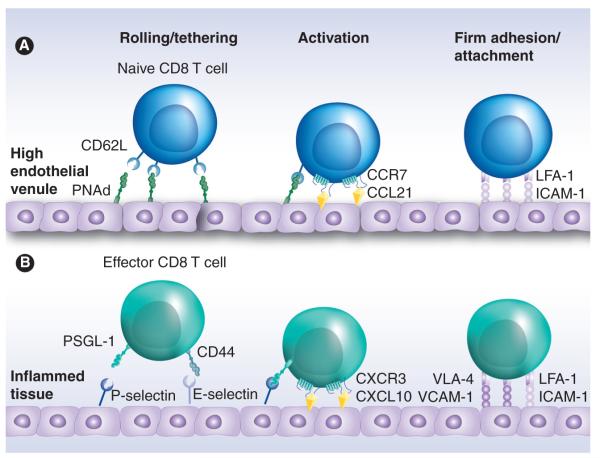
Figure 1. Trafficking of naive and effector CD8 T cells.
(A) Naive CD8 T cells enter lymph nodes through the high endothelial venule. Rolling and tethering is mediated by interactions between L-selectin (CD62L) and PNAd, which allows the chemokine receptor CCR7 to interact with CCL19 or CCL21 displayed by the endothelial cells. CCR7 ligation results in polarization and activation of the integrin LFA-1, which will bind to its ligand ICAM-1 and cause the naive CD8 T cell to firmly adhere to the surface of the high endothelial venule before transmigrating across the endothelial layer. (B) Following antigenic stimulation, effector CD8 T cells express functional ligands such as PSGL-1 and CD44, that bind P-selectin and E-selectin expressed by inflamed tissues. Effector T cells also express chemokine receptors (such as CXCR3), which interact with inflammatory chemokines (such as CXCL10) that are produced by the inflamed tissues. This causes activation and polarization of LFA-1 on the effector T cell, as well as newly expressed integrins, such as VLA-4, which will bind to ICAM-1 and VCAM-1 on the inflamed tissue, respectively. These interactions result in firm adhesion, allowing the effector CD8 T cell to then transmigrate through the endothelial layer and into the inflamed tissue.
LFA: Lymphocyte function-associated antigen; PNAd: Peripheral node addressin; PSGL: P-selectin glycoprotein ligand; VLA: Very-late antigen.
Naive
CD8 T cells Naive CD8 T cells move in a linear fashion from the blood to the lymph nodes, into the lymphatics and back into the systemic circulation via the thoracic duct. The process by which T cells move from the blood and into lymph nodes through the HEV involves the coordination of several steps (Figure 1a). The first step involves the interaction between CD62L and PNAd expressed by the naive CD8 T cell and the HEV endothelium, respectively. These molecules help bring circulating naive T cells from the lumen of the HEV towards the endothelium. Owing to the shear stress within the vessel and the high avidity/low affinity nature of the CD62L–PNAd interaction, naive CD8 T cells appear to ‘roll’ across the endothelium [31,32]. During the rolling process, CCR7 on the T cell can be stimulated by the homeostatic chemokines (i.e., CCL19 and/or CCL21) that are immobilized on the surface of the HEV [33-35]. This stimulation polarizes and induces a conformational change in LFA-1 that increases its affinity for its ligand, ICAM-1 expressed by the HEV endothelium [27,36]. The enhanced interaction between LFA-1 and ICAM-1 after CCR7 stimulation firmly attaches the T cell to the endothelium, thereby enabling it to transmigrate the HEV and enter the lymph node to search for DCs expressing cognate antigens.
Egress of CD8 T cells from the lymph node is controlled by sphingosine 1-phosphate (S1P), which serves as a ligand for the S1P receptor, S1PR1 (known as S1P1) [37]. Similar to chemokine receptors, S1PR1 is a GPCR and activates signaling pathways that result in both cell polarity [38] and migration [39]. Expression of S1PR1 is required for CD8 T cells to exit the lymph node [40] as it directs them across a naturally occurring gradient of increasing S1P concentration from the lymph nodes and into the efferent lymphatics [41-44]. However, if T cells should encounter a reactive lymph node, proinflammatory cytokines can temporarily inhibit the egress of CD8 T cells by reducing S1PR1 expression. Specifically, Type I interferons (IFN-α and IFN-β) will induce expression of CD69, which directly inhibits the surface expression of S1PR1 [45]. This ‘innate-like’ retention of naive CD8 T cells in reactive lymph nodes is thought to promote DC–T cell interactions, thereby increasing the likelihood of CD8 T cells to be stimulated by cognate antigens. Should the T cell encounter a DC presenting cognate antigen, TCR stimulation also substantially reduces S1PR1 transcription, resulting in increased retention of antigen-specific CD8 T cells [42,46,47]. As a result of either complete activation or loss of inflammation, the presence of S1PR1 at the surface of the T cell is regained through renewed transcription and downregulation of CD69 [40]. Once S1PR1 surface expression returns (on either naive or activated CD8 T cells), these cells are then able to exit the lymph node and re-enter the systemic circulation.
