Abstract
Natural killer (NK) cells contribute to not only innate but also to adaptive immunity by interacting with dendritic cells (DCs) and T cells. All activated human NK cells express HLA-DR and can initiate MHCII-dependent CD4+ T-cell proliferation; however, the expression of MHCII by mouse NK cells and its functional significance are controversial. In this study, we show that NK–DC interactions result in the emergence of MHCII-positive NK cells. Upon in vitro or in vivo activation, mouse conventional NK cells did not induce MHCII transcripts, but rapidly acquired MHCII protein from DCs. MHCII H2-Ab1–deficient NK cells turned I-Ab-positive when adoptively transferred into wild-type mice or when cultured with WT splenic DCs. NK acquisition of MHCII was mediated by intercellular membrane transfer called “trogocytosis,” but not upon DAP10/12- and MHCI-binding NK cell receptor signaling. MHCII-dressed NK cells concurrently acquired costimulatory molecules such as CD80 and CD86 from DCs; however, their expression did not reach functional levels. Therefore, MHCII-dressed NK cells inhibited DC-induced CD4+ T-cell responses rather than activated CD4+ T cells by competitive antigen presentation. In a mouse model for delayed-type hypersensitivity, adoptive transfer of MHCII-dressed NK cells attenuated footpad swelling. These results suggest that MHCII-dressed NK cells generated through NK–DC interactions regulate T cell-mediated immune responses.
Natural killer (NK) cells have long been known to play important roles in innate immunity, but recently their contributions to adaptive immunity have also been reported (1). Following immunization or infection, NK cells migrate to draining lymphoid organs, where they interact with dendritic cells (DCs) and/or T cells (2). By production of cytokines such as IFN-γ and TNF-α and direct cell–cell contact, NK cells activate DCs to induce T-cell proliferation and differentiation (3, 4). However, NK cells can also negatively regulate adaptive immune responses (2, 5). For example, in mouse models of autoimmune diseases such as rheumatoid arthritis and experimental autoimmune encephalomyelitis, depletion of NK cells by anti-NK1.1 mAb exacerbates these diseases (5). However, the molecular mechanisms underlying negative immune regulation by NK cells are poorly understood (5). Although conventional mouse NK cells do not express MHCII, subpopulations of activated mouse NK cells have been found to express MHCII (6–9), suggesting that NK cells may directly regulate CD4+ T-cell responses. Of note, activated human NK cells express HLA-DR and can induce MHCII-dependent CD4+ T-cell proliferation (6, 10–13).
MHCII molecules are crucial for the presentation of peptides processed from extracellular proteins to CD4+ T cells, and they shape T-cell receptor repertoire development during T-cell maturation and lineage commitment. The constitutive expression of MHCII is restricted to professional antigen-presenting cells (APCs) such as DCs, macrophages, and B cells (14). In addition to professional APCs, basophils also express MHCII and play a crucial role as APCs (15). MHCII expression is transcriptionally regulated by the class II transactivator (CIITA) in APCs, including basophils (14, 15). However, the regulation of MHCII expression in murine NK cells and the mechanism by which MHCII+ NK cells are generated remain unclear.
A wide variety of immune cell lineages communicate with each other through direct cell–cell contact or cytokine production to establish appropriate immune responses. Several recent studies have shown that during direct cell–cell interactions, plasma membrane fragments of one cell are transferred to the opposite cell (16, 17). This phenomenon is currently called “trogocytosis” (17, 18), and it may generate novel cell populations that result from the interaction between two different types of immune cells. Indeed, Wakim and Bevan have recently reported that DC–DC interactions generate a novel DC subset, called “cross-dressed” DCs, which acquire peptide–MHCI complexes from donor DCs to drive memory CD8+ T-cell activation (19). Therefore, immune responses can be regulated not only by lineage-committed cell populations but also by cell populations generated independently of transcription, by trogocytosis. Whether NK–DC interactions produce novel cell populations in a similar manner has not been explored. Therefore, we investigated whether MHCII-positive NK cells could be generated through interactions between mouse conventional NK cells and splenic DCs, and asked whether the resulting MHCII-positive NK cells could regulate CD4+ T-cell responses.
Results
Activated NK Cells Express MHCII Protein, Although Not the Transcript, in Vivo.
