Microscopic counts of microbial cells in deep sediment cores retrieved by scientific ocean drilling have revealed the largest living ecosystem on earth: the deep biosphere (1, 2). Hidden beneath the seafloor, a large part of all prokaryotic cells on earth persist under conditions so highly energy-limited that it seems to violate the constraints to life. This deep biosphere is the most understudied ecosystem, inhabited by organisms only distantly related to known laboratory strains. It is not even clear whether the cells are alive and metabolically active, or are dormant or perhaps dead. The report by Morono et al. in PNAS (3) attacks this very basic question with new convincing experiments.
The idea of Morono et al. has been that, even though the deep biosphere is highly energy-starved under natural conditions, the organisms may still have the capacity to feed very actively if served a rich and varied diet of organic substrates. The authors therefore performed experiments with sediment from several hundred meters’ depth in the seabed of the Japan Sea and fed the inhabiting microorganisms a mixture of stable isotope-labeled carbon and nitrogen compounds. After 2 mo, they extracted the cells from the mud and analyzed the increase in cell numbers and fraction of all cells that had taken up the isotopes, 13C or 15N. For this purpose, they used a nanometer-scale secondary ion mass spectrometer with which they could identify individual isotope-labeled cells and even determine how much of the substrates each cell had taken up and incorporated into its biomass.
The novelty of this study is not so much that it reveals the mode of life of these deep-living microorganisms in their natural environment. In that respect, the substrate concentrations offered to the cells were orders of magnitude too high compared with what these cells may experience in the million-year-old sediment in which they subsist. The novelty is the test of whether the deeply buried organisms maintain the potential to metabolize and grow, i.e., whether they are still alive and physiologically intact. Morono et al. (3) show that most, and perhaps all, of the cells are indeed alive. As many as 76% of the cells assimilated isotope-labeled substrate such as glucose or amino acids, some more than 1,000 times faster than the mean rates of organic carbon assimilation that are typical for the deep biosphere.
Why is this result so important? Is it really so hard to determine whether microorganisms are alive and active? To appreciate the challenge of the new study, it is necessary to envision life at an energy flux many orders of magnitude lower than anything studied in bacterial cultures in the laboratory so far. Deep subsurface sediments have ages of many millions of years. However, they are inhabited by highly diverse communities of microorganisms with densities of 103 to 108 cells/cm3 (1, 4). These microorganisms have as their main food the remains of organic material that sank out of the paleo-ocean in the geological past, became buried deep down into the seabed, and has been slowly degraded ever since at steadily decreasing rates. It is truly surprising that sufficient organic material still remains for the organisms to feed on and for the large microbial communities to be maintained.
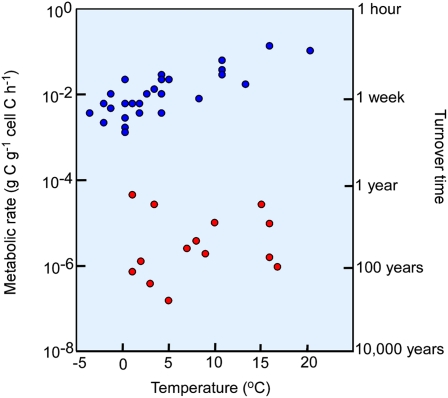
The explanation is that deep life is able to proceed in extreme slow motion. This was illustrated by Price and Sowers (5), who compiled data from a wide range of environments. A typical metabolic rate of microorganisms in ecosystems on the surface of our planet, such as soil, lake water, or seawater, is 0.1 to 10 fmol C⋅cell−1⋅d−1, corresponding to 10−3 to 10−1 g C metabolized per gram cell C per hour (Fig. 1). The mean metabolic rate for deep subsurface bacteria is typically four orders of magnitude lower: 10−5 to 10−3 fmol C⋅cell−1⋅d−1 (6, 7), corresponding to 10−7 to 10−5 g C⋅g−1 cell C⋅h−1. Such numbers are calculated by counting all the microorganisms in a deep sediment core and dividing by the rate at which the main metabolic substrates or products are turning over in the bulk sediment. The process rates are determined from transport-reaction models of pore-water constituents or from direct experimental process measurements using sensitive radiotracer methods. The rationale for taking a mean of the entire microbial community is the assumption that most of the cells are actively engaged in the energy metabolism. This assumption is now strongly supported by the findings of Morono et al. (3).
Fig. 1.