Effector CD8 T cells
When a naive CD8 T cell receives sufficient antigenic and costimulatory signals, it will undergo considerable proliferation and acquire effector functions such as the ability to produce cytokines and lyse pathogen-infected cells in a TCR-dependent manner. In addition, activated or ‘effector’ CD8 T cells undergo a dramatic shift in the expression of surface proteins that regulate cellular trafficking. Importantly, effector CD8 T cells lose expression of both CD62L and CCR7, which prevents these cells from gaining access to lymph nodes through the HEV. Instead, activated CD8 T cells gain expression of a new cohort of trafficking molecules including selectin ligands, chemokine receptors and integrins (Figure 2). This overall change in expression (or post-translational modification) of trafficking regulators results in localization and transmigration of effector CD8 T cells into inflamed tissues (Figure 1b).
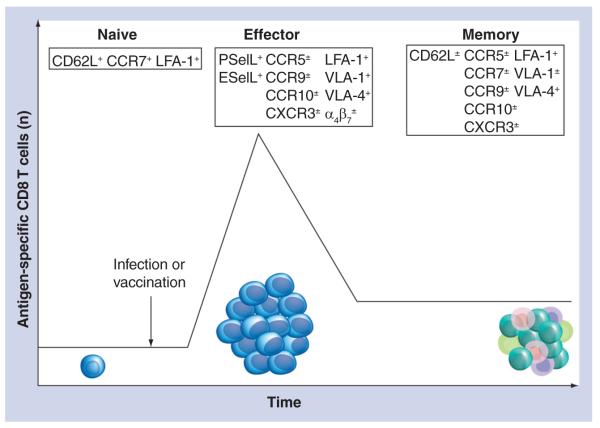
Figure 2. Expression of various trafficking molecules dynamically changes during the course of CD8 T-cell activation and progression into memory cells.
Naive CD8 T cells express relatively few trafficking molecules (e.g., CD62L, CCR7 and LFA-1). However, following sufficient antigenic and costimulatory activation, CD8 T cells will rapidly proliferate and expand. In addition to this marked expansion, effector CD8 T cells lose expression of CD62L and CCR7, while gaining the expression of P- and E-selectin ligands, inflammatory chemokine receptors (e.g., CXCR3 and CCR5) and additional integrins (e.g., VLA-1, VLA-4, α4 and β7), resulting in efficient recruitment of these cells to inflamed tissues. Following contraction, 90–95% of the effector cells die via apoptosis. However, the CD8 T cells that survive the contraction phase are maintained long term and progress into memory cells. Collectively, memory CD8 T-cell populations contain a heterogeneous mixture of cells that express a variety of trafficking molecules, shared with both naive and effector CD8 T cells.
ESelL: E-selectin ligands; LFA: Lymphocyte function-associated antigen; PSelL: P-selectin ligands; VLA: Very-late antigen.
Effector CD8 T cells can express functional ligands for both P- and E-selectin, defined as the ability for such protein to bind to either P- or E-selectin expressed on inflamed endothelium. As previously mentioned, the formation of functional P- and E-selectin ligands (on molecules such as PSGL-1, CD44 and E-selectin ligand-1) relies heavily upon the proper post-translational modification of proteins via glycosylation. Indeed, TCR-stimulation of CD8 T cells results in the expression of at least two glycotransferases that are critical for transforming these molecules into P- and E-selectin ligands, core 2 β1-6-N-glucosaminyltransferase-I and α-(1,3)-fucosyltransferase-VII [8]. In addition, other studies have suggested that inflammatory cytokines (e.g., IL-12 and IL-2) enhance expression of selectin ligands following TCR stimulation [48,49], although this idea remains controversial [50]. Nevertheless, the formation of functional P- and E-selectin ligands following activation enables effector CD8 T cells to efficiently roll on inflamed endothelium expressing their c ounter-receptors P- and E-selectin.