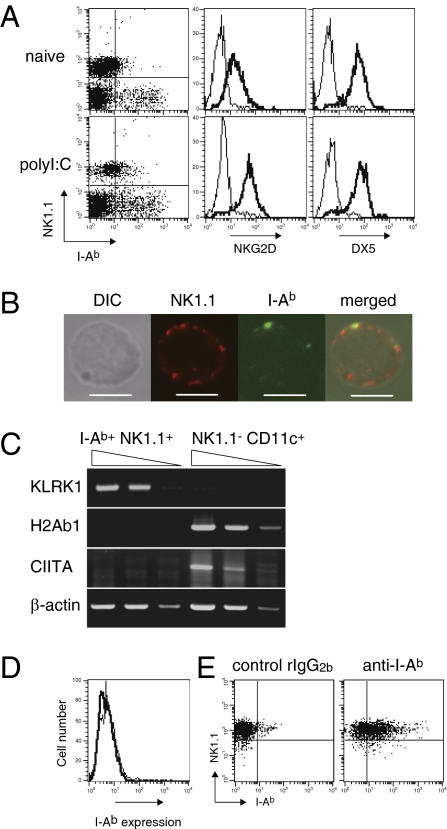
MHCII expression has been observed on human NK cells prepared from mixed lymphocyte cultures or pathogen-infected organs, suggesting that activated NK cells induce MHCII (10, 11). Consistent with these reports, we observed MHCII I-Ab expression on splenic NK1.1+ cells in C57BL/6 mice injected i.v. with the double-stranded RNA synthetic analog polyI:C, but not in naïve mice (Fig. 1A). These NK1.1+ cells also expressed NKG2D and DX5 (Fig. 1A), indicating that these cells were conventional NK cells. Expression levels of MHCII on splenic B cells and T cells were not altered by polyI:C administration (Fig. S1). To confirm that I-Ab+ NK1.1+ cells were a single NK cell population and not merely conjugates of NK cells and I-Ab+ cells such as DCs, we sort-purified these cells and analyzed them by confocal microscopy. Fig. 1B shows that NK1.1+ cells per se express I-Ab on their cell surface.
Fig. 1.
Activated NK cells express MHCII protein but not the transcript in vivo. (A) NK1.1 and I-Ab expression on a non-T/B-cell population in spleen from naive or polyI:C (100 μg per mouse)-treated mice was analyzed (Left). Expression of NKG2D (Center) and DX5 (Right) on NK1.1+ cells from splenocytes was analyzed using isotype control mAbs (thin lines) and specific mAbs (thick lines). (B) Purified I-Ab+ DX5+ cells were stained by AF488-anti–I-Ab mAb and biotinylated anti-NK1.1 mAb, followed by DyLight 594-streptavidin. (Scale bars, 5 μm.) (C) Expression of the indicated transcripts in purified splenic I-Ab+ NK1.1+ cells or NK1.1− CD11c+ cells was analyzed by semiquantitative RT-PCR using 10-fold serially diluted cDNA templates. (D) Splenic NK cells were purified and then cultured with IL-2 (1,000 U/mL) for 5 d, and then stained with control rat IgG2b (thin line) or anti–I-Ab mAb (thick line). (E) NK cells cultured as described in D were labeled with CFSE and then transferred into mice. The following day, I-Ab and NK1.1 expression level on CFSE+ cells in spleen was analyzed. Similar results were obtained in three (A, D, and E) or two (B and C) independent experiments.
To explore whether I-Ab expression on NK cells depended on transcriptional regulation, we next performed semiquantitative PCR on mRNA from sort-purified NK cells. Unexpectedly, we observed neither MHCII H2-Ab1 transcript nor the transactivator CIITA transcript in I-Ab+ NK1.1+ cells, whereas we did detect the Klrk1 gene transcript that encodes NKG2D protein in these cells (Fig. 1C). Thus, it appeared that the expression of MHCII protein on NK cells occurred independently of transcriptional regulation in vivo.
We next addressed whether activated NK cells express MHCII in vitro. We purified conventional NK cells from naïve mouse spleens and cultured them with IL-2 for 5 d. Unexpectedly, these conventional NK cells remained MHCII-negative in vitro (Fig. 1D). Interestingly, when we labeled the cells with 5- (and 6-) carboxyfluorescein diacetate succinimidyl ester (CFSE) and transferred them into naïve mice, these NK cells turned MHCII-positive in the spleens of recipient mice (Fig. 1E). Nevertheless, we were unable to detect H2-Ab1 or CIITA transcripts in transferred I-Ab+ NK cells purified from the spleens of recipient mice (Fig. S2). Thus, our findings suggest that activated NK cells become MHCII-positive in vivo, and do so by transcription-independent mechanisms.