Metabolic rates and turnover times of natural communities of microorganisms. Blue indicates nutrient-rich environments such as soil, lake water, or seawater. Red indicates nutrient-starved environments such as subsurface sediments. Left axis shows metabolized organic carbon per cell carbon per unit time. Right axis shows the corresponding turnover time of cell carbon, approximately corresponding to minimum potential generation times. Data are redrawn from Price and Sowers (5) and supplemented with unpublished deep subseafloor data.
A cell must metabolize a certain amount of carbon relative to its own biomass before it can double its cell size and divide. This relationship is called the growth yield and has limited variability among microorganisms of similar physiological traits. If we now assume a growth yield typical of a laboratory culture of anaerobic bacteria, then we can ask about the minimum generation time possible at a metabolic rate of 10−5 fmol C⋅cell−1⋅d−1. The answer is that the average cell may divide once in 1,000 y. Such a slow growth means relatively more energy spent on maintenance metabolism, however, so the growth yield is probably lower than for laboratory cultures and the potential generation time therefore as long as several thousand years.
This longevity is such an enigmatic property of the deep biosphere that it is difficult to reconcile with our current understanding of microbial life. However, there are no biological constraints violated. There are no mechanisms of aging known in prokaryotes. If the mortality of a population is near zero because of the absence of predators, the cells need not divide and multiply to maintain a steady-state community size. In the absence of cell division in a sparse community packed in fine-porous clay, virus attack is probably also insignificant for cell death. The calculated mean rates of carbon metabolism do not translate into growth and cell division in a simple manner. The microorganisms could theoretically be turning over their biomass slowly without ever dividing. Therefore, turnover time rather than generation time is indicated in Fig. 1. In the absence of mortality, the size of the microbial community may in the past have grown in size until the cellular rate of metabolism decreased to the bare minimum required for maintenance. At that stage, there may be insufficient energy for growth, and the community size is balanced against the ambient energy flux.
An alternative scenario could be that most of the deeply buried microorganisms are in a dormant state (8) and that only few cells are metabolically active and growing. Morono et al. (3) show that, if they were dormant, they certainly wake up when they have been fed. Let us assume, hypothetically, that only 1% of the cells are active at any given time whereas the rest are dormant and can persist for very long periods without a vegetative stage. This would imply that the small active fraction has a potential generation time of 1 y, which is still longer than reflected by most data calculated for the more active surface layers of the seabed (Fig. 1). The remaining inactive cells, however, would need to maintain cell integrity in dormancy for a correspondingly much longer time, maybe 1 million years.
There are only few studies available to help us distinguish between these possibilities. There are reports that intact bacterial cells and their genome may survive for millions of years in deep marine sediments (9). For the oldest records, the cells are apparently in a dormant stage, such as spores that are highly resistant to the destructive effect of natural radiation (10). Dormancy, however, does not completely arrest the slow degradation of DNA and of other vital cell components as a result of spontaneous chemical or radiolytic reactions. Over geological timescales, dormancy may therefore not be an optimal survival strategy. In fact, there is evidence from Siberian permafrost soil that, over hundreds of thousands of years, the maintenance of a low metabolic level that
Intact bacterial cells and their genome may survive for millions of years in deep marine sediments.
enables continuous DNA repair is superior to long-term dormancy for survival (11). On this background, reports that microbial cells and DNA may be preserved for millions of years are an even greater challenge to our understanding of longevity than the mean generation times calculated here.
There is other evidence to show that dormancy is probably not a general explanation for the extremely low calculated metabolic rates. Most deep subsurface cells can be hybridized and stained by fluorescent oligonucleotide probes (i.e., FISH) that bind to ribosomal RNA, thereby showing that the majority of the cells have ribosomes in addition to cell walls that can be penetrated by molecular probes (12, 13). True resting stages such as bacterial endospores may indeed occur in high numbers, but these are not included in fluorescent cell counts or FISH and have not been considered in the energy calculations described earlier. Furthermore, there is a positive correlation between the cell densities in subseafloor sediments and the energy flux available, which indicates that a universal minimum cellular energy flux ultimately regulates the size of the deep microbial communities (14).