In addition to expressing selectin ligands, effector CD8 T cells also express different integrins and chemokine receptors compared with naive cells, which enables them to complete the extravasation process into inflamed tissues. Significantly, following activation, the expression of various trafficking molecules on CD8 T cells can occur in both a transient or permanent fashion. Surface expression of CD44, LFA-1 and VLA-4 is elevated following CD8 T-cell activation, and remains high on memory cells. By contrast, expression of other trafficking molecules, especially those that are thought to localize effector CD8 T cells to specific tissues, are lost around the peak of the expansion process. For example, effector CD8 T cells that are found in gut express the α4β7 integrin [51], whereas cells on the skin express E-selectin ligands [52,53], both of which are downregulated as activated CD8 T cells transition to memory cells. In agreement with this, memory CD8 T cells (which lose α4β7) localize poorly to the gut. Thus, the transient expression of various trafficking molecules on effector CD8 T cells is critical to localize these cells toward specific inflamed tissues.
Although T cells undergo a programmed modulation of trafficking molecules following activation, the secondary lymphoid environment can fine-tune their homing properties. It has been recently demonstrated that bone marrow-derived DCs can induce unique chemokine receptor (CXCR3, CCR3, CCR4, CCR5, CCR6 and CCR9) and selectin ligand expression profiles on responding antigen-specific populations, depending on the route of immunization [54,55]. Similar results were also found when bone marrow-derived DCs were replaced with vaccinia virus infection. Other studies have also demonstrated that priming of CD8 T cells in the mesenteric lymph nodes promotes migration to the gut by increasing α4β7 integrin and CCR9 expression, while priming in cutaneous lymph nodes promotes migration to the skin by increasing E-selectin ligands and CCR10 expression [56-58]. These data together promote the idea that draining lymph node environments can dictate the migratory properties of activated CD8 T cells in order to direct them toward specific sites of infection.
Although CD8 T-cell ‘imprinting’ can significantly influence the expression of tissue-specific homing factors, this process does not permanently alter their trafficking capabilities. It has been demonstrated that when peptide-coated peripheral lymph node DCs are incubated with CD8 T cells, P- and E-selectin ligand expression is induced and migrating to the skin is significantly increased [58]. However, this effect can be ameliorated with the addition of gut-derived DC. Interestingly, CD8 T cells already imprinted in the skin or gut can be ‘re-educated’ following an encounter with the DC from the opposite anatomical site. Owing to these results, it appears that T-cell imprinting is not static and can be defined by the net effect of local DC stimuli.
In addition, it was recently demonstrated that CD4 T helper cells can also regulate the recruitment of effector CD8 T cells. Specifically, recruitment of effector CD8 T cells to the vaginal musosa, following infection with herpes virus, is impaired in the absence of CD4 T cells [59]. Without CD4 T cells, the localized production of the IFN-γ-inducible chemokines CXCL9 and CXCL10 was significantly impaired, suggesting that local recognition of virus by CD4 T cells resulted in IFN-γ-dependent production of these chemokines. Since effector CD8 T cells express ample amounts of CXCR3 (the receptor for CXCL9 and CXCL10) [60], this results in more efficient recruitment of these cells to the infected tissue. A role for CD4 T cells in the recruitment of CD8 T cells into tumors has also been suggested [61]. However, the role that CD4 T cells play in the recruitment of effector CD8 T cells to other tissues is currently unknown.
Memory CD8 T cells
Following activation and contraction in response to an acute infection or vaccination, approximately 5–10% of the responding antigen-specific CD8 T cells will persist as memory cells [62]. Although this population is stably maintained, it is not homogenous [63], and the heterogeneity can be demonstrated by its diverse expression of trafficking molecules (Figure 2). In spite of this diversity, the most general and widely accepted classification of memory CD8 T cells relies on their expression (or lack of expression) of the lymph node homing molecules CCR7 and CD62L [64,65]. As previously discussed, expression of both CD62L and CCR7 are dramatically downregulated following CD8 T-cell activation. However, as memory cells are formed, CD62L and CCR7 eventually become re-expressed on individual cells within the bulk memory population and the percentage of cells expressing these receptors continues to increase over time. Memory CD8 T cells that express low levels of these molecules are designated as effector memory (Tem) cells; while those that have high coexpression are known as central memory (Tcm) cells. Owing to their differential expression of CD62L and CCR7, lymph node homing of these subsets is significantly impacted [64,66]. While both Tem and Tcm cells are commonly found throughout the blood, spleen and peripheral tissues, Tcm cells are highly enriched in lymph nodes relative to Tem cells. This bifurcation of lymph node trafficking enables memory CD8 T cells to maintain immunity in both the lymph nodes and in the periphery, where infection may first take hold. Significantly, exposure to high inflammation during the priming phase significantly diminishes the rate of CD62L and CCR7 re-expression on the memory CD8 T-cell population, whereas a low inflammatory environment results in rapid reacquisition of these lymph node-homing molecules [67,68]. Therefore, the overall inflammatory cytokine milieu present during CD8 T-cell activation can have long-lasting effects on the trafficking patterns of the ensuing memory CD8 T-cell population.