Activated NK Cells Acquire MHCII from DCs Through NK–DC Interaction.
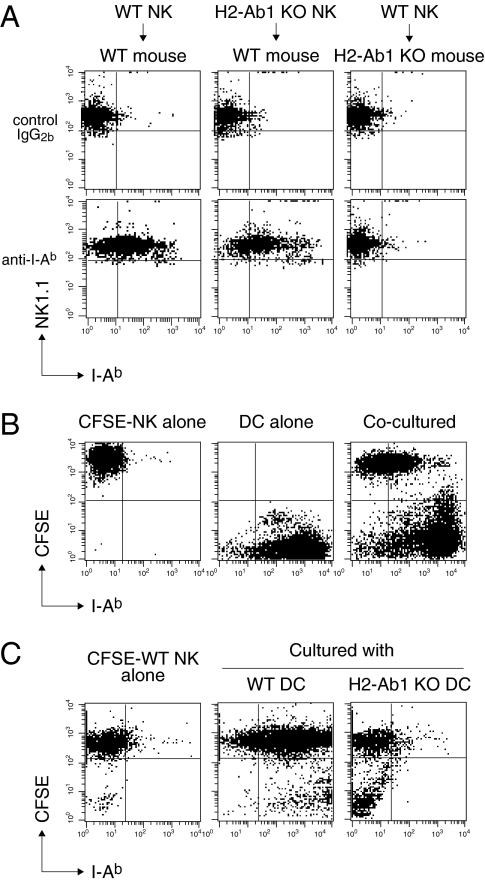
To further elucidate the pathway for MHCII+ NK cell generation, we next adoptively transferred IL-2-activated MHCII H2-Ab1 gene-deficient NK cells into wild-type (WT) mice. Surprisingly, we found that MHCII was expressed at high levels on H2-Ab1–deficient NK cells in WT mouse spleens within 1 d of transfer (Fig. 2A). In contrast, WT NK cells transferred into H2-Ab1–deficient mice remained MHCII-negative (Fig. 2A). Given that activated NK cells interact with DCs in vivo (2), we hypothesized that NK cells acquire MHCII from DCs. To address this possibility, we cocultured CFSE-labeled NK cells and splenic DCs at a 1:1 ratio. We observed that IL-2-activated NK cells turned MHCII-positive within 1 h of coculture (Fig. 2B). In contrast, IL-2-activated WT NK cells remained MHCII-negative after coculture with H2-Ab1–deficient DCs (Fig. 2C), providing evidence that NK cells acquire MHCII from WT DCs. The acquired MHCII protein remained on NK cells for at least 12 h after removal of DCs (Fig. S3A). Freshly isolated splenic NK cells did not acquire MHCII protein from DCs effectively (Fig. S3B), and thus NK cells gain this ability once activated.
Fig. 2.
Activated NK cells acquire MHCII from DCs. (A) WT or H2-Ab1–deficient NK cells prepared as described in Fig. 1E were adoptively transferred into WT or H2-Ab1–deficient mice. The following day, NK1.1 and I-Ab expression level on CFSE+ cells in spleen was analyzed. (B) CFSE-labeled NK cells prepared as described in Fig. 1D were cocultured with splenic CD11c+ cells at a 1:1 ratio for 1 h. I-Ab expression on these cells was analyzed. (C) CFSE-labeled WT NK cells were cocultured with WT or H2-Ab1–deficient splenic DCs, and I-Ab expression on these cells was analyzed as described in B. Similar results were obtained in three independent experiments.