Further research is needed to clarify what may be the physiological or biochemical traits that enable subsurface microorganisms to subsist under such low energy flux. It is interesting that, although bacteria are the predominant prokaryotic domain of life in most environments on the surface of earth, the other prokaryotic domain, the archaea, are very common and even seem to rule the deep subsurface (13, 15). The archaea have a selective advantage in that their cell membrane is much less permeable toward passive diffusion of protons or other ions (16). This may enable the archaea to generate an energized membrane with a 100-fold lower energy loss than the bacteria. Morono et al. (3) applied FISH probes in an attempt to identify the actively metabolizing archaea. Most isotope-labeled cells, however, did not hybridize with common FISH probes. This probably reflects a combination of the methodological difficulty of this technique and the largely uncharacterized phylogenic diversity of deep subseafloor archaea (17).
Deep biosphere research is still a young science with exciting challenges ahead.
Acknowledgments
This work was supported by the Danish National Research Foundation and the German Max Planck Society.
Footnotes
The author declares no conflict of interest.
See companion article on page 18295.
References
- 1.Parkes RJ, Cragg BA, Wellsbury P. Recent studies on bacterial populations and processes in subseafloor sediments: A review. Hydrogeol J. 2000;8:11–28. [Google Scholar]
- 2.Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: The unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morono Y, et al. Carbon and nitrogen assimilation in deep subseafloor microbial cells. Proc Natl Acad Sci USA. 2011;108:18295–18300. doi: 10.1073/pnas.1107763108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Hondt S, et al. Subseafloor sedimentary life in the South Pacific Gyre. Proc Natl Acad Sci USA. 2009;106:11651–11656. doi: 10.1073/pnas.0811793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price PB, Sowers T. Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc Natl Acad Sci USA. 2004;101:4631–4636. doi: 10.1073/pnas.0400522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jørgensen BB, D'Hondt S. Ecology. A starving majority deep beneath the seafloor. Science. 2006;314:932–934. doi: 10.1126/science.1133796. [DOI] [PubMed] [Google Scholar]
- 7.D'Hondt S, Rutherford S, Spivack AJ. Metabolic activity of subsurface life in deep-sea sediments. Science. 2002;295:2067–2070. doi: 10.1126/science.1064878. [DOI] [PubMed] [Google Scholar]
- 8.Lennon JT, Jones SE. Microbial seed banks: The ecological and evolutionary implications of dormancy. Nat Rev Microbiol. 2011;9:119–130. doi: 10.1038/nrmicro2504. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki F, Okada H, Tsapin AI, Nealson KH. Microbial survival: The paleome: A sedimentary genetic record of past microbial communities. Astrobiology. 2005;5:141–153. doi: 10.1089/ast.2005.5.141. [DOI] [PubMed] [Google Scholar]
- 10.Kminek G, Bada JL, Pogliano K, Ward JF. Radiation-dependent limit for the viability of bacterial spores in halite fluid inclusions and on Mars. Radiat Res. 2003;159:722–729. doi: 10.1667/0033-7587(2003)159[0722:rlftvo]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson SS, et al. Ancient bacteria show evidence of DNA repair. Proc Natl Acad Sci USA. 2007;104:14401–14405. doi: 10.1073/pnas.0706787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schippers A, et al. Prokaryotic cells of the deep sub-seafloor biosphere identified as living bacteria. Nature. 2005;433:861–864. doi: 10.1038/nature03302. [DOI] [PubMed] [Google Scholar]
- 13.Biddle JF, et al. Heterotrophic Archaea dominate sedimentary subsurface ecosystems off Peru. Proc Natl Acad Sci USA. 2006;103:3846–3851. doi: 10.1073/pnas.0600035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parkes RJ, et al. Deep sub-seafloor prokaryotes stimulated at interfaces over geological time. Nature. 2005;436:390–394. doi: 10.1038/nature03796. [DOI] [PubMed] [Google Scholar]
- 15.Lipp JS, Morono Y, Inagaki F, Hinrichs KU. Significant contribution of Archaea to extant biomass in marine subsurface sediments. Nature. 2008;454:991–994. doi: 10.1038/nature07174. [DOI] [PubMed] [Google Scholar]
- 16.van de Vossenberg JLCM, Ubbink-Kok T, Elferink MGL, Driessen AJM, Konings WN. Ion permeability of the cytoplasmic membrane limits the maximum growth temperature of bacteria and archaea. Mol Microbiol. 1995;18:925–932. doi: 10.1111/j.1365-2958.1995.18050925.x. [DOI] [PubMed] [Google Scholar]
- 17.Teske A, Sørensen KB. Uncultured archaea in deep marine subsurface sediments: Have we caught them all? ISME J. 2008;2:3–18. doi: 10.1038/ismej.2007.90. [DOI] [PubMed] [Google Scholar]