Besides their ability to perform effector functions, memory CD8 T cells also possess the ability to populate peripheral tissues and provide a ‘first-line of defense’ against pathogen reinfection. Significantly, memory CD8 T cells are also rapidly recruited to localized areas of inflammation in an antigen-independent fashion, and can provide additional host protection against pathogens expressing cognate antigens [69,70]. In addition, it has recently been demonstrated that memory CD8 T cells recruited to the lung during viral infection exhibit increased expression of granzyme B, which enhances the cytolytic activity of these cells [71]. The molecular mechanisms that regulate antigen-independent memory CD8 T-cell trafficking are not completely understood, although expression of CCR5 appears to be critical for memory cell recruitment to the lung [70]. Nevertheless, these data demonstrate that memory CD8 T cells adopt ‘innate-like’ trafficking characteristics that allow recruitment into inflamed tissues, without the need for antigenic restimulation by DCs.
In general, memory CD8 T cells are able to freely circulate between the blood, spleen, lymph nodes and some peripheral tissues (but not others, such as the skin and gut) [72]. However, recent studies have identified a specialized subset of memory CD8 T cells that appear to be retained long term in these tissues and have therefore been defined as ‘resident memory’ CD8 T cells [51,73]. Interestingly, the molecular signature of the trafficking molecules required to localize effector CD8 T cells into these tissues is transient and is quickly lost on the bulk memory population. This suggests that the establishment of ‘resident memory’ CD8 T cells in tissues such as the skin requires the recruitment of these cells during the effector phase of the T-cell response. Interestingly, ‘resident-memory’ CD8 T cells of the skin have been shown to express CD69 and the αEβ7 integrin [73], which may assist in their retention. However, it is also worth noting that although α4β7 integrin expression assists in recruiting CD8 T cells to the gut, it is not required for their long-term retention [51]. Therefore, a combination of molecules for both recruitment and retention may collectively result in the establishment of ‘resident memory’ CD8 T cells in various tissues.
Finally, recent studies demonstrate that the overall expression of trafficking molecules on memory CD8 T cells is significantly altered following multiple, subsequent antigenic stimulations [74-76]. As antigen experience increases, memory CD8 T cells drastically downregulate the expression of both CD62L and CCR7. Interestingly, an inverse correlation can be found for the expression of trafficking molecules associated with homing to peripheral tissue [74]. For example, with additional ‘antigen experience’, memory CD8 T cells significantly increase their expression of molecules associated with homing to the lung, such as CCR5 and VLA-1 [70,77]. As implied by these expression patterns, memory CD8 T cells that have been exposed to multiple infections are enriched within peripheral tissue, such as the liver and lung, indicating that antigen experience shifts memory CD8 T-cell-mediated immunity away from secondary lymphoid organs and to peripheral tissues that have high propensity for reinfection (e.g., the lung). These data demonstrate that antigen experience enhances the peripheral tissue localization of memory CD8 T cells and concurrently, inhibits localization of these memory cells to lymph nodes.
CD8 T-cell trafficking during chronic infection
As described previously, the trafficking patterns of CD8 T cells change, depending on the activation state of the individual cells, as they progress from naive to effector to memory cell following an acute stimulation, whereby the vaccination agent or pathogen is rapidly and efficiently cleared by the immune system. However, in a number of human diseases, the infectious agent is not cleared, resulting in the establishment of a prolonged chronic infection. This results in CD8 T-cell populations exhibiting characteristics of ‘functional exhaustion’, such as impaired cytokine production and a requirement for antigen-driven proliferation for cell survival [78,79]. Not surprisingly, this continuous exposure to antigens also results in the differential anatomical distribution of CD8 T cells compared with memory CD8 T cells that are formed following acute infection or vaccination [80,81].