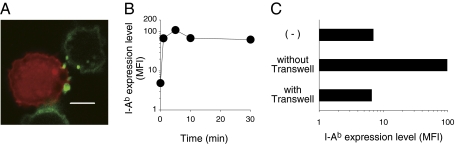
Intercellular protein transfer between immune cells is mediated by several pathways, including membrane nanotubes (transient long-distance connections), trogocytosis (a rapid, cell contact-dependent transfer of membrane fragment), and exosomes (secreted membrane nanovesicles of <100 nm) (16–18, 20). By confocal microscopy, we could not observe nanotubes between NK cells and DCs, but we found I-Ab-containing membrane fragments (∼1 μm) from DCs on NK cells (Fig. 3A). Although DCs have been reported to secrete MHCII-containing exosomes (20), we did not detect exosomes in culture supernatants until 12 h after incubation (Fig. S4). In contrast, MHCII-containing membrane fragment transfer occurred within minutes (Fig. 3B). Furthermore, MHCII acquisition was completely abrogated by culturing NK cells and DCs in transwell plates (Fig. 3C), indicating that acquisition was dependent upon cell–cell contact. These results are consistent with the transfer of MHCII being mediated through trogocytosis rather than exosome secretion.
Fig. 3.
Intercellular MHCII transfer is mediated by trogocytosis. (A) TAMRA-labeled NK cells cocultured with DCs were stained with AF488-anti–I-Ab mAb and analyzed by confocal microscopy. (Scale bar, 5 μm.) (B) CFSE-labeled NK cells were cocultured with DCs as described in Fig. 2B for the indicated periods of time. The mean fluorescence intensity (MFI) of I-Ab expression on NK cells was analyzed by flow cytometry. (C) CFSE-labeled NK cells were cocultured with DCs for 1 h together (no transwell) or separated by a semipermeable membrane (transwell). The MFI of I-Ab expression on NK cells was analyzed by flow cytometry. Similar results were obtained in three (A and B) and two (C) independent experiments.
Trogocytosis is generally thought to be triggered by receptor signaling, and it has been reported that NK cells acquire MHCI and MHCI-related chain B from target cells by using NK cell receptors (NKRs) (21–23). Therefore, we conducted further studies to determine whether NKR signaling is required for MHCII acquisition. However, DAP10/12-deficient NK cells as well as WT NK cells acquired MHCII from DCs (Fig. S5A). WT NK cells also acquired MHCII from β2-microglobulin–deficient DCs that lacked cell surface expression of MHCI, indicating that MHCI-binding NKRs are not involved in this process (Fig. S5A). Consistent with these results, MHCII acquisition was not inhibited by an inhibitor of Syk family kinase, PI3K, or Src kinase (Fig. S5B). Neither did we observe the involvement of NK effector molecules such as perforin, IFN-γ, FasL, or TRAIL (24) in this process (Fig. S5). Interestingly, pretreatment of NK cells or DCs with cytochalasin D, an inhibitor of actin polymerization, or sodium azide, which depletes intracellular ATP, substantially inhibited MHCII transfer (Fig. S5B). Fixation of either NK cells or DCs with paraformaldehyde completely inhibited MHCII transfer (Fig. S5B). Collectively, these results suggest that activated NK cells acquire MHCII-containing DC membranes through a process that is dependent on plasma membrane and actin cytoskeleton interactions, but not NKR signaling or NK effector molecules.
MHCII-Dressed NK Cells Negatively Regulate CD4+ T-Cell Proliferation.
To explore whether MHCII-dressed NK cells could act as APCs to stimulate naïve T cells, we examined whether NK cells could also acquire costimulatory molecules from DCs by an in vitro coculture assay. Activated NK cells acquired CD80 and CD86, but the levels of these molecules on NK cells were minimal (Fig. S6).
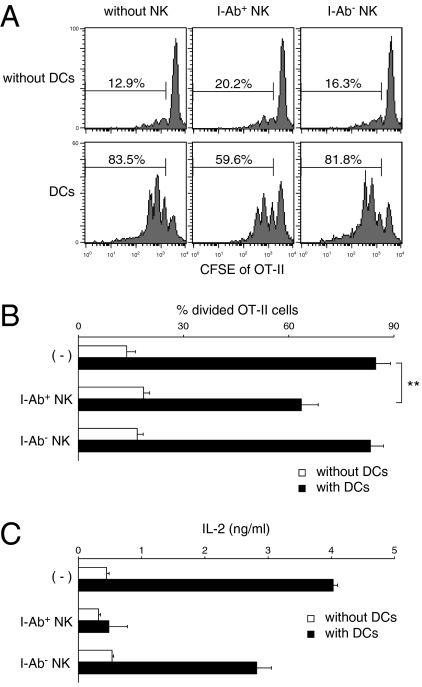
We next examined whether MHCII-dressed NK cells could influence CD4+ T-cell responses. We performed an antigen-presentation study using OT-II CD4+ T cells specific for OVA323–339/I-Ab. After coculture with WT or H2-Ab1–deficient DCs, I-Ab-dressed (I-Ab+) or I-Ab-negative (I-Ab−) NK cells were sort-purified. Purified I-Ab+ NK cells did not induce naïve OT-II CD4+ T-cell proliferation (Fig. 4 A and B), possibly due to insufficient expression of costimulatory molecules (Fig. S6). Interestingly, purified I-Ab+ NK cells suppressed OT-II CD4+ T-cell proliferation induced by DCs, whereas I-Ab– NK cells cocultured with H2-Ab1–deficient DCs did not (Fig. 4 A and B). Moreover, I-Ab+ NK cells suppressed IL-2 production from OT-II CD4+ T cells more effectively than I-Ab− NK cells (Fig. 4C). These results indicate that MHCII-dressed NK cells regulate CD4+ T-cell responses to DCs via antigen presentation on MHCII without sufficient costimulation.