Functionally exhausted CD8 T cells exhibit a genetic signature that is unique compared with either effector or memory CD8 T cells [82]. Antigen-specific CD8 T cells in both mouse models of chronic infection and in humans chronically infected with HIV, Epstein–Barr virus or cytomegalovirus, do not express (or re-express) CD62L or CCR7, and are therefore impaired in lymph node trafficking and localization [83]. Interestingly, most HIV and Epstein–Barr virus replication occurs within the lymph nodes, which may suggest that these pathogens have coevolved with the human immune system to replicate in anatomical locations that continually stimulated CD8 T cells are unable to access. As well as lymph node homing receptors (i.e., CD62L and CCR7), exhausted CD8 T cells also abnormally express other trafficking molecules such as CXCR3, CD49d, CD29 and β7 integrin [82]. Both effector and memory CD8 T cells following acute infection express the chemokine receptor CXCR3. However, it is not expressed on exhausted CD8 T cells, and in addition, HIV-infected individuals also have decreased numbers of CXCR3-expressing CD8 T cells [84]. Therefore, it is possible that exhausted CD8 T cells may also be impaired in trafficking to tissues that express CXCL9 and CXCL10, the cognate ligands of CXCR3, following localized exposure to IFN-γ. It is clear that chronic infections impact the trafficking capabilities of antigen-specific CD8 T cells, but the overall biological consequences remain to be elucidated.
Conclusion
By contrast to cells of the innate immune system, trafficking patterns of CD8 T cells can vary dramatically depending on the state of activation, which in-turn dictates expression of various trafficking molecules. Through disparate expression of selectins and selectin ligands, chemokine receptors and integrins, CD8 T cells differentially home to various tissues as they progress from naive to effector to memory cells. This results in the localization of cells into specific tissues where they can be activated (lymph nodes for naive and memory CD8 T cells) or limit pathogen burden (peripheral tissues for effector and memory CD8 T cells). Importantly, the collective integration of external stimuli, provided by DCs and the inflammatory environment during activation, results in both short- and long-term effects that impact the trafficking of CD8 T cells. A more thorough understanding of the mechanisms regulating the differential expression of trafficking molecules on CD8 T cells could therefore potentially aid in therapies against specific human diseases.
Future perspective
Although it is clear that antigenic stimulation results in both transient and permanent alterations in the trafficking molecules expressed by CD8 T cells, a number of questions still remain. For example, it is currently unclear how DCs from different tissues and lymphoid organs stimulate CD8 T cells to express trafficking molecules that will direct them to specific tissues. Although many studies have clearly demonstrated that imprinted CD8 T-cell subsets become enriched in particular target organs several hours following transfer, they have not gone further to demonstrate how well these cells maintain their homing properties beyond effector differentiation and into memory [54-58]. It would seem beneficial for imprinting to be permanent throughout memory progression, since pathogens tend to have a high specificity for particular host tissues. By maintaining residence in previously infected tissues (i.e., resident memory), memory CD8 T cells could help to limit pathogen burden during future reinfection. However, the mechanisms leading to the establishment of resident memory are currently unknown. In addition, it has only recently been demonstrated that CD4 helper T cells can assist in the recruitment of effector CD8 T cells to areas of infection; but it is currently unknown whether CD4 T-cell help is also required for memory CD8 T-cell recruitment to inflamed tissues in either an antigen-dependent- or -independent fashion. It it now also clear that memory CD8 T cells that have gone through multiple rounds of antigenic stimulation differ considerably with regards to expression of trafficking molecules, compared with memory cells that have only been stimulated by antigens once. This finding is of considerable importance, as it is probable that most memory CD8 T-cell populations in humans will have been exposed to multiple rounds of antigenic stimulation. Therefore, future studies uncovering the molecular mechanisms that regulate CD8 T-cell trafficking (and potentially, its manipulation) will be critical for the design and implementation of various c ellular-based immunotherapies.
Executive summary.
-
■
The ‘rolling and tethering’, ‘activation’ and ‘firm adhesion’ of immune cells are regulated by the interactions between selectin–selectin ligands, chemokines–chemokine receptors and integrins–integrin ligands, respectively, which ultimately results in the transmigration of cells from the circulation, through blood vessels and into tissues.
-
■
Naive CD8 T cells are generally confined to the circulation and lymph nodes and express CD62L, CCR7 and LFA-1, which allow them to enter the lymph node through the high endothelial venule.