Fig. 4.
MHCII+ NK cells suppress CD4+ T-cell proliferation induced by DCs. (A) NK cells were purified by cell sorting after coculture with WT DCs (I-Ab+ NK) or H2-Ab1–deficient DCs (I-Ab– NK). CFSE-labeled OT-II CD4+ T cells were cocultured with purified NK cells at a 1:10 ratio (NK:T), DCs at a 1:10 ratio (DC:T), or NK cells and DCs at a 1:1:10 ratio (NK:DC:T) in the presence of OVA323–329 peptides (10 ng/mL) for 3 d. The CFSE intensity of OT-II CD4+ cells was then analyzed. The percentages of divided OT-II cells are shown as means ± SD of triplicates in B (**P < 0.01 compared with DCs alone). (C) Production of IL-2 in the culture supernatants at 48 h after coculture was measured by ELISA. Similar results were obtained in three independent experiments.
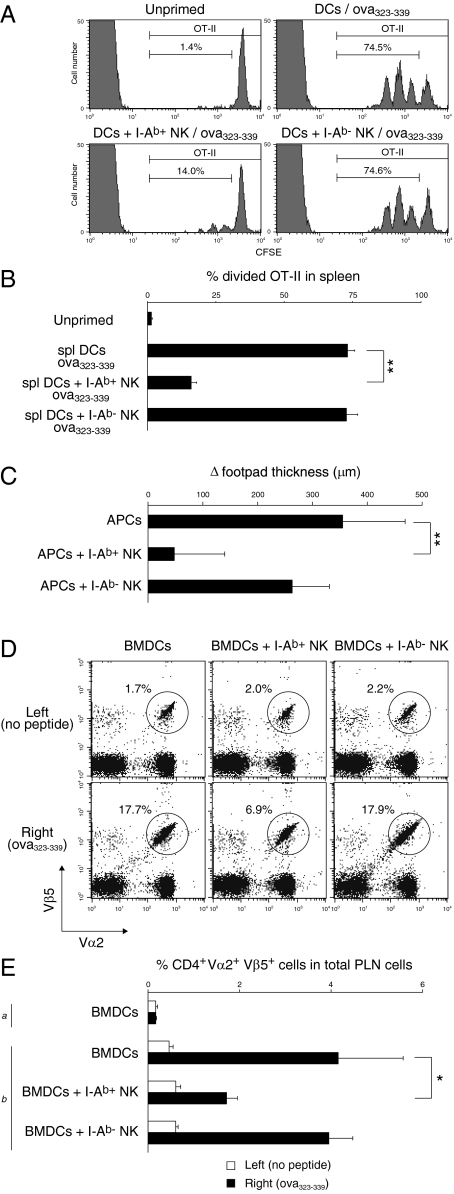
Furthermore, we addressed whether I-Ab+ NK cells could suppress CD4+ T-cell responses in vivo. Interestingly, I-Ab+ NK cells significantly suppressed OT-II CD4+ T-cell proliferation induced by DCs loaded with OVA323–339 peptides in spleen, although I-Ab− NK cells did not affect the proliferation (Fig. 5 A and B). In a mouse model for delayed-type hypersensitivity (DTH) where activated OT-II CD4+ T cells were i.v. transferred followed by s.c. injection with OVA323–339-loaded APCs, I-Ab+ NK cells attenuated footpad swelling (Fig. 5C) and reduced OT-II CD4+ T-cell accumulation in the draining popliteal lymph nodes (PLNs) (Fig. 5 D and E). Taken together, these results suggest that MHCII-dressed NK cells suppress CD4+ T-cell responses in vivo.