-
■
Following antigenic stimulation, effector CD8 T cells then express a new cohort of trafficking molecules, resulting in efficient recruitment of these cells into areas of inflammation.
-
■
Different dendritic cell subsets and vaccination route can influence the expression of various trafficking molecules on effector CD8 T cells.
-
■
Memory CD8 T-cell populations are quite heterogenous and different subpopulations exhibit differential localization, presumably to confer maximum host protection against reinfection.
-
■
Additional antigen experience drives memory CD8 T-cell populations away from lymph nodes and towards peripheral tissues.
Acknowledgements
The authors would like to acknowledge members of the Harty laboratory for helpful discussion. We also offer apologies to the many investigators whose contributions we were unable to discuss owing to space limitations.
Financial & competing interests disclosure
This work is supported by grants from the NIH (JT Harty) and a Career Development Award from the Leukemia and Lymphoma Society (JC Nolz); grant no AI050073, AI042767, AI085515, AI046653 and AI059752. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. ■■A comprehensive review on the molecular regulation of leukocyte transmigration.
- 2.Butler NS, Nolz JC, Harty JT. Immunologic considerations for generating memory CD8 T cells through vaccination. Cell Microbiol. 2011;13(7):925–933. doi: 10.1111/j.1462-5822.2011.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33(4):451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2002;2(4):251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 5.Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol. Rev. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 6.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 7.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111(6):837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 8.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 2004;4(5):325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 9.Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995;374(6522):539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- 10.Finger EB, Puri KD, Alon R, Lawrence MB, Von Andrian UH, Springer TA. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996;379(6562):266–269. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
- 11.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat. Rev. Immunol. 2008;8(11):874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruehl RE, Bertozzi CR, Rosen SD. Minimal sulfated carbohydrates for recognition by L-selectin and the MECA-79 antibody. J. Biol. Chem. 2000;275(42):32642–32648. doi: 10.1074/jbc.M001703200. [DOI] [PubMed] [Google Scholar]
- 13.Hemmerich S, Bistrup A, Singer MS, et al. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity. 2001;15(2):237–247. doi: 10.1016/s1074-7613(01)00188-1. [DOI] [PubMed] [Google Scholar]
- 14.Carlow DA, Gossens K, Naus S, Veerman KM, Seo W, Ziltener HJ. PSGL-1 function in immunity and steady state homeostasis. Immunol. Rev. 2009;230(1):75–96. doi: 10.1111/j.1600-065X.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- 15.Ellies LG, Sperandio M, Underhill GH, et al. Sialyltransferase specificity in selectin ligand formation. Blood. 2002;100(10):3618–3625. doi: 10.1182/blood-2002-04-1007. [DOI] [PubMed] [Google Scholar]
- 16.Yago T, Fu J, McDaniel JM, Miner JJ, McEver RP, Xia L. Core 1-derived O-glycans are essential E-selectin ligands on neutrophils. Proc. Natl Acad. Sci. USA. 2010;107(20):9204–9209. doi: 10.1073/pnas.1003110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu. Rev. Immunol. yr2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 18.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 19.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296(5573):1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 20.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat. Immunol. 2001;2(2):123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 21.Campbell JJ, Pan J, Butcher EC. Cutting edge: developmental switches in chemokine responses during T cell maturation. J. Immunol. 1999;163(5):2353–2357. [PubMed] [Google Scholar]
- 22.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat. Rev. Immunol. 2006;6(2):127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 23.Gunn MD, Kyuwa S, Tam C, et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 1999;189(3):451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat. Rev. Immunol. 2005;5(7):546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 25.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 26.Grakoui A, Bromley SK, Sumen C, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285(5425):221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 27.Constantin G, Majeed M, Giagulli C, et al. Chemokines trigger immediate β2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13(6):759–769. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- 28.Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J. Cell Biol. 1995;128(6):1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agace WW. Tissue-tropic effector T cells: generation and targeting opportunities. Nat. Rev. Immunol. 2006;6(9):682–692. doi: 10.1038/nri1869. [DOI] [PubMed] [Google Scholar]
- 30.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol. 2009;9(3):153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 31.Kansas GS, Ley K, Munro JM, Tedder TF. Regulation of leukocyte rolling and adhesion to high endothelial venules through the cytoplasmic domain of L-selectin. J. Exp. Med. 1993;177(3):833–838. doi: 10.1084/jem.177.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ley K, Tedder TF, Kansas GS. L-selectin can mediate leukocyte rolling in untreated mesenteric venules in vivo independent of E- or P-selectin. Blood. 1993;82(5):1632–1638. [PubMed] [Google Scholar]
- 33.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl Acad. Sci. USA. 1998;95(1):258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279(5349):381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 35.Baekkevold ES, Yamanaka T, Palframan RT, et al. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J. Exp. Med. 2001;193(9):1105–1112. doi: 10.1084/jem.193.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J. Exp. Med. 1998;187(2):205–216. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee MJ, van Brocklyn JR, Thangada S, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279(5356):1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 38.Lee JF, Ozaki H, Zhan X, Wang E, Hla T, Lee MJ. Sphingosine-1-phosphate signaling regulates lamellipodia localization of cortactin complexes in endothelial cells. Histochem. Cell Biol. 2006;126(3):297–304. doi: 10.1007/s00418-006-0143-z. [DOI] [PubMed] [Google Scholar]
- 39.Dorsam G, Graeler MH, Seroogy C, Kong Y, Voice JK, Goetzl EJ. Transduction of multiple effects of sphingosine 1-phosphate (S1P) on T cell functions by the S1P1 G protein-coupled receptor. J. Immunol. 2003;171(7):3500–3507. doi: 10.4049/jimmunol.171.7.3500. [DOI] [PubMed] [Google Scholar]
- 40.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. doi: 10.1038/nature02284. ■■ Using conditional knockout mice, the authors provide direct evidence that S1PR1 regulates T lymphocyte egress from lymph nodes.
- 41.Graeler M, Shankar G, Goetzl EJ. Cutting edge: suppression of T cell chemotaxis by sphingosine 1-phosphate. J. Immunol. 2002;169(8):4084–4087. doi: 10.4049/jimmunol.169.8.4084. [DOI] [PubMed] [Google Scholar]
- 42.Lo CG, Xu Y, Proia RL, Cyster JG. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J. Exp. Med. 2005;201(2):291–301. doi: 10.1084/jem.20041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 2002;16(14):1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- 44.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 2005;5(7):560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 45.Shiow LR, Rosen DB, Brdickova N, et al. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 46.Chi H, Flavell RA. Cutting edge: regulation of T cell trafficking and primary immune responses by sphingosine 1-phosphate receptor 1. J. Immunol. 2005;174(5):2485–2488. doi: 10.4049/jimmunol.174.5.2485. [DOI] [PubMed] [Google Scholar]
- 47.Graler MH, Huang MC, Watson S, Goetzl EJ. Immunological effects of transgenic constitutive expression of the type 1 sphingosine 1-phosphate receptor by mouse lymphocytes. J. Immunol. 2005;174(4):1997–2003. doi: 10.4049/jimmunol.174.4.1997. [DOI] [PubMed] [Google Scholar]
- 48.Cerwenka A, Morgan TM, Harmsen AG, Dutton RW. Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection. J. Exp. Med. 1999;189(2):423–434. doi: 10.1084/jem.189.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie H, Lim YC, Luscinskas FW, Lichtman AH. Acquisition of selectin binding and peripheral homing properties by CD4(+) and CD8(+) T cells. J. Exp. Med. 1999;189(11):1765–1776. doi: 10.1084/jem.189.11.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlow DA, Williams MJ, Ziltener HJ. Inducing P-selectin ligand formation in CD8 T cells: IL-2 and IL-12 are active in vitro but not required in vivo. J. Immunol. 2005;174(7):3959–3966. doi: 10.4049/jimmunol.174.7.3959. [DOI] [PubMed] [Google Scholar]
- 51.Masopust D, Choo D, Vezys V, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 2010;207(3):553–564. doi: 10.1084/jem.20090858. ■■ Along with [73], provides evidence of ‘resident memory’ CD8 T cells that persist long term in the skin and gut.