Fig. 5.
MHCII+ NK cells suppress CD4+ T-cell responses in vivo. (A) B6 mice (n = 3) adoptively transferred with CFSE-labeled OT-II CD4+ T cells were i.v. injected with OVA323–339-loaded DCs or a mixture of these DCs and NK cells precultured with DCs together (I-Ab+ NK cells) or separated (I-Ab− NK cells) as described in Fig. 3C. Two days later, CFSE intensity of CD4+ Vα2+ OT-II cells in spleen was analyzed. The percentage of divided OT-II cells is shown in B. B6 mice [n = 4 (C) or n = 3 (D and E)] adoptively transferred with activated OT-II CD4+ T cells were s.c. injected with OVA323–339-loaded APCs or a mixture of these APCs and I-Ab+ NK or I-Ab− NK cells into the right footpads. The left footpads were injected with the same cell population without OVA323–339 as controls. The following day, Δfootpad thickness was calculated by subtracting the left hind footpad thickness from the right (C). Three days after the s.c. injection, the percentage of Vα2+ Vβ5+ cells in total CD4+ T cells in PLNs was analyzed (D) and the percentage of CD4+ Vα2+ Vβ5+ cells in total PLN cells is calculated in E. (a) Naive mice. (b) OT-II CD4+ T-cell transferred mice. *P < 0.05, **P < 0.01 compared with APCs alone. Similar results were obtained in two (A and B) or three (C and D) independent experiments. BMDCs, bone marrow-derived DCs.
Discussion
In this study, we provide evidence that conventional murine NK cells do not transcriptionally induce MHCII but instead rapidly acquire MHCII protein from DCs through NK–DC interactions. These MHCII-dressed NK cells suppress CD4+ T-cell responses to DCs by presenting antigen–MHCII complexes without sufficient costimulation, which might induce anergy in CD4+ T cells. In addition, adoptive transfer of MHCII-dressed NK cells attenuated dermal DTH. Therefore, our findings may provide a mechanistic explanation for the negative immune regulation of T-cell immunity by NK cells.
Several recent studies have identified NK/DC hybrid-phenotype cells, which have functional properties characteristic of both NK cells and DCs (11, 25–28). IFN-producing killer DCs (IKDCs), also called B220+ NK1.1+ DCs, were identified as a novel DC subset harboring killer activity (27, 28). On the contrary, more recent studies have proposed that these killer DCs are functionally and developmentally activated NK cells (7–9). It remains unclear whether the MHCII-dressed NK cells we describe here are identical to the NK/DC hybrid-phenotype cells described in previous studies (25–28), although at least IKDCs and MHCII-dressed NK cells have similar antigenic phenotypes: CD11c+ B220+ MHCII+ NKG2D+ CD86dull+ Gr1− (Fig. S7). Interestingly, in a mouse model of type 1 diabetes, CD11c+ DX5+ cells, which are functionally and phenotypically similar to MHCII-dressed NK cells, were found to negatively regulate pathogenic T-cell activation (25). Of note, we observed that a small population of DCs cocultured with IL-2-activated NK cells became Ly49G2-positive (Fig. S8), suggesting that DCs could also acquire NK cell surface proteins. Although this observation might also account for the generation of NK/DC hybrid-phenotype cells described in previous studies (25, 26), further studies will be necessary to characterize these hybrid-phenotype cells.
In addition, we observed that activated murine NK cells acquired MHCII from B cells in coculture experiments. However, the acquisition level of MHCII on these NK cells was lower than that on NK cells cocultured with DCs (Fig. S9), suggesting that activated NK cells preferentially acquired MHCII from DCs.
In contrast to mouse NK cells, after activation, all human NK cells synthesize HLA-DR as well as costimulatory molecules including CD80, CD86, and OX40 ligand (6, 11, 12). Unlike in humans, activation of mouse NK cells apparently does not induce the endogenous expression of MHCII.