- 52.Erdmann I, Scheidegger EP, Koch FK, et al. Fucosyltransferase VII-deficient mice with defective E-, P-, and L-selectin ligands show impaired CD4+ and CD8+ T cell migration into the skin, but normal extravasation into visceral organs. J. Immunol. 2002;168(5):2139–2146. doi: 10.4049/jimmunol.168.5.2139. [DOI] [PubMed] [Google Scholar]
- 53.Hirata T, Furie BC, Furie B. P-, E-, and L-selectin mediate migration of activated CD8+ T lymphocytes into inflamed skin. J. Immunol. 2002;169(8):4307–4313. doi: 10.4049/jimmunol.169.8.4307. [DOI] [PubMed] [Google Scholar]
- 54.Ferguson AR, Engelhard VH. CD8 T cells activated in distinct lymphoid organs differentially express adhesion proteins and coexpress multiple chemokine receptors. J. Immunol. 2010;184(8):4079–4086. doi: 10.4049/jimmunol.0901903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dudda JC, Simon JC, Martin S. Dendritic cell immunization route determines CD8+ T cell trafficking to inflamed skin: role for tissue microenvironment and dendritic cells in establishment of T cell-homing subsets. J. Immunol. 2004;172(2):857–863. doi: 10.4049/jimmunol.172.2.857. [DOI] [PubMed] [Google Scholar]
- 56.Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 2003;198(6):963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mora JR, Bono MR, Manjunath N, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424(6944):88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 58.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 2005;201(2):303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462(7272):510–513. doi: 10.1038/nature08511. ■■ Along with [61], demonstrates a role for activated CD4 T cells in recruiting effector CD8 T cells into tissues.
- 60.Groom JR, Luster AD. CXCR3 in T cell function. Exp. Cell Res. 2011;317(5):620–631. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marzo AL, Kinnear BF, Lake RA, et al. Tumor-specific CD4+ T cells have a major ‘post-licensing’ role in CTL mediated anti-tumor immunity. J. Immunol. 2000;165(11):6047–6055. doi: 10.4049/jimmunol.165.11.6047. ■ Along with [59], demonstrates a role for activated CD4 T cells in recruiting effector CD8 T cells into tissues.
- 62.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 2008;8(2):107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 63.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31(6):859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 65.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4(3):225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 66.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 67.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25(1):19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 68.Butler NS, Harty JT. The role of inflammation in the generation and maintenance of memory T cells. Adv. Exp. Med. Biol. 2010;684:42–56. doi: 10.1007/978-1-4419-6451-9_4. [DOI] [PubMed] [Google Scholar]
- 69.Ely KH, Cauley LS, Roberts AD, Brennan JW, Cookenham T, Woodland DL. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J. Immunol. 2003;170(3):1423–1429. doi: 10.4049/jimmunol.170.3.1423. ■ Along with [70] demonstrates antigen-independent localization of memory CD8 T cells to the lung following viral infection.
- 70.Kohlmeier JE, Miller SC, Smith J, et al. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29(1):101–113. doi: 10.1016/j.immuni.2008.05.011. ■ Along with [69] demonstrates antigen-independent localization of memory CD8 T cells to the lung following viral infection.
- 71.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33(1):96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20(5):551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 73.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009;10(5):524–530. doi: 10.1038/ni.1718. ■■ Along with [51], provides evidence of ‘resident memory’ CD8 T cells that persist long term in the skin and gut.
- 74.Wirth TC, Xue HH, Rai D, et al. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33(1):128–140. doi: 10.1016/j.immuni.2010.06.014. ■■ Along with [75,76], describes functional and phenotypic changes that occur when memory CD8 T cell populations undergo multiple antigenic stimulation.
- 75.Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J. Exp. Med. 2006;203(4):919–932. doi: 10.1084/jem.20052237. ■■ Along with [74,76], describes functional and phenotypic changes that occur when memory CD8 T cell populations undergo multiple antigenic stimulation.
- 76.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J. Immunol. 2006;177(2):831–839. doi: 10.4049/jimmunol.177.2.831. ■■ Along with [74,75], describes functional and phenotypic changes that occur when memory CD8 T cell populations undergo multiple antigenic stimulation.
- 77.Ray SJ, Franki SN, Pierce RH, et al. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20(2):167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- 78.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr. Opin Immunol. 2007;19(4):408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 79.Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 2007;204(4):941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003;77(8):4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He XS, Rehermann B, Lopez-Labrador FX, et al. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl Acad. Sci. USA. 1999;96(10):5692–5697. doi: 10.1073/pnas.96.10.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27(4):670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 83.Chen G, Shankar P, Lange C, et al. CD8 T cells specific for human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood. 2001;98(1):156–164. doi: 10.1182/blood.v98.1.156. [DOI] [PubMed] [Google Scholar]
- 84.Brainard DM, Tager AM, Misdraji J, et al. Decreased CXCR3+ CD8 T cells in advanced human immunodeficiency virus infection suggest that a homing defect contributes to cytotoxic T-lymphocyte dysfunction. J. Virol. 2007;81(16):8439–8450. doi: 10.1128/JVI.00199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]