Given that many cell types store a large excess of membrane on their cell surface (16), intercellular membrane transfer might occur frequently during immune cell–cell interactions. Recently, DCs have been reported to acquire peptide–MHCI complexes from distinct donor DCs and subsequently drive memory CD8+ T-cell activation (19). T cells have also been reported to acquire CD80 and CD86 proteins from DCs by CTLA-4, thereby down-modulating the delivery of costimulatory signals (29). Our findings show that activated NK cells can acquire MHCII from DCs and regulate T-cell immune responses in vitro and in vivo. Taken together, it is possible that immune cells acquire additional functions and/or alter their intrinsic functions through intercellular transfer of immune regulatory molecules such as MHCII in lymphoid organs. Such newly generated cell populations could play important roles in the regulation of immune responses through their effects on other cell types.
Materials and Methods
Further details are available in SI Materials and Methods.
Mice.
C57BL/6 mice were obtained from CLEA Japan. MHCII H2-Ab1–deficient mice (30) were kindly provided by D. Mathis (Harvard Medical School, Boston, MA). OT-II transgenic/rag-1 knockout mice were obtained from Taconic. These mice were maintained under specific pathogen-free conditions, and used according to the guidelines of the Institutional Animal Care and Use Committee established at Tohoku University.
RT-PCR.
Total RNA was purified from cells using Sepasol (Nacalai). Complementary DNAs were synthesized from total RNAs by using oligo(dT) primer (Invitrogen). PCR was performed by using 10-fold serially diluted cDNA templates, AmpliTaq poly (Applied Biosystems), and primers listed in SI Materials and Methods.
NK–DC Interaction.
Mouse splenic NK cells and DCs were prepared as described previously (31, 32). CFSE (0.5 μM)-labeled IL-2 (1,000 U/mL)-activated NK cells (5 × 105 per well) and splenic DCs (5 × 105 per well) were cocultured in a 96-well flat-bottom plate for the indicated periods at 37 °C. Then cells were stained with APC-labeled anti–I-Ab mAb (BioLegend) and analyzed on a FACSCanto II (BD Biosciences).
Confocal Microscopy.
Cells were stained with 5- (and 6-) carboxytetramethylrhodamine succinimidyl ester (TAMRA; Invitrogen) or the following mAbs: AF488-anti–I-Ab, biotinylated anti-NK1.1 mAbs, and streptavidin-DyLight 594 (BioLegend). Then these cells were analyzed on a Carl Zeiss LSM510 confocal laser-scanning microscope equipped with a 63× objective lens as described previously (33).
In Vitro Antigen Presentation Assay.
The antigen presentation assay was performed as described previously (32) with some modifications. Bone marrow-derived DCs (5 × 103 per well) and/or sort-purified NK cells (5 × 103 per well) were cocultured with CFSE (10 μM)-labeled OT-II CD4+ T cells (5 × 104 per well) in a 96-well U-bottom plate for 3 d in the presence of OVA323–339 peptides (10 ng/mL; Abgent). CFSE fluorescence intensity of OT-II CD4+ T cells was analyzed by flow cytometry. Production of IL-2 in the culture supernatant at 48 h postaddition of OT-II CD4+ T cells was measured by ELISA (eBioscience).
In Vivo OT-II Proliferation Assay.
CFSE-labeled OT-II CD4+ T cells (3 × 106 per mouse) were adoptively transferred into B6 mice. The following day, mice were i.v. injected with OVA323–339 (1 μg/mL)-loaded splenic DCs (3 × 106 per mouse) or a mixture of these DCs (3 × 106 per mouse) and NK cells (3 × 106 per mouse). Two days later, mice were killed, and CFSE dilution of CD4+ Vα2+ splenocytes was analyzed by flow cytometry.
Dermal DTH.
B6 mice adoptively transferred with activated OT-II CD4+ T cells (6 × 106 per mouse) were s.c. injected with 50 μL of OVA323–339-loaded APCs (2 × 107 per mouse) or a mixture of these APCs (2 × 107 per mouse) and NK cells (1 × 107 per mouse) into the right footpad. The left footpads were injected with the same cell population without OVA323–339 as controls. Footpad thickness and infiltration of OT-II cells into PLNs were analyzed.
Supplementary Material
Acknowledgments
We thank Hiromi Yoshida for cell sorting; Shota Endo and Hisaya Akiba for advice on OT-II experiments; and Chika Takahashi, Misato Tsugita, and Kazusa Ishizaki for technical assistance. This work was supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (to M.N., K.T., and K.O.); by Grants-in-Aid for Scientific Research from the Ministry of Health, Labour and Welfare of Japan H22-meneki-ippan-004 (to K.O.), and H22-meneki-ippan-005 (to M.N.); by a grant from the Takeda Science Foundation (to M.N.); and by a grant from the Mochia Memorial Foundation for Medical and Pharmaceutical Research (to M.N.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110584108/-/DCSupplemental.
References
- 1.Vivier E, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Zitvogel L. Dendritic and natural killer cells cooperate in the control/switch of innate immunity. J Exp Med. 2002;195(3):F9–F14. doi: 10.1084/jem.20012040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25(1):47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Shi FD, Van Kaer L. Reciprocal regulation between natural killer cells and autoreactive T cells. Nat Rev Immunol. 2006;6:751–760. doi: 10.1038/nri1935. [DOI] [PubMed] [Google Scholar]
- 6.Spits H, Lanier LL. Natural killer or dendritic: What's in a name? Immunity. 2007;26(1):11–16. doi: 10.1016/j.immuni.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Blasius AL, Barchet W, Cella M, Colonna M. Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells. J Exp Med. 2007;204:2561–2568. doi: 10.1084/jem.20070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vosshenrich CA, et al. CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. J Exp Med. 2007;204:2569–2578. doi: 10.1084/jem.20071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caminschi I, et al. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. J Exp Med. 2007;204:2579–2590. doi: 10.1084/jem.20071351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips JH, Le AM, Lanier LL. Natural killer cells activated in a human mixed lymphocyte response culture identified by expression of Leu-11 and class II histocompatibility antigens. J Exp Med. 1984;159:993–1008. doi: 10.1084/jem.159.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanna J, et al. Novel APC-like properties of human NK cells directly regulate T cell activation. J Clin Invest. 2004;114:1612–1623. doi: 10.1172/JCI22787. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Zingoni A, et al. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173:3716–3724. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 13.Roncarolo MG, et al. Natural killer cell clones can efficiently process and present protein antigens. J Immunol. 1991;147:781–787. [PubMed] [Google Scholar]
- 14.Mach B, Steimle V, Martinez-Soria E, Reith W. Regulation of MHC class II genes: Lessons from a disease. Annu Rev Immunol. 1996;14:301–331. doi: 10.1146/annurev.immunol.14.1.301. [DOI] [PubMed] [Google Scholar]
- 15.Sokol CL, et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7:238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 17.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 18.Rechavi O, Goldstein I, Kloog Y. Intercellular exchange of proteins: The immune cell habit of sharing. FEBS Lett. 2009;583:1792–1799. doi: 10.1016/j.febslet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–632. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 21.Roda-Navarro P, Vales-Gomez M, Chisholm SE, Reyburn HT. Transfer of NKG2D and MICB at the cytotoxic NK cell immune synapse correlates with a reduction in NK cell cytotoxic function. Proc Natl Acad Sci USA. 2006;103:11258–11263. doi: 10.1073/pnas.0600721103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlin LM, Eleme K, McCann FE, Davis DM. Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J Exp Med. 2001;194:1507–1517. doi: 10.1084/jem.194.10.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sjöström A, et al. Acquisition of external major histocompatibility complex class I molecules by natural killer cells expressing inhibitory Ly49 receptors. J Exp Med. 2001;194:1519–1530. doi: 10.1084/jem.194.10.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 25.Homann D, et al. CD40L blockade prevents autoimmune diabetes by induction of bitypic NK/DC regulatory cells. Immunity. 2002;16:403–415. doi: 10.1016/s1074-7613(02)00290-x. [DOI] [PubMed] [Google Scholar]
- 26.Pillarisetty VG, Katz SC, Bleier JI, Shah AB, Dematteo RP. Natural killer dendritic cells have both antigen presenting and lytic function and in response to CpG produce IFN-γ via autocrine IL-12. J Immunol. 2005;174:2612–2618. doi: 10.4049/jimmunol.174.5.2612. [DOI] [PubMed] [Google Scholar]
- 27.Taieb J, et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 28.Chan CW, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 29.Qureshi OS, et al. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosgrove D, et al. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 31.Ogasawara K, et al. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18(1):41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama M, et al. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113:3821–3830. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama M, et al. Paired Ig-like receptors bind to bacteria and shape TLR-mediated cytokine production. J Immunol. 2007;178:4250–4259. doi: 10.4049/jimmunol.178.7.4250